Abstract
Background:
A risk-based model of care for managing patients with chronic kidney disease (CKD) using the Kidney Failure Risk Equation (KFRE) has been successfully integrated into nephrology care pathways in several jurisdictions. However, as most patients with CKD can be managed in primary care, the next pertinent steps would be to integrate the KFRE into primary care pathways.
Objective:
Using a risk-based approach for guiding CKD care in the primary care setting, the objective of the study is to develop, implement, and evaluate tools that can be used by patients and providers.
Design:
This study is a multicenter cluster randomized control trial.
Setting:
Thirty-two primary care clinics belonging to the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) across Manitoba and Alberta.
Patients:
All patients at least 18 years old or older with CKD categories G3-G5 attending the participating clinics; we estimate each clinic will have an average of 185 patients with CKD.
Methods:
Thirty-two primary care clinics will be randomized to receive either an active knowledge translation intervention or no intervention. The intervention involves the addition of the KFRE and decision aids to clinics’ Data Presentation Tool (DPT), as well as patient-facing visual aids, a medical detailing visit, and sentinel feedback reports. Control clinics will only be exposed to current guidelines for CKD management, without active dissemination.
Measurements:
Data from the CPCSSN repository will be used to assess whether a risk-based care approach affected management of CKD. Primary outcomes are as follows: the proportion of patients with measured urine albumin-to-creatinine ratio, and the proportion of patients being appropriately treated with angiotensin-converting enzyme inhibitor or angiotensin receptor blockers. Secondary outcomes are as follows: the optimal management of diabetes (hemoglobin A1C <8.5%, and the use of sodium-glucose cotransporter-2 inhibitors in CKD G3 patients), hypertension (office blood pressure <130/80 for patients with diabetes, 140/90 for those without), and cardiovascular risk (statin prescription); prescriptions of nonsteroidal anti-inflammatory drugs; and decline in estimated glomerular filtration rate (eGFR). In addition, in a substudy, we will measure CKD-specific health literacy and trust in physician care via surveys administered in the clinic post-visit. At the provider level, we will measure satisfaction with the risk prediction tools. Lastly, at the health system level, outcomes include cost of CKD care, and appropriate referrals for patients at high risk of kidney failure based on provincial guidelines. Primary and secondary outcomes will be measured at the patient level and enumerated at the clinic level 1 year after the intervention implementation, except for decline in eGFR, which will be measured 2 years postintervention.
Limitations:
Limitations include scalability of the proposal in other health care systems.
Conclusions:
If successful, this intervention has the potential to improve the management of patients with CKD within Canadian primary care settings, leading to health and economic benefits, and influencing practice guidelines.
Trial Registration:
ClinicalTrials.gov identifier: NCT03365063
Keywords: patient-oriented research, cluster randomized trial, randomized controlled trial, knowledge translation, chronic kidney disease, kidney failure risk
Abrégé
Contexte:
Un modèle de soins intégrant la prévision du risque d’évolution vers l’insuffisance rénale par la KFRE (Kidney Failure Risk Equation) a été incorporé avec succès aux protocoles de soins des patients atteints d’insuffisance rénale chronique (IRC) de plusieurs provinces. Comme la plupart des patients souffrant d’IRC peuvent être pris en charge en première ligne, l’étape suivante serait d’intégrer la KFRE aux protocoles de soins de première ligne.
Objectifs:
Avec une approche intégrant la prévision des risques dans les soins en IRC, l’étude vise à élaborer, mettre en œuvre et évaluer les outils qui pourraient être utilisés par les patients et les fournisseurs de soins en contexte de soins de première ligne.
Type d’étude:
Il s’agit d’un essai multicentrique, contrôlé et à répartition aléatoire en grappes.
Cadre:
L’étude se tiendra dans trente-deux cliniques de soins de première ligne du Manitoba et de l’Alberta faisant partie du Réseau canadien de surveillance sentinelle en soins primaires (RCSSSP).
Sujets:
Tous les patients adultes atteints d’IRC de stades G3-G5 fréquentant les cliniques participantes. Nous estimons que chaque clinique fournira une moyenne de 185 patients à l’étude.
Méthodologie:
Les trente-deux cliniques seront réparties aléatoirement pour recevoir ou non une intervention active de transmission des connaissances. L’intervention comprendra l’ajout de la KFRE et d’outils d’aide à la décision à l’outil actuel de présentation des données de la clinique; de même que du support visuel pour les patients, une consultation médicale détaillée et des rapports de rétroaction sentinelle. Les cliniques contrôles, quant à elles, ne seront exposées qu’aux lignes directrices actuelles pour la prise en charge de l’IRC, sans diffusion active.
Mesures:
Les données du registre du RCSSSP seront employées pour évaluer l’impact de l’approche intégrant la prévision du risque sur la gestion de l’IRC. Les critères de jugement principaux seront la proportion de patients pour lesquels on aura une mesure du rapport albumine/créatinine urinaire (RAC) et la proportion de patients traités adéquatement avec un inhibiteur de l’enzyme de conversion de l’angiotensine ou d’antagonistes des récepteurs de l’angiotensine. Les critères de jugement secondaires incluront la gestion optimale du diabète (hémoglobine A1C < 8,5 %, et l’emploi d’inhibiteurs de SGLT2 chez les patients de stade G3), de l’hypertension (pression sanguine en cabinet à < 130/80 pour les diabétiques et à < 140/90 pour les non-diabétiques) et du risque de maladies cardiovasculaires (prescription de statines); ainsi que la prescription d’anti-inflammatoires non stéroïdiens et un déclin du débit de filtration glomérulaire estimé (DFGe). Parallèlement, dans une étude secondaire, nous examinerons les connaissances des patients sur l’IRC et leur confiance envers les soins médicaux par le biais de sondages menés à la clinique après la consultation. Nous mesurerons également la satisfaction des fournisseurs de soins à l’égard des outils de prévention du risque. Enfin, du point de vue du système de santé, nous examinerons les coûts associés aux soins en IRC et l’aiguillage adéquat des patients dont le risque d’évolution vers l’insuffisance rénale est jugé élevé selon les lignes directrices provinciales. Les critères de jugement primaires et secondaires seront mesurés du point de vue des patients et recensés à l’échelle de la clinique un an après la mise en œuvre de l’intervention, à l’exception du déclin du DFGe qui sera mesuré deux ans après l’intervention.
Limites:
Les limites de l’étude incluent notamment l’extensibilité de la proposition à d’autres systèmes de santé.
Conclusions:
Si elle réussit, cette intervention pourrait améliorer la prise en charge des patients atteints d’IRC dans les établissements canadiens de première ligne, et ainsi entraîner des retombées positives en matière de santé et d’économie en plus d’influencer les lignes directrices de pratique.
What was known before
Most patients with chronic kidney disease (CKD) can be managed within the primary care setting. However, as many primary care physicians may be unaware of recommended treatment pathways,1,2 there is currently a misalignment between the care that is provided and the care that is required. Introducing risk-based care approaches into CKD treatment pathways can allow clinicians to identify and appropriately treat high-risk patients, while safely monitoring those who are low risk.3
What this adds
Risk-based care for patients with CKD will be conveyed to primary care providers through the integration of the Kidney Failure Risk Equation (KFRE) into CKD care pathways in clinics belonging to the Canadian Primary Care Sentinel Surveillance Network. Based on risk, providers will be informed of risk-appropriate clinical care guidelines and prompted to use our patient-facing visual aids to discuss individualized risk and disease management strategies with their patients. Success of this study has the potential to improve the patient-provider dialogue, quality of care, health literacy, and trust in care, for patients with CKD in the primary care setting.
Background
Chronic kidney disease (CKD) and end-stage kidney failure are major public health problems in Canada and worldwide.4,5 Chronic kidney disease is a significant risk factor for several patient-oriented adverse outcomes, including an increased risk of hospitalizations, cardiovascular disease, and early mortality.6 Once patients reach kidney failure and require dialysis, direct health care costs can rise to over Can$80 000 CAD per patient annually.7 However, as less than 3% of Canadian patients diagnosed with CKD will reach kidney failure,7,8 most patients with CKD can generally be managed in the primary care setting.9,10
Progression of CKD to kidney failure can be reduced with upstream risk factor reduction, and in patients with known CKD, early detection and management of CKD in the primary care setting is crucial for those at high risk of progression.11-14 To ideally manage patients with CKD, it is imperative to distinguish between those patients who are at higher versus lower risk of kidney failure. For example, in high-risk patients, aggressive blood pressure control and treatment with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) can reduce the risk of progression substantially.15 In contrast, patients at lower risk receive smaller benefits from ACEi, but equal or higher rates of potential harm. Likewise, the process of dialysis planning, including creation of an arteriovenous fistula for hemodialysis access, is important for patients who are likely to progress to kidney failure, but carries unnecessary surgical risks and costs for those who will not.16,17
The Kidney Failure Risk Equation (KFRE) is a validated predictive model for progression of CKD to kidney failure. The 4-variable equation incorporates age, sex, and the readily available biomarkers—estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio (ACR).18,19 Using the KFRE, clinicians can accurately stratify CKD patients according to their risk of progression to kidney failure, thereby appropriately treating or referring high-risk patients, while safely monitoring low-risk patients.3,20
Currently, use of the KFRE remains largely concentrated in nephrology practices. As most patients with CKD are managed in primary care, integrating the KFRE into primary care settings has the potential to achieve improved risk-based care for patients with CKD. However, the integration of the KFRE needs to be executed with clear management strategies that would benefit physicians, patients, and the health care system.
Methods
Study Design
We are conducting a multicenter, 2-arm parallel design cluster randomized trial in primary care clinics. Through the use of an active knowledge translation (KT) strategy, we will integrate the KFRE and associated visual aids into usual care practices within the intervention clinics. We will subsequently evaluate the effect our approach has on the management of patients with CKD in primary care clinics, when compared with usual care alone.
The cluster randomized trial design was adopted due to the type of intervention being implemented. As it is not feasible to apply the intervention at the patient level without experimental contamination (ie, physicians who care for different patients but are located at the same clinic will share information regarding clinical practice and patient care), we are applying the intervention at the clinic level. At this level, the study interventions and data collection processes pose minimal risk, and the anonymized data we require are already routinely collected. Therefore, informed consent will only be sought for the patient-reported outcomes portion of this study. This study has been approved by the Health Research Ethics Boards at both the University of Manitoba and the University of Calgary, and meets the Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials.21
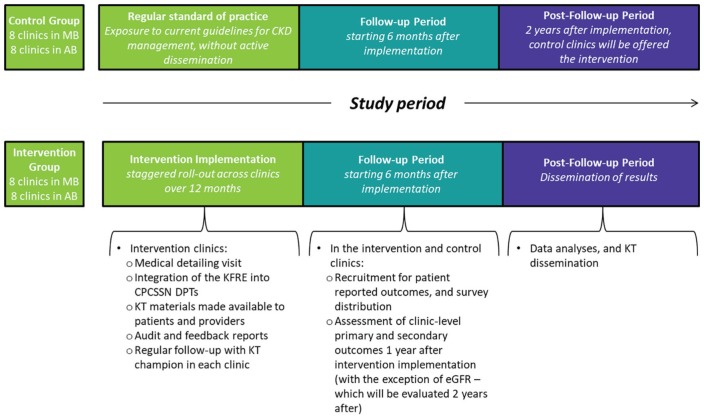
Figure 1.
Study intervention and timeline.
Note. AB = Alberta; MB = Manitoba; CKD = chronic kidney disease; KFRE = Kidney Failure Risk Equation; KT = knowledge translation; CPCSSN = Canadian Primary Care Sentinel Surveillance Network; DPT = Data Presentation Tool; eGFR = estimated glomerular filtration rate.
Study Population
The study will enroll eligible primary care clinics across Alberta and Manitoba, who are part of the Canadian Primary Care Sentinel Surveillance Network (CPCSSN), and who provide care to adults 18 years old and older. The CPCSSN is a pan-Canadian disease surveillance system representing more than 1200 family physicians and nurse practitioners who provide primary care to 1.8 million patients across Canada.22,23 The network consists of 12 primary care research networks that collect data from the electronic medical records (EMRs) of “sentinels” (ie, individual primary care physicians or nurse practitioners). Age- and sex-adjusted prevalence rates of the CPCSSN population have been shown to be fairly well representative of the general primary care population,24 and practices representative of other primary care practices that use EMRs.25
Data are anonymized, coded, and processed, and then merged into the national CPCSSN repository. Patient data on sociodemographics, medications, laboratory results, and comorbidities are routinely collected. Validated algorithms are used to monitor an array of chronic diseases and neurological conditions.22
Clinics will be recruited through regular CPCSSN mail-outs and recruitment sessions. From the 32 clinics that will be recruited, 16 will be randomized to receive the intervention and 16 to the control group. All adult patients with CKD categories G3-G5 at the eligible clinics will be included in the clinic-reported outcomes. We estimate that each clinic will have an average of 185 patients with CKD.
Data Sources
For the clinic-reported outcomes component of the study, routinely collected data from each CPCSSN clinic enrolled in the study will be analyzed for all patients with CKD in the clinics. A substudy surveying 320 patients (160 from the intervention clinics and 160 from the control clinics) will be conducted to assess the patient-reported outcomes.
Study Objective, Aims, and Hypothesis
The objective of this study is to develop, implement, and evaluate tools to guide the care of patients with CKD in the community, using a risk-based approach for managing CKD in the primary care setting. Through the integration of the KFRE within the CPCSSN Data Presentation Tool (DPT), accompanied by infographics and care pathway guidelines, the primary aims of this study are the following:
To determine whether providing patients and primary care providers with a patient’s predicted risk for kidney failure and risk-based criteria for referral increases appropriate management of and referral for patients at low risk of kidney failure (<3% in 5-year risk), medium risk (3%-10% in 5-year risk), and high risk (>10% in 5-year risk)—as based on the KFRE, compared with usual care without personalized risk information.
To determine whether providing patients with individualized information on their risk of progression increases CKD-specific health literacy and improves trust in the patient-provider relationship.
To determine the costs associated with the risk-based care paradigm and its comparator.
We hypothesize that clinics randomized to receive the intervention (the integration of the KFRE with provider- and patient-facing visual aids, accompanied by an active knowledge strategy), alongside usual care, will see an improvement in their patient-provider dialogue, an improvement in patients’ CKD-specific health literacy and their trust in physician care, and improvements to the management of CKD and its risk factors, as well as to nephrology specialist referral patterns.
Cluster Randomization
In this matched cluster randomized study, the unit of observation is the patient, while the unit of randomization is the primary care clinic. Clusters will be matched based on regions to account for additional clustering within regions and to facilitate subgroup analyses. As well, clusters will be matched based on the size to help reduce imbalances that might be related to cluster-level characteristics. Randomization will be carried out using software for random number generation, with allocation concealment and stratification by province/region, and size of the clinic. A simulation of the randomization will be completed to ensure balance on prespecified cluster-level variables. Approximately 1000 iterations will be performed, and a randomization scheme that results in good balance on all measured cluster factors will be selected. Randomization assignments will be concealed until the planned implementation of the intervention for a given region.
Active KT Intervention Group
The active KT intervention is comprised of multiple components:
Integration of the KFRE and accompanying visual aids into primary care pathways
The CPCSSN DPT is a sentinel feedback-reporting tool for a panel of chronic conditions and quality improvement targets which can be customized for physicians and practices.26 The DPT will report the output from the KFRE for all patients with CKD categories G3-G5 whenever urine ACR and an eGFR are available within a 3-month period. The risk output will include interpretation and automated categorization (low/medium/high) of risk of progression to kidney failure, and will recommend actions (eg, management of elevated urine ACR, appropriate medications for progression prevention) based on our risk-based care pathway.
Clinicians will also be prompted to discuss the KFRE results with their patients, using our patient-facing visual aids. Via our interactive website, patients will receive their own customized KFRE results with an explanation regarding what implications the results will have. Based on their KFRE result, patients will also receive information via videos, and infographics on appropriate management strategies on how to reduce their risk of progression to kidney failure—for example, information on appropriate CKD diet and medications that can harm or improve kidney function—will be provided. Information provided will be tailored based on the patient’s category of risk (low vs medium vs high). Patients will also receive an introduction to CKD categories, eGFR, causes of CKD, statistics on CKD, and the KFRE.
All patient-facing materials will go through extensive rounds of development by using feedback gathered from focus groups involving patient panels in Canada and the United States. This will ensure that the tools are patient-focused and reflect what patients need and want to know during the early stages of being diagnosed with CKD.
Medical detailing
Just prior to the KFRE being integrated into the clinic DPTs, the clinics will receive an in-person visit from a nephrologist and family physician from our study team, who will provide the evidence for the accuracy of the KFRE and guidance on implementation, as well as detailed information on provincial guidelines, in a standardized 1-hour presentation to clinic medical and allied health staff. Furthermore, the detailing physicians will describe the supporting visual aids and provide a forum for discussion on the appropriate management of CKD, with a simulation of the patient-provider discussion.
Detailing by a medical expert or key opinion leader has been shown to greatly enhance the effectiveness of audit and feedback interventions, and is a key component of our active KT strategy.27 In addition, at the visit, a local KT champion or “super-user” (a family physician from the clinic) who is willing to report practice-level data using the DPT, and who will continue to advocate for risk-based care with colleagues, will be identified. They will be the point person with whom we will review adherence and barriers to implementation.
Audit and feedback
Providers at all participating CPCSSN clinics receive sentinel feedback reports quarterly from CPCSSN on a panel of chronic conditions and quality improvement targets. For clinics involved in this study, CKD status, KFRE risk distribution and urine ACR measurement, as well as appropriate management of elevate urine ACR will be added to the feedback reports; providers will also receive detailed feedback on the risk profiles of their practices, as well as the proportion of patients meeting risk factor and referral targets.
Control Group
For clinics randomized to the control group, the KFRE will not be integrated into their CPCSSN DPT, information on personalized risk and risk-based referral will not be provided, and medical detailing will not be provided. However, the study materials will be made available to primary care clinics in the control arm at the end of the study period.
Outcomes
Primary outcomes
Outcomes for this trial will be measured at the patient level and enumerated at the clinic level. The primary outcome is the percentage of adult patients with urine ACR measured within 3 months of a lab-reported eGFR less than 60 mL/min/1.73 m2. The second primary outcome is the percentage of patients who are appropriately managed with an ACEi or ARB, and who either have CKD and diabetes, or who have CKD and a urine ACR >30 mg/mmol. Currently, the rate of urine ACR testing among CKD patients in the primary care setting is low; in order for the DPTs to output the KFRE results for each patient, it is essential that urine ACR testing occur to facilitate this. Part of the intervention involves stressing the importance of urine ACR testing and management for patients with CKD, and thus, our primary outcomes facilitate our secondary outcomes.
Secondary outcomes
Management of comorbidities will be determined by management of diabetes (hemoglobin A1C <8.5%, and the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors in patients with CKD G3), hypertension (office blood pressure <130/80 for patients with diabetes, 140/90 for those without), and cardiovascular risk (statin prescription). Decline in eGFR (which will be defined as an eGFR decline of 30% or more during the follow-up period, or a difference in slopes), 2 years after baseline, will also be evaluated, as well as the presence/absence of any nonsteroidal anti-inflammatory drug (NSAID) prescriptions. Appropriateness of referral will be cross-checked against the provincial guidelines for the province that the clinic is in.
Data for the primary and secondary outcomes will be pulled from the CPCSSN repository at baseline (before intervention implementation), as well as 1 year after the intervention has been implemented for most outcomes, except for eGFR which will be evaluated 2 years after implementation.
For the patient-reported outcomes, clinic staff from patients’ existing circle of care will approach patients to participate. Data required will be collected through the Kidney Knowledge Survey, a CKD-specific health literacy survey, as well as the Trust in Physician Scale.28,29 Convenience sampling of patients with CKD will begin 6 months following the introduction of the KFRE and administration of the KT intervention.
For the provider-reported outcomes, data will be collected through a Likert scale to measure provider satisfaction with the risk prediction tools. These data will be collected using a paper-based data collection form at each individual clinic.
In addition, we will evaluate relevant costs related to the risk-based paradigm and status quo care. We will include costs relevant to the public health payer, including costs related to diagnostic testing (urine ACR, eGFR), primary care visits, nephrology consultations, the costs of relevant medications (ACEis, ARBs, statins, NSAIDs, and SGLT2s), and relevant complications of mismanaged diabetes, hypertension, cardiovascular disease, and kidney disease.
Power Calculation
Practice-level intervention
To calculate the sample size, we assumed a level of significance of 0.05, with 85% power, and an intracluster correlation coefficient (ICC) of 0.0485. There are 42 CPCSSN clinics in Alberta and Manitoba, with an average of 185 patients with CKD categories G3-G5 at each clinic with 2 eGFR < 60 mL/min/1.73 m2 tests per year. Assuming a conservative treatment effect of 10% improvement (baseline urine ACR testing in 18% of patients), we would need 16 clusters in each of the control and intervention arms. Given that all patients with CKD categories G3-G5 will contribute data to the EMR-reported outcomes, we are confident that the design and availability of patients will yield a viable study.
Patient-reported outcomes
Given an ICC of 0.02, an effect size of 0.5 standard deviation improvement in CKD-specific health literacy,30 and 90% power, we require at least 210 patient participants.
Statistical Analyses
For continuous mixed-effects, linear models will be used. These models will allow us to examine the effects of the intervention while adjusting for potential confounding variables and accounting for the clustering of observations within clinics. For dichotomous outcomes, we will use a paired t test to compare the event rate for our matched-pair design. Despite the robustness of this test, we recognize some of the required assumptions may not be strictly satisfied. As such, we will conduct sensitivity analyses using the Wilcoxon signed ranked test, the one-sample permutation test, and Liang adjusted χ2 test.31 If the cluster sizes are highly variable, we will also apply a weighted t test.
To simultaneously control for individual- and cluster-level covariates, as well as clustering, we will use the generalized estimating equations approach (an extension of standard logistic regression using robust variance estimation). This method determines a more accurate variance associated with the odds of success for bivariate outcomes. In this analysis, variables upon which randomization was matched will be included (region and cluster size). Analyses will be performed using SAS. A 2-sided P value less than .05 will be considered statistically significant, with adjustments for multiple comparisons also conducted.
Discussion
In collaboration with CPCSSN, we have designed a novel approach that has the potential to improve the way that CKD care is delivered in primary care clinics for patients and providers. With the completion of this study, we anticipate that the KFRE will be integrated into the workflow of CKD care in family medicine/primary care practices, providing practitioners with a prediction of each patient’s kidney failure risk. According to risk level, providers will be prompted to have a data-informed, patient-centered discussion with their patients, using decision aids and infographics that we are developing with individuals who have lived experience of CKD. Our intervention—implemented using an active KT approach—will result in a meaningful change in patient-provider communication, patient and provider awareness of CKD, as well as appropriate control of risk factors and referrals to nephrology specialists.
It is clear that there is currently a clinical uncertainty for primary care physicians in terms of CKD management. For example, when automated eGFR reporting was implemented in Ontario in 2006 and Manitoba in 2010, a sudden and sustained increase in nephrology referrals was noted. When faced with a potentially significant health issue without a clear management strategy, front-line providers typically seek consultation; in some practices, all patients with CKD, including those at very low risk of progression, were referred to a nephrologist, and in others, referral was often delayed even for the highest risk individuals.32 For low-risk individuals, this creates considerable unnecessary anxiety about the likelihood of kidney failure and places an undue burden on the health system in terms of testing and referrals. Conversely, when high-risk individuals go underrecognized, there are limited opportunities to learn about CKD, as well as limited opportunities to control risk factors and potentially delay or prevent the progression to kidney failure. If interventions are delayed for the highest risk individuals, there may also be adverse consequences such as rapid disease progression, higher risk of hospitalizations and early mortality, as well as inadequate preparation for dialysis and/or kidney transplant. Together, this misalignment of intensity of care with the risk of kidney failure results in adverse consequences for patients and the health care system. If the intervention described here is successful, patients with CKD will receive care that is better tailored to their risk of progression to kidney failure.
Shared Decision-Making
The shared decision-making concept is the idea that patients and providers share information and jointly decide upon the best treatment course, by using evidence-based information. By providing both parties with appropriate evidence and knowledge, this approach to care has the potential to improve treatment satisfaction and disease knowledge.33 An important aspect of shared decision-making is the presence of patient decision aids, which are tools that help guide the decision-making process by summarizing the evidence. Limited health literacy is common in patients with CKD and has been consistently associated with poor control of CKD risk factors (blood pressure, glycemic control), decreased adherence to important process measures (arteriovenous fistula use and transplant referral), as well as major adverse patient outcomes (all-cause mortality).28,34-36
Patient-Researcher Engagement
In March 2014, the Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease network (Can-SOLVE CKD) was formed under the Strategies for Patient-Oriented Research initiative, through the Canadian Institute for Health Research.37 Can-SOLVE CKD is a pan-Canadian patient-oriented network that aims to meaningfully engage CKD patients as research partners. Our study is one of the 18 projects under the Can-SOLVE CKD umbrella. Our panel of patient partners from across Canada will be meaningfully involved throughout this project, to ensure that the project is patient-focused and that the needs of patients are addressed within the trial and beyond. In particular, patient partners will be involved with refinement of the decision aids, infographics, and website, as well as with developing the intervention. The importance of engaging patient partners to produce effective patient-facing aids that are accessible to patients at all levels of health literacy cannot be overstated, and indeed, international guidelines for the development of decision aids encourage the participation of patients within the development process.
Limitations
However, one limitation of this study—scalability of the intervention—will be a challenge. The active KT strategy requires an in-person visit from a member of the study team. In the future, presentations may be given at primary care conferences, rather than to individual clinics. However, our novel approach of leveraging existing CPCSSN infrastructure and EMR resources, and using it as a platform for the intervention and for outcome evaluation, will result in increased sample sizes and significant cost savings when compared with primary data collection.
Conclusion
Together, our approach and the findings from this study have the potential to strengthen the case for routine integration of the KFRE in primary clinics across North America and worldwide. By introducing risk-based care into primary care clinics, this proposed project has the potential to have a transformative impact on the quality of care provided to patients. Knowledge users will use our integrated clinical visual aids to appropriately share information about the risk of kidney failure in each patient encounter, and decision makers will use our findings to inform clinical practice guidelines for management of CKD in primary care clinics.
Footnotes
Ethics Approval and Consent to Participate: Informed consent will be sought from patients participating in the patient-reported outcomes component of this trial. This study has been approved by the Health Research Ethics Boards at the University of Manitoba and the University of Calgary.
Consent for Publication: Not applicable.
Availability of Data and Materials: Data have not yet been collected and analyzed; there are no data to share.
Author Contributions: O.H. prepared and edited the article, and conducted study activities. N.D., A.S., A.B., P.K., C.R., J.L., D.S., and T.W.F. contributed to the design of the trial, reviewed and edited the article, and conducted study activities. N.T. contributed to the design of the trial, reviewed, edited, and approved the article, and conducted study activities.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded by the Canadian Institutes of Health Research through the Strategy for Patient-Oriented Research, as well as Research Manitoba, the Manitoba Renal Program, and the Chronic Disease Innovation Centre.
References
- 1. Tahir MA, Dmitrieva O, deLusignan S, et al. Confidence and quality in managing CKD compared with other cardiovascular diseases and diabetes mellitus: a linked study of questionnaire and routine primary care data. BMC Fam Pract. 2011;12:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shardlow A, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Chronic kidney disease in primary care: outcomes after five years in a prospective cohort study. PLoS Med. 2016;13(9):e1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Q, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8(117):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canadian Institute for Health Information. Annual statistics on organ replacement in Canada: dialysis, transplantation and donation, 2006 to 2015; February 2017. https://secure.cihi.ca/free_products/CORR-snapshot-en-web.pdf. Accessed March 20, 2019.
- 6. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. [DOI] [PubMed] [Google Scholar]
- 7. Manns BJ, Mendelssohn D, Taub K. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. Int J Health Care Finance Econ. 2007;7(2-3):149-169. [DOI] [PubMed] [Google Scholar]
- 8. Komenda P, Copland M, Makwana J, Djurdjev O, Sood MM, Levin A. The cost of starting and maintaining a large home hemodialysis program. Kidney Int. 2010;77(11):1039-1045. [DOI] [PubMed] [Google Scholar]
- 9. Manns B, Tonelli M, Culleton B, et al. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(4):565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bello AK, Ronksley PE, Tangri N, et al. Prevalence and demographics of CKD in Canadian primary care practices: a cross-sectional study. Kidney Int Reports. 2019;4(4):561-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860. [DOI] [PubMed] [Google Scholar]
- 12. Appel LJ, Middleton J, Miller E, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14(7, suppl 2):S166-S172. [DOI] [PubMed] [Google Scholar]
- 13. Tobe SW, Clase CM, Gao P, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation. 2011;123(10):1098-1107. [DOI] [PubMed] [Google Scholar]
- 14. Grill S, Brimble A. Approach to the detection and management of chronic kidney disease: what primary care providers need to know. Canadian Family Physician|Le Médecin de famille canadien. 2018;64:728-735. [PMC free article] [PubMed] [Google Scholar]
- 15. Strippoli GFM, Bonifati C, Craig M, SD Navaneethan, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;(4):CD006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Hare AM, Bertenthal D, Walter LC, et al. When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration. Kidney Int. 2007;71(6):555-561. [DOI] [PubMed] [Google Scholar]
- 17. O’Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59(4):513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tangri N, Stevens L, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553-1559. [DOI] [PubMed] [Google Scholar]
- 19. Tangri N, Kitsios GD, Inker LA, et al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med. 2013;158(8):596-603. [DOI] [PubMed] [Google Scholar]
- 20. Inston N, Lok CE. Improving precision in prediction: using kidney failure risk equations as a potential adjunct to vascular access planning. J Vasc Access. 2018;20(1):95-97. [DOI] [PubMed] [Google Scholar]
- 21. Weijer C, Grimshaw JM, Eccles MP, et al. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9(11):e1001346-e1100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williamson T, Green ME, Birtwhistle R, et al. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med. 2014;12(4):367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birtwhistle R. Canadian Primary Care Sentinel Surveillance Network: a developing resource for family medicine and public health. Can Fam Physician. 2011;57(10):1219-1220. [PMC free article] [PubMed] [Google Scholar]
- 24. Queenan JA, Williamson T, Khan S, et al. Representativeness of patients and providers in the Canadian Primary Care Sentinel Surveillance Network: a cross-sectional study. CMAJ Open. 2016;4(1):E28-E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birtwhistle R, Queenan JA. Update from CPCSSN. Can Fam Physician|Le Médecin Fam Can. 2016;62:851. [PMC free article] [PubMed] [Google Scholar]
- 26. Greiver M, Drummond N, Birtwhistle R, Queenan J, Lambert-Lanning A, Jackson D. Using EMRs to fuel quality improvement. Can Fam Physician. 2015;61(1):92, e68-e69. [PMC free article] [PubMed] [Google Scholar]
- 27. Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):1-227. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000259.pub3/full. Accessed March 20, 2019. [DOI] [PMC free article] [PubMed]
- 28. Wright JA, Wallston KA, Elasy TA, Ikizler TA, Cavanaugh KL. Development and results of a kidney disease knowledge survey given to patients with CKD. Am J Kidney Dis. 2011;57(3):387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thom DH, Ribisl KM, Stewart AL, Luke DA. Further validation and reliability testing of the Trust in Physician Scale. Med Care. 1999;37(5):510-517. [DOI] [PubMed] [Google Scholar]
- 30. Littenberg B, MacLean CD. Intra-cluster correlation coefficients in adults with diabetes in primary care practices: the Vermont Diabetes Information System field survey. BMC Med Res Methodol. 2006;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fox CH, Brooks A, Zayas LE, McClellan W, Murray B. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) Study. J Am Board Fam Med. 2006;19(1):54-61. [DOI] [PubMed] [Google Scholar]
- 32. Hingwala J, Bhangoo S, Hiebert B, et al. Evaluating the implementation strategy for estimated glomerular filtration rate reporting in Manitoba: the effect on referral numbers, wait times, and appropriateness of consults. Can J Kidney Health Dis. 2014;1:9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff. 2013;32(2):276-284. [DOI] [PubMed] [Google Scholar]
- 34. Cavanaugh KL, Wingard RL, Hakim RM, et al. Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol. 2010;21(11):1979-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dageforde LA, Cavanaugh KL. Health literacy: emerging evidence and applications in kidney disease care. Adv Chronic Kidney Dis. 2013;20(4):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(suppl 3):268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levin A, Adams E, Barrett BJ, et al. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD): form and Function. Can J Kidney Health Dis. 2018;5:1-12. doi: 10.1177/2054358117749530. [DOI] [PMC free article] [PubMed] [Google Scholar]