Abstract
Background.
Growing evidence suggests that neuroimmune signaling via Toll-like receptors (TLRs) alters brain circuitry related to alcohol use disorders. Both ethanol exposure and the TLR3 agonist, poly(I:C), increase brain TLR3 expression in neurons and glia. Furthermore, previous studies have shown that cortical TLR3 expression is correlated with lifetime ethanol intake in humans.
Methods.
The current experiments investigated the consequences of poly(I:C) treatment on gene expression in two brain regions contributing to alcohol reinforcement, the insular cortex (IC) and nucleus accumbens (Acb) and on operant ethanol self-administration, in Long Evans rats.
Results.
TLR3 activation increased mRNA levels of neuroimmune genes (TLR3, COX2), glutamatergic genes (mGluR2, mGluR3, GLT1), and the trophic factor BDNF in Acb and IC. Furthermore, increases in each of these genes were correlated with increases in TLR3 mRNA, suggesting that TLR3 induction of these genes may impact excitatory transmission in IC and Acb. TLR3 activation also increased ethanol self-administration 18 days post-injection, and enhanced the effects of the mGluR2/3 agonist LY379268 to reduce ethanol self-administration following poly(I:C).
Conclusions.
Together, these findings suggest lasting consequences of TLR3 activation on gene expression including increases in Group II mGluRs in the Acb. Furthermore, we show an important role for TLR3 signaling in ethanol intake, and a functional involvement of Group II mGluRs.
Keywords: neuroimmune, mGluR, TLR3, alcohol, alcohol reinforcement, abstinence
Introduction
There are numerous factors that lead to the development of alcohol use disorders (AUDs) and there is growing evidence that neuroimmune activation may play an important role in all stages of addiction (Coller and Hutchinson 2012; Crews et al. 2011). Neuroimmune signaling includes a large number of secreted proteins and their associated receptors, first discovered as immune signaling molecules that through paracrine and autocrine amplification mediate responses to infections and trauma (Crews et al. 2017a; Warden et al. 2016). Emerging studies in the brain, which is generally sterile, indicate neuroimmune signals contribute to normal brain development and growth, neuroplasticity, neurodegeneration, and addiction (Crews et al. 2017a; Crews and Vetreno 2014; 2016; Crews et al. 2017b). Toll-like receptors (TLRs) are an integral part of this immune signaling and are activated by a variety of innate immune cells in response to pathogens. Animal studies find ethanol exposure increases expression of neuroimmune genes and human studies find expression of cortical TLRs, particularly TLR3 in frontal cortex, are positively correlated with lifetime ethanol drinking (Crews et al. 2013; Crews et al. 2017b). Recent studies have found endogenous agonists activate these receptors in the brain contributing to changes in gene expression, neurocircuitry, and neurodegeneration (Crews and Vetreno 2016; Okun et al. 2011). Interestingly, TLR expression is increased in response to stress, alcohol, and other drugs of abuse in both humans and animals (Crews et al. 2013; Crews and Vetreno 2014; 2016; Frank et al. 2015; Hutchinson et al. 2010; Northcutt et al. 2015). Among TLR receptors TLR3 is somewhat unique in signaling specific kinases, inducing interferons and interferon receptors. In addition, both ethanol and TLR3 agonists increase TLR3 expression in neurons and glia (Lawrimore and Crews, 2017) that could alter brain circuitry.
Moreover, TLR3 activates glial signaling; an effect that is disrupted by naltrexone, a drug used in the treatment of alcohol use disorders (Crews et al. 2013; Qin and Crews 2012). Interestingly, cyclooxygenase 2 (COX2), an enzyme that is rapidly induced in response to immune activation (Appleby et al. 1994) is also increased by TLR3 activation (Lee et al. 2011; Pindado et al. 2007; Symensma et al. 2003) and by both acute and chronic ethanol intake (Knapp and Crews 1999). Further, TLR activation can lead to glutamatergic disruptions such as enhanced NMDA excitability and ultimately excitotoxicity, an effect also observed in ethanol dependence (For review, see (Crews and Vetreno 2014)). Indeed, mice lacking TLRs do not show ethanol-induced changes in cortical glutamate receptor expression (Montesinos et al. 2016). Together, this suggests an important interplay between TLR signaling and glutamatergic function following ethanol exposure. This is especially relevant to the present work given that glutamatergic dysregulation can contribute to escalations in ethanol drinking and is a feature of excessive ethanol intake (Hwa et al. 2017).
To this end, there is interest in understanding the potential contribution of Group II metabotropic glutamate receptors (mGluRs), along with excitatory amino acid transporter 2 (EAAT2) also known as solute carrier family 1 member 2(SLC1A2) and glutamate transporter 1 (GLT1), for their role in AUDs (Joffe et al. 2018). These Gi-coupled receptors are located both pre- (mGluR2 and mGluR3) and post- (mGluR3) synaptically and modulate glutamate release, in part by preventing excitotoxicity (Cartmell and Schoepp 2000; Conn and Pin 1997; Forsythe and Barnes-Davies 1997; Scanziani et al. 1997; Schoepp 2001). Moreover, functional activity of mGluR2/3 receptors in cortical regions has been shown to be altered in ethanol dependence and pharmacological activation of mGluR2/3 can reduce ethanol reinstatement (Kufahl et al. 2011; Sidhpura et al. 2010; Zhao et al. 2006), and restore sensitivity to alcohol following stress exposure (Jaramillo et al. 2015). Further, activation of mGluR2/3 in the nucleus accumbens (Acb) has been shown to decrease ethanol self-administration and ethanol-seeking behaviors (Griffin et al. 2014). Moreover, we have recently shown that the insular cortex (IC) and its projections to nucleus accumbens core (AcbC), which are presumed to be predominantly glutamatergic (Leong et al. 2016; Seif et al. 2013), modulate various aspects of ethanol-related behavior. For example, silencing of IC projections to AcbC decreases self-administration (Jaramillo et al. 2018). In addition, sensitivity to an interoceptive ethanol drug cue is enhanced when IC or IC→AcbC projections are chemogenetically silenced (Jaramillo et al. 2017).
Based on previous work from our lab and others, we hypothesized that TLR3 activation induces glutamatergic adaptations in mGluR2/3 in the Acb and IC that contribute to escalations in ethanol self-administration. To this end, the first goal of the present work was to identify and assess changes in expression of TLRs and cellular markers related to TLR function along with levels of expression of mGluR2/3 in IC and AcbC following treatment with the TLR3 agonist polyinosinic-polycytidylic acid (poly(I:C)). These experiments assessed both immediate changes in expression and persistence of changes 18 days later. Next, we assessed the effects of poly(I:C) on ethanol self-administration and the functional role of mGluR2/3. Together, these experiments lay the groundwork for better understanding how neuroimmune activation, through the actions of TLR3, impacts ethanol self-administration.
Methods
Animals
Adult Male Long-Evans rats (n=85) were used for these experiments. Rats used to assess poly(I:C) time course effects (n=29) were ethanol naïve. Rats used for assessing poly(I:C) effects on ethanol self-administration (n=16) and the role of mGluR2/3 (n=40) had an ethanol self-administration history (>5 months). Rats were single-housed in ventilated cages (Techniplast) with ad-libitum food and water. Rats were maintained in a temperature and humidity controlled colony with a 12hr light/dark cycle (lights on at 07:00). All experiments were conducted during the light cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Comparative Medicine (DCM) at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
Drugs
Ethanol (95% v/v; Pharmco-AAPER, Shelbyville, KY, USA) was diluted in distilled water. Poly(I:C) (GE Healthcare and Life Sciences, Lot#QI2122) was dissolved in sterile PBS which was also used for control injections. Poly(I:C) was injected intraperitoneal (IP) at a volume of 1 ml/kg. The mGluR2/3 agonist LY379268 (Tocris Bioscience) was dissolved in saline which was also used for control injections and was administered IP at a volume of 1 ml/kg.
Apparatus
Self-administration chambers (Med Associates Inc, St. Albans, VT, USA) were individually located within sound attenuating chambers with an exhaust fan to circulate air and mask outside sounds. Chambers were fitted with a retractable lever on the opposite walls (left and right) of the chamber. There was a cue light above each lever and liquid receptacles in the center panels adjacent both levers. Responses on the left (i.e. active) lever resulted in cue light illumination, stimulus tone and delivery of 0.1 ml of solution across 1.66 sec via a syringe pump into the left receptacle once the response requirement was met. Responses on the right (inactive) lever had no programmed consequence. The chambers also had infrared photobeams which divided the floor into four zones to record general locomotor activity throughout each session.
Ethanol self-administration training
Self-administration sessions (30 min) took place 5 days per week (M-F) with the active lever on a fixed ratio 2 (FR2) schedule of reinforcement such that every second response resulted in delivery of ethanol. A sucrose fading procedure was used in which ethanol was gradually added to the 10% (w/v) sucrose solution. The exact order of exposure was as follows: 2% (v/v) ethanol/10% (w/v) sucrose (2E/10S), 5E/10S, 10E/10S, 10E/5S, 15E/5S, 15E/2S, 15E, 15E/2S. Following sucrose fading, sweetened ethanol (15E/2S) was the reinforcer for the remainder of the study. At the end of each session, wells were inspected to ensure that rats had consumed all fluid. A sweetened alcohol reinforcer was used as we find this results in stable alcohol self-administration in these long-term studies (Randall et al., 2015, Randall et al., 2017, Jaramillo et al., 2018).
RNA extraction and reverse transcription polymerase chain reaction (RTPCR)
Rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH: 7.4). IC and Acb tissue was dissected based on the atlas of Paxinos and Watson (1998) and total mRNA extracted from individual samples by homogenization in TRI reagent (Sigma-Aldrich, St. Louis, MO) following the single step method (Chomczynski and Sacchi, 2006). Total mRNA was reverse transcribed as previously described (Vetreno and Crews, 2012; Vetreno et al., 2013). SYBER green PCR Master Mix (Life Technologies, Carlsbad, CA) was used for the RTPCR. The real-time PCR was run with an initial activation for 10 min at 95 °C, followed by 40 cycles of denaturation (95 °C, 15 s), annealing/extension (57–58 °C, 1 min), and finally a melt curve. The primer sequences are presented in Table 1. The threshold cycle (CT) of each target product was determined and the ΔΔCT method was used to calculate the percent change relative to control (CON).
Table 1:
List of primers for RT-PCR.
| Primer | Forward | Reverse |
|---|---|---|
| mGluR2 | 5’-AAA CCC AAC GCT GCT TCC TA-3’ | 5’-CCC TTG CCT CAC CAT TTC CT-3’ |
| mGluR3 | 5’-ATT CTC AGT CCT CTG CAA GC-3’ | 5’-AGA CCC TGT CAC CAA TGC TC-3’ |
| mGluR5 | 5’-CTG GCC TTC GTG CCT ATC TA-3’ | 5’-TTT CCG TTG GAG CTT AGG GTT-3’ |
| TLR3 | 5’-GCA ACA ACA ACA TAG CCA AC-3’ | 5’-CCT TCA GGA AAT TAA CGG GAC-3’ |
| TLR7 | 5’-AGC TCT GTT CTC CTC CA C CA-3’ | 5’-CAT GGG TGT TTG TGC TAT CG-3’ |
| COX2 | 5’-CCG GGT TGC TGG GGG AAG GA-3’ | 5’-CCA CCA GCA GGG CGG GAT ACA G-3’ |
| GLT1 | 5’-GGA CTG GCT GCT GGA TAG AA-3’ | 5’-ATG GTAAGAATG GAT GCA GGG G-3’ |
| BDNF | 5’-ATG ACC ATC CTT TTC CTT ACT ATG GT-3’ | 5’-TCT TCC CCT TTT AAT GGT CAG TGT AC-3’ |
| β-actin | 5’-CTA CAA TGA GCT GCG TGT GGC-3’ | 5’-CAG GTC CAG ACG CAG GAT GGC-3’ |
mGluR2/3 Immunohistochemistry
Rats were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (PBS, pH: 7.4) followed by 4% paraformaldehyde. Brains were collected and stored in 30% sucrose cryprotectant solution until sectioning. Brains were sliced into 40μm coronal sections on a freezing microtome. Free-floating sections were rinsed in 0.1M PBS. Inhibition of endogenous peroxidase was achieved with a single 5 minute wash in 30% H2O2. Sections underwent antigen retrieval in citra buffer for 30 minutes at 70oC and were allowed to cool to room temperature. Sections were incubated in rabbit anti-mGluR2/3 primary antibody (1:1000, Millipore Sigma 06–676) for 48 hours at 4oC. Sections were then incubated in HRP goat anti-rabbit secondary (1:200, Vector Labs) for 1 hour at room temperature. Tissue was treated with Diaminobenzidine (DAB; Polysciences) and then mounted on slides for imaging. Images were acquired utilizing Olympus CX41 light microscope (Olympus America) and analyzed utilizing Image-Pro Premier image analysis soft-ware (Media Cybernetics, MD). Immunoreactivity (IR) data (mGluR2/3-positive pixels/mm2) were acquired from a minimum of three sections/brain region/animal, by an experimenter blind to group assignment, and the data were averaged to obtain a single value per subject. The regions examined were the anterior insular cortex (IC; +2.8 to +1.9 mm) and nucleus accumbens core (AcbC; AP to −2.3 to −1.3). The mGlu2/3 antibody was used to verify mGlu2/3-specific staining in 10 micrograms of brain tissue from the dorsal medial striatum (1.25 micron tissue punch) using western blot. Immunoblots showed labeling at around 97 kDa and 210 kDa (Figure 4C), which correspond to the monomer and dimer of mGluR2/3, respectively (Qian et al. 2018).
Experiments:
Time course study of the effects of Poly(I:C).
Ethanol naïve rats (n=29) were injected with poly(I:C; 0 or 3 mg/kg, IP). To examine the rapid consequences of poly(I:C) treatment, rats (n=10 poly(I:C), n=6 control) were sacrificed 6 hours post-injection. The remaining rats were monitored daily until weights of the poly(I:C) group (n=10) were no longer significantly different from the control group (n=6). As such, rats were sacrificed 18 days post-poly(I:C) injection. Brain tissue was collected for RT-PCR analysis as described above.
Effects of poly(I:C) on ethanol self-administration following a period without self-administration.
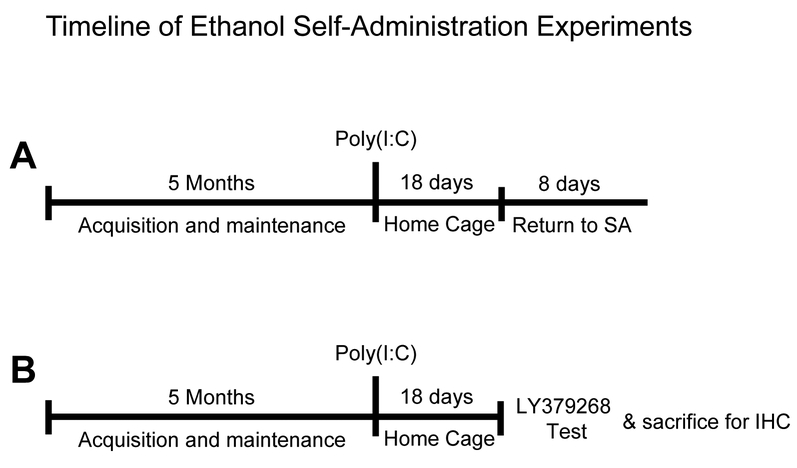
Figure 1A shows a timeline of this experiment. Self-administration trained rats were injected with poly(I:C) (0 or 3 mg/kg, IP; n=8/group) and remained in the home cage for 18 days. During this time rats were weighed daily and self-administration was withheld. Following this period, rats returned to self-administration training for 8 days.
Figure 1.
Timeline of self-administration experiments.
Intersection of mGluR2/3 and the effects of poly(I:C) treatment.
Figure 1B shows a timeline of this experiment. Self-administration trained rats were injected with poly(I:C) (0 or 3 mg/kg, IP, n=19 and 20/group, respectively) and remained in the home cage for 18 days. To test the effect of mGluR2/3 activation, rats were administered the mGluR2/3 agonist LY379268 (0, 3 mg/kg, IP, n = 26 and 13/group, respectively), 20 min prior to their first self-administration session following the18 days in the home cage. To examine the effects of poly(I:C) on expression of mGluR2/3, the following day, 10 rats (n=5 saline/saline) and n=5 (poly(I:C)/saline) were sacrificed for mGluR2/3 immunohistochemistry. Immunohistochemistry analysis was used such that we could examine subregional mGluR2/3 expression (e.g., core vs. shell of the nucleus accumbens; anterior insular cortex).
Statistical Analyses
PCR results were assessed using one-way analysis of variance (ANOVA) with treatment condition as the between groups factor. In addition, Pearsons correlations between gene expression levels for all genes within each brain region (IC or Acb) for each time point (6 hours or 18 days) were also computed. For self-administration experiments, ethanol lever responses, inactive lever responses, estimated ethanol intake (g/kg; estimated from the number of reinforcers received) and locomotor activity were analyzed with two-way repeated measures analysis of variance (RM-ANOVA) with poly(I:C) dose and session as factors. We made the a priori decision to make comparisons between the poly(I:C) group and control for the first two sessions following poly(I:C) treatment. In addition, cumulative ethanol lever responses on the first two days post-poly(I:C) were analyzed with two-way RM-ANOVA with dose and session time as factors. Ethanol intake between doses for the first two sessions post-poly(I:C) was assessed with independent samples t-test. For the LY379268 experiment, t-tests were used to compare mGluR2/3 positive pixels/mm2 between poly(I:C) and control for each brain region. In addition, ethanol lever responses, estimated ethanol intake (g/kg) and locomotor activity for this were assessed using two-way ANOVA with poly(I:C) dose and LY379268 dose as factors. For each experiment, rat weights (grams) were recorded each day and was analyzed with two-way RM-ANOVA with dose and day as factors. In all cases, post-hoc analysis (Tukey) was used to determine specific differences between conditions. For all analyses, significance was set at 0.05.
Results
Poly(I:C) induces time-point dependent changes in expression of several markers.
To determine how TLR3 agonists alter gene expression in rat Acb and IC, two brain regions regulating ethanol self-administration, rats were treated with 3 mg/kg poly(I:C). As expected, an acute “sickness behavior” caused a transient loss of body weight. Mean weight change in the first 24 hours post-injection was +2.0±0.8 (0.6%) and −13±7.2 (−4.0%) grams for control and poly(I:C) groups, respectively. At 18 days post-poly(I:C) injection, weights did not differ between groups [control: 372±18 grams; poly(I:C): 365±17 grams]. We assessed both acute (e.g., 6 hrs) and persistent (e.g., 18 days) mRNA changes including synaptic markers (mGluR, GLT1), as well as neuroimmune (TLR3, TLR7 and COX2) and trophic (BDNF) signals in the Acb and IC using rtPCR. mRNA in the vehicle condition at the two time points did not differ, and were pooled.
IC mRNA Analyses
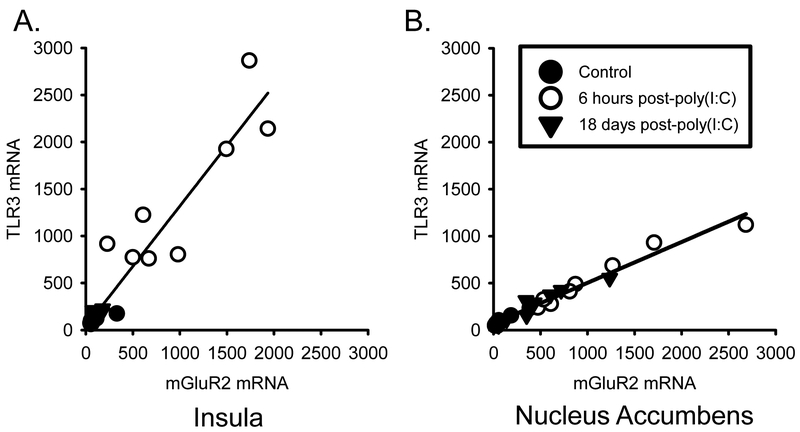
As shown in Table 2, there were significant changes in gene expression of mGluR2, mGluR3, TLR3, COX2, GLT-1 and BDNF in the IC following poly(I:C) administration. Post-hoc analyses showed that at 6 hours post-injection with poly(I:C), there was a 10-fold increase in mGluR2, a 1.5-fold decrease in mGluR3, a 14-fold increase in TLR3, a 2-fold increase in COX2, a 2-fold increase in GLT1, and an 5.5-fold decrease in BDNF mRNA expression (p<0.05). 18 days post-poly(I:C) there was a 1.3-fold increase in GLT1. At the 6 h time point, TLR3 mRNA levels were positively correlated with increases in mGluR2 (r=0.94, p < 0.001; Figure 2A), TLR7 (r=0.57, p < 0.01), COX2 (r=0.88, p < 0.001) and GLT1 (r=0.63, p < 0.01), and TLR3 mRNA levels were correlated with decreases in levels of mGluR3 (r = −0.6, p < 0.05) and BDNF (r= −0.76, p < 0.05, Table 3). 18 days post-injection, TLR3 mRNA levels were positively correlated with mGluR2 (r=0.61, p < 0.01; Table 4/Figure 1A) and GLT1 (r=0.68, p < 0.01; Table 6).
Table 2:
Gene expression in the insula 6 hours and 18 days following administration of Poly(I:C).
| Gene | CON | 6 hour | 18 day | |
|---|---|---|---|---|
| mGluR2 | 100 ± 34 | 1019 ± 222** | 112 ± 14 | F[2,21] = 16.432, p < 0.001 |
| mGluR3 | 100 ± 7 | 63 ± 5** | 103 ± 6 | F[2,21] = 13.321, p < 0.001 |
| mGluR5 | 100 ± 2 | 101 ± 9 | 104 ± 3 | F[2,21] =0.116, n.s. |
| TLR3 | 100 ± 13 | 1428 ± 280** | 159 ± 15 | F[2,21] = 21.426, p < 0.001 |
| TLR7 | 100 ± 4 | 161 ± 34 | 121 ± 6 | F[2,21] = 2.474, n.s. |
| COX2 | 100 ± 10 | 204 ± 32** | 137 ± 12 | F[2,21] = 6.624, p < 0.01 |
| GLT-1 | 100 ± 5 | 220 ± 11** | 133 ± 10* | F[2,21] = 50.802, p < 0.001 |
| BDNF | 100 ± 8 | 18 ± 2** | 107 ± 10 | F[2,21] = 52.270, p < 0.001 |
Adult male Long-Evans rats were treated with 3.0 mg/kg (i.p.) Poly(I:C) or naïve controls (CON) and then sacrificed either 6 hours or 18 days later. Dunnett Posthoc T-test:
p < 0.05,
p < 0.01, relative to CON. Data are presented as mean % CON mRNA expression ± SEM.
Figure 2.
(A) Correlation plot of insular cortex TLR3 mRNA expression and mGluR2 mRNA expression. (B) Correlation plot of nucleus accumbens TLR3 mRNA expression and mGluR2 mRNA expression. Controls (filled circles), 6 hours post-poly(I:C) (open circles) and 18 days post-poly(I:C) (triangles). TLR3 was positively correlated with mGluR2 in nucleus accumbens: r = 0.98, p < 0.01.
Table 3:
Correlations of gene expression in the insular cortex 6 hr following poly(I:C) treatment.
| mGluR3 | mGluR5 | TLR3 | TLR7 | COX2 | GLT1 | BDNF | |
|---|---|---|---|---|---|---|---|
| mGluR2 | −0.43 | −0.07 | 0.94** | 0.28 | 0.73** | 0.64** | −0.70** |
| mGluR3 | 0.07 | −0.60* | −0.56* | −0.50 | −0.64** | 0.85** | |
| mGluR5 | −0.02 | 0.29 | 0.27 | 0.11 | −0.05 | ||
| TLR3 | 0.57* | 0.88** | 0.63** | −0.76** | |||
| TLR7 | 0.79** | 0.21 | −0.43 | ||||
| COX2 | 0.44 | −0.57* | |||||
| GLT1 | −0.89** |
Table 4:
Correlations of gene expression in the insular cortex 18 days following poly(I:C) treatment.
| mGluR3 | mGluR5 | TLR3 | TLR7 | COX2 | GLT1 | BDNF | |
|---|---|---|---|---|---|---|---|
| mGluR2 | 0.46 | 0.17 | 0.61* | −0.04 | −0.07 | −0.40 | 0.01 |
| mGluR3 | 0.05 | 0.02 | 0.14 | −0.08 | 0.21 | 0.62* | |
| mGluR5 | 0.32 | 0.65** | 0.12 | 0.70** | −0.05 | ||
| TLR3 | 0.39 | 0.42 | 0.68** | −0.27 | |||
| TLR7 | 0.29 | 0.70** | −0.05 | ||||
| COX2 | 0.44 | 0.34 | |||||
| GLT1 | 0.11 |
R values of correlations in gene expression in insular cortex in rats that were treated with 3mg/kg poly(I:C) and sacrificed 18 days later.
p < 0.01 or
p < 0.001, significantly correlated.
Table 6:
Correlations of gene expression in the nucleus accumbens 6 hr following poly(I:C) treatment.
| mGluR3 | mGluR5 | TLR3 | TLR7 | COX2 | GLT1 | BDNF | |
|---|---|---|---|---|---|---|---|
| mGluR2 | −0.40 | −0.57* | 0.99** | −0.10 | 0.82** | 0.36 | 0.44 |
| mGluR3 | 0.42 | −0.41 | −0.05 | −0.39 | −0.24 | −0.06 | |
| mGluR5 | −0.60* | 0.18 | −0.60* | −0.46 | −0.47 | ||
| TLR3 | −0.07 | 0.83** | 0.4 | 0.39 | |||
| TLR7 | 0.34 | −0.01 | 0.30 | ||||
| COX2 | 0.53* | 0.63** | |||||
| GLT1 | 0.09 |
R values of correlations in gene expression in nucleus accumbens in rats that were treated with 3mg/kg poly(I:C) and sacrificed 6 hours later.
p < 0.01 or
p < 0.001, significantly correlated.
Acb mRNA Analyses
In the Acb, there were also significant poly(I:C)-induced changes in gene expression of mGluR2, mGluR3, mGluR5, TLR3, COX2, GLT1 and BDNF (See Table 5). Post hoc analyses showed, that 6 hours post-injection of poly(I:C), there was an 11-fold increase in mGluR2 and an almost 1 fold decrease in mGluR5, a 5.5-fold increase in TLR3, a 2.5-fold increase in COX2, and a 1.5-fold increase in GLT1 mRNA expression (p<0.05). 18 days post-injection, there was a 1.7-fold increase in mGluR3, and a 2.4-fold increase in BDNF. At the 6 hour time point, TLR3 mRNA levels were positively correlated with increases in levels of mGluR2 (r=0.99, p < 0.001; Figure 2B) and COX2 (r=0.83, p < 0.001), and TLR3 levels were negatively correlated levels of mGluR5 (r= −0.60, p < 0.01; Table 6). At the 18-day time point, TLR3 mRNA levels were positively correlated with mGluR2 (r=0.96, p < 0.001; Figure 1B), mGluR3 (r=0.56, p < 0.01), TLR7 (r=0.72, p < 0.001), COX2 (r=0.85, p < 0.001), GLT1 (r=0.75, p < 0.001) and BDNF (r=0.55, p < 0.01).
Table 5:
Gene expression in the nucleus accumbens 6 hours and 18 days following administration of Poly(I:C).
| Gene | CON | 6 hour | 18 day | |
|---|---|---|---|---|
| mGluR2 | 100 ± 45 | 1119 ± 267** | 486 ± 132 | F[2,21] = 8.745, p < 0.01 |
| mGluR3 | 100 ± 14 | 70 ± 7 | 177 ± 31* | F[2,21] = 7.634, p < 0.01 |
| mGIuR5 | 100 ± 9 | 76 ± 8* | 99 ± 4 | F[2,21] = 3.819, p < 0.05 |
| TLR3 | 100 ± 23 | 561 ± 114** | 285 ± 60 | F[2,21] = 9.392, p < 0.01 |
| TLR7 | 100 ± 7 | 98 ± 22 | 166 ± 53 | F[2,21] = 1.376, n.s. |
| COX2 | 100 ± 16 | 250 ± 29** | 170 ± 26 | F[2,21] = 9.718, p < 0.01 |
| GLT-1 | 100 ± 4 | 163 ± 12** | 123 ± 7 | F[2,21] = 13.404, p < 0.001 |
| BDNF | 100 ± 33 | 142 ± 30 | 246 ± 54* | F[2,21] =3.472, p = 0.05 |
Adult male Long-Evans rats were treated with 3.0 mg/kg (i.p.) Poly(I:C) or naïve controls (CON) and then sacrificed either 6 hours or 18 days later. Dunnett Posthoc T-test:
p < 0.05,
p < 0.01, relative to CON. Data are presented as mean % CON mRNA expression ± SEM.
Thus, the TLR3 agonist poly(I:C) rapidly increases mRNA expression of glutamatergic (mGluR2, GLT1) and neuroimmune (TLR3, COX2) targets in both the IC and Acb while decreasing trophic (BDNF) targets and mGluR5 only in the IC. Interestingly, the increased expression of glutamatergic (mGluR3, GLT1) targets persists for at least 18 days in the IC or Acb while the trophic target (BDNF) recovers in IC, but is increased in Acb 18 days after poly(I:C).
TLR3 agonist poly(I:C) increases ethanol self-administration
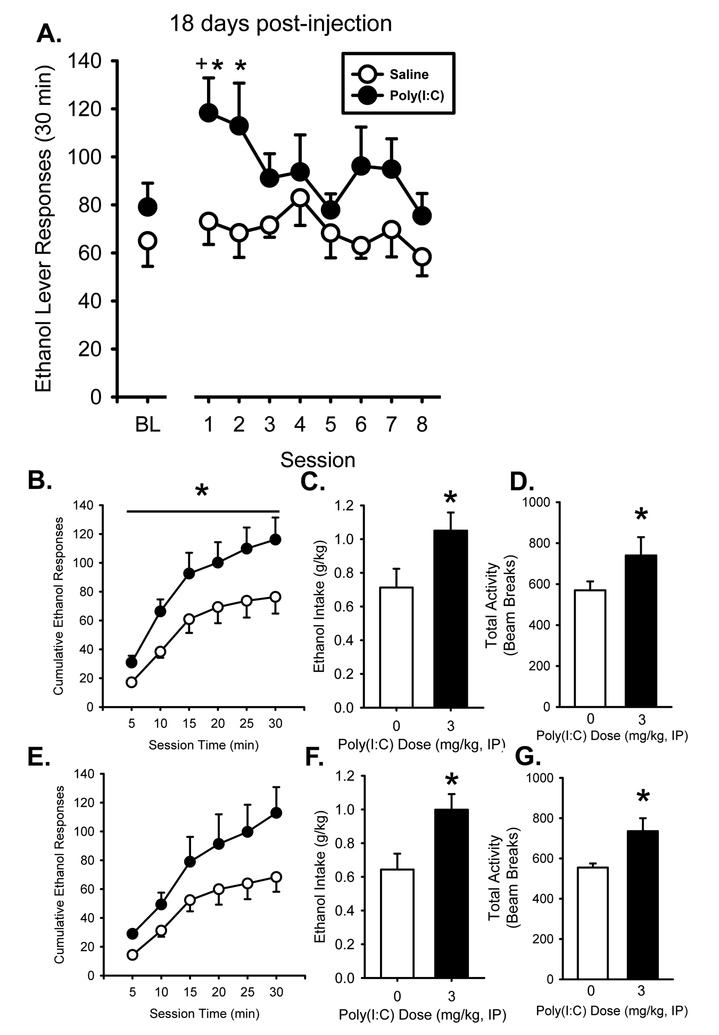
To determine if the persistent effects of poly(I:C) in IC and Acb were associated with changes in ethanol drinking behavior, rats with a history of ethanol self-administration were studied. Baseline ethanol self-administration prior to poly(I:C) injection is shown to the left of the axis break in Figure 3A. There was no difference in self-administration at the baseline time point between either vehicle or poly(I:C) treated rats (t[10] = 0.075, n.s.). The poly(I:C) group showed significant weight loss compared to control, confirming our findings from the time course experiment. Mean weight change in the first 24 hours post-injection was +2.0±1.2 (+0.5%) and −20±5.1 (−4.2%) grams for control and poly(I:C) groups, respectively. By 18 days post-poly(I:C), weights between poly(I:C) and saline treated groups did not differ (t[10] = 2.00, n.s.). 18 days post-injection, weights were 372±18 and 365±17 grams for the control and poly(I:C) group, respectively.
Figure 3.
Poly(I:C) increases alcohol self-administration following an 18 day period of abstinence. Following poly(I:C) treatment (3 mg/kg), alcohol lever responses (mean±SEM) were significantly higher than control and baseline on sessions 1 and 2 (A). Rats treated with poly(I:C) responded more throughout the first session following abstinence compared to controls (B). Alcohol intake (g/kg) was significantly higher on the first two sessions following 18 days in the home cage in the poly(I:C) treated group (C/F). Total activity (beam breaks) was also significantly higher on the first two sessions in the poly(I:C) group (D/G). * - p < 0.05, significantly different from control. + - p < 0.05, significantly different from baseline.
There was a significant increase in ethanol self-administration in the poly(I:C) group once rats were returned to ethanol self-administration (Figure 3), whereas controls returned to previous baseline ethanol self-administration levels. There was a main effect of dose (F[1,14] = 5.81, p < 0.05), and session (F[7,98] = 2.90, p < 0.01) on TLR3 agonist induced increases in ethanol lever responses. Ethanol lever responses on the first and second sessions were significantly greater in the poly(I:C) treated group compared to control (t[14] = 2.94, p < 0.05 and t[14] = 2.65, p < 0.05, respectively). Furthermore, ethanol lever responding on the first session was significantly greater in the poly(I:C) treated rats compared to pre-poly(I:C) baseline (t[7] = 3.63, p < 0.05). Inactive lever responses were not affected by poly(I:C) treatment (Table 8). Thus, in rats with a history of ethanol self-administration that were abstinent through the initial TLR3 agonist induced “sickness-like behavior” increased ethanol self-administration upon the return to self-administration, whereas controls return to previous baseline response levels.
Table 8:
Inactive Lever Respouses – Effects of poly(I:C) on ethanol self-adinmistration
| Session post-poly(I:C) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| saline | 1.33 ± 0.40 | 1.33 ± 0.72 | 0.44 ± 0.33 | 0.44 ± 0.77 | 0.77 ± 0.32 | 0.88 ± 0.65 | 0.44 ±0.24 | 0.33 ± 0.23 |
| poly(I:C) | 3.71 ± 0.91 | 2.71 ± 1.53 | 1.71 ± 1.08 | 1.42 ± 0.71 | 2.71 ± 1.53 | 1.42 ±0.97 | 1.28 ±0.68 | 1.28 ±0.71 |
Mean ± SEM responses on the inactive lever for the eight sessions post-poly(I:C). Inactive lever responses did not differ between the saline and poly(I:C) treated rats.
Analysis of the pattern of ethanol lever responding in the first two sessions showed that on the first session post-poly(I:C), there was a main effect of poly(I:C) treatment (F[1,40] = 6.47, p < 0.05) with poly(I:C) treated rats responding on the ethanol lever more than controls throughout the session (p < 0.05; Figure 3B) but not on the second session (Figure 3E). Furthermore, the poly(I:C) group showed significantly higher ethanol intake (g/kg) on both the first and second sessions (Figure 3C/F) following 18 days in the home cage compared to controls (t(14) = 2.20, p = 0.05 and t(14) = 2.63, p < 0.05, respectively). In addition, locomotor activity was significantly higher in the poly(I:C) treated rats on days one and two compared to saline (p < 0.05, Figure 3D/G) suggesting that poly(I:C) treated rats maintained a heightened level of activity across the first two sessions. It is important to note though that this heightened level of activity did not increase non-specific responding as inactive lever presses were not affected (Table 8). These findings suggest that TLR3 agonist treatment induces long lasting changes that enhance ethanol self-administration.
TLR3 agonist induction of mGluR2/3 expression and enhanced mGluR2/3 agonist inhibition of self-administration
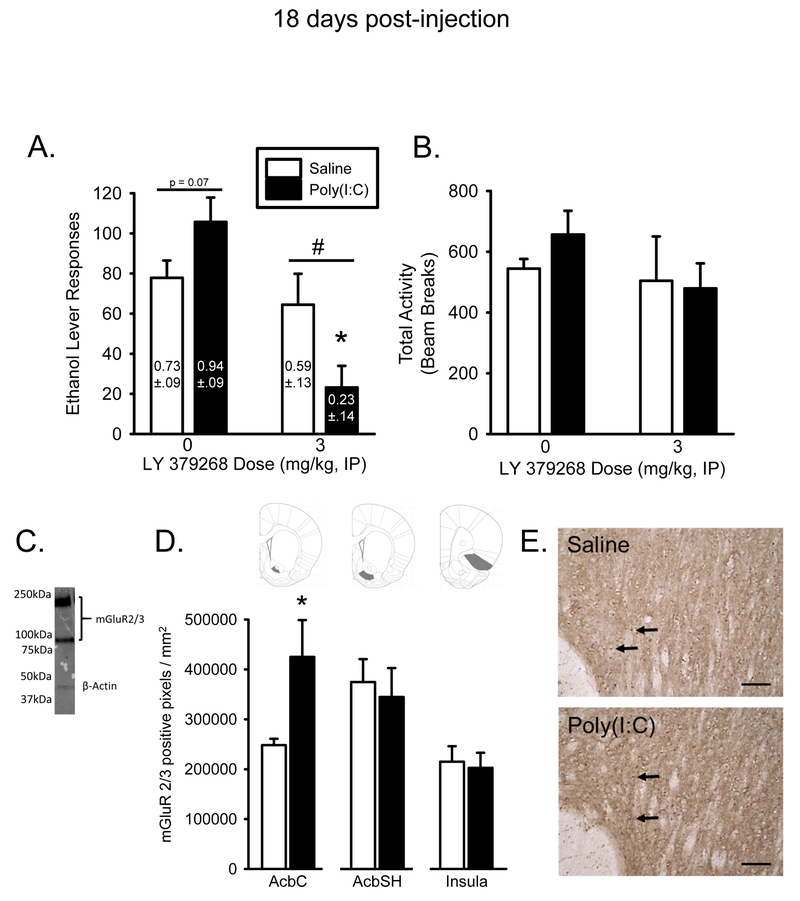
To determine whether the TLR3 agonist-induced changes in glutamatergic markers are related to ethanol self-administration, mGluR2/3-IHC and the impact of the mGluR2/3 agonist LY379268 (3 mg/kg) post-poly(I:C) were assessed. As shown in Figure 4A, the saline controls were not affected by mGluR2/3 activation. In contrast, in the poly(I:C) group, LY379268 treatment significantly reduced lever responses compared to both controls and poly(I:C) alone. For ethanol lever responses, there was a main effect of LY379268 dose (F[1,35] = 14.424, p < 0.001) and a poly(I:C) dose by LY379268 dose interaction (F[1,35] = 7.484, p < 0.05). Post hoc analysis showed that LY379268 significantly decreased ethanol responses in the poly(I:C) treated rats, (p < 0.001). Similarly, in assessing ethanol intake (g/kg), there was a main effect of LY379268 dose (F[1,35] = 13.287, p < 0.001) and a poly(I:C) dose by LY379268 dose interaction (F[1,35] = 5.948, p < 0.05). Post hoc analysis showed that ethanol intake in the poly(I:C) treated rats was significantly decreased by LY379268 (p < 0.001). There were no significant effects of poly(I:C) or LY379268 on inactive lever responses or total activity (beam breaks) (Figure 4B/Table 9). Figure 4C shows immunoblot labeling of the mGluR2/3 antibody that was used for immunohistochemistry. As shown in Figure 4D and 4E, mGluR2/3 protein expression as measured by immunohistochemistry was nearly doubled in AcbC compared to control (t(8) = 2.36, p < 0.05). Expression in nucleus accumbens shell (AcbSH) and IC did not differ between poly(I:C) and control. Together, these findings suggest that the persistent long lasting induction of mGluR2/3 protein in the Acb following TLR3 activation, is related to the increase in ethanol self-administration and in turn, sensitizes response to the mGluR2/3 agonist (i.e., decrease in self-administration only in the poly(I:C) treated group).
Figure 4.
Poly(I:C) alters mGluR2/3 expression and it’s behavioral effects are mitigated by mGluR2/3 activation. LY379268 (3 mg/kg) significantly decreased alcohol lever responses in poly(I:C) treated rats suggesting that mGluR2/3 activation counteracts the effects of poly(I:C). Alcohol intake (g/kg) is denoted on each bar (A). Total activity (beam breaks) was not affected by poly(I:C) or LY379268 administration (B). mGluR2/3-specific staining was verified with western blots, the mGluR2/3 antibody was shown to label around 97kDa and 210kDa bands in striatal tissue, corresponding to the monomer and dimer of mGluR2/3, respectively (C). 18 days post- poly(I:C) treatment, expression of mGluR2/3 is increased in nucleus accumbens core (AcbC), but not the shell (AcbSH) or insular cortex (D). Atlas images show where images for quantification were taken (D). Representative photomicrographs of AcbC following saline or poly(I:C) at 20X magnification. (E). Arrows indicate example mGluR2/3 staining. Scale bars are 1000 μm. * - p < 0.05, significantly different from control, # - p < 0.05, poly(I:C) treated rats significantly different from saline.
Table 9:
Inactive Lever Responses -Effects of mGluR2/3 activation on ethanol self-administration following poly(I:C)
| LY379268 Dose (mg/kg IP) | ||
|---|---|---|
| 0 | 3 | |
| saline | 1.15 ± 0.33 | 0.28 ± 0.12 |
| poly(I:C) | 2.30 ± 0.69 | 0 ± 0 |
Mean ± SEM responses on the inactive lever. Inactive lever responses did not differ between the saline and poly(I:C) treated rats
Discussion
In this study, we evaluated the effects of the TLR3 agonist poly(I:C) on mRNA levels of neuroimmune genes (TLR3, TLR7, COX2), glutamatergic genes (mGluR2, mGluR3, mGluR5, GLT1) along with the trophic factor BDNF, as well as the effects of TLR3 activation on operant ethanol self-administration. Our results indicate that acute activation of TLR3 via poly(I:C) induces rapid (6 hours post-injection) and long-term (18 days post-injection) changes in gene expression. In both IC and Acb, TLR3 agonist treatment rapidly (6 hrs) increased mGluR2, TLR3, COX2 and GLT1, while decreasing BDNF mRNA in the IC and decreasing the predominantly postsynaptic-expressing mGluR3 and mGluR5 in the IC and Acb, respectively. BDNF, TLR3 and COX2 are expressed in neurons and glia (Bsibsi et al. 2002; Huang et al. 1987; Luo et al. 1998), and GLT1 in astrocytes (Rothstein et al. 1994). mGluR2/3 is expressed in neurons (Jin et al. 2017; Luo et al. 1998; Ohishi et al. 1993; Tanabe et al. 1992) and typically provides negative feedback on glutamate synapses presynaptically, although mGlu3 has been found on glia as well (Aronica et al. 2003). Additionally, in our previous studies we found ethanol induced TLR3 expression in both neurons and microglia (Qin and Crews 2012). Together, the present findings suggest TLR3 agonist-induced alterations in mRNA and signaling across these brain regions, and likely across multiple cell types. Indeed, correlations between increased expression of TLR3 mRNA and mGluR2 mRNA among other genes are consistent with altered synaptic signaling. Additionally, treatment with the TLR3 agonist resulted in an increase in ethanol self-administration 18 days after injection, and increased sensitivity to mGluR2/3 activation-induced inhibition of drinking. Together these findings suggest that TLR3 activation induces long lasting changes in IC and Acb gene and protein expression that may be associated with potentiated alcohol self-administration.
The mechanisms of TLR3 induced increases in gene expression and ethanol drinking are complex. Figure 5 depicts a possible mechanism for these TLR3-ethanol effects based on previous studies and our current findings. Moreover, emerging studies suggest endogenous TLR agonists in brain contribute to glial-neuronal plasticity (Crews et al. 2017b). Previous studies have found poly(I:C) treatment in mice increases TNFα in serum and brain, with the brain response persisting long after the serum response declines. Moreover, IL6 and MCP1 levels were also increased in blood and brain by poly(I:C) (Qin and Crews 2012). In addition to TLR activation, ethanol exposure also increases TLR expression. Ethanol pretreatment induces TLR3 and potentiates poly(I:C) response, consistent with ethanol increasing TLR3 signaling in the brain and periphery (Qin and Crews 2012). Furthermore, in vitro studies find both ethanol and poly(I:C) increase TLR3 mRNA in neuron-like cells (Lawrimore and Crews 2017) while emerging studies suggest endogenous TLR agonists in brain may contribute to ethanol induction of TLR receptors (Coleman and Crews 2018; Coleman et al. 2017). Importantly, poly(I:C) induction of brain TLR3 and other genes needs additional mechanistic studies to determine if systemic responses have direct effects on brain (i.e., peripheral nerve activation of brain), as one would not expect large amounts of poly(I:C) to cross the blood brain barrier (Table 7).
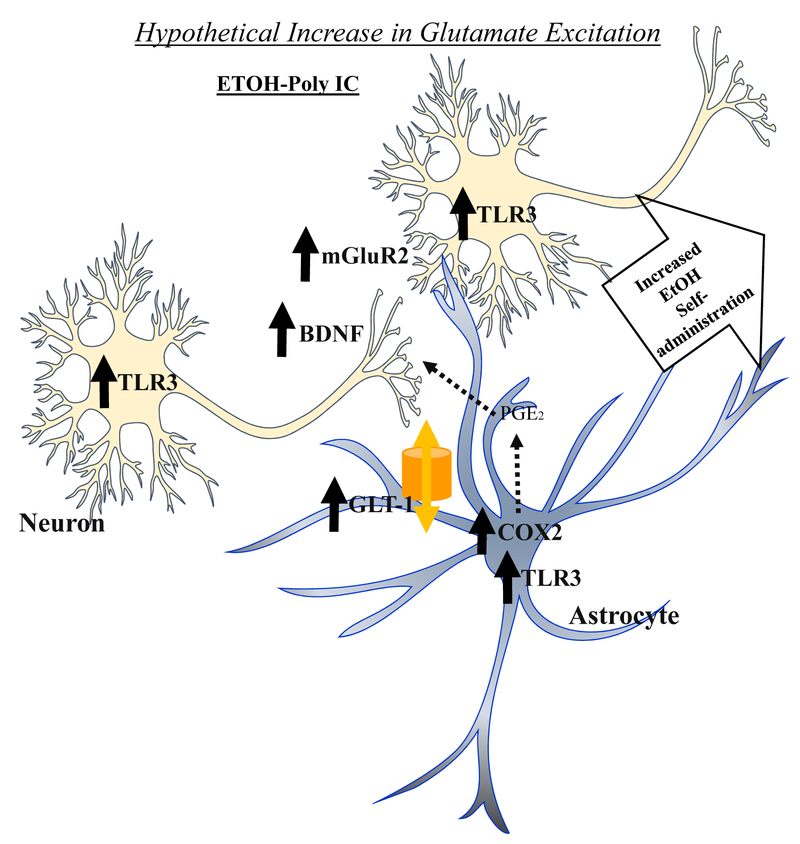
Figure 5.
Hypothetical mechanism of poly(I:C)-TLR3 and ethanol exposure induced increases in nucleus accumbens excitation and responding for ethanol. TLR3 is expressed in both neurons and glia, and immune signaling is linked to astrocyte-neuronal increases in synaptic glutamate (Haroon, et.al. 2017). Poly(I:C)-TLR3 activation can prime calcium signaling and release mechanisms (Park et al. 2016). TLR3 activation in astrocytes increases intracellular calcium stimulating astrocyte exocytosis (Beckel et al. 2018). Multiple studies have found Acb excitation is linked to increased operant responding for ethanol. We find poly(I:C) increases responding for ethanol and induces TLR3 mRNA as well as several proteins known to modulate glutamate synapses. Presynaptic mGLuR2 receptors regulate presynaptic glutamate release and increased mGLuR2 mRNA is consistent with larger or more excitatory synapses. COX2 is expressed in astrocytes and increases PGE2 that stimulates astrocyte glutamate release and reduces uptake by transporters increasing excitation (Sang et al. 2005). The trophic factor BDNF is increased consistent with it promoting new excitatory synapse formation (Parkhurst et al. 2013). Together these changes are consistent with poly(I:C)-TLR3 signaling increasing ethanol responding through increases in glutamate synapse excitation. However, additional studies are needed to test these hypotheses.
Table 7:
Correlations of gene expression in the nucleus accumbens 18 days following poly(I:C) treatment.
| mGluR3 | mGluR5 | TLR3 | TLR7 | COX2 | GLT1 | BDNF | |
|---|---|---|---|---|---|---|---|
| mGluR2 | 0.54* | −0.03 | 0.96** | 0.84** | 0.90** | 0.66** | 0.60* |
| mGluR3 | −0.05 | 0.56* | 0.24 | 0.52* | 0.25 | 0.39 | |
| mGluR5 | 0.03 | −0.12 | −0.27 | −0.09 | −0.33 | ||
| TLR3 | 0.72** | 0.85** | 0.75** | 0.55** | |||
| TLR7 | 0.82** | 0.55* | 0.45 | ||||
| COX2 | 0.69** | 0.76** | |||||
| GLT1 | 0.41 |
R values of correlations in gene expression in nucleus accumbens in rats that were treated with 3mg/kg poly(I:C) and sacrificed 18 days later.
p < 0.01 or
p < 0.001, significantly correlated.
Glial-neuronal signaling regulates synapses through multiple mechanisms including synapse elimination and formation, changes in glial glutamate transporters and gliotransmitter release processes. Poly(I:C) has been shown to increase hippocampal extracellular glutamate sensitizing mice to seizures (Hunsberger et.al., 2017). Here, we found increased ethanol self-administration weeks after TLR3 agonist treatment on the same time scale with increased mRNA expression of mGluR3, GLT-1 and a 4-fold increase in mGluR2 (albeit a trend), and a significant increase in mGluR2/3 protein (quantified via immunohistochemistry) in AcbC. For the immunohistochemistry analysis an antibody that labels both mGluR2 and 3 was used, and the observed poly(I:C)-induced increase in mGluR2/3 immunoreactivity in the AcbC was in animals with a history of ethanol self-administration, which provides evidence of a glutamatergic adaptation within the context of ethanol self-administration. Furthermore, expression of GLT1 was increased in both Acb and IC at 6 hours and 18 days later in the IC following poly(I:C). As both mGluR2 and GLT1 act to decrease glutamatergic transmission, this upregulation may suggest the emergence of increased glutamatergic tone in the IC and Acb. Furthermore, as gene expression changes were the primary variable for these studies, it will be interesting for follow-up studies to measure protein expression for the targets. Together, these changes are relevant to ethanol self-administration as IC to AcbC projections have been shown to directly modulate ethanol drinking (Jaramillo et al. 2018; Seif et al. 2013). Consistent with Acb TLR3 contributing to drug reinforcement are studies finding transgenic mice lacking TLR3 or TLR3 inhibitors injected directly into Acb reduce cocaine conditioned place preference that is restored by selective viral expression of Acb TLR3 (Zhu et al. 2018). In sum, these changes could represent increased glutamatergic signaling due to TLR3 induced plasticity. As such, the poly(I:C)-induced neuroimmune response through TLR3 promotes persistent changes in brain circuitry that are consistent with neuronal-glial signaling altering glutamatergic excitation through one or more of these mechanisms (Crews et al. 2017a; Haroon et al. 2017), which in turn may contribute to the escalations in ethanol self-administration.
Activation of TLRs has been shown to produce lasting increases in ethanol intake (Blednov et al. 2011). In the current experiments, TLR3 activation by poly(I:C) significantly increased ethanol self-administration 18 days post-injection. This increase was the most dramatic on the first two sessions and was accompanied by a general increase in locomotor activity that was elevated in the poly(I:C) treated rats through the second session. Following the second session, self-administration in the poly(I:C) treated rats gradually returned to control levels. Interestingly, although TLR3 mRNA was elevated at 6 hours following poly(I:C) exposure, but not elevated at 18 days this pattern is consistent with a time related loss of TLR3 sensitization that might be related to the decline in self-administration. It will be important for future studies to assess whether repeated administration of poly(I:C) would further potentiate these effects. In addition, studies using the bacterial endotoxin lipopolysaccharide (LPS), a potent agonist for TLR4 (Kawai and Akira 2007), have shown increases in home cage ethanol intake more than a month after treatment (Blednov et al. 2011) so it will be interesting for future studies to assess later time points to determine if poly(I:C) is capable producing such lasting changes. In addition, Blednov et al. (2011) showed that mice lacking a key component of TLR4, rendering it non-functional, did not show an increase in ethanol intake following LPS treatment.
An important consideration of the current experiments is time-course. TLR3 systemic responses decline within 24 hours whereas brain responses can persist for longer periods (Qin and Crews 2012).There is evidence for changes in sensitization of neuroimmune signals over time that mature. For example, negative affect used to model depression has been shown to emerge at later time points (see (Remus and Dantzer 2016) for review). Our protocol used an extended period of abstinence, e.g. 18 days, to allow initial sickness-like responses to subside and body weight to return to control levels. It is possible this home-cage abstinence acts as an “incubation period”, contributing to increased self-administration.
An important consideration for the current experiments is that these rats were all males. There is evidence that both acute and chronic ethanol intake induces greater TLR expression and signaling activity in TLR-related pathways in female mice compared to males (Fulham and Mandrekar 2016; Wagnerberger et al. 2013). Additionally, females (human and rodents) tend to show increased activation of pro-inflammatory response following TLR activation (see (Klein and Flanagan 2016) for review). Therefore, it will be interesting to determine whether there are sex differences in response to the poly(I:C)-induced changes in gene expression and self-administration. Another consideration is that a sweetened ethanol reinforcer was used in the self-administration studies. LPS has been shown to decrease taste response to sucrose, an effect absent in TLR4-KO mice (Zhu et al. 2014). However, in the current experiments, poly(I:C) increased intake of the sweetened ethanol solution. Regardless, it will be important for future studies to assess whether these changes in self-administration following poly(I:C) are specific to ethanol or generalize to other drugs of abuse or non-drug reinforcers such as sucrose.
The current experiments show that TLR3 activation induces lasting changes at glutamatergic targets. As such, the final set of experiments sought to assess the impact of mGluR2/3 activation on ethanol self-administration following poly(I:C) treatment. Here we show that the mGluR2/3 agonist LY379268 blocked the effects of TLR3 activation on ethanol self-administration, but did not affect self-administration on its own (Figure 4A). That is, following mGluR2/3 activation (poly(I:C)/LY379268 treated), self-administration was significantly decreased compared to poly(I:C)/saline or saline/LY379268 treated rats. Similar patterns of decreases in ethanol intake have been observed following mGluR2/3 activation in studies assessing ethanol dependence (Griffin et al. 2014; Windisch and Czachowski 2018). Moreover, saline treated rats in the current experiments were not affected by treatment with LY379268, suggesting that it does not affect self-administration in control rats (non-poly(I:C)). One limitation of this finding was that poly(I:C) did not significantly increase self-administration compared to saline in this experiment (trend for an increase p = 0.07; Figure 4A). Even with this consideration, the finding that LY379268 significantly decreased self-administration in the poly(I:C) group, but not the saline treated group, suggests differential sensitivity to the agonist. This finding further supports that neuroimmune activation leads to increases in glutamatergic function that may affect sensitivity to ethanol, an effect that is ameliorated by mGluR2/3 activation.
Taken together, these findings demonstrate the functional impact of TLR3 neuroimmune activation on ethanol self-administration and the interaction with mGluR2/3. Numerous studies have suggested that neuroimmune activation may play an important role in the development of addiction (for review, see (Coleman and Crews 2018)). Further, the identified interaction with mGlu2/3 is significant given the growing interest in mGluR2/3 as a therapeutic target of AUDs (Joffe et al. 2018). Furthermore, ethanol induces many of the same effects in the brain that a viral pathogen would (i.e., increases in TLRs and other markers of inflammation; (Crews et al. 2013; Crews and Vetreno 2014; 2016; Frank et al. 2015; Hutchinson et al. 2010; Knapp and Crews 1999; McCarthy et al. 2017; Northcutt et al. 2015)). Based on these findings, future experiments should assess the effects of chronic TLR3 activation and the effects of activating other TLRs and potential interaction with mGluRs on ethanol self-administration, as gaining a better understanding of the underlying neurobiology of neuroimmune activation may ultimately lead to the development of more effective treatments for AUDs.
Acknowledgements:
This work was funded in part by NIH grants AA011605, AA026537, and by the Bowles Center for Alcohol Studies. VHM was supported by NS007431–18.
References
- Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T (1994) Structure of the human cyclo-oxygenase-2 gene. Biochem J 302(Pt 3): 723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D (2003) Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci 17: 2106–18. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Gomez NM, Lu W, Campagno KE, Nabet B, Albalawi F, Lim JC, Boesze-Battaglia K, Mitchell CH (2018) Stimulation of TLR3 triggers release of lysosomal ATP in astrocytes and epithelial cells that requires TRPML1 channels. Sci Rep 8: 5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25 Suppl 1: S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61: 1013–21. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889–907. [DOI] [PubMed] [Google Scholar]
- Coleman LG Jr., Crews FT (2018) Innate Immune Signaling and Alcohol Use Disorders. Handb Exp Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Zou J, Crews FT (2017) Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134: 219–45. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–37. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr. (2017a) The role of neuroimmune signaling in alcoholism. Neuropharmacology 122: 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73: 602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2014) Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol 118: 315–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2016) Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 233: 1543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Walter TJ, Coleman LG Jr. Vetreno RP (2017b) Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 234: 1483–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25 Suppl 1: S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M (1997) Synaptic transmission: well-placed modulators. Curr Biol 7: R362–5. [DOI] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF (2015) Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun 48: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulham MA, Mandrekar P (2016) Sexual Dimorphism in Alcohol Induced Adipose Inflammation Relates to Liver Injury. PLoS One 11: e0164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC (2014) Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 39: 707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Tsai CC, Adams SP, Huang JS (1987) Neuron localization and neuroblastoma cell expression of brain-derived growth factor. Biochem Biophys Res Commun 144: 81–7. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa L, Besheer J, Kash T (2017) Glutamate plasticity woven through the progression to alcohol use disorder: a multi-circuit perspective. F1000Res 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J (2017) Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Frisbee S, Fisher KR, Besheer J (2015) Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol 49: 525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, Besheer J (2018) Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology 130: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LE, Wang M, Yang ST, Yang Y, Galvin VC, Lightbourne TC, Ottenheimer D, Zhong Q, Stein J, Raja A, Paspalas CD, Arnsten AFT (2017) mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions. Mol Psychiatry 22: 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Centanni SW, Jaramillo AA, Winder DG, Conn PJ (2018) Metabotropic glutamate receptors in alcohol use disorder: physiology, plasticity, and promising pharmacotherapies. ACS Chem Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S (2007) Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13: 460–9. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16: 626–38. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT (1999) Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res 23: 633–43. [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F (2011) Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology 36: 2762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrimore CJ, Crews FT (2017) Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2. Alcohol Clin Exp Res 41: 939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Gai WW, Cheung TK, Peiris JS (2011) Antiviral effect of a selective COX-2 inhibitor on H5N1 infection in vitro. Antiviral Res 91: 330–4. [DOI] [PubMed] [Google Scholar]
- Leong JK, Pestilli F, Wu CC, Samanez-Larkin GR, Knutson B (2016) White-Matter Tract Connecting Anterior Insula to Nucleus Accumbens Correlates with Reduced Preference for Positively Skewed Gambles. Neuron 89: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Lang JA, Miller MW (1998) Transforming growth factor beta1 regulates the expression of cyclooxygenase in cultured cortical astrocytes and neurons. J Neurochem 71: 526–34. [DOI] [PubMed] [Google Scholar]
- McCarthy GM, Warden AS, Bridges CR, Blednov YA, Harris RA (2017) Chronic ethanol consumption: role of TLR3/TRIF-dependent signaling. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J, Guerri C (2016) Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun 53: 159–171. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR (2015) DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry 20: 1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N (1993) Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 53: 1009–18. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP (2011) Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34: 269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Kim SH, Das A, Yang SN, Jung KH, Kim MK, Berggren PO, Lee Y, Chai JC, Kim HJ, Chai YG (2016) TLR3-/4-Priming Differentially Promotes Ca(2+) Signaling and Cytokine Expression and Ca(2+)-Dependently Augments Cytokine Release in hMSCs. Sci Rep 6: 23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155: 1596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindado J, Balsinde J, Balboa MA (2007) TLR3-dependent induction of nitric oxide synthase in RAW 264.7 macrophage-like cells via a cytosolic phospholipase A2/cyclooxygenase-2 pathway. J Immunol 179: 4821–8. [DOI] [PubMed] [Google Scholar]
- Qian Z, Wu X, Qiao Y, Shi M, Liu Z, Ren W, Han J, Zheng Q (2018) Downregulation of mGluR2/3 receptors during morphine withdrawal in rats impairs mGluR2/3- and NMDA receptor-dependent long-term depression in the nucleus accumbens. Neurosci Lett 690: 76–82. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT (2012) Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation 9: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus JL, Dantzer R (2016) Inflammation Models of Depression in Rodents: Relevance to Psychotropic Drug Discovery. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW (1994) Localization of neuronal and glial glutamate transporters. Neuron 13: 713–25. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C (2005) Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci 25: 9858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA (1997) Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature 385: 630–4. [DOI] [PubMed] [Google Scholar]
- Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12–20. [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16: 1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R (2010) Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry 67: 804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symensma TL, Martinez-Guzman D, Jia Q, Bortz E, Wu TT, Rudra-Ganguly N, Cole S, Herschman H, Sun R (2003) COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J Virol 77: 12753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S (1992) A family of metabotropic glutamate receptors. Neuron 8: 169–79. [DOI] [PubMed] [Google Scholar]
- Wagnerberger S, Fiederlein L, Kanuri G, Stahl C, Millonig G, Mueller S, Bischoff SC, Bergheim I (2013) Sex-specific differences in the development of acute alcohol-induced liver steatosis in mice. Alcohol Alcohol 48: 648–56. [DOI] [PubMed] [Google Scholar]
- Warden A, Erickson E, Robinson G, Harris RA, Mayfield RD (2016) The neuroimmune transcriptome and alcohol dependence: potential for targeted therapies. Pharmacogenomics 17: 2081–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch KA, Czachowski CL (2018) Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol 66: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006) Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 26: 9967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Bu Q, Fu D, Shao X, Jiang L, Guo W, Chen B, Liu B, Hu Z, Tian J, Zhao Y, Cen X (2018) Toll-like receptor 3 modulates the behavioral effects of cocaine in mice. J Neuroinflammation 15: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, He L, McCluskey LP (2014) Ingestion of bacterial lipopolysaccharide inhibits peripheral taste responses to sucrose in mice. Neuroscience 258: 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]