Abstract
Although remote ischemic preconditioning (RIPC) is an organ-protective maneuver from subsequent ischemia reperfusion injury (IRI) by application of brief ischemia and reperfusion to other organs, its mechanism remains unclear. However, it is known that RIPC reduces the heart, brain, and liver IRI, and that nitric oxide (NO) is involved in the mechanism of this effect. To identify the role of NO in the protective effect of RIPC in renal IRI, this study examined renal function, oxidative status, and histopathological changes using N-nitro-L-arginine methyl ester (L-NAME), an NO synthase inhibitor. Remote ischemic preconditioning was produced by 3 cycles of 5 minutes ischemia and 5 minutes reperfusion. Blood urea nitrogen, creatinine (Cr), and renal tissue malondialdehyde levels were lower, histopathological damage was less severe, and superoxide dismutase level was higher in the RIPC + IRI group than in the IRI group. The renoprotective effect was reversed by L-NAME. Obtained results suggest that RIPC before renal IRI contributes to improvement of renal function, increases antioxidative marker levels, and decreases oxidative stress marker levels and histopathological damage. Moreover, NO is likely to play an important role in this protective effect of RIPC on renal IRI.
Keywords: ischemia reperfusion injury, nitric oxide, remote ischemic preconditioning
Introduction
Ischemia is defined as a state in which the blood flow is partially or completely blocked in a tissue or organ. When ischemia persists, necrosis and cell death occur. On the other hand, reperfusion is the restoration of the blood flow. Paradoxically, reperfusion may be even more harmful than ischemic injury. During the reperfusion, reactive oxygen species (ROS) cause endothelial damage, increase microvascular permeability, produce tissue edema, activate adhesion molecules, release cytokines, and lead to systemic inflammatory response syndrome.1–4 The sequence of damage that occurs with ischemia and reperfusion is termed ischemia reperfusion injury (IRI).
Renal IRI is one of the main causes of acute kidney injury and occurs in various clinical situations, such as partial nephrectomy, renal transplantation, aortic cross clamping surgery, cardiopulmonary resuscitation, sepsis, and shock.5–8
Hydrogen sulfide, superoxide dismutase (SOD), apocynin, allopurinol, hypothermia, ischemic preconditioning (IPC), and remote ischemic preconditioning (RIPC) were found to reduce IRI.9–11 Among these interventions aimed to attenuate IRI, RIPC is known as a secure, noninvasive, and low cost method, and is therefore a clinically compatible procedure.12,13 Remote ischemic preconditioning of the limb is easily applicable and attenuates IRI of the heart, lungs, and other organs in humans and animals.14–16 Ischemic preconditioning is an organ-protective maneuver produced by cycles of sublethal ischemia and reperfusion before prolonged ischemia, subsequently attenuating IRI of the organ.17 Remote ischemic preconditioning, in which the IPC of one organ protects another distant organ against prolonged IRI, may attenuate renal injury induced by IRI.18
Brief ischemia and reperfusion produce large amounts of oxygen-derived free radicals, which in turn release antioxidants that act as free radical scavengers.19 Although the underlying protective mechanisms of RIPC have not been fully understood, nitric oxide (NO) generated by RIPC is considered to have a protective effect against IRI of the heart, brain, and liver.20–22 Regarding the cardioprotective effect of RIPC, a previous study showed that NO is released at the RIPC application site and is subsequently transferred to the target organ in the form of nitrite (NO2 −) to generate protective signaling by protective regulation of mitochondrial functions and reduced ROS.23 In the brain and liver, IRI is reduced by the RIPC-mediated endothelial nitric oxide synthase (eNOS)-NO pathway.24,25 Although the effects of RIPC and its mechanisms, including the role of NO, in the heart, brain, and liver IRI have been studied, they remain unclear in the kidney.
In this study, a nonselective nitric oxide synthase (NOS) inhibitor, N-nitro-L-arginine methyl ester (L-NAME), was used to investigate whether NO is involved in the limb RIPC-mediated attenuation of the renal IRI. Moreover, the effect of RIPC on the renal function, oxidative status, and histopathological changes in a rat model of hind limb RIPC and subsequent renal IRI was evaluated.
Materials and Methods
Ethical Approval
The experimental protocols were approved by the Institutional Animal Care and Use Committee (2017–0086) and in accordance with the National Institute of Health guidelines (Bethesda, Maryland) on laboratory animal welfare.
Experimental Animals
In this study, 30 male Sprague-Dawley rats (weight, 280-320 g) were adapted in a temperature-controlled environment with a 12 hours light/dark photoperiod and free access to food and water. After the rats were housed in separate cages and closely observed for 1 week, the experiment was started. The experimental protocols were approved by the Kyungpook National University Institutional Animal Care and Use Committee (IRB No. 2017 to 0086).
Experimental Protocol and Allocating Animals to Experimental Groups
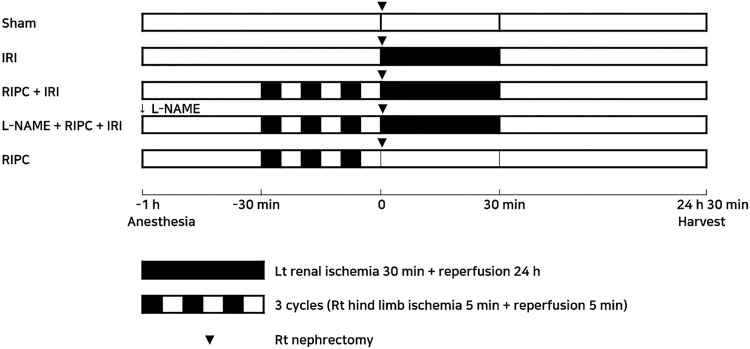
The rats (n = 30) were randomly divided into the following 5 groups: (1) sham group; (2) IRI group (30-minute renal ischemia followed by 24 hours of reperfusion); (3) RIPC (3 cycles of 5 minutes of the right hind limb ischemia and 5 minutes of reperfusion) + IRI group; (4) L-NAME (10 mg/kg, intraperitoneally 1 hour before IRI) + RIPC + IRI group; (5) RIPC group (Figure 1). Subsequently, the rats were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylene (10 mg/kg). After the depth of anesthesia was evaluated, a right flank incision and right nephrectomy were performed. The dosage of L-NAME was chosen based on previous publications. Inhibition of NOS by L-NAME (10 mg/kg) attenuated the effects of NO not only in a neuropathic pain model26 but also in renal IRI.25
Figure 1.
Schematic overview of the study design and experimental groups. The number of rats per group is 6. IRI indicates ischemia reperfusion injury; L-NAME, N-nitro-L-arginine methyl ester; RIPC, remote ischemic preconditioning.
Ischemia Reperfusion Injury
During the operation, the body temperature was obtained using a rectal probe and maintained at approximately 37°C. After the right flank incision and right nephrectomy, a left flank incision was performed and the left renal pedicles were clamped with an atraumatic vascular clamp with 50 to 110 g of pressure (Roboz Surgical Instrument; Gaithersburg, Maryland). After 30 minutes of ischemia, the clamp was released to allow reperfusion. Ischemia was verified visually based on the color change of the kidney to pale color, and reperfusion, to fresh red color.
Remote Ischemic Preconditioning
Remote ischemic preconditioning was induced by applying a pneumatic cuff around the right thigh, inflated to 300 mm Hg.27 A protocol of 5 minutes of limb ischemia followed by 5 minutes of reperfusion for a total of 3 cycles was used. Successful occlusion of the bloodstream was confirmed if a color change to purple was observed in the foot and femoral pulse was absent.28
Harvest
Twenty-four hours after the reperfusion, blood samples were obtained via intracardiac puncture with minimal hemolysis and the left kidney was harvested under anesthesia. For biochemical analysis, plasma was separated from the blood samples through centrifugation at 3000 rpm for 15 minutes. The kidney was divided into 4 sections. One section was processed for histopathological examination, and the other sections were placed in liquid nitrogen and stored at −80°C for malondialdehyde (MDA) and SOD analyses.
Plasma Blood Urea Nitrogen and Creatinine Measurement
Plasma blood urea nitrogen (BUN) and creatinine (Cr) levels were measured spectrophotometrically using a Vitros 250 analyzer (Johnson and Johnson; New Brunswick, New Jersey).
Renal Tissue MDA Level and SOD Activity Measurement
The MDA levels in renal tissues were determined spectrophotometrically using thiobarbituric acid reactive substances.29 Renal tissue (300 mg) was homogenized with 1 mL of lysis buffer using a tissue homogenizer (Kontes Glass Co; Vineland, New Jersey). To 0.1 mL of the sample solution, 0.375% thiobarbituric acid (Alfa Aesar; Ward Hill, Massachusetts), 15% trichloroacetic acid (Sigma; St Louis, Missouri), and 0.25 N HCl were added. The mixture was placed in glass test tubes, sealed with foil, and heated in boiling water for 15 minutes. After cooling at room temperature, the samples were centrifuged at 12 000 rpm for 10 minutes. The absorbance was measured spectrophotometrically at a 535-nm wavelength. Protein concentration was determined using the Bradford assay. The values were expressed as “nmol/mg” protein.
The SOD activity assay was performed using the pyrogallol method.30 Tris–HCl and pentetic buffer were used as a reaction medium, and the decrease in pyrogallol absorbance was monitored spectrophotometrically at 420 nm. Tris–HCl (50 mM) to 900 μL of 1 mM pentetic acid (Sigma) buffer was adjusted to a pH = 8.2 and placed in a 1.5 mL cuvette. Pyrogallol (10 μL of 20 mM) in 10 μM HCl (Sigma) buffer and 10 μL of 0.1 M EDTA buffer were added in the same cuvette and mixed well. The increase in absorbance was measured for 1 minute at a wavelength of 420 nm. The optical density value was derived from approximately 0.1. Next, the 300 mg of renal tissue sample for analysis was homogenized with 1 mL of lysis buffer using a tissue homogenizer (Kontes Glass Co; Vineland, New Jersey). The sample was placed in a 10-μL sample solution and controlled in a 1.5-mL cuvette. The abovementioned steps were repeated. Protein concentration was determined using the Bradford assay. Superoxide dismutase activity was evaluated as the amount of enzyme that reduced the color change by 50% and calculated as “μ/mg” protein.
Histopathological Analysis
Histological damage scores were analyzed as described previously.31 Kidney specimens were fixed immediately in 4% paraformaldehyde, embedded in paraffin, and cut in 3-μm sections using a microtome. The sections were stained with periodic acid-Schiff and observed under light microscopy at a magnification of ×200. The degree of damage was examined in 10 fields per slide for histopathological analysis. To quantify renal damage scoring, 50 outer medulla tubules were observed and rated as follows: 0, no damage; 1, mild damage with rounding of epithelial cells and dilated tubular lumen; 2, severe damage with flattened epithelial cells, loss of nuclear staining, and dilated and congested lumen; and 3, destroyed tubules with flat epithelial cells lacking nuclear staining and lumen congestion.32,33
Sample Size
Sample size calculation was performed based on the study of Hussein and colleagues29 using PASS version 08.0.6 (NCSS LLC, Kaysville, Utah). In the study, the means (standard deviation [SD]) of the serum Cr levels between the IRI group and the RIPC + IRI group were 3.5 (0.8) and 1.69 (0.4). The significance level α = .017 was corrected by Bonferroni multiple comparison procedure. Group sample sizes of 6 achieved 87% power.
Statistical Analysis
Data analysis was performed using the statistical software SPSS version 23.0 for Windows (SPSS Inc, Chicago, Illinois). Data were summarized as mean ± standard error of mean. To analyze the data, 1-way analysis of variance was used for repeated measurements of the same variable, followed by Bonferroni test for post hoc comparisons. Between-group differences were considered statistically significant at P values < .05.
Results
Renal Function
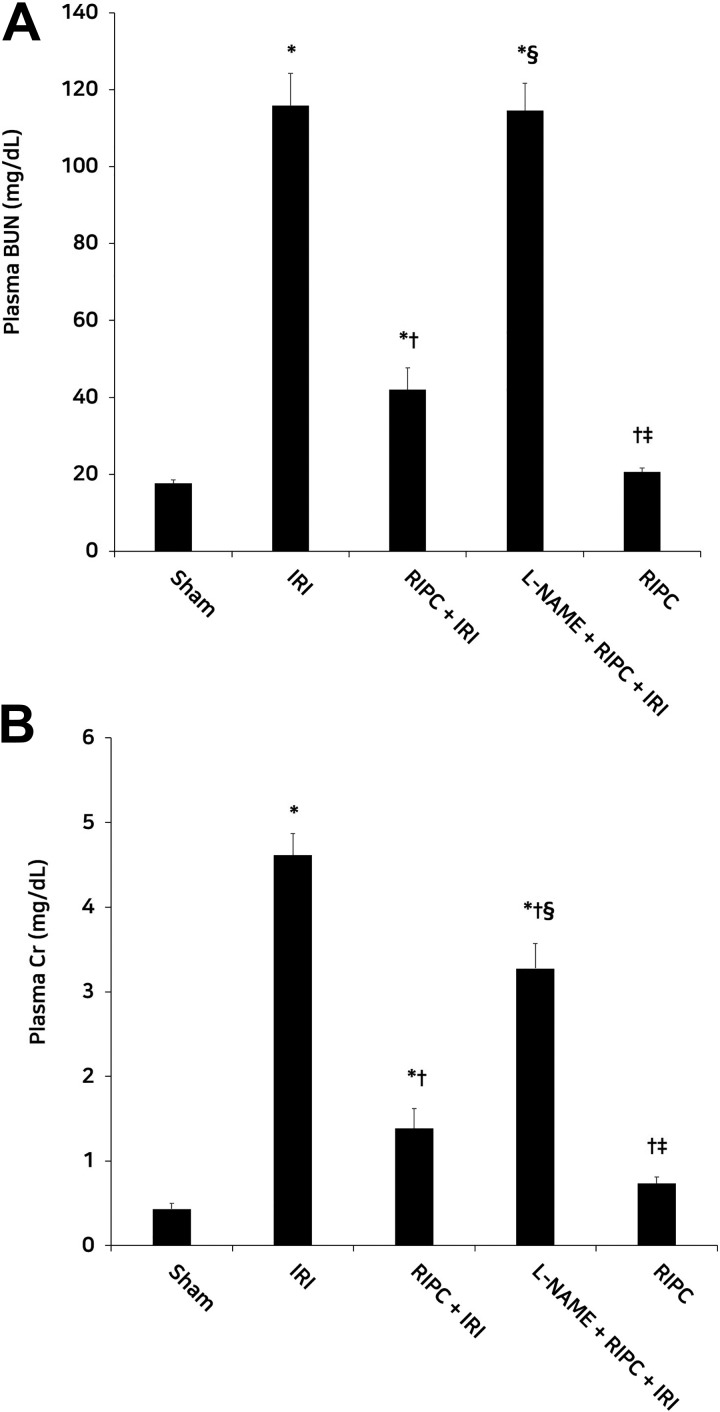
Blood urea nitrogen and Cr levels were significantly higher in the IRI group than in the sham group (P < .05 for both), but lower in the RIPC + IRI group than in the IRI group (P < .05 for both). Compared to the RIPC + IRI group, the L-NAME + RIPC + IRI group showed significantly higher BUN and Cr levels (P < .05 for both). Blood urea nitrogen and Cr levels were significantly lower in the RIPC group than in the L-NAME + RIPC + IRI group (P < .05 for both). No significant difference in plasma BUN level was observed between the IRI and L-NAME + RIPC + IRI groups. In the RIPC group, the BUN and Cr levels were not affected, unlike in the sham group (Figure 2).
Figure 2.
Plasma BUN (A) and Cr (B) levels in the experimental groups. Plasma BUN and Cr levels were significantly lower in the RIPC + IRI group than in the IRI group. Compared with the RIPC + IRI group, the L-NAME + RIPC + IRI group showed significantly high plasma BUN and Cr levels. *P < .05 versus the sham group; † P < .05 versus the IRI group; § P < .05 versus the RIPC + IRI group; ‡ P < .05 versus the L-NAME + RIPC + IRI group. Data are expressed as mean ± SEM (n = 6 rats/group). BUN indicates blood urea nitrogen; Cr, creatinine; IRI, ischemia reperfusion injury; L-Name, N-nitro-l-arginine methyl ester; RIPC, remote ischemic preconditioning; SEM, standard error of the mean.
Renal Oxidative Stress
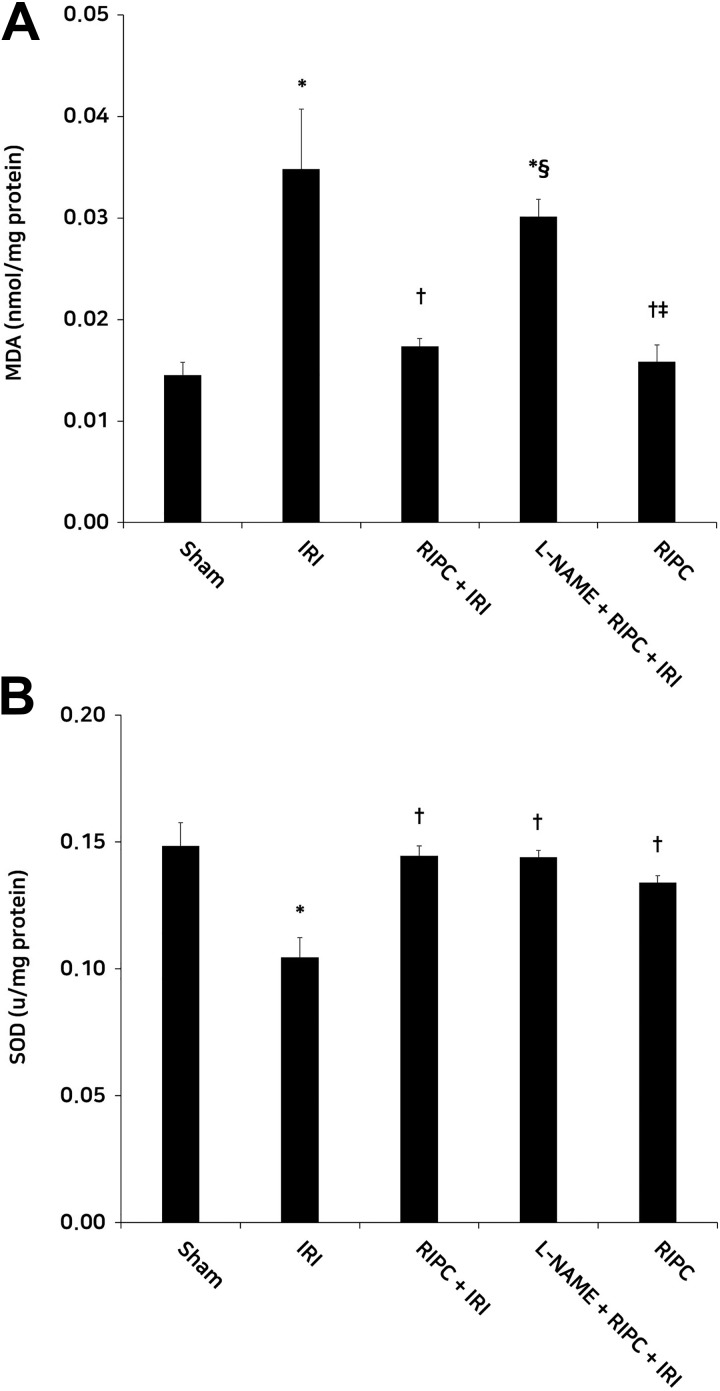
The IRI group showed significantly higher MDA levels than the sham group, suggesting an increase in lipid peroxidation (P < .05). Malondialdehyde levels in the RIPC + IRI group were significantly lower than those in the IRI group (P < .05). The MDA levels in the L-NAME + RIPC + IRI group were significantly higher than those in the RIPC + IRI group (P < .05). Malondialdehyde levels in the RIPC group were significantly lower than those in the L-NAME + RIPC + IRI group (P < .05). No significant differences in MDA levels were observed among the sham, RIPC + IRI, and RIPC groups. The IRI group showed significantly lower SOD levels in contrast to the sham group, suggesting a decrease in antioxidant defense mechanism (P < .05). The SOD levels in the RIPC + IRI, L-NAME + RIPC + IRI, and RIPC groups were significantly higher than those in the IRI group (P < .05). No significant differences in SOD level were observed among all groups, except the IRI group (Figure 3).
Figure 3.
Renal tissue MDA (A) and SOD (B) levels in the experimental groups. (A) the RIPC + IRI group showed lower MDA levels than the IRI group. The MDA levels in the L-NAME + RIPC + IRI group were higher than in the RIPC + IRI group. (B) Compared with the IRI group, the RIPC + IRI group showed significantly high SOD levels. *P < .05 and .001 for MDA and SOD, respectively, versus the sham group; † P < .05 for MDA and SOD, versus the IRI group; § P < .05 for MDA, versus the RIPC + IRI group; P < .05 for MDA, versus the L-NAME + RIPC + IRI group. Data are expressed as mean ± SEM (n = 6 rats/group). MDA, malondialdehyde; SOD, superoxide dismutase; IRI, ischemia reperfusion injury; RIPC, remote ischemic preconditioning; l-name, N-nitro-l-arginine methyl ester; SEM, standard error of the mean.
Renal Histopathology
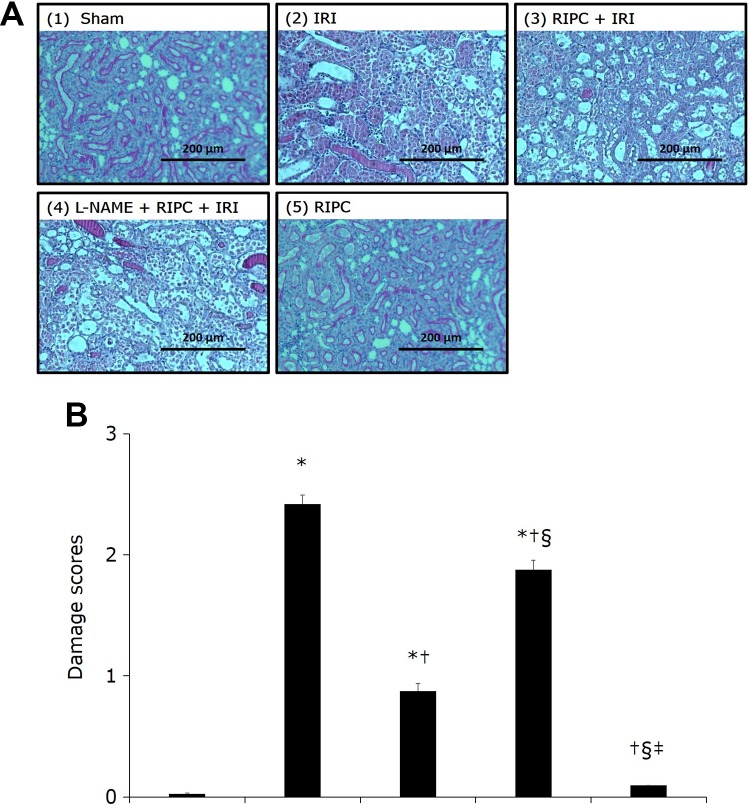
Histopathological examination revealed no renal tissue damage in the sham and RIPC groups (damage score [mean (SD)]: 0.03 [0.01] and 0.09 [0.01], respectively). In the IRI group (2.42 [0.18]), an increased number of destroyed tubules with flat epithelial cells lacking nuclear staining and higher lumen congestion were observed, in contrast to the sham group (P < .05). The RIPC + IRI group (0.87 [0.15]), however, showed mild damage with rounding of epithelial cells and dilated tubular lumen, compared to the IRI group (P < .05), suggesting attenuated IRI-induced damage. The L-NAME + RIPC + IRI group (1.88 [0.19]) showed severe damage with flattened epithelial cells, loss of nuclear staining, dilated lumen, and lumen congestion, compared to the RIPC + IRI group (P < .05; Figure 4).
Figure 4.
Histopathological photographs of renal tissue in the experimental groups. A, Periodic acid-Schiff (PAS) stain, original magnification × 100. (1) Sham group: no damage; (2) IRI group: destroyed tubules with flat epithelial cells lacking nuclear staining and lumen congestion; (3) RIPC + IRI group: mild damage with rounding of epithelial cells and dilated tubular lumen; and (4) L-NAME + RIPC + IRI group: severe damage with flattened epithelial cells, loss of nuclear staining, dilated lumen, and lumen congestion; (5) RIPC group: no damage. B, Damage score on the basis of the PAS staining. Renal tissue damage was lower in the RIPC + IRI group than in the IRI group. In the L-NAME + RIPC + IRI group, renal tissue damage was significantly higher than in the RIPC + IRI group. *P < .05 versus the sham group; † P <.05 versus the IRI group; § P <.05 versus the RIPC + IRI group; ‡ P < .05 versus the RIPC + IRI group. Data are expressed as mean ± SEM (n = 6 rats/group). IRI indicates ischemia reperfusion injury; L-NAME, N-nitro-L-arginine methyl ester; PAS, periodic acid-Schiff; RIPC, remote ischemic preconditioning; SEM, standard error of the mean.
Discussion
In this study, RIPC before renal IRI reduced BUN, Cr, and MDA levels, as well as histopathological damage, and preserved SOD level in renal IRI. Inhibition of NO production using L-NAME before RIPC attenuated the renoprotective effects of RIPC on renal IRI.
Remote ischemic preconditioning releases endogenous molecules, such as adenosine, bradykinin, opioids, interleukins, stromal cell-derived factor, hypoxia-inducible factor 1α, and NO.13,30 These molecules are transmitted by 3 major pathways: humoral, neurogenic, and immunological.13,30 Although its organ protective action mechanism has not yet been elucidated, some reports have indicated that RIPC increases shear stress.31 Owing to the increase in shear stress, reperfusion is a strong physiological stimulus of eNOS production.31–33 The increased shear stress during RIPC-mediated reperfusion will increase eNOS activation and subsequently enhance NO production. The eNOS-mediated NO production has been known as a potent modulator of vascular smooth muscle tone and organ perfusion.34–37
Generally, NO has important roles in homeostasis and host-defense mechanisms.38 Nitric oxide itself is a stable, but highly reactive molecule. Thus, NO oxidation to nitrite occurs rapidly in the systemic circulation. Based on the half-life of sodium nitrite (35 minutes),39 the nitrite in the systemic circulation has enough time to be transported in an organ before prolonged ischemia.
Nitrite, a stable NO oxidation product, may become an alternative source of NO under hypoxic and acidic conditions by xanthine oxidase (XO) pathway, which is a clinically relevant environment of ischemia.40–42 In normal oxygen concentration, NO is produced through the classical L-arginine-NOS-NO signaling pathway. However, in the hypoxic state, it is impossible to generate NO through this process because of oxygen deficiency.43 Xanthine oxidase, an oxidized form of xanthine oxidoreductase, is a key source of superoxide production during IRI.44 Previous studies on IRI have shown increased XO activity and negative clinical or biological outcomes.10,25 Both human and bovine XO have also been reported to exert NO-generating nitrite reductase activities in chemical reaction systems, with these reactivities possibly being relevant during tissue ischemia and inflammation.45,46 The molybdopterin cofactor is the site of nitrite reduction under acidic pH and hypoxic conditions.42,47 This nitrite reduction, even not proven in vivo, suggests a paradigm shift on the redox pathomechanism of IRI.
The nitrite transferred to the target organ is chemically reduced back to bioactive NO at lower oxygen concentration and pH by several mechanisms, including reaction with deoxymyoglobin in the heart and with other heme proteins in organs and the blood.48–50 In this process, the function of cardiac myoglobin changes from an oxygen storage and NO scavenger to an NO producer by reducing nitrite to bioactive NO.31,51,52 This can protect cardiomyocytes from cardiac IRI.53 Hemoglobin (Hb) also has enzymatic characteristics of a nitrite reductase, particularly when Hb is in its allosteric deoxyHb form.49
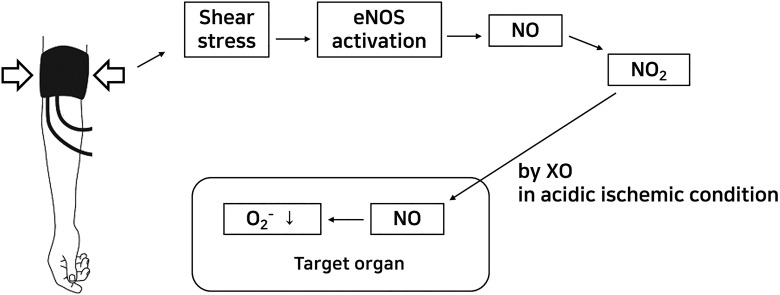
Nitric oxide is well-known as a major ROS of IRI with superoxide.54,55 Although the effects of NO on IRI remain controversial, it is generally accepted that the proper amount of NO is helpful for IRI. Inhibition of massively overexpressed NO, such as NO from inducible NOS, attenuates IRI-mediated organ dysfunction.10,25,54,56 On the other hand, other reports have indicated that NO or NO donors increase IRI.57 The interaction between NO and superoxide produces peroxynitrite, which acts as a strong oxidant. Instead of peroxynitrite production, NO can reduce the bioavailability of superoxide, which is also a strong oxidant, by interaction with superoxide.58,59 Thus, balanced control of ROS, especially the interaction of NO with superoxide, is important to attenuate IRI. As a consequence of the nitrite reduction to NO, the net redox change during IRI will decrease superoxide production and increase NO production. It seems that RIPC-mediated renoprotection is related to these roles of NO (Figure 5).
Figure 5.
Hypothesis of RIPC-NO-mediated organ protection. eNOS indicates endothelial nitric oxide synthase; NO, nitric oxide; NO2 −, nitrite; O2 −, superoxide; RIPC, remote ischemic preconditioning; XO, xanthine oxidase.
The RIPC-eNOS-mediated NO production was blocked using the NO blockade induced by L-NAME, which reduced the renoprotective effect of RIPC as expected. The role of NO was confirmed using an NOS inhibitor (L-NAME) in this study. An NO scavenger, such as carboxy-2-(4-carboxyphenyl)−4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, which reacts with NO to yield nitrogen dioxide and finally removes generated NO,60 might be also a candidate to confirm the role of NO. When an NO scavenger is used, NO generated by other mechanisms besides the RIPC-mediated pathway may be also blocked; therefore, an NOS inhibitor may be appropriate for confirming the effect of RIPC.
Malondialdehyde is a biomarker used to measure the level of oxidative stress induced by lipid peroxidation. Superoxide dismutase, a key endogenous antioxidant enzyme, scavenges ROS and protects the function of mitochondria. The results of this study demonstrated that while MDA concentration and SOD activity were normal in the sham group, MDA concentration increased and SOD activity decreased, due to oxidative stress, in the IRI group. Further, when RIPC was applied, these changes shown in the IRI group did not occur and the levels of these 2 measures were as normal as in the sham group. In fact, the application of RIPC has been shown to alleviate increased MDA and decreased SOD, indicating reduced oxidative stress induced by IRI. Specifically, these findings are consistent with the result of Ozturk and colleagues,61 which showed that NO attenuated MDA levels. However, blocking of RIPC-eNOS-mediated NO using L-NAME increased MDA and induced no changes in SOD. Based on obtained results on MDA and SOD, the authors suggest that an NO-mediated mechanism during RIPC is, in part, involved in attenuating the oxidative stress induced by IRI.
Some issues about the methodology used in this study should be acknowledged. An ischemic time between 30 and 60 minutes in the rat renal IRI model is usually used and known to lead to acute renal dysfunction.62–65 It is also known that this damage does not last long and is recovered within 7 days.62,64,65 Increased ischemic time can lead to more damage and delayed recovery, which can cause death in the long term.66,67 In previous studies,10,25 30-minute ischemia was selected as a reversible response when treated. In the rat renal IRI model with 30 minutes of ischemia, the damage tended to increase up to 24 hours after reperfusion and then decreased.62 In this study, the blood and kidney samples were obtained 24 hours after reperfusion to proceed with the experiment at maximum damage and before the degree of damage was reduced.
Wang and colleagues applied RIPC using a small pneumatic cuff inflated to 300 mm Hg around the right proximal thigh.22 A protocol of 5 minutes of limb ischemia followed by 5 minutes of reperfusion for a total of 3 cycles was used. In the present study, 3 cycles of a RIPC protocol of 5-minute ischemia and 5-minute reperfusion with a pneumatic cuff applied at the thigh were used 30 minutes before renal IRI. Johnsen and colleagues evaluated the cardioprotective effects of RIPC, especially the number and duration of RIPC cycles and the effector organ mass of RIPC.68 Two cycles of RIPC, rather than 4, 6, 8 cycles, and 2 and 5 minutes ischemia during RIPC, rather than 10 minutes ischemia, were more effective 30 to 90 minutes before IRI. No difference was found between 1-limb and 2-limb RIPC. Hussein and colleagues reported suppression of inflammatory cytokine genes, as well as activation of antioxidative and antiapoptotic genes, with the same protocol as that used in this experiment.29,69
Owing to high metabolic activity, biotransformation of enzyme activities, and oxygen consumption in the outer medulla, this area is more sensitive to ischemic damage than the cortex.70,71 Based on the results of previous studies,10,25 this study used the tubules of the outer medulla for histopathological analysis.
This study has some limitations. First, the most accurate method for determining the NO role in the renoprotective effect of RIPC is to directly measure the NO level. As NO is extremely reactive with other substances, direct NO measurement is difficult and many studies have been conducted using NOS inhibitors or NO scavengers. Alternatively, the level of nitrite, a stable reaction product of NO with molecular oxygen, was determined using the Griess reaction.72 To clarify this mechanism, further research with direct NO or nitrite measurement is recommended. Second, it would also be possible to selectively block RIPC-eNOS-mediated NO using an eNOS-specific inhibitor, as opposed to a nonspecific NOS inhibitor. In this way, the eNOS-NO-mediated effect may be more specific. Third, L-arginine, the precursor of NO, may be used to determine the effect of NO. Further research could be done to confirm that NO is involved in the renoprotective effect of RIPC using L-arginine. Forth, if the urine output, the basic test that evaluates kidney function, was measured, it would have provided better insight.
In conclusion, our results show that renal damage is attenuated by RIPC. The renoprotective effect is reversed by NO inhibition by a nonspecific NOS inhibitor, L-NAME. Nitric oxide seems to play an important role in the protective effects of RIPC on renal IRI.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hoon Jung  https://orcid.org/0000-0003-2488-5221
https://orcid.org/0000-0003-2488-5221
References
- 1. Huang S-S, Wei F-C, Hung L-M. Ischemic preconditioning attenuates postischemic leukocyte—endothelial cell interactions: role of nitric oxide and protein kinase C. Circ J. 2006;70(8):1070–1075. [DOI] [PubMed] [Google Scholar]
- 2. Olguner C, Koca U, Kar A, et al. Ischemic preconditioning attenuates the lipid peroxidation and remote lung injury in the rat model of unilateral lower limb ischemia reperfusion. Acta Anaesthesiol Scand. 2006;50(2):150–155. [DOI] [PubMed] [Google Scholar]
- 3. Gueler F, Park J-K, Rong S, et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170(4):1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xia Z, Li H, Irwin MG. Myocardial ischaemia reperfusion injury: the challenge of translating ischaemic and anaesthetic protection from animal models to humans. Br J Anaesth. 2016;117(suppl 2):ii44–ii62. [DOI] [PubMed] [Google Scholar]
- 5. Matin SF, Novick AC. Renal dysfunction associated with staged bilateral partial nephrectomy: the importance of operative positioning. J Urol. 2001;165(3):880–881. [PubMed] [Google Scholar]
- 6. Anaya-Prado R, Toledo-Pereyra LH. The molecular events underlying ischemia/reperfusion injury. Transplant Proc. 2002;34(7):2518–2519. [DOI] [PubMed] [Google Scholar]
- 7. Landry GJ, Lau IH, Liem TK, Mitchell EL, Moneta GL. Adjunctive renal artery revascularization during juxtarenal and suprarenal abdominal aortic aneurysm repairs. Am J Surg. 2010;199(5):641–645. [DOI] [PubMed] [Google Scholar]
- 8. Nieuwenhuijs-Moeke GJ, Nieuwenhuijs VB, Seelen MAJ, et al. Propofol-based anaesthesia versus sevoflurane-based anaesthesia for living donor kidney transplantation: results of the VAPOR-1 randomized controlled trial. Br J Anaesth. 2017;118(5):720–732. [DOI] [PubMed] [Google Scholar]
- 9. Dai H, Ji X, Zhu S, et al. Hydrogen sulphide and mild hypothermia activate the CREB signaling pathway and prevent ischemia-reperfusion injury. BMC Anesthesiol. 2015;15(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi EK, Jung H, Kwak KH, et al. Effects of allopurinol and apocynin on renal ischemia-reperfusion injury in rats. Transplant Proc. 2015;47(6):1633–1638. [DOI] [PubMed] [Google Scholar]
- 11. Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13(4):193–209. [DOI] [PubMed] [Google Scholar]
- 12. Veighey K, MacAllister R. Ischemic conditioning in kidney transplantation. J Cardiovasc Pharmacol Ther. 2017;22(4):330–336. [DOI] [PubMed] [Google Scholar]
- 13. Totzeck M, Hendgen-Cotta U, Rassaf T. Concepts of hypoxic NO signaling in remote ischemic preconditioning. World J Cardiol. 2015;7(10):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia Z, Herijgers P, Nishida T, Ozaki S, Wouters P, Flameng W. Remote preconditioning lessens the deterioration of pulmonary function after repeated coronary artery occlusion and reperfusion in sheep. Can J Anesth. 2003;50(5):481–488. [DOI] [PubMed] [Google Scholar]
- 15. Küntscher MV, Kastell T, Sauerbier M, Nobiling R, Gebhard MM, Germann G. Acute remote ischemic preconditioning on a rat cremasteric muscle flap model. Microsurgery. 2002;22(6):221–226. [DOI] [PubMed] [Google Scholar]
- 16. Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106(23):2881–2883. [DOI] [PubMed] [Google Scholar]
- 17. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. [DOI] [PubMed] [Google Scholar]
- 18. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–899. [DOI] [PubMed] [Google Scholar]
- 19. Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vlasov TD, Korzhevskii DE, Polyakova EA. Ischemic preconditioning of the rat brain as a method of endothelial protection from ischemic/repercussion injury. Neurosci Behav Physiol. 2005;35(6):567–572. [DOI] [PubMed] [Google Scholar]
- 21. Duan Y-F, An Y, Zhu F, Jiang Y. Remote ischemic preconditioning protects liver ischemia-reperfusion injury by regulating eNOS-NO pathway and liver microRNA expressions in fatty liver rats. Hepatobiliary Pancreat Dis Int. 2017;16(4):387–394. [DOI] [PubMed] [Google Scholar]
- 22. Wang T, Zhou Y-T, Chen X-N, Zhu A-X, Wu B-H. Remote ischemic postconditioning protects against gastric mucosal lesions in rats. World J Gastroenterol. 2014;20(28):9519–9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. [DOI] [PubMed] [Google Scholar]
- 24. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–474. [DOI] [PubMed] [Google Scholar]
- 25. Choi EK, Jung H, Kwak KH, et al. Inhibition of oxidative stress in renal ischemia-reperfusion injury. Anesth Analg. 2017;124(1):204–213. [DOI] [PubMed] [Google Scholar]
- 26. Kwak KH, Han CG, Lee SH, et al. Reactive oxygen species in rats with chronic post-ischemia pain. Acta Anaesthesiol Scand. 2009;53(5):648–656. [DOI] [PubMed] [Google Scholar]
- 27. Kim J, Park J-W, Park KM. Increased superoxide formation induced by irradiation preconditioning triggers kidney resistance to ischemia-reperfusion injury in mice. Am J Physiol-Ren Physiol. 2009;296(5):F1202–F1211. [DOI] [PubMed] [Google Scholar]
- 28. Seok YM, Kim J, Choi KC, et al. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney ischemia/reperfusion injury in mice: a role for antioxidant enzymes and heat-shock proteins. J Ethnopharmacol. 2007;112(2):333–340. [DOI] [PubMed] [Google Scholar]
- 29. Hussein AM, Harraz AM, Awadalla A, Barakat N, Khater S, Shokeir AA. Remote limb ischemic preconditioning (RIPC) activates antioxidant and antiapoptotic genes and inhibits proinflammatory cytokine genes in renal ischemia/reperfusion injury. Gen Physiol Biophys. 2016;35(1):77–86. [DOI] [PubMed] [Google Scholar]
- 30. Menting TP, Wever KE, Ozdemir-van Brunschot DM, Van der Vliet DJ, Rovers MM, Warle MC. Ischaemic preconditioning for the reduction of renal ischaemia reperfusion injury. Cochrane Database Syst Rev. 2017;4(3):CD010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114(10):1601–1610. [DOI] [PubMed] [Google Scholar]
- 32. Rassaf T, Preik M, Kleinbongard P, et al. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest. 2002;109(9):1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sriram K, Laughlin JG, Rangamani P, Tartakovsky DM. Shear-induced nitric oxide production by endothelial cells. Biophys J. 2016;111(1):208–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toda N, Toda H. Nitric oxide-mediated blood flow regulation as affected by smoking and nicotine. Eur J Pharmacol. 2010;649(1):1–13. [DOI] [PubMed] [Google Scholar]
- 35. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rochette L, Lorin J, Zeller M, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther. 2013;140(3):239–257. [DOI] [PubMed] [Google Scholar]
- 37. Ekeloef S, Larsen MHH, Schou-Pedersen AMV, Lykkesfeldt J, Rosenberg J, Gögenür I. Endothelial dysfunction in the early postoperative period after major colon cancer surgery. Br J Anaesth. 2017;118(2):200–206. [DOI] [PubMed] [Google Scholar]
- 38. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. [DOI] [PubMed] [Google Scholar]
- 39. Hon YY, Sun H, Dejam A, Gladwin MT. Characterization of erythrocytic uptake and release and disposition pathways of nitrite, nitrate, methemoglobin, and iron-nitrosyl hemoglobin in the human circulation. Drug Metab Dispos. 2010;38(10):1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. [DOI] [PubMed] [Google Scholar]
- 41. Totzeck M, Hendgen-Cotta UB, Kelm M, Rassaf T. Crosstalk between nitrite, myoglobin and reactive oxygen species to regulate vasodilation under hypoxia. In Jourd’heuil D, ed. PLoS One. 2014;9(8):e105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kelley EE. A new paradigm for XOR-catalyzed reactive species generation in the endothelium. Pharmacol Rep. 2015;67(4):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rassaf T, Bryan NS, Maloney RE, et al. NO adducts in mammalian red blood cells: too much or too little? Nat Med. 2003;9(5):481–482. [DOI] [PubMed] [Google Scholar]
- 44. Abdelrahman M, Mazzon E, Bauer M, et al. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23(2):107–114. [DOI] [PubMed] [Google Scholar]
- 45. Godber BLJ, Doel JJ, Sapkota GP, et al. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275(11):7757–7763. [DOI] [PubMed] [Google Scholar]
- 46. Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276(27):24482–24489. [DOI] [PubMed] [Google Scholar]
- 47. Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide. 2013;34:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100(12):1749–1754. [DOI] [PubMed] [Google Scholar]
- 49. Huang Z, Shiva S, Kim-Shapiro DB, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia–reperfusion damage. Proc Natl Acad Sci. 2004;101(37):13683–13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Totzeck M, Hendgen-Cotta UB, Rammos C, et al. Assessment of the functional diversity of human myoglobin. Nitric Oxide. 2012;26(4):211–216. [DOI] [PubMed] [Google Scholar]
- 52. Totzeck M, Hendgen-Cotta UB, Luedike P, et al. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126(3):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hendgen-Cotta UB, Merx MW, Shiva S, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105(29):10256–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res. 2005;129(2):236–241. [DOI] [PubMed] [Google Scholar]
- 55. Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;(132):7–21. [DOI] [PubMed] [Google Scholar]
- 56. Viñas JL, Sola A, Genescà M, Alfaro V, Pí F, Hotter G. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med. 2006;40(6):992–1003. [DOI] [PubMed] [Google Scholar]
- 57. Chatterjee PK, Patel NSA, Sivarajah A, et al. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63(3):853–865. [DOI] [PubMed] [Google Scholar]
- 58. Berges A, Van Nassauw L, Bosmans J, Timmermans J-P, Vrints C. Role of nitric oxide and oxidative stress in ischaemic myocardial injury and preconditioning. Acta Cardiol. 2003;58(2):119–132. [DOI] [PubMed] [Google Scholar]
- 59. Iwase H, Robin E, Guzy RD, et al. Nitric oxide during ischemia attenuates oxidant stress and cell death during ischemia and reperfusion in cardiomyocytes. Free Radic Biol Med. 2007;43(4):590–599. [DOI] [PubMed] [Google Scholar]
- 60. Yoshida M, Akaike T, Wada Y, et al. Therapeutic effects of imidazolineoxyl N-oxide against endotoxin shock through its direct nitric oxide-scavenging activity. Biochem Biophys Res Commun. 1994;202(2):923–930. [DOI] [PubMed] [Google Scholar]
- 61. Ozturk H, Tuncer MC, Ozturk H, Buyukbayram H. Nitric oxide regulates expression of sonic hedgehog and hypoxia-inducible factor-1alpha in an experimental model of kidney ischemia-reperfusion. Ren Fail. 2007;29(3):249–256. [DOI] [PubMed] [Google Scholar]
- 62. Rao K, Sethi K, Ischia J, et al. Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One. 2017;12(7):e0180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jo SK, Yun SY, Chang KH, et al. α-MSH decreases apoptosis in ischaemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dial Transplant. 2001;16(8):1583–1591. [DOI] [PubMed] [Google Scholar]
- 64. Ysebaert DK, De Greef KE, Vercauteren SR, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15(10):1562–1574. [DOI] [PubMed] [Google Scholar]
- 65. Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35(3):198–204. [DOI] [PubMed] [Google Scholar]
- 66. Zager RA. Adenine nucleotide changes in kidney, liver, and small intestine during different forms of ischemic injury. Circ Res. 1991;68(1):185–196. [DOI] [PubMed] [Google Scholar]
- 67. Zager RA. Partial aortic ligation: a hypoperfusion model of ischemic acute renal failure and a comparison with renal artery occlusion. J Lab Clin Med. 1987;110(4):396–405. [PubMed] [Google Scholar]
- 68. Johnsen J, Pryds K, Salman R, Løfgren B, Kristiansen SB, Bøtker HE. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol. 2016;111(2):10. [DOI] [PubMed] [Google Scholar]
- 69. Hussein AM, Sakr HF, Alenzi FQ. Possible underlying mechanisms of the renoprotective effect of remote limb ischemic preconditioning against renal ischemia/reperfusion injury: a role of osteopontin, transforming growth factor-beta and survivin. Nephron. 2016;134(2):117–129. [DOI] [PubMed] [Google Scholar]
- 70. Sabbahy ME, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med. 2011;3(5):606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66(2):480–485. [DOI] [PubMed] [Google Scholar]
- 72. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. [DOI] [PubMed] [Google Scholar]