Abstract
Pulmonary hypertension (PH) is classified into five groups based on disease etiology but there is only limited information on the prognostic value of exercise testing in non-group 1 PH. In group 1 PH, the incremental shuttle walking test (ISWT) distance has been shown to correlate with pulmonary hemodynamics and predict survival without a ceiling effect. This study assessed the ISWT in non-group 1 PH. Data were retrieved from the ASPIRE Registry (Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre) for consecutive patients diagnosed with PH. Patients were required to have been systematically assessed as group 2–5 PH and to have a baseline ISWT within three months of cardiac catheterization. Patients were stratified according to incremental shuttle walk test distance (ISWD) and ISWT distance percent predicted (ISWD%pred). A total of 479 patients with non-group 1 PH were identified. ISWD and ISWD%pred correlated significantly with symptoms and hemodynamic severity. ISWD and ISWD%pred predicted survival with no ceiling effect. The test was prognostic in groups 2, 3, and 4. ISWD and ISWD%pred and change in ISWD and ISWD%pred at one year were all significant predictors of outcome. In patients with non-group 1 PH the ISWT is a simple non-invasive test that is easy to perform, is predictive of survival at baseline and follow-up, reflects change, and can be used in the assessment of PH of any etiology.
Keywords: exercise testing, disease severity, prognosis
Introduction
Many mechanisms can lead to an elevation of resting mean pulmonary arterial pressure (mPAP). Pulmonary hypertension (PH) is classified into five groups according to distinct pathophysiological characteristics.1 Group 1 (pulmonary arterial hypertension [PAH]) has been the most studied but PH due to other causes is more common.2,3 Whatever the cause, PH places a substantial burden on the patient, contributing to the severity of disease and affecting quality of life.4–9 As exercise limitation is a significant symptom in PH, it is important that exercise tests used for the assessment of physical functioning reflect the severity of disease. Currently, two exercise tests are mainly used in patients with PAH: the 6-min walking test (6MWT), which is an unencouraged field test; and the cardiopulmonary exercise test (CPET), which is an incremental maximal test.
Because of its simplicity, the 6MWT is the test most frequently used to monitor patients in the clinic and assess the efficacy of new therapies. However, concerns have been raised regarding the use of the 6MWT.10–13 In milder disease, a “ceiling effect” has been noted where 6-min walking distance (6MWD) no longer reflects maximal oxygen aerobic capacity (VO2peak)14–16 or disease severity;17 the test has been shown to be unresponsive to clinically important changes in hemodynamic parameters,17–20 while change in 6MWD does not explain a large proportion of observed treatment effect.21–23 Very few studies look specifically at the prognostic significance of the 6MWT distance in non-group 1 patients.24–26
CPET is a more complex test. It provides information on the etiology of exercise limitation,27 provides prognostic information,28 reflects hemodynamic severity and, unlike the 6MWT, reflects VO2 peak even in mild disease. However, due to its complexity, routine use of CPET is challenging and when used in a randomized controlled trial, it was unable to demonstrate a treatment effect.29 In addition, the prognostic utility of CPET variables may also differ according to the etiology of PAH.30
The ISWT is an externally paced symptom-limited test requiring the patient to walk around a 10 -m course. The speed increases every minute and patients continue walking until they can no longer keep pace with the signal. Strong correlations are found between distance walked and VO2 peak in patients with different diseases, including heart failure.31,32 In addition to correlating more strongly than the 6MWT with VO2 peak, the ISWT has been found to be a better predictor of survival than the 6MWT in chronic heart failure.33
The ISWT shares benefits of both the 6MWT and CPET. It is simple and quick to perform but also has the benefits of an incremental nature to maximum exercise capacity. In addition, as the test uses a 10 -m course rather than the 30 -m corridor required for the 6MWT, it is easy to perform in the clinical environment. Because of these advantages, the ISWT has been proposed as an alternative field walking test to the 6MWT in PAH,34,35 and a small study looking specifically at patients with PAH noted that ISWT distance (ISWD) correlated more closely with peak oxygen consumption than 6MWT distance.36
Using data from the ASPIRE Registry, we recently demonstrated in patients with PAH that distance walked during the ISWT correlated with pulmonary hemodynamics, World Health Organization (WHO) functional class (FC) and resting Borg score, and predicted prognosis both at baseline and at subsequent follow-up, all without a ceiling effect.37 This study now assesses the usefulness of the ISWT in patients with non-group 1 PH.
Methods
Data were retrieved from the ASPIRE Registry for consecutive patients diagnosed with PH, defined as having a mPAP ≥ 25 mmHg, during 2001–2010, as previously described.38
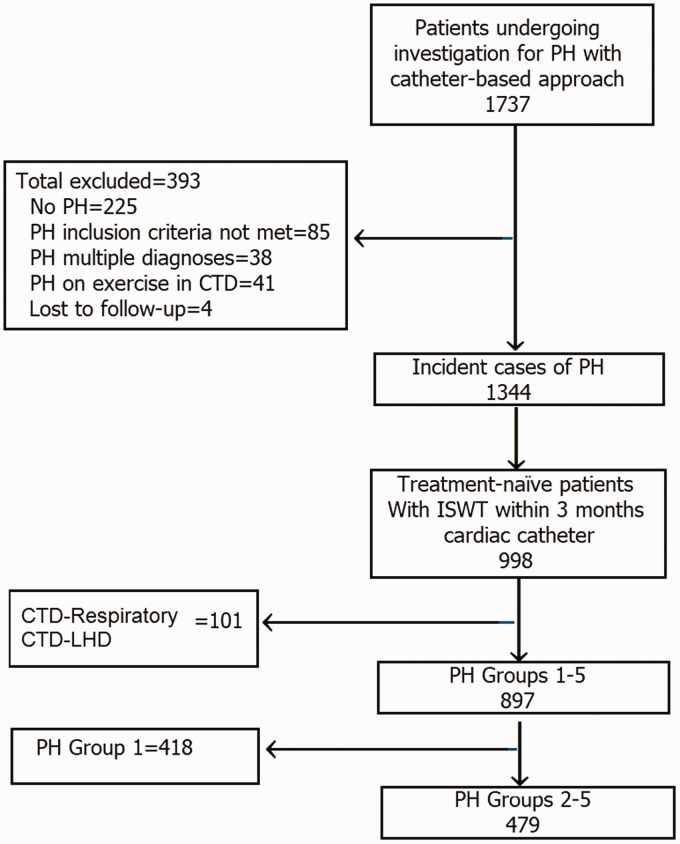
For inclusion, patients were required to have been systematically assessed as group 2–5 PH (PH due to: left heart disease = group 2; lung disease and/or hypoxemia = group 3; chronic thromboembolic PH [CTEPH] = group 4; and unclear or multifactorial etiologies = group 5) and to have a baseline ISWT within three months of cardiac catheterization. Patients with multiple potential causes of PH were excluded to allow comparisons between single phenotypes, e.g. patients with connective disease with left heart disease or lung disease or patients with CTEPH and significant co-existing left heart disease or lung disease, were excluded (Fig. 1). Ethical approval was granted by the North Sheffield Research Ethics Committee (reference no. 06/Q2308/8).
Fig. 1.
The study cohort. PH, pulmonary hypertension; ISWT, incremental shuttle walk test; CTD, connective tissue disease; PAH, pulmonary arterial hypertension.
Incremental shuttle walk test
The ISWT was performed according to the method of Singh et al.39 Using a standardized recording, patients were asked to walk as far as possible around the 10-m course keeping in time to the audio signal until they were too breathless or could no longer keep up with the speed. The initial walking speed was 0.50 m/s and this increased incrementally every minute to a maximum of 2.37 m/s. The primary measure was distance walked. In addition, breathlessness was measured at rest before the ISWT and at the end of the test. Percent predicted ISWT distance (ISWT%pred) was calculated for each patient based on sex, age, and body mass index using the equation derived by Probst et al.40 Patients were recorded as walking 0 m if they could not complete 1 shuttle length in the required time while breathing room air. A small proportion of patients who were on high flow O2 who were unable to remove their oxygen and attempt to walk along the corridor were assigned a walking distance of 0 m, if in the opinion of the physiologist they were unable to complete 1 shuttle length.
Follow-up
Survival status was ascertained at the census point via the NHS enhanced reporting service. Data were collected for patients who had had a repeat assessment at 12 months (±2 months) after diagnosis.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v19 (SPSS, Chicago, IL, USA). Continuous variables were described by mean ± standard deviation (SD). If data were not normally distributed, results were presented as median ± interquartile range (IQR). Categorical variables are presented as n (%). Pearson’s correlation test was used to assess correlations between ISWD or ISWD%pred and hemodynamic parameters and was tested for two-sided significance. Student’s t-test and Mann–Whitney U test were used to compare groups. Multiple comparisons between groups were performed using ANOVA. Event (death or transplantation)-free survival from date of diagnosis was estimated using the Kaplan–Meier method with comparison between groups performed by the log-rank test. The accuracy of the prognostic parameters was estimated using receiver operating characteristic (ROC) curves. A P value < 0.05 was deemed statistically significant.
Stratification of patients
A model with five pre-specified bands based on walking distance/maximal walking speed was used as previously described for group 1 PAH:37 band 1 = 0–30 m; band 2 = 40–120 m; band 3 = 130–250 m; band 4 = 260–420 m; and band 5 = 430–1020 m. ISWD%pred was similarly stratified into five bands: band 1 = 0–10%; band 2 > 10–20%; band 3 > 20–35%; band 4 > 35–60% and band 5 > 60%. At follow-up, improvement in ISWD was defined as an increase >40 m, decline was defined as a decrease >20 m, and the remaining patients were defined as stable. Improvement in ISWD%pred was defined as an increase in ISWD%pred of >10%, decline was defined as a decrease of >4% ISWD%pred, and the remaining patients were defined as stable. Patients were also grouped by age to assess the utility of the ISWT in more elderly patients.
Results
A total of 479 patients with non-group 1 PH were identified (Fig. 1). Patient characteristics are shown in Table 1. Data completeness was high (>90%). Median distance walked at baseline was 120 m and median time to complete the walk was 3.2 min. Details of treatment are given in Supplementary Table S1A.
Table 1.
Patient characteristics.
| Overall (n = 479) | Group 2 LHD (n = 103) | Group 3 Lung (n = 147) | Group 4 CTEPH (n = 202) | Group 5 Unclear (n = 27) | |
|---|---|---|---|---|---|
| Female (n (%)) | 240 (50) | 69 (67) | 51 (35) | 105 (52) | 15 (56) |
| Age (years) | 65 ± 13 | 70 ± 10 | 66 ± 11 | 61 ± 15 | 58 ± 15 |
| WHO FC I + II/III + IV (%) | 17/83 | 28/72 | 12/88 | 15/85 | 15/85 |
| mRAP (mmHg)* | 10.0 (8.0) | 14.0 (9.0) | 9.0 (6.0) | 10.0 (7.0) | 11.0 (10.0) |
| mPAP (mmHg) | 43 ± 11 | 39 ± 13 | 43 ± 12 | 47 ± 11* | 43 ± 12 |
| PWP (mmHg) | 13.8 ± 6.7 | 23.2 ± 4.3 | 11.9 ± 5.0 | 10.6 ± 4.4 | 10.4 ± 4.2 |
| CI (L/min/m2)* | 2.6 (1.1) | 2.9 (0.8) | 2.8 (1.3) | 2.4 (1.0)* | 2.8 (1.5) |
| PVR (Wood Unit)* | 5.8 (6.7) | 2.7 (2.3) | 5.3 (6.0) | 8.4 (6.1)* | 6.0 (6.8) |
| SmvO2 (%) | 62 ± 9 | 64 ± 9 | 65 ± 8 | 60 ± 8* | 59 ± 11 |
| FEV1 (%pred) | 69 ± 23 | 69 ± 21 | 55 ± 24 | 79 ± 18 | 68 ± 25 |
| FVC (%pred) | 83 ± 24 | 78 ± 23 | 74 ± 27 | 91 ± 20 | 81 ± 19 |
| FEV1/FVC (%) | 68 ± 13 | 71 ± 10 | 60 ± 17 | 71 ± 12 | 68 ± 17 |
| Tlco (%pred) | 53 ± 21 | 62 ± 17 | 34 ± 17 | 63 ± 16 | 41 ± 21 |
| ISWT distance (m)* | 120 (160) | 120 (150) | 80 (130) | 140 (210) | 120 (160) |
| ISWT time (min)* | 3.2 (2.9) | 3.2 (2.8) | 2.4 (2.6) | 3.5 (3.4) | 3.2 (2.8) |
Values are presented as mean ± SD for parametric data and *median (IQR) for non-parametric data.
mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; PWP, pulmonary wedge pressure; LHD, left heart disease; CTEPH, chronic thromboembolic pulmonary hypertension; CI, cardiac index; PVR, pulmonary vascular resistance; SmvO2, mixed venous oxygen saturation; FEV1, forced expiratory volume in 1 s; %pred, % predicted; FVC, forced vital capacity; TLco, transfer factor of the lung for carbon monoxide; ISWT, incremental shuttle walk test.
Disease severity
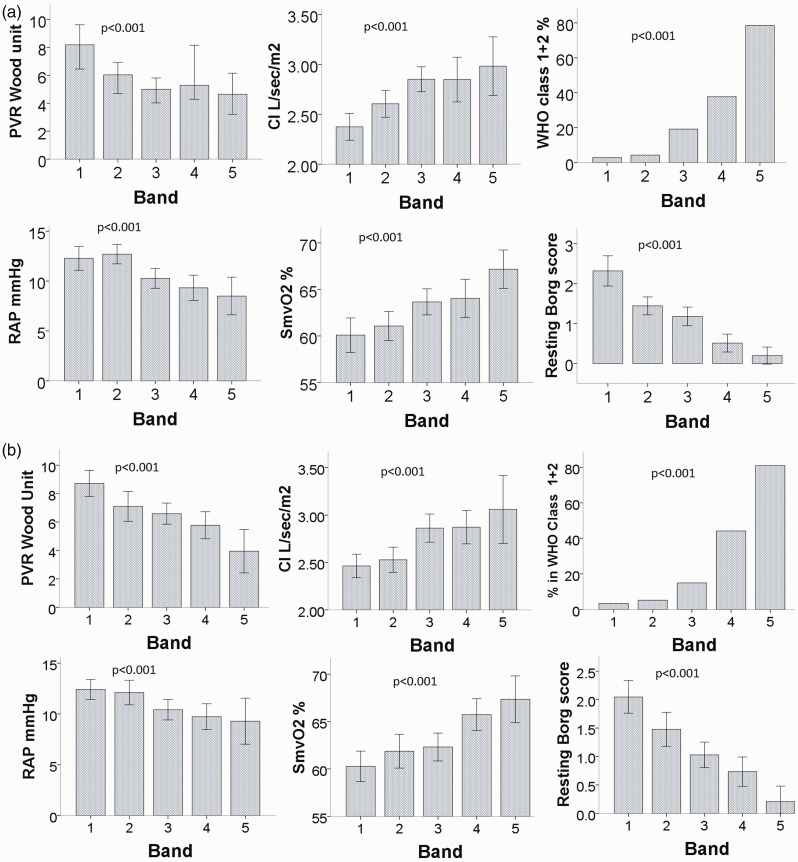
ISWD and ISWD%pred correlated significantly both with the severity of symptoms (Borg score and WHO FC) and with hemodynamic parameters including right atrial pressure (RAP), pulmonary vascular resistance (PVR), cardiac output, cardiac index, and mixed venous oxygen saturation (all P < 0.001). mPAP also correlated with ISWD (P = 0.025) and ISWD%pred (<0.001). One-way ANOVA demonstrated highly significant relationships (P < 0.001) across all ISWD and ISWD%pred bands with WHO FC, baseline resting Borg score, and baseline pulmonary hemodynamic parameters (Fig. 2). Analyzing etiological groups separately, in groups 3 and 4, ISWD and ISWD%pred correlated significantly with mPAP, mRAP, CI, PVR, and WHO FC. In addition, in group 4, ISWD and ISWD%pred also correlated with SmVO2. In group 2, ISWD correlated significantly with only SmVO2 and WHO FC while ISWD%pred correlated with mPAP and WHO FC (Supplementary Table S2).
Fig. 2.
Relationship between baseline ISWT bands. (a) Band 1 ISWD m = 10–30; band 2 = 40–120; band 3 = 130–250; band 4 = 260–420; band 5 = 430–1020; (b) ISWD%predicted band 1 = 0–10; band 2 > 10–20; band 3 > 20–35; band 4 > 35–60; band 5 > 60. CI, cardiac index; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SmVO2, mixed venous oxygen saturation; %WHO Class 1 + 2, percentage of patients in World Health Organization functional class I and II (all P < 0.001).
Baseline ISWD and survival
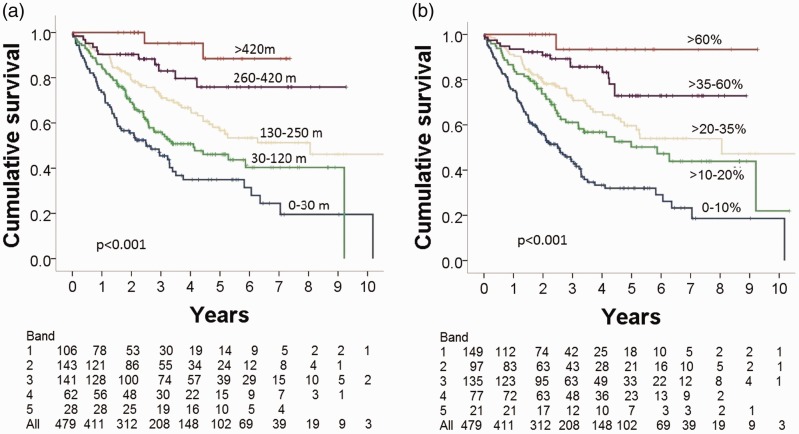
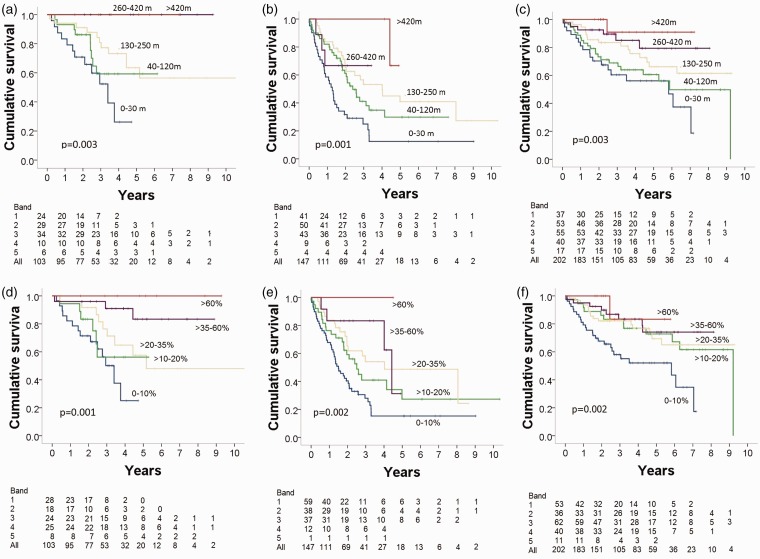
Kaplan–Meier survival analysis demonstrated that both baseline ISWD and baseline ISWD%pred predicted survival with no ceiling effect across the five bands (both P < 0.001) (Fig. 3). ROC analysis found no significant difference in the predictive power for long-term survival between ISWD and ISWD%pred (area under the ROC curve [AUC] = 0.693, 95% confidence interval [CI] = 0.646–0.739 and 0.703, 95% CI = 0.657–0.750, respectively). Analyzing the PH groups separately, Kaplan–Meier survival analysis demonstrated that both baseline ISWD and baseline ISWD%pred predicted survival in groups 2, 3, and 4 (all P < 0.005); analysis was not performed in group 5 due to small numbers (n = 27) (Fig. 4). In addition to baseline ISWD predicting long-term outcome in all patients with CTEPH, it also predicted outcome in patients undergoing (n = 94, P = 0.045) and not undergoing surgery (n = 108, P = 0.008) during the study period. ISWD%pred also predicted outcome for all CTEPH patients (P = 0.002) and patients not undergoing surgery (P = 0.009) but was not a significant predictor in those who had had surgery,
Fig. 3.
Kaplan–Meier survival analysis stratified according to baseline. (a) ISWD and (b) ISWD%predicted.
Fig. 4.
Kaplan–Meier survival analysis stratified according to baseline ISWD in patients with PH due to (a) left heart disease, (b) lung disease, and (c) CTEPH, and stratified by ISWD%predicted in patients with PH due to (d) left heart disease; (e) lung disease, and (f) CTEPH.
Follow-up
Follow-up data were available for 150 patients at one-year follow-up. Mean change in ISWD (ΔISWD) was +37 ±128 m (P = 0.001, paired t-test). Kaplan–Meier analysis showed that the ISWD at one year and ISWD%pred at one year were significant predictors of outcome (both P < 0.001). Sub-analysis of data on CTEPH patients (n = 93, 49 having endarterectomy) showed that patients ISWD predicted long-term survival (P = 0.04).
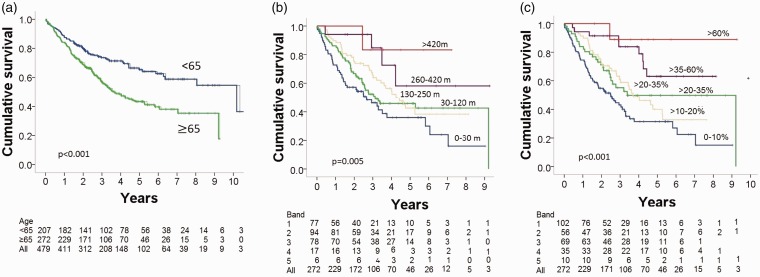
Age
Of the patients, 57% were aged ≥65 years. Patient characteristics stratified by age are shown in Table 2. At baseline, mPAP, CI, and SmvO2 were significantly lower in the older patients while there was no significant difference in PVR and mRAP. Those aged ≥65 years presented with more severe functional decline, with only 12% being in WHO FC 1 or 2 compared to 24% of patients aged <65 years (P = 0.002). This was reflected by a significantly lower ISWD and lower ISWD%pred. The distribution of patients in the distance bands also differed: of the older patients, 9% walked >250 m and 17% walked >35% predicted ISWD compared with 32% and 26%, respectively, in the younger patients. Nonetheless, although overall survival in older patients was worse than in patients aged <65 years (Fig. 5a), ISWD and ISWD%pred were significant predictors of long-term survival (Fig. 5b and c) and were as effective predictors of one-year survival in the elderly (AUC = 0.670, 95% CI = 0.557–0.776 and AUC = 0.701, 95% CI = 0.600–0.802, respectively) as for the younger patients (AUC = 0.678, 95% CI = 0.587–0.770 and AUC = 0.707, 95% CI = 0.621–0.792, respectively). Cox multivariate survival analysis, including either ISWD or ISWD%pred, hemodynamic parameters mRAP, mPAP, PVR, CI, and SmVO2, age, sex, and WHO FC I, II versus WHO FC III, IV, showed both ISWD or ISWD%pred to be independent predictors of outcome in both age groups. As well as predicting survival in older patients, ISWD and ISWD%pred also reflected disease severity in the elderly group, correlating significantly (all P < 0.005) with mRAP, mPAP, PVR, CI, and SmVO2 and WHO FC (Supplementary Table S2).
Table 2.
Patient characteristics in patients aged <65 and ≥65 years.
| <65 years n = 207 | ≥65 years n = 272 | p | |
|---|---|---|---|
| Female n (%) | 102 (49) | 138 (51) | >0.05 |
| WHO FC I + II n (%) | 49 (24) | 32 (12) | <0.001 |
| mRAP mmHg* | 10.0 (9.0) | 11.0 (7.0) | >0.05 |
| mPAP mmHg | 45 ± 12 | 42 ± 10 | 0.001 |
| CI L/min/m2* | 2.8 (0.8) | 2.6 (0.7) | 0.002 |
| PVR Wood Unit* | 6.0 (6.9) | 5.8 (6.7) | >0.05 |
| SmvO2 (%) | 64 ± 9 | 62 ± 9 | 0.019 |
| Wedge mmHg | 13 ± 7 | 14 ± 7 | 0.029 |
| ISWD* m | 180 (203) | 80 (130) | <0.001 |
| ISWD%pred* | 24 (24) | 16 (24) | <0.001 |
WHO FC: World Health Organization functional class; mRAP: mean right atrial pressure; mPAP: mean pulmonary artery pressure: CI: cardiac index; PVR: pulmonary vascular resistance; SmvO2: mixed venous oxygen saturation; ISWD: incremental shuttle walk distance
Fig. 5.
Kaplan–Meier survival curve (a) by age, (b) by ISWD in patients aged ≥65 years, and (c) by ISWD%pred in patients aged ≥65 years.
Discussion
This study is the first to show that in non-group 1 PH patients that distance walked using the ISWT, whether absolute or expressed as a percentage of predicted walking distance, correlates with baseline pulmonary hemodynamics, WHO FC, and resting Borg score, and predicts prognosis both at baseline and at subsequent follow-up. Furthermore, this test is prognostic of survival when the data are analyzed as a whole group, in separate etiological groups and older patients aged ≥65 years. Follow-up ISWTT distance had prognostic value, emphasizing the need for assessment at multiple timepoints.41
Patients with group 2–5 PH represent a substantial percentage of the patients referred to specialist PH centres;26,38 however, with the exception of group 4 (CTEPH), little has been published previously on the predictive significance of exercise tests in non-group 1 PH. There have been some studies of the predictive significance of the 6MWT in single non-group 1 PH sub-groups. A study of patients with PH related to heart failure with preserved ejection fraction found that 6MWD was an independent predictor of hospitalization and/or death for cardiac reasons.24 It has been shown that shown that 6MWD reflected the clinical and hemodynamic severity of disease in patients with CTEPH and the change in the 6MWD correlated with the observed clinical and hemodynamic improvement.26 One small study looking at the full range of group 2–5 PH found, in 60 patients, that a 6MWD < 400 m was associated with a higher risk of death. Numbers were too small to analyze etiological groups separately and follow-up data were not available.25 Analysis of survival data from the Giessen Pulmonary Hypertension Registry, the largest single-center PH registry, found that 6MWD was a predictor of outcome across all groups of PH, although the results were most robust in group 1.26 In groups 2, 3, and 4, they found that survival of the top quartile of patients who walked >390 m was not significantly better than patients in the third quartile who walked 311–390 m. Our study demonstrates the utility of a field exercise test in different PH diagnostic groups and across the range of disease severity with no ceiling effect.
Currently, guidelines do not recommend any PAH-specific drugs for the treatment of patients with group 2–5 PH, but to consider trials of therapy in carefully selected patients with severe PH.43 Nonetheless, studies have shown a significant number of these patients do receive PAH-targeted therapies44,45 and the need to evaluate therapies in groups 2 and 3 has been highlighted.44–47 We have demonstrated that the ISWT is a useful tool for assessing exercise capacity for clinical monitoring and may have utility as an endpoint in trials of targeted therapies for non-group 1 PH.
Limitations of study
The models of prediction using the ISWT were based on retrospective data from a single specialist PH center. Although the numbers were high at baseline (n = 479) and allowed analysis of groups 2–4, there were insufficient numbers to allow sub-group analysis. In addition, only 150 patients were re-evaluated at one year, limiting follow-up analysis, since it is not the policy of the center to follow-up group 2–5 patients unless they are receiving specific interventions. For patients with CTEPH, surgery is a potential confounding factor influencing outcome. Nonetheless, at baseline, ISWT distance predicted outcome for patients both undergoing and not undergoing surgery.
The data in this Registry were collected when PH was defined as a mPAP ≥ 25 mmHg. The definition of PH has recently been updated to mPAP > 20 mmHg.48 Further work is required to evaluate the utility of the ISWT in patients with mPAP > 20 mmHg and <25 mmHg.
In addition, the nature of this study precludes any comparison with the current standard field walking test used in the evaluation of PH (6MWT). However, this study further highlights the utility of the ISWT in the evaluation of patients with PH.
Conclusion
The ISWT is a simple non-invasive test that is easy to perform in patients with non-group 1 PH, is predictive of survival at baseline and follow-up, and reflects change. We have demonstrated that the ISWT can be used to predict outcome in both group 1 and non-group 1 PH.
Supplementary Material
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
AR was supported by a Wellcome Trust Clinical Research Career Development Fellowship (206632/Z/17/Z). RL was supported by a University of Sheffield Donald Heath Clinical Fellowship, JAH ‘s research fellowship during the period of the study was part-funded by an unrestricted educational grant from Actelion Pharmaceuticals Ltd. AL is a British Heart Foundation Senior Basic Sciences Research Fellow (FS/13/48/30453). AJS is supported by a Wellcome Trust Clinical Research Development Fellowship (205188/Z/16/Z). AART is supported by a BHF Intermediate Clinical Fellowship (FS/18/13/3328).
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D34–D41. [DOI] [PubMed] [Google Scholar]
- 2.Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013; 173(10): 887–893. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4(4): 306–322. [DOI] [PubMed] [Google Scholar]
- 4.Abramson SV, Burke JF, Kelly JJ, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med 1992; 116: 888–895. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Barbera JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009; 54(1 Suppl): S85–S96. [DOI] [PubMed] [Google Scholar]
- 6.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31: 373–380. [DOI] [PubMed] [Google Scholar]
- 7.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura M, Taniguchi H, Kondoh Y, et al. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration 2013; 85: 456–463. [DOI] [PubMed] [Google Scholar]
- 9.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011; 4(3): 257–265. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S97–S107. [DOI] [PubMed] [Google Scholar]
- 11.Rich S. The 6-minute walk test as a primary endpoint in clinical trials for pulmonary hypertension. J Am Coll Cardiol 2012; 60: 1202–1203. [DOI] [PubMed] [Google Scholar]
- 12.Faber HW. Validation of the 6-minute walk in patients with pulmonary arterial hypertension: trying to fit a square peg into a round hole? Circulation 2012; 126: 258–260. [DOI] [PubMed] [Google Scholar]
- 13.Gaine S, Simonneau G. The need to move from 6-minute walk distance to outcome trials in pulmonary hypertension. Eur Respir Rev 2013; 22: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipkin DP, Scriven AJ, Crake T, et al. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J 1986; 292: 653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deboeck G, Taboada D, Hagan G, et al. Maximal cardiac output determines 6 minutes walking distance in pulmonary hypertension. PLoS One 2014; 9: e92324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Plas MN, Duffels MG, Ponse D, et al. Bosentan in mild pulmonary hypertension. Lancet 2008; 372: 1730. [DOI] [PubMed] [Google Scholar]
- 17.Degano B, Sithon O, Savale L, et al. Characterization of pulmonary arterial hypertension patients walking more than 450 m in 6 min at diagnosis. Chest 2010; 137: 1297–1303. [DOI] [PubMed] [Google Scholar]
- 18.Galièn N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 37: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 19.Tiede H, Sommer N, Milger K, et al. Short-term improvement in pulmonary haemodynamics is strongly predictive of long-term survival in patients with pulmonary arterial hypertension. Pulm Circ 2013; 3: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost AE, Langleben D, Oudiz R, et al. for the STRIDE-1 Study Group. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: Demonstration of a ceiling effect. Vasc Pharmacol 2005; 43: 36–39. [DOI] [PubMed] [Google Scholar]
- 21.Macchia A, Marchioli R, Marfisi RM, et al. A meta-analysis of trials of pulmonary hypertension: A clinical condition looking for drugs and research methodology. Am Heart J 2007; 153: 1037–1047. [DOI] [PubMed] [Google Scholar]
- 22.Gabler NB, French B, Strom BL, et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary hypertension trials. Circulation 2012; 126: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savarese G, Paolillo S, Costanzo P, et al. Do changes in 6-minute walk distance predict clinical events in patients in pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J Am Coll Cardiol 2012; 60: 1192–1201. [DOI] [PubMed] [Google Scholar]
- 24.Zotter-Tufaro C, Mascherbauer J, Duca F, et al. Prognostic significance and determinants of the 6-Min Walk Test in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2015; 3(6): 459–466. [DOI] [PubMed] [Google Scholar]
- 25.Golpe R, Castro-Añón O, Pérez-de-Llano LA, et al. Prognostic significance of six-minute walk test in non-group 1 pulmonary hypertension. Heart Lung 2014; 43: 42–46. [DOI] [PubMed] [Google Scholar]
- 26.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36(9): 957–967. [DOI] [PubMed] [Google Scholar]
- 27.Sun X-G, Oudiz RJ, Hansen JE, et al. Exercise pathophysiology in primary pulmonary vascular hypertension. Circulation 2001; 104: 429–435. [DOI] [PubMed] [Google Scholar]
- 28.Wensel R, Francis DP, Meyer FJ, et al. Incremental prognostic value of cardiopulmonary testing and resting haemodynamics in pulmonary arterial hypertension. Int J Cardiol 2013; 167: 1193–1198. [DOI] [PubMed] [Google Scholar]
- 29.Oudiz RJ, Barst RJ, Hansen JE, et al. Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol 2006; 97: 123–126. [DOI] [PubMed] [Google Scholar]
- 30.Deboeck G, Scoditti C, Huez S, et al. Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur Respir J 2012; 40(6): 1410–1419. [DOI] [PubMed] [Google Scholar]
- 31.Morales FJ, Montemayor T, Martinez A. Shuttle versus six-minute walk test in the prediction of outcome in chronic heart failure. Int J Cardiol 2000; 76: 101–105. [DOI] [PubMed] [Google Scholar]
- 32.Parreira VF, Janaudis-Ferreira T, Evans RA, et al. Measurement properties of the incremental shuttle walk test. Chest 2014; 145: 1357–1369. [DOI] [PubMed] [Google Scholar]
- 33.Irisawa H, Takeuchi K, Inui N, et al. Incremental shuttle walk test as a valuable assessment of exercise performance in patients with pulmonary hypertension. Circ J 2014; 78: 215–221. [DOI] [PubMed] [Google Scholar]
- 34.Roberts K, Preston I, Hill NS. Pulmonary hypertension trials. Current end points are flawed, but what are the alternatives? Chest 2006; 130: 934–935. [DOI] [PubMed] [Google Scholar]
- 35.Scharf ML, Bagga S. A call to apply the minimal important difference in pulmonary arterial hypertension beyond the flawed 6-minute-walk test. Am J Respir Crit Care Med 2013; 187: 659. [DOI] [PubMed] [Google Scholar]
- 36.Irisawa H, Takeuchi K, Inui N, et al. Incremental shuttle walk test as a valuable assessment of exercise performance in patients with pulmonary hypertension. Circ J 2014; 78: 215–221. [DOI] [PubMed] [Google Scholar]
- 37.Billings CG, Hurdman JA, Condliffe R, et al. Incremental shuttle walk test distance and autonomic dysfunction predict survival in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 36(8): 871–879. [DOI] [PubMed] [Google Scholar]
- 38.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a Referral centre. Eur Resp J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 39.Singh SJ, Morgan MD, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Probst VS, Hernandes NA, Teixeira DC, et al. Reference values for the incremental shuttle walking test. Respir Med 2012; 106: 243–248. [DOI] [PubMed] [Google Scholar]
- 41.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 39: 589–596. [DOI] [PubMed] [Google Scholar]
- 42.Reesink HJ, van der Plas MN, Verhey NE, et al. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2007; 133: 510–516. [DOI] [PubMed] [Google Scholar]
- 43.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 44.Trammell AW, Pugh ME, Newman JH, et al. Use of pulmonary arterial hypertension-approved therapy in the treatment of non-group 1 pulmonary hypertension at US referral centers. Pulm Circ 2015; 5: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prins KW, Duval S, Markowitz J, et al. Chronic use of PAH-specific therapy in World Health Organization Group III Pulmonary Hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7(1): 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papathanasiou A, Nakos G. Why there is a need to discuss pulmonary hypertension other than pulmonary arterial hypertension? World J Crit Care Med 2015; 4(4): 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heresi GA, Platt DM, Wang W, et al. Healthcare burden of pulmonary hypertension owing to lung disease and/or hypoxia. BMC Pulm Med 2017; 17(1): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019; 53(1): 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.