Abstract
Determination of biventricular dimensions, function, and ventricular–ventricular interactions (VVI) is an essential part of the echocardiographic examination in adults with pulmonary hypertension (PH); however, data from according pediatric studies are sparse. We hypothesized that left and right heart dimensions/function and VVI variables indicate disease severity and progression in children with PH. Left heart, right heart, and VVI variables (e.g. end-systolic LV eccentricity index [LVEI], right ventricular [RV]/left ventricular [LV] dimension ratio) were echocardiographically determined in 57 children with PH, and correlated with New York Heart Association (NYHA) functional class (FC), N-terminal-pro brain natriuretic peptide (NT-proBNP), and invasive hemodynamic variables (e.g. pulmonary vascular resistance index [PVRi]). Clinically sicker patients (higher NYHA FC) had lower LV ejection fraction (LVEF) and higher LVEI – a surrogate of LV compression. In PH children, the ratio of systolic pulmonary arterial pressure divided by systolic systemic arterial pressure (sPAP/sSAP) and the PVRi correlated well with the LVEI (P < 0.001). Patients with more severe PH (sPAP/sSAP ratio, PVRi) had increased RV/LV and right-to-left atrial dimension ratios (P < 0.01). When stratified using NYHA-FC, sicker PH children had greater RV and right atrial dimensions with lower exercise capacity, while the tricuspid annular plane systolic excursion as surrogate for longitudinal systolic RV function decreased. Consistent with previous studies, serum NT-proBNP correlated with both, sPAP/sSAP ratio (P < 0.001) and NYHA FC (P < 0.01). Taken together, the VVI variables LVEI and RV/LV dimension ratio are associated with lower FC, worse hemodynamics, and higher NT-proBNP levels, thus highlighting the importance of ventricular interdependence in pediatric PH.
Keywords: left ventricular eccentricity index, New York Health Association functional class, pediatric pulmonary hypertension, ventricular–ventricular interactions
Introduction
Children and adults with pulmonary hypertension (PH) share some pathogenic and clinical features but the feasibility of diagnostic modalities differs between these age groups.1–3 Echocardiography is used as a first-line tool to detect PH and to assess left ventricular (LV) and right ventricular (RV) dimensions and function.4,5 In children and adults with significant RV pressure overload, the importance of biventricular size and function determination has increasingly been recognized.6–13 RV enlargement, due to pressure loading such as in PH, can lead to subsequent LV compression, which can easily be visualized by echocardiography.14
Biventricular size and function variables and ventricular–ventricular interaction (VVI) are emerging in pediatric PH research.9–13 As RV pressure overload progresses, direct or sequential RV–LV interaction leads to impaired LV filling. During direct interaction, the LV cavity is compressed owing to leftward bowing of the ventricular septum, causing impaired LV filling, reduced LV preload, and low cardiac output.9–13 In the sequential mechanism, an increased RV afterload decreases RV output, with consequently decreased LV preload and output.17 Here, we used echocardiographic imaging, together with hemodynamically obtained variables (i.e. indexed pulmonary vascular resistance [PVRi]) and disease severity classifications (New York Heart Association [NYHA] functional class [FC]/modified ROSS score) to evaluate biventricular dimensions or function in pediatric PH patients.
We hypothesized that VVI variables correlate with surrogate variables of clinical outcome in children with PH.
Materials and methods
Study group
Our study group consisted of 57 children and adolescents with PH; 26 patients had PH secondary to congenital heart disease (PH-CHD) (12 boys, 14 girls; median age = 6.6 years; age range = 116 days–18.0 years; body surface area [BSA] = 0.31–1.59 m2); 12 patients had idiopathic (I)PAH (9 boys, 3 girls; median age = 14.4 years; age range = 309 days–17.9 years; BSA = 0.42–1.92 m2); and 19 patients had PH due to bronchopulmonary dysplasia (PH-BPD) (12 boys, 7 girls; median age = 1.0 year; age range = 104 days–7.6 years; BSA = 0.2–1.05 m2). The PH-CHD patients included patients with post-tricuspid left-to right shunts such as ventricular septal defect (VSD) and atrioventricular septal defects but no patients with isolated atrial septal defect (ASD). The respective CHDs were surgically repaired in all patients at a mean age of 6.1 months (age range = 0.45–14.3 months). None of our patients had Eisenmenger syndrome. The baseline characteristics and the type of congenital heart defects in our pediatric PH cohort are provided in Table 1. Patients with severe atrioventricular valve regurgitation or conduit regurgitation were excluded from the study. Children and adolescents with left atrial hypertension or pulmonary venous obstruction were excluded from the study.
Table 1.
Demographic data of our 57 patients with PH.
| All PH patients | |
| Fulfilled inclusion criteria (n) | 57 |
| Female (n (%)) | 24 (42) |
| Age at baseline (years) (range) | 5.7 (0.6–18.2) |
| Body weight (kg) (range) | 20.8 (4.9–86.5) |
| Body length (cm) (range) | 127 (50–187) |
| BSA range (m2) | 0.2–1.92 |
| NYHA-FC/ROSS score | |
| I (n) | 18 |
| II (n) | 27 |
| III (n) | 12 |
| PH medication (n) | |
| Sildenafil (single) | 13 |
| Bosentan | 4 |
| Macitentan | 8 |
| Bosentan, sildenafil (comb) | 10 |
| Macitentan, sildenafil | 18 |
| Selexipag (triple comb) | 4 |
| Calcium antagonists | 3 |
| PAH-CHD (n = 26) | |
| TRV (m/s) | 3.9 (3.0–4.9) |
| sPAP/sSAP (%) | 71 (39–102) |
| mPAP (mmHg) (mean ± range) | 37 (27–55) |
| PVRi (WU) (mean ± range) | 3.9 (2.2–15.3) |
| TAPSE (mm) | 1.54 (1.21–1.74) |
| PAAT (ms) | 75 ± 19 |
| Diagnosis (n) | |
| AVSD | 12 |
| VSD | 10 |
| PA with VSD | 4 |
| IPAH (n = 12) | |
| TRV (m/s) | 4.4 (3.3–5.6) |
| sPAP/sSAP (%) | 88 (44–118) |
| mPAP (mmHg) (mean ± range) | 47 (32–91) |
| PVRi (WU) (mean ± range) | 8.9 (3.1–20.4) |
| TAPSE (mm) | 1.32 (1.24–1.66) |
| PAAT (ms) | 70 ± 16 |
| PH-BPD (n = 19) | |
| TRV (m/s) | 3.4 (2.9–4.3) |
| sPAP/sSAP (%) | 62 (38–85) |
| mPAP (mmHg) (mean ± range) | 35 (27–54) |
| PVRi (WU) (mean ± range) | 5.6 (3.0–15.8) |
| TAPSE (mm) | 1.12 (0.85–1.55) |
| PAAT (ms) | 65 ± 16 |
Values are presented as n or median (range) unless otherwise specified.
AVSD, atrioventricular septal defect; BSA, body surface area; BPD, bronchopulmonary dysplasia; IPAH, idiopathic pulmonary arterial hypertension; mPAP, mean pulmonary arterial pressure; NYHA, New York Heart Association; PA, pulmonary atresia; PAAT, pulmonary artery acceleration time; PH-CHD, pulmonary hypertension secondary to congenital heart disease; PVRi, pulmonary vascular resistance indexed; RV, right ventricle; sPAP/sSAP, systolic pulmonary arterial pressure/systolic systemic arterial pressure; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion; TRV, tricuspid regurgitation velocity; VSD, ventricular septal defect; WU, Wood Units.
NYHA FC and the modified ROSS score18,19 were determined by two pediatric cardiologists who were responsible for the daily medical care of the patients. At the time of enrolment, all patients were in a clinically stable condition without any changes in medications over the preceding four months. PH-targeted treatment was performed in accordance with the treatment algorithm for pediatric PH during the study period (Table 1).
This study complies with all institutional guidelines related to patient confidentiality and research ethics including institutional review board approval of the Ethics Board of the Medical University of Graz.
Echocardiography
An institutional study protocol for transthoracic echocardiography, including predefined two-dimensional (2D) variables and M-Mode variables, was designed and performed in all children with PH who visited our referral center. Echocardiograms were performed by two specifically trained cardiac sonographers (AG, MK), using commercially available echocardiographic system (Sonos iE33, Philips, Andover, MA, USA) using transducers of 5-1, 8-3, and 12-4 MHz depending on patient size and weight. Images were recorded digitally and later analyzed by one of the investigators using off-line software (Xcelera Echo; Philips Medical Systems, Eindhoven, The Netherlands). Echocardiographic images were considered to be of sufficient quality only if the entire endocardial surface of the cavities was clearly visualized in the apical four-chamber (4C) view at end-systole. All measurements were performed according to the pediatric guidelines of the American Society of Echocardiography (ASE).20
Imaging parameters of the left heart
LA dimension: As per ASE guidelines, the measurements of LA size and area were performed at end-systole.20 The area was obtained by tracing the endocardial border, excluding the pulmonary vein entrance.
LV dimension: The left ventricular internal dimensions at end-diastole (LVEDd) were measured in the apical 4C view at the level of the mitral valve leaflet tips obtained from 2D echocardiographic images. End-diastole was defined as measured at the end of the R wave.
LVEF: The biplane method of disks (modified Simpson method) as a 2D echocardiographic technique requiring area tracings of the LV cavity was used. This is the method usually recommended by the ASE for measuring LVEF.20
Imaging parameters of the right heart
RA dimension: The RA area is traced at the end of ventricular systole from the lateral aspect of the tricuspid annulus to the septal aspect, excluding the area between the leaflets and annulus, following the RA endocardium, excluding the IVC and SVC and the RA appendage.
RV dimension: In the apical 4C view, the RV internal diameter at the base (RVEDd) was measured just apical to the tricuspid annulus at end-systole as a horizontal line from endocardium of the RV free wall to endocardium of the interventricular septum.
Pulmonary artery acceleration time (PAAT): The PAAT was measured as the interval between the onset of ejection and the peak flow velocity, defined as the time from the onset to maximal velocity.21 The PAAT is the interval in microseconds from the onset of ejection to the peak flow velocity and can be used for the estimation of RV pressure and sPAP.
Systolic RV function: The tricuspid annular plane systolic excursion (TAPSE) reflects the longitudinal excursion of the tricuspid annulus toward the apex, i.e. longitudinal RV systolic function, and is measured with M-mode in the apical 4C view.22
Imaging parameters of ventricular–ventricular interaction
RV/LV ratio: The ratio of RV basal diameter of LV basal diameter measured from a parasternal short-axis image was calculated and measured three times. The RV/LV ratio was derived to combine a measure of RV size with septal shift secondary to elevated RV pressure.
RV end-diastolic to LV end-diastolic diameter ratio: The ratio of RVEDd to LVEDd was measured three times in the 4C view and their average was reported.
RV end-diastolic to LV end-diastolic area ratio: The ratio of RVED/LVED area was calculated and measured in the apical 4C view three times in end-diastole and their average was reported.
RA/LA area ratio: The RA/LA ratio was derived to combine a measure of RA size with possible interatrial septal shift secondary to elevated RA pressure. The ratio of RA to LA area was calculated and measured three times and their average was reported.
LV end-systolic eccentricity index (LVEI): The LVEI was defined as the ratio of the anterior–inferior and septal–posterolateral cavity dimensions at the mid-ventricular level, in the parasternal short-axis view, determined at end-systole. The LVEI is the ratio of the minor axis of the LV parallel to the septum divided by the minor axis perpendicular to the septum.6 The LVEI has limited power in postoperative CHD-PH patients due to an unpredictable effect of an e.g. VSD repair on the long-time performance of the interventricular septum. Of note, interpretation of LVEI is difficult or nearly impossible in patients with unrepaired CHD, e.g. children with a large, pressure-unrestrictive VSD.
Hemodynamic data
Patients in whom right heart catheterization was performed within two months from the baseline echo-study were included in analysis to evaluate the correlation between echocardiography and invasively obtained hemodynamic measures, previously reported to confirm prognosis in pediatric PH, i.e. PVRi, mean PAP (mPAP), and sPAP/sSAP ratio.23 Left-sided filling pressure and cardiac output data were obtained to rule out entities that can elevate PAP other than pulmonary vascular disorder, such as pulmonary venous hypertension. PH was defined as a mPAP ≥ 25 mmHg at rest, a pulmonary capillary wedge pressure (PCWP) < 15 mmHg, and a pulmonary vascular resistance (PVR) ≥ 3 mm (WU.m2). Catheterization data are provided in Table 1.
NYHA/modified ROSS score
The use of NYHA FC is limited in young children because of its subjective nature, although several pediatric studies have now shown NYHA FC to be a useful predictor of outcome also in pediatric PAH. An adapted functional classification for children (the modified ROSS score) has therefore been proposed and is now commonly used in children.18,19
N-terminal-pro brain natriuretic peptide (NT-proBNP)
NT-proBNP was analyzed within a routine blood sample. Therefore, blood was taken from the vein and was collected into lithium heparin tubes. The NT-proBNP assay was performed with Cobas 8000 from Roche Diagnostics (Mannheim, Germany).
Statistics
All data were measured from two well-trained observers. For data analysis, SPSS 24.0.0.0 (IBM Corp., Armonk, NY, USA) and the R package ggplot2 (version 3.1.0) was used. Data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). To account for systematic changes in the measured parameters in children and adolescents, values were z-transformed whenever possible. TAPSE were z-transformed using an age-specific formula18) and RVED area, LA area, and RA were z-transformed using BSA-specific formulas.20 Associations between age, sPAP, and PVRi with parameters of the left atrial, left ventricular, and ventricular interdependence were analyzed using Pearson's correlation coefficient (r) or Spearman's rank correlation (ρ). For each correlation, 95% confidence intervals (95% CI) were calculated using bootstrapping techniques. For visualizing, correlation coefficients and their 95% CI forest plots are given in the Supplemental Section, as Supplemental Figs. 3 and 4. Differences between WHO FC groups were analyzed and adjusted for patient's age using ANCOVA. Not normally distributed variables were rank transformed before performing ANCOVA. If the overall test showed a significant difference between groups, the significant results of the post-hoc analysis using Bonferroni adjustment is reported. Otherwise the overall test is reported.
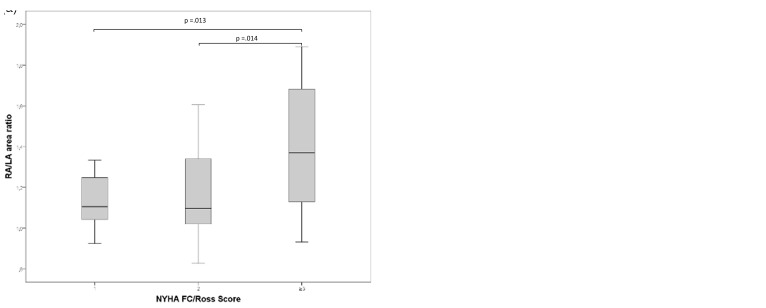
Fig. 3.
(a) Differences in RA/LA area ratio according to NYHA FC/Ross Score and (b) Differences in RV/LV dimension ratio according to NYHA FC/Ross Score. FC, functional class; LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
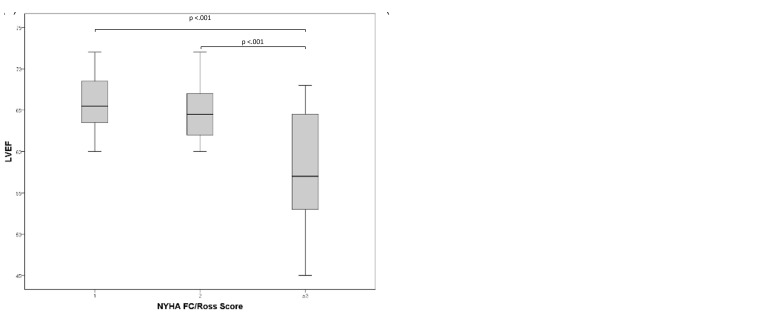
Fig. 4.
(a) Differences according to NYHA FC/Ross Score and the LVEF and (b) Differences according to NYHA FC/Ross Score and the LVEI. FC, functional class; LVEF, left ventricular ejection fraction; LVEI, left ventricular eccentricity index.
Results
Left heart parameters
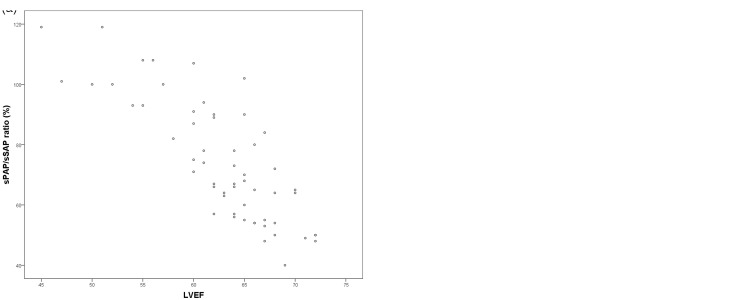
LVEF negatively correlated with sPAP/sSAP ratio (r = −0.810, P < 0.001) in the PH patients, with 41 of 57 patients (72%) still having a LVEF > 60%. Increasing PVRi was associated with decreasing LVEF (ρ = −0.479, P = 0.001) and with increasing LVEI (ρ = 0.508, P < 0.001) (Fig. 1). No significant correlation between LA area versus sPAP/sSAP ratio (ρ = 0.046, P = 0.733) or versus PVRi (ρ = −0.120, P = 0.432) could be detected.
Fig. 1.
(a) Association of the LVEF with the sPAP/sSAP ratio (r = −0.810, P < 0.001) and (b) Association of the LVEF with the PVRi (ρ = −0.479, P = 0.001). LVEF, left ventricular ejection fraction; PVRi, indexed pulmonary vascular resistance; sPAP, systolic pulmonary arterial pressure, sSAP, systolic systemic arterial pressure.
Right heart parameters
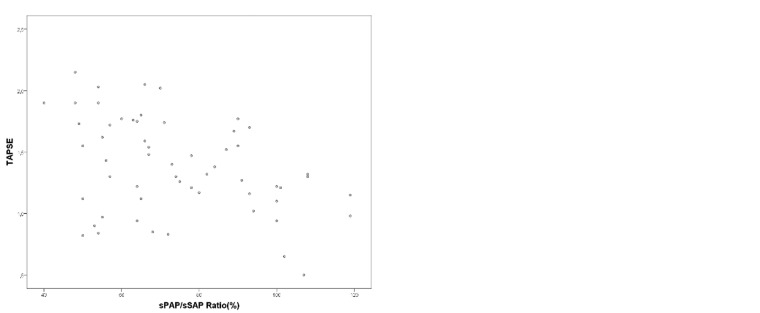
Higher RVED area values (ρ = 0.916, P < 0.001) and RA area values (ρ = 0.921, P < 0.001) were detected with an increasing disease severity (higher NYHA FC/ROSS score) in our PH patients. The systolic function parameter TAPSE decreased with an increasing sPAP/sSAP ratio (r = −0.392, P = 0.003), an increasing PVRi (ρ = −0.477, P < 0.001), and an increasing age (ρ = 0.631, P < 0.001). Age-specific TAPSE z-scores decreased with an increasing NYHA FC (FC I = −1.12 ± 1.62, FC II = −2.52 ± 1.51, FC III = −4.29 ± 1.58; FC I vs. FC III, P < 0.001; FC II vs. FC III, P < 0.005) in our patients (Fig. 2).
Fig. 2.
(a) Association of the TAPSE with the sPAP/sSAP ratio and (b) Differences in TAPSE according to the NYHA FC/Ross Score. FC, functional class; sPAP, systolic pulmonary arterial pressure, sSAP, systolic systemic arterial pressure; TAPSE, tricuspid annular plane systolic excursion.
Interdependence parameters
An increasing sPAP/sSAP ratio was associated with an increasing RV/LV dimension ratio (r = 0.752, P < 0.001), with an increasing RVED/LVED area ratio (r = 0.700, P < 0.001), and with an increasing end-systolic RA/LA area ratio (r = 0.735, P < 0.001). With an increasing LVEI, the sPAP/sSAP ratio increased (r = 0.636, P < 0.001). An increasing PVRi was associated with an increasing ratio of the echocardiographically derived variables RVEDd/LVEDd (r = 0.459, P < 0.005), RVED/LVED area (ρ = 0.471, P < 0.001), and RV/LV dimension ratio (ρ = 0.399, P < 0.01). The invasively measured parameter PVRi positively correlated with an increasing RA/LA area ratio (ρ = 0.513, P < 0.001), (Supplemental Fig. 1).
NYHA/modified ROSS score
The echocardiographically measured RA/LA area ratio increased with increasing NYHA FC in our pediatric PH patients (FC1 = 1.13 ± 0.13, FC2 = 1.15 ± 0.20, FC3 = 1.39 ± 0.32 [FC I vs. FC III, P < 0.02; FC II vs. FC III, P < 0.02]). Similarly, the RV/LV dimension ratio increased with higher NYHA FC (FC I = 0.87 [0.79–1.03], FC II = 0.96 [0.85–1.03], FC III = 1.18 [1.02–1.30] [FC I vs. FC III, P < 0.001]) (Fig. 3). Accordingly, increasing NYHA FC was associated with decreasing LVEF (FC I = 65.9 ± 3.7, FC II = 64.7 ± 3.6, FC III = 57.6 ± 6.8 [FC I vs. FC III, P < 0.001, FC II vs. FC III, P < 0.001]), while the LVEI (FC I = 1.29 ± 0.15, FC II = 1.45 ± 0.21, FC III = 1.64 ± 0.26 [FC I vs. FC III, P < 0.001, FC II vs. FC III, P < 0.05]) significantly increased (Fig. 4).
Biomarker NT-proBNP
NT-proBNP values of our PH patients significantly increased with an increasing sPAP/sSAP ratio (ρ = 0.509, P < 0.001). With increasing disease severity (NYHA FC), the NT-proBNP values significantly increased (FC I = 160 [93–299], FC II = 226 [87–399], FC III = 981 [227–2060] [FC I vs. FC III, P < 0.005, FC II vs. FC III, P < 0.01]) (Supplemental Fig. 2).
Discussion
Herein, we investigated echocardiographic variables of biventricular dimensions/function, VVI, and their correlations with hemodynamic values, disease severity, and NT-proBNP values in a pediatric PH cohort.
Previous studies on the use of echocardiography in pediatric PH mostly focused on detection of elevated PAP as a means to either detect PH or to provide a non-invasive tool for follow-up in PH patients.15,16,25 Measurements of left and right heart dimensions and functional variables are part of the pediatric guidelines of the ASE.20 In addition, recently published pediatric nomograms for various chamber diameters showed that the most reproducible measurements of left heart and right heart variables can be derived from the apical 4C view.24 The role of left heart size dimensions in diseases such as PH is currently increasingly recognized.26,27 The physiology of VVIs is based on the in-series circulation and common pericardium, interventricular septum, and myocardial tracts running between the ventricles.28 When an RV is enlarged due to pressure loading, such as in PH, it compresses the LV. The impact of the hypertensive RV is related to underfilling and dysfunction of the compressed LV, which reduces the LV ability to generate appropriate output.29 Previous data on adult IPAH patients suggested that LV mechanics are altered, due to VVIs.30
In our pediatric PH cohort, we found that a decreasing LVEF (in patients with severe PH) is associated with an increasing sPAP/sSAP ratio and also with an (invasively determined) increasing PVRi. With increasing disease severity in our patients, the LVEF was found to be decreased. Usually, due to the underfilling and compression of the LV in suprasystemic PH, the end-systolic volume of the LV in adults is described to be often very small, making the measured LVEF normal or high normal, although the LV stroke is likely decreased. However, we find different association in our cohort probably because most of our patients had sub-systemic RV pressure and were postoperative CHD-PH patients. Similar to our pediatric findings, Driessen et al.31 reported the more pronounced LV compression, the more distended and dysfunctional the RV becomes and the lower its transverse shortening due to leftward systolic septal shift gets.
In 100 children with PH, the RVEF as well as the LV stroke volume measured by cardiac MR have been shown to correlate with clinical status and prognosis in children with PH.11 These findings underlie the clinically relevant concept of VVIs in PH. Smaller volumes of the left atrium were shown to be a marker of disease severity in adults, which are associated with higher PVRi, higher sPAP/sSAP ratio, and higher BNP.32 It is under discussion whether the reduced LV dimensions in advanced PH are more a result of an imbalanced RV/LV ratio because of compression by a dilated high-pressure RV or whether these reduced LV dimensions could also be due to ventricular interdependence-related change of the LV structure.
The LVEI measures the LV lateral dimension as a ratio over the anterior–posterior dimension in the short axis and is used to evaluate LV compression for hemodynamic assessment in PH patients.33 The end-systolic LVEI has been shown to be a useful variable of RV mechanics in infants with bronchopulmonary dysplasia and may be incorporated in routine protocols when there is a concern for PH in neonates.34,35 The LVEI can help guide clinicians as to whether relief of RV pressure overload (e.g. due to new medications) is adequate. We found that our pediatric PH patients with increased LVEI values have significantly smaller LA areas (P = 0.001) and that the LVEI increased with an increasing sPAP/sSAP ratio and also with an increasing PVRi, showing that the degree of LV compression due to significant RV afterload can be visualized, at least in PH-CHD patients with an intact ventricular septum.
The RV/LV end-systolic diameter ratio, as a different VVI variable, is measured from a parasternal short-axis image at the LV papillary muscle level as the ratio of the anterior–posterior dimension of RV and LV diameters at end-systole. The RV/LV ratio has been shown to correlate well with invasive hemodynamic measures and to be higher in pediatric PH patients compared to controls.16 In our pediatric PH cohort, the RV/LV ratio positively correlated with an increasing sPAP/sSAP ratio and with an increasing NYHA FC. This suggests that these VVI variables (RV/LV ratio and the LVEI) might be able to reflect the disease severity of PH also in children suffering from PH.
The systolic longitudinal RV function parameter TAPSE is vastly independent of heart rate (HR) and, thus, is especially useful in children18 and accepted to assess systolic RV function in children with PH.21 In this study, TAPSE decreased with an increasing sPAP/sSAP ratio and an increasing PVRi. TAPSE also decreased with increasing NYHA FC in our pediatric PH patients. Therefore, our findings are in line with a previous report showing a correlation between elevated sPAP and decreased TAPSE values in pediatric PH.21
Recently, children with PH were investigated by three-dimensional (3D) echocardiography: the ratio of stroke volume/end-systolic volume, as a surrogate of RV-arterial coupling ratio, correlates with RV strain data and is a strong predictor of adverse clinical events in pediatric PH. Furthermore, 3D-measured RVEF and free wall RV strain were reported as outcome predictors in children with PH.10,36
NT-proBNP, a surrogate marker of RA stretch, is a well-established biomarker used in adult patients with PAH,37 while in pediatric PH experience is relatively limited.38 Pediatricians have to consider changing normative values of NT-proBNP throughout childhood.39 Nir et al.39 have shown that the levels of NT-proBNP are highest in the first days of life and decrease thereafter. Ploegstra et al.6 identified the NT-proBNP as a significant prognostic factor in pediatric PH. NT-proBNP was also found to be a useful biomarker for both respiratory exacerbations and mortality in pediatric PH patients.37 In our PH children, the NT-proBNP values positively correlated with the sPAP/sSAP ratio and with increasing NYHA FC/modified ROSS score. Thus, our data further support the use of NT-proBNP as a prognostic marker in children with PH.
We here provide a comprehensive overview of echocardiographic biventricular dimension and function and VVI variables in our pediatric PH cohort. We show that these echocardiographic variables significantly correlate with disease severity (NYHA FC/modified ROSS score), NT-proBNP values, and hemodynamic values. Thus, we suggest that a significantly increased RV afterload causes RV volume loading, which may paradoxically reduce LVEF because of direct VVI.
Limitations
The heterogeneity in a patient's diagnosis (IPAH, PH-BPD, PH-CHD) may limit the interpretation of the findings. It is important to note that even slight differences in the echocardiographic plan can result in substantial differences in area and diameter quantification.40 From an imaging point of view, LVEF was calculated by the modified Simpson method that can be inaccurate in abnormally shaped ventricles. As the prognostic value of 6-min walking distance (6MWD) in young children is not clear, with a minority of children being able to perform a reliable 6-min walking test we did not include this parameter in our study. We think this is justified due to data of a pediatric registry, where 6MWD was available in only one-third of children in a global cohort.1 Furthermore, we found an association between increased sPAP/SAP and lower LVEF values. It is well-known that, due to a relative reduction in end-systolic LV size, LVEF artificially increases in beginning PH. Therefore, our findings likely represent more advanced disease states with all patients already receiving PH-targeted medication. Further studies are required to validate our data and to evaluate other parameters such as strain parameters concerning biventricular dimensions and function as well as VVI variables in pediatric PH.
Supplemental Material
Supplemental Material for Ventricular–ventricular interaction variables correlate with surrogate variables of clinical outcome in children with pulmonary hypertension by Martin Koestenberger, Hannes Sallmon, Alexander Avian, Massimiliano Cantinotti, Andreas Gamillscheg, Stefan Kurath-Koller, Sabrina Schweintzger and Georg Hansmann in Pulmonary Circulation
Acknowledgments
The authors thank Bettina Leschnik for her technical and laboratory support.
Conflict of interest
GH currently receives grant support from the German Research Foundation (DFG; HA 4348/6-1, KFO311). MK is a consultant an is on the Advisory Board of Actelion.
Ethics
This study complies with all institutional guidelines related to patient confidentiality and research ethics including institutional review board approval of the Ethics Board of the Medical University of Graz.
Funding
This study was supported by intramural funding and travel grants from the European Pediatric Pulmonary Vascular Disease Network.
References
- 1.Beghetti M, Berger RM, Schulze-Neick I, et al. TOPP Registry Investigators Diagnostic evaluation of paediatric pulmonary hypertension in current clinical practice. Eur Respir J 2013; 42: 689–700. [DOI] [PubMed] [Google Scholar]
- 2.Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013; 62: D117–D126. [DOI] [PubMed] [Google Scholar]
- 4.Mertens LL, Friedberg MK. Imaging the right ventricle–current state of the art. Nat Rev Cardiol 2010; 7: 551–563. [DOI] [PubMed] [Google Scholar]
- 5.Koestenberger M, Friedberg MK, Ravekes W, et al. Non-invasive imaging for congenital heart disease: recent innovations in transthoracic echocardiography. J Clin Exp Cardiolog 2012Suppl 8 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ploegstra MJ, Roofthooft MT, Douwes JM, et al. Echocardiography in pediatric pulmonary arterial hypertension: early study on assessing disease severity and predicting outcome. Circ Cardiovasc Imaging 2014; 8: e000878. [DOI] [PubMed] [Google Scholar]
- 7.Kassem E, Humpl T, Friedberg MK. Prognostic significance of 2-dimensional, M-mode, and Doppler echo indices of right ventricular function in children with pulmonary arterial hypertension. Am Heart J 2013; 165: 1024–1031. [DOI] [PubMed] [Google Scholar]
- 8.Okumura K, Slorach C, Mroczek D, et al. Right ventricular diastolic performance in children with pulmonary arterial hypertension associated with congenital heart disease: correlation of echocardiographic parameters with invasive reference standards by high-fidelity micromanometer catheter. Circ Cardiovasc Imaging 2014; 7: 491–501. [DOI] [PubMed] [Google Scholar]
- 9.Friedberg MK. Imaging right-left ventricular interactions. JACC Cardiovasc Imaging 2018; 11: 755–771. [DOI] [PubMed] [Google Scholar]
- 10.Jone PN, Schäfer M, Pan Z, et al. 3D echocardiographic evaluation of right ventricular function and strain: a prognostic study in paediatric pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018; 19(9): 1026–1033. [DOI] [PubMed] [Google Scholar]
- 11.Moledina S, Pandya B, Bartsota M, et al. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging 2013; 6(3): 407–414. [DOI] [PubMed] [Google Scholar]
- 12.Burkett DA, Slorach C, Patel SS, et al. Impact of pulmonary hemodynamics and ventricular interdependence on left ventricular diastolic function in children with pulmonary hypertension. Circ Cardiovasc Imaging 2016; 9(9): e004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer M, Collins KK, Browne LP, et al. Effect of electrical dyssynchrony on left and right ventricular mechanics in children with pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37(7): 870–878. [DOI] [PubMed] [Google Scholar]
- 14.Ten Kate CA, Tibboel D, Kraemer US. B-type natriuretic peptide as a parameter for pulmonary hypertension in children. A systematic review. Eur J Pediatr 2015; 174: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 15.Koestenberger M, Nagel B, Ravekes W, et al. Tricuspid annular peak systolic velocity (S') in children and young adults with pulmonary artery hypertension secondary to congenital heart diseases, and in those with repaired tetralogy of Fallot: echocardiography and MRI data. J Am Soc Echocardiogr 2012; 25: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 16.Jone PN, Hinzman J, Wagner BD, et al. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr 2014; 27: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurudevan SV, Malouf PJ, Auger WR, et al. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension—true diastolic dysfunction or left ventricular underfilling?. J Am Coll Cardiol 2007; 49: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 18.Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol 2012; 33: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 19.Masarone D, Valente F, Rubino M, et al. Pediatric heart failure: a practical guide to diagnosis and management. Pediatr Neonatol 2017; 58: 303–312. [DOI] [PubMed] [Google Scholar]
- 20.Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010; 23: 465–495. [DOI] [PubMed] [Google Scholar]
- 21.Koestenberger M, Grangl G, Avian A, et al. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ Cardiovasc Imaging 2017; 10: e005336. [DOI] [PubMed] [Google Scholar]
- 22.Koestenberger M, Ravekes W, Everett A, et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr 2009; 22: 715–719. [DOI] [PubMed] [Google Scholar]
- 23.Rosenzweig EB, Abman S, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019; 53(1): 1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantinotti M, Scalese M, Murzi B, et al. Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J Am Soc Echocardiogr 2014; 27: 1279–1292. [DOI] [PubMed] [Google Scholar]
- 25.Koestenberger M, Apitz C, Abdul-Khaliq H, et al. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102(Suppl 2): ii14–22. [DOI] [PubMed] [Google Scholar]
- 26.Tonelli AR, Conci D, Tamarappoo BK, et al. Prognostic value of echocardiographic changes in patients with pulmonary arterial hypertension receiving parenteral prostacyclin therapy. J Am Soc Echocardiogr 2014; 27: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatzoulis MA, Alonso-Gonzalez R, Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev 2009; 18: 154–161. [DOI] [PubMed] [Google Scholar]
- 28.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 2014; 129: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 29.Marcus JT, Gan CT, Zwanenburg JJ, et al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008; 51: 750–757. [DOI] [PubMed] [Google Scholar]
- 30.Tonelli AR, Plana JC, Heresi GA, et al. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141: 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driessen MM, Hui W, Bijnens BH, et al. Adverse ventricular-ventricular interactions in right ventricular pressure load: Insights from pediatric pulmonary hypertension versus pulmonary stenosis. Physiol Rep 2016; 4: e12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014; 63: 493–505. [DOI] [PubMed] [Google Scholar]
- 33.Jensen AS, Broberg CS, Rydman R, et al. Impaired right, left, or biventricular function and resting oxygen saturation are associated with mortality in Eisenmenger syndrome: a clinical and cardiovascular magnetic resonance study. Circ Cardiovasc Imaging 2015; 8: e003596. [DOI] [PubMed] [Google Scholar]
- 34.Ehrmann DE, Mourani PM, Abman SH, et al. Echocardiographic measurements of right ventricular mechanics in infants with bronchopulmonary dysplasia at 36 weeks postmenstrual age. J Pediatr 2018; 203: 210–217.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham S, Weismann CG. Left ventricular end-systolic eccentricity index for assessment of pulmonary hypertension in infants. Echocardiography 2016; 33(6): 910–915. [DOI] [PubMed] [Google Scholar]
- 36.Jone PN, Schäfer M, Pan Z, et al. Right ventricular-arterial coupling ratio derived from 3-dimensional echocardiography predicts outcomes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging 2019; 12(1): e008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frantz RP, Farber HW, Badesch DB, et al. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL Registry. Chest 2018; 154: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amdani SM, Mian MUM, Thomas RL, et al. NT-pro BNP-A marker for worsening respiratory status and mortality in infants and young children with pulmonary hypertension. Congenit Heart Dis 2018; 13: 499–505. [DOI] [PubMed] [Google Scholar]
- 39.Nir A, Lindinger A, Rauh M, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009; 30: 3–8. [DOI] [PubMed] [Google Scholar]
- 40.Cantinotti M. Current pediatric nomograms are only one source of error for quantification in pediatric echocardiography: what to expect from future research. J Am Soc Echocardiogr 2013; 26: 919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Ventricular–ventricular interaction variables correlate with surrogate variables of clinical outcome in children with pulmonary hypertension by Martin Koestenberger, Hannes Sallmon, Alexander Avian, Massimiliano Cantinotti, Andreas Gamillscheg, Stefan Kurath-Koller, Sabrina Schweintzger and Georg Hansmann in Pulmonary Circulation