Abstract
Study Design:
A nonrandomized, two-armed prospective study.
Objective:
Water-tight dural closure is paramount to the prevention of cerebrospinal fluid (CSF) leakage and associated complications. Synthetic polyethylene glycol (PEG) hydrogel has been used as an adjunct to sutured dural repair; however, its expansion postoperatively is a concern for neurological complications. A low-swell formulation of PEG sealant was introduced as DuraSeal Exact Spine Sealant System (DESS). A Post-Approval Study was performed primarily to evaluate the safety and efficacy of DESS for spinal dural repair compared to current alternatives, in a large patient population, reflecting a real-world practice.
Methods:
A total of 36 sites in the United States enrolled 429 patients treated with DESS as an adjunct to dural repair in the spinal sealant group and 406 patients treated with all other modalities in the control arm, from October 2011 to June 2016. The primary endpoint was the incidence of CSF leak within 90 days of operation. The secondary endpoints evaluated were deep surgical site infection and neurological serious adverse events.
Results:
The CSF leakage in the DESS group (6.6%) was not significantly different from the control group (6.5%) (p = .83), and there was no significant difference in the time to first leak. The two groups had no significant differences in deep surgical site infection (1.6% versus control 2.1%, p = .61) or proportion of subjects with neurological serious adverse events (2.9% versus control 1.6%, p = .516).
Conclusions:
DuraSeal Exact Spinal Sealant is safe when compared to current alternatives for spinal dural repair.
Keywords: dural tears, epidural, cauda equina syndrome, dural repair, spine sealant, DuraSeal, pseudomeningocele, CSF leak
Introduction
Dural opening in spinal operations, either intentional or incidental, is associated with a risk of cerebrospinal fluid (CSF) leak postoperatively.1,2 Clinically, CSF leak manifests as postural headache, often associated with nausea, vomiting, dizziness, and photophobia. Persistent CSF leak often leads to collection of a fluid pocket outside the confines of the dura mater, creating a pseudomeningocele or a dural cutaneous fistula. This predisposes to infectious sequelae such as meningitis, or abscesses.3 Less common but more serious complications associated with persistent CSF drainage include cerebellar hemorrhage and intracranial subdural hematoma.4 Progressive pseudomeningocele formation can lead to nerve root compression or even herniation of neural elements causing neurological deficits.3
A watertight closure after dural openings can be an elusive target, though critical in the prevention of complications. Primary closure of spinal dural layer is usually achieved by suture, sometimes with the concomitant use of fat graft, muscle and/or fascia.1,2,5 Commonly used adjuncts to sutured repair may include the use of a collagen-based dural implant and/or a sealant.
DuraSeal (Integra Life Sciences, Plainsboro, NJ) is a synthetic, absorbable polyethylene glycol (PEG) hydrogel, developed for use as a sealant in cranial and spinal dural repair.6-8 DuraSeal, being hydrophilic in nature, has an intrinsic property to swell after application. Reports of neurological complications due to mass effect have been reported with use of DuraSeal in the spine.9-14 The original PEG hydrogel of DuraSeal was therefore modified to a low-swell formulation, known as DuraSeal Exact Spine Sealant (DESS). DESS is a synthetic, bioabsorbable hydrogel and has desirable characteristics such as the ease of application, rapid in situ polymerization, greater mechanical strength and elasticity. A pivotal multicenter randomized trial evaluated the safety and efficacy of DESS, leading to Food and Drug Administration (FDA) approval for dural closure in the spine.15
Study Objective
The objective of this postapproval study was to evaluate the safety and efficacy of DESS as dural closure adjunct compared with the available alternatives, in a real-world practice model. This study was designed to evaluate incidence of postoperative CSF leak and adverse events in patients receiving DESS for treatment of intentional or incidental durotomies, and to compare that to the corresponding rates of alternative dural repair adjuncts.
Material and Methods
Study Design
This was a multicenter, nonrandomized, 2-arm postapproval study designed to evaluate and compare postoperative CSF leakage rates in subjects who received DESS and control subjects who received other products and/or treatments to close durotomies (both incidental and intentional). The DESS arm consisted of subjects who underwent a spinal procedure where DESS was administered, in addition to any other methods of dural closure. Eligible subjects were enrolled prospectively within 24 hours after completion of the spine surgery. The control arm consisted of subjects who underwent a spinal procedure where the standard of care other than DESS was administered. The subjects meeting study criteria were enrolled either prospectively or retrospectively in this arm. Sites were not required to enroll in both study arms.
The study consisted of 2 study visits: a screening visit/baseline data collection and a 90-day postoperative follow-up visit (±30 days). The follow-up included a physical examination, complete neurologic examination, and wound healing evaluation. Any reported adverse events were documented. All data was collected on study-specific case report forms (CRFs) and housed in the study-specific, 21 CFR Part 11 compliant, database.
The protocol was approved by the institutional review board of all participating sites. Thirty-six centers participated in this clinical investigation. All subjects provided written informed consent prior to participation. The study has been registered on http://clinicaltrials.gov (NCT01410864).
Participants
Patients were adults at least 18 years of age who had any spinal operation where a dural opening (either intentional or incidental) occurred that needed repair. Pregnant and breastfeeding females were excluded from the study as well as those subjects who the investigator determined were not able to comply with the required follow-up visits.
Treatment Response Assessments and Outcomes Measures
The primary endpoint of the study was the occurrence of postoperative CSF leak within 90 days after spine surgical procedure. A CSF leak was defined as a CSF fistula or a pseudomeningocele confirmed by clinical examination or diagnostic testing (ie, magnetic resonance imaging [MRI] or computed tomography [CT]), whether or not treatment such as surgical repair or drainage was required. The secondary endpoints of the study were the occurrence of deep surgical site infection (DSSI) and neurological serious adverse event (SAE) within 90 days after the surgical procedure. Serious adverse events included, but were not limited to, those leading to a life-threatening illness or injury or those that required inpatient hospitalization.
Statistical Analysis
The statistical analyses were produced using Statistical Analysis System version 9.3 (SAS Inc, Cary, NC). Unless otherwise specified, the statistical comparisons were carried out using 2-tailed tests, with type 1 error level set at 5%. Continuous data was summarized using descriptive statistics, specifically the number of observations (N), mean, standard deviation, median, minimum, and maximum. Categorical data was summarized using frequency counts and percentages.
Results
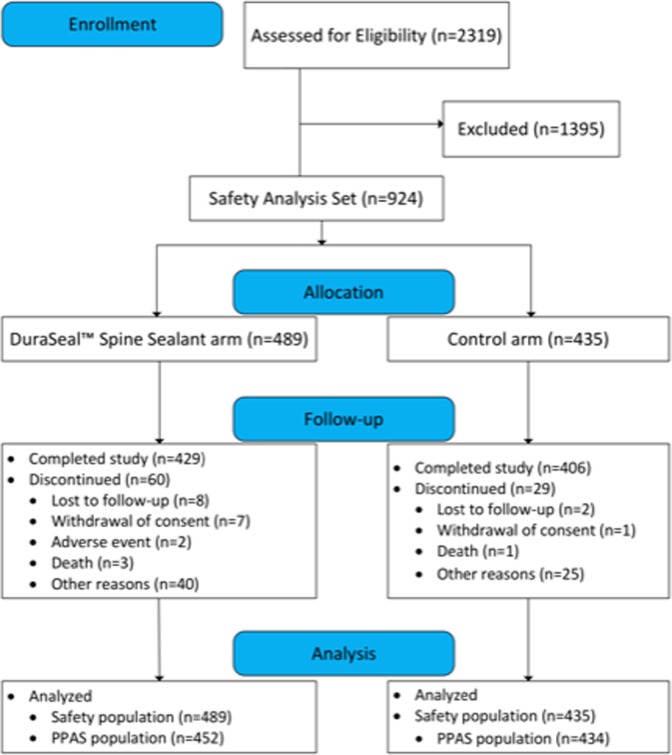
Between October 5, 2011 and June 7, 2016, a total of 924 subjects underwent a spinal procedure during which 489 subjects received DuraSeal Exact Spine Sealant (DESS arm) and 435 subjects received other products and/or treatments (control arm) to close the dura (Figure 1).
Figure 1.
Enrollment and study participation schematic.
Median age of the enrolled subjects was 61 and 501 (54.2%) were female (Table 1). Of the total, 817 (90.1%) subjects were white. There was a statistically significant difference in gender and height between the two treatment arms before controlling for the propensity score (gender, P < .0001; height, P = .0002). After controlling for the propensity scores, no statistically significant difference in any of the subject characteristics was reported between the treatment arms.
Table 1.
Baseline Patient Characteristics and Surgical Information (Safety Analysis Set).
| Parameter | Spine Sealant | Control | All | P |
|---|---|---|---|---|
| Age (n) | 489 | 435 | 924 | .1983 |
| Mean (SD) (years) | 58.8 (15.14) | 57.5 (16.32) | 58.2 (15.71) | |
| Median (years) | 61.0 | 61.0 | 61.0 | |
| Range (min, max | 18, 92 | 19, 89 | 18, 92 | |
| Sex, n (%) | 489 | 435 | 924 | <.0001 |
| Male | 257 (52.6) | 166 (38.2) | 423 (45.8) | |
| Female | 232 (47.4) | 269 (61.8) | 501 (54.2) | |
| Body mass index (n) | 441 | 360 | 801 | .2797 |
| Mean (SD) (kg/m2) | 29.1 (6.36) | 29.7 (6.83) | 29.4 (6.58) | |
| Median (kg/m2) | 28.0 | 29.0 | 29.0 | |
| Range (min, max) | 16, 55 | 16, 74 | 16, 74 | |
| Spinal levels involved, n (%) | ||||
| Lumbar | 364 (74.4) | 295 (67.8) | 659 (71.3) | .0230 |
| Sacral | 138 (28.2) | 132 (30.3) | 270 (29.2) | .4910 |
| Thoracic | 115 (23.5) | 87 (20.0) | 202 (21.9) | .1909 |
| Cervical | 65 (13.3) | 113 (26.0) | 178 (19.3) | <.0001 |
A higher proportion of subjects had involvement of a lumbar level followed by sacral, thoracic, and cervical levels of the spine. A statistically significant higher proportion of subjects in the DESS arm underwent a procedure in the lumbar spine (P = .0230); in contrast, a statistically higher proportion of subjects in the control arm underwent a procedure in the cervical spine (P < .0001) compared with other levels (Table 1).
As demonstrated in Table 2, there was no statistically significant difference in the duration of the procedures (P = .0521) or the use of suture/nonsuture repairs (P = .4481) between the DESS arm and the control arm). The length of the suture repair was longer for patients in the DESS arm (P = .0139). There was a statistically higher rate of incidental dural openings as compared to intentional dural openings in the control arm (P = .0059). Also, there was a statistically higher percentage of shunts and drains used in the control group (P = .0039).
Table 2.
Surgical Procedure Characteristics (Safety Analysis Set).a
| Parameter | Spine Sealant | Control | All | P |
|---|---|---|---|---|
| Duration of procedure (h) | 489 | 432 | 921 | .0521 |
| Mean (SD) | 3.6 (2.03) | 3.9 (2.16) | 3.7 (2.09) | |
| Median | 3.2 | 3.4 | 3.3 | |
| Range (min, max) | 1, 13 | 1, 15 | 1, 15 | |
| Cause of dural opening, n (%) | 488 | 430 | 918 | .0059 |
| Incidental | 309 (63.3) | 309 (71.9) | 618 (67.3) | |
| Intentional | 179 (36.7) | 121 (28.1) | 300 (32.7) | |
| Sutures used for dural opening? n (%) | 486 | 428 | 914 | .4481 |
| Yes | 411 (84.6) | 354 (82.7) | 765 (83.7) | |
| No | 75 (15.4) | 74 (17.3) | 149 (16.3) | |
| Total length of dural incision (cm) | 146 | 52 | 198 | .0139 |
| Mean (SD) | 1.5 (1.75) | 1.0 (1.22) | 1.4 (1.64) | |
| Shunts/drains placed, n (%) | 489 | 435 | 924 | .0039 |
| Yes | 221 (45.2) | 238 (54.7) | 459 (49.7) | |
| No | 268 (54.8) | 197 (45.3) | 465 (50.3) |
a Percentages are based on the number of subjects having a non-missing value for the parameter.
The data set was subdivided into the Per Protocol Analysis Set (PPAS) and the Safety Analysis Set (SAS) endpoint analysis. The primary efficacy endpoint was analyzed in both PPAS and SAS subgroups and the secondary endpoints were analyzed using only the SAS.
The SAS included all subjects who were enrolled in the study (N = 924). The PPAS (N = 886) is a subset of the SAS, which included subjects from the SAS who did not deviate significantly from the protocol.
Primary Endpoint Results
Ninety-Day Postoperative CSF Leak
For the primary endpoint, 58 (6.6%) subjects (DESS arm, 30 [6.6%]; control arm, 28 [6.5%]) in PPAS experienced a CSF leak within 90 days after the spine surgical procedure (Table 3). The difference estimates and 95% CI (%) between treatment arms were reported as −0.9 (−3.7, 2.0) from nonjustified analysis and as −0.5 (−3.4, 2.3) from a propensity score justified analysis (Table 4). The upper limit of a one-sided 95% CI for the difference in estimated probability of CSF leak between the treatment arms 90 days postoperatively (DESS arm − control arm) was 2.0% from the unstratified analysis and 2.3% from the stratified analysis by propensity score. This which is far less than the 5% noninferiority margin) and thus the non-inferiority conclusion was confirmed, as also noted with SAS group.
Table 3.
Postoperative Cerebrospinal Fluid (CSF) Leak (Per Protocol Analysis Set).a
| Spine Sealant, n (%) | Control, n (%) | All, n (%) | |
|---|---|---|---|
| Did subject experience a postoperative CSF leak? | 452 | 434 | 866 |
| Yes | 30 (6.6) | 28 (6.5) | 58 (6.6) |
| No | 399 (88.3) | 382 (88.0) | 781 (88.1) |
| Not noted | 0 | 16 (3.7) | 16 (1.8) |
| Assessment not done | 23 (5.1) | 8 (1.8) | 31 (3.5) |
| CSF leak was manifested by | 30 | 28 | 58 |
| Incisional | 12 (40.0) | 14 (50.0) | 26 (44.8) |
| Neuropathy exam | 3 (10.0) | 2 (7.1) | 5 (8.6) |
| Imaging study | 17 (56.7) | 10 (35.7) | 27 (46.6) |
| Other | 7 (23.3) | 4 (14.3) | 11 (19.0) |
| Was fluid tested for CSF? | 30 | 28 | 58 |
| Yes | 5 (16.7) | 2 (7.1) | 7 (12.1) |
| No | 25 (83.3) | 26 (92.9) | 51 (87.9) |
| Fluid CSF tested result | 5 | 2 | 7 |
| Positive | 5 (100.0) | 2 (100.0) | 7 (100.0) |
| Negative | 0 | 0 | 0 |
| Inconclusive | 0 | 0 | 0 |
| Imaging study result | 17 | 10 | 27 |
| Confirmed CSF leak | 12 (70.6) | 8 (80.0) | 20 (74.1) |
| Not confirmed as a CSF leak | 3 (17.6) | 2 (20.0) | 5 (18.5) |
| Other | 2 (11.8) | 0 (0.0) | 2 (7.4) |
a Percentages are based on the number of subjects having a non-missing value for the parameter.
Table 4.
Primary Endpoint: Estimated Probability of Cerebrospinal Fluid (CSF) Leak at 90-Day Postoperative (Per Protocol Analysis Set).
| Spine Sealant Arm (N = 452) | Control Arm (N = 434) | Difference in Estimated Probability of CSF Leak Between Treatment Groups at 90 Days Postoperative (DuraSeal − Control) and 95% CI | ||
|---|---|---|---|---|
| Unstratified | Stratified by Propensity Score Quintile | |||
| No. (%) of subjects had CSF leak | 30 (6.6) | 28 (6.5) | ||
| Estimated probability of CSF leak at 90 days and 95% CI (%) | 6.1 (4.2, 8.0) | 7.0 (4.9, 9.1) | −0.9 (−3.7, 2.0) | −0.5 (−3.4, 2.3) |
Time to First CSF Leak
Time to first CSF leak was defined as the number of days between the date of surgery and the date of CSF leak. Per Kaplan-Meier analysis, lack of CSF leak was censored at the date of last contact for subjects who were lost to follow up. There was no statistically significant difference in the time to first CSF leak between the DESS arm and the control arm (P = .836).
Secondary Endpoint Results
There was no statistically significant difference in the proportion of subjects with DSSIs between DESS and Control arms (1.6% vs 2.1%; P = .6160) (Table 5). Nor was there any statistically significant difference in the proportion of subjects with a neurological SAE between DESS and control arms (Table 5). Ninety-two subjects experienced at least 1 AE (DuraSeal Exact Spine Sealant arm, 51 [10.4%] subjects; control arm, 41 [9.4%] subjects). There was no statistically significant difference in the proportion of subjects with at least 1 AE between the DESS arm and control arm (P = .6109).
Table 5.
Adverse Events (Safety Analysis Set).
| Spine Sealant, n (%) | Control, n (%) | P | |
|---|---|---|---|
| Subjects with any surgical site infections, n | 463 | 426 | .2295 |
| No | 443 (95.7) | 414 (97.2) | |
| Yes | 20 (4.3) | 12 (2.8) | |
| Type of surgical site infections | |||
| Deep | 7 (1.6) | 9 (2.1) | .6160 |
| Superficial | 13 (2.8) | 2 (0.5) | .0076 |
| Organ space | 0 (0.0) | 1 (0.2) | .4792 |
| Neurological serious adverse events | |||
| Subjects with any neurological serious adverse event | 14 (2.9) | 7 (1.6) |
A total of 75 SAEs were reported during the study, with no unanticipated adverse device effects (DESS arm, 42 [8.6%] subjects; control arm, 33 [7.6%] subjects) and no statistically significant difference in the proportion of subjects with at least 1 SAE between the DESS arm and the control arm (8.6% vs 7.6%; P = .5755).
Finally, there was no statistically significant difference in the proportion of subjects between the treatment groups in terms of AE relatedness to the study device (P = .5370) and AE severity (P = .5979).
Discussion
In many clinical situations, primary dural closure is either not feasible or inadequate to ensure watertight dural closure. CSF leakage rate after spinal surgery may vary depending on patient age, surgical technique, prior spinal operations, and the indication for surgery.16 The reported CSF leakage rate in intentional durotomies varies from 10% in intradural spinal tumor resection to even higher rate for surgeries for tethered spinal cord syndrome or Chiari malformation.17 Retrospective series in the literature report failure rates between 5% and 10% in incidental durotomies, requiring reoperations.1,2 Postoperative CSF leak can first be managed by conservative strategies such as the use of brace, bedrest, lumbar intrathecal drain, or epidural blood patches.3 Persistent leakage may be addressed by reoperation, in addition to conservative measures.18
DuraSeal Exact Spine Sealant System (DESS) is the only FDA-approved secondary agent for dural closure in spine.19 In addition to its low-swell property, DESS has other key advantages. It remains in situ for about 4 to 8 weeks, much longer compared with other sealants such as a fibrin sealant (5-7 days).20 DESS is entirely synthetic unlike a fibrin product, obviating the risk of any disease transmission. It is easy to apply and a blue dye enables easy distinction from blood and CSF.
This postapproval study evaluated DESS in both intentional and incidental spinal durotomies in comparison to a myriad of currently used alternatives. Patients of all demographics undergoing spinal operations for variety of indications were included in this study to reflect a real-world scenario. The sample size and follow-up were robust to capture potential adverse effects associated with its use. The primary endpoint was occurrence of postoperative CSF leaks within 90 days of the operation. This analysis was restricted to a subgroup of patients who adhered to the protocol of the study. There was no significant difference in the postoperative CSF leakage rate between the 2 groups and no significant difference in the time to first leak.
Unlike previous studies that favored a PEG sealant, the control group had similar CSF leakage rate to the DESS group.8,15 Previous studies were randomized and limited to intentional durotomies whereas this study was not randomized and also included incidental durotomies, which are often more challenging to address. In addition, for the control group, the subjects were both prospectively and retrospectively enrolled.
Similar to previous randomized studies, the safety of DESS was confirmed in this study. No significant difference in deep surgical site infection rate (1.6% vs 2.1%, P = .61) or neurological serious adverse events between the 2 groups (P = .53) were found. There was no statistically significant difference in the proportion of subjects with at least 1 adverse event between the 2 groups (10.4% sealant arm vs 9.4% in control, P = .61). Regardless of the intrinsic differences in the groups such as the indication for surgery and the type of operation, the rates of neurological serious adverse events were similar.
This study did find a significantly higher rate of superficial skin infection with DESS arm (2.8% vs 0.5% in control arm). Several risk factors are known to be predictive of surgical site infection such as diabetes, preoperative steroid use, high body mass index, female sex and inpatient status.21,22 We did not find any such difference between the 2 groups after adjusting for propensity scores. Two cases of transcutaneous exudation of DuraSeal have been reported in the literature but no similar incidents were noted in this study.23 The most plausible explanation for the difference in superficial skin infection is the fact that for much of the control group, the data for infection was recorded retrospectively. Retrospective review of medical records is not expected to capture all adverse events as would be the case with the prospective data collection in the DESS arm.
More realistic and consequential concern is the reported neurological complication from the expansion of the original formulation of DuraSeal. In a canine model study, the peak expansion of DuraSeal was noted between 3 and 14 days after application, and complete resolution was noted at 8 weeks.20 The onset of complication due to expansion in clinical settings range from few hours to days. In a case of anterior cervical decompression and fusion, when DuraSeal was used as the only treatment for incidental durotomy, progressive quadriparesis developed within 3 hours.24 In a patient who underwent posterior fossa decompression for Chiari malformation, DuraSeal reportedly led to spinal cord compression with worsening quadriparesis noted couple of weeks after the surgery.25 In the lumbar spine, patients who underwent discectomy and interbody fusion developed symptomatic thecal sac compression 2 and 9 days after the operations.9,10,14 One patient with cervical spinal cord compression after anterior cervical discectomy and instrumented fusion did not undergo revision surgery and had symptomatic and radiographic resolution at 8 weeks.26 All of these reported complications resulted from the use of the original formulation of DuraSeal known to swell 38% more than the low swell formulation.
Duraseal Exact Spine Sealant has a very low expansion rate of 19%, substantially reducing the risk of neural compression. To date, no clinical case of neurologic complication due to expansion of DESS has been reported in the literature. As a general rule, DESS should not be used in very confined space such as in anterior cervical discectomy, and only a thin layer should be applied when used elsewhere. Adequate hemostasis should be achieved before closure to prevent additional mass effect from hematoma.12
The nonrandomized, multicenter design of the present study is associated with several confounding variables. Although a direct comparison of the efficacy of DESS is difficult, this study confirmed its safety. Neither were cases of swelling reported nor were any subsequent complications from swelling noted in the prospectively enrolled DESS arm. Large sample size across multiple surgical indications and demographics reflecting a real-world clinical practice led to our conclusion of its safety. It is the only FDA-approved adjunct to closure of dura mater in the spine. Duraseal Exact Spinal Sealant System (PEG spinal sealant) should be used instead of the conventional PEG sealant (DuraSeal) in the spine.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The device is approved by the Food and Drug Administration. Although the authors have not received or will receive benefits for person or professional use from a commercial party related directly or indirectly to the subject of this manuscript, benefits have been or will be received but are directed solely to a research fund, foundation, educational institution, or other nonprofit organization with which the authors have been associated. The study and the preparation of the manuscript was supported by a commercial party related to the subject of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Hodges SD, Humphreys SC, Eck JC, Covington LA. Management of incidental durotomy without mandatory bed rest. A retrospective review of 20 cases. Spine (Phila Pa 1976). 1999;24:2062–2064. [DOI] [PubMed] [Google Scholar]
- 2. Cammisa FP, Jr, Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS. Incidental durotomy in spine surgery. Spine (Phila Pa 1976). 2000;25:2663–2667. [DOI] [PubMed] [Google Scholar]
- 3. Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000;9:e5. [DOI] [PubMed] [Google Scholar]
- 4. Lu CH, Ho ST, Kong SS, Cherng CH, Wong CS. Intracranial subdural hematoma after unintended durotomy during spine surgery. Can J Anaesth. 2002;49:100–102. doi:10.1007/BF03020428. [DOI] [PubMed] [Google Scholar]
- 5. Black P. Cerebrospinal fluid leaks following spinal or posterior fossa surgery: use of fat grafts for prevention and repair. Neurosurg Focus. 2000;9:e4. [DOI] [PubMed] [Google Scholar]
- 6. Cosgrove GR, Delashaw JB, Grotenhuis JA, et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg. 2007;106:52–58. doi:10.3171/jns.2007.106.1.52. [DOI] [PubMed] [Google Scholar]
- 7. Osbun JW, Ellenbogen RG, Chesnut RM, et al. A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (Duraseal Dural Sealant System) as a dural sealant in cranial surgery. World Neurosurg. 2012;78:498–504. doi:10.1016/j.wneu.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 8. Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976). 2011;36:1906–1912. doi:10.1097/BRS.0b013e3181fdb4db. [DOI] [PubMed] [Google Scholar]
- 9. Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine (Phila Pa 1976). 2009;34:E144–E148. doi:10.1097/BRS.0b013e31818d5427. [DOI] [PubMed] [Google Scholar]
- 10. Neuman BJ, Radcliff K, Rihn J. Cauda equina syndrome after a TLIF resulting from postoperative expansion of a hydrogel dural sealant. Clin Orthop Relat Res. 2012;470:1640–1645. doi:10.1007/s11999-011-2071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SH, Park CW, Lee SG, Kim WK. Postoperative cervical cord compression induced by hydrogel dural sealant (DuraSeal®). Korean J Spine. 2013;10:44–46. doi:10.14245/kjs.2013.10.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee G, Lee CK, Bynevelt M. DuraSeal-hematoma: concealed hematoma causing spinal cord compression. Spine (Phila Pa 1976). 2010;35:E1522–E1524. doi:10.1097/BRS.0b013e3181edfe2c. [DOI] [PubMed] [Google Scholar]
- 13. Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine (Phila Pa 1976). 2010;35:E25–E26. doi:10.1097/BRS.0b013e3181b9fc45. [DOI] [PubMed] [Google Scholar]
- 14. Tseng WL, Xiao F. Duraseal thecal sac compression after lumbar discectomy causing radiculopathy. Spine J. 2015;15:1892–1893. doi:10.1016/j.spinee.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 15. Wright NM, Park J, Tew JM, et al. Spinal sealant system provides better intraoperative watertight closure than standard of care during spinal surgery: a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976). 2015;40:505–513. doi:10.1097/BRS.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 16. Liu V, Gillis C, Cochrane D, Singhal A, Steinbok P. CSF complications following intradural spinal surgeries in children. Childs Nerv Syst. 2014;30:299–305. doi:10.1007/s00381-013-2276-4. [DOI] [PubMed] [Google Scholar]
- 17. Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 2006;104(1 suppl):16–20. doi:10.3171/ped.2006.104.1.16. [DOI] [PubMed] [Google Scholar]
- 18. Kerr EE, Panchal RR, Kim KD. Refractory cervical spinal pseudomeningocele successfully Treated with a Polycarbonate Face Mask: Technical Note. JSM Neurosurg Spine. 2014;2:1011. [Google Scholar]
- 19. US Food & Drug Administration. Premarket Approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P080013. Accessed September 22, 2017.
- 20. Preul MC, Campbell PK, Bichard WD, Spetzler RF. Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model. J Neurosurg. 2007;107:642–650. doi:10.3171/JNS-07/09/0642. [DOI] [PubMed] [Google Scholar]
- 21. Lieber B, Han B, Strom RG, et al. Preoperative predictors of spinal infection within the National Surgical Quality Inpatient Database. World Neurosurg. 2016;89:517–524. doi:10.1016/j.wneu.2015.12.085. [DOI] [PubMed] [Google Scholar]
- 22. Elsamadicy AA, Adogwa O, Vuong VD, et al. Patient body mass index is an independent predictor of 30-day hospital readmission after elective spine surgery. World Neurosurg. 2016;96:148–151. doi:10.1016/j.wneu.2016.08.097. [DOI] [PubMed] [Google Scholar]
- 23. Siman H, Techy F. Transcutaneous drainage of gel-like substance after application of hydrogel dural sealant: report of two cases. Global Spine J. 2016;6:e7–e10. doi:10.1055/s-0035-1550088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine (Phila Pa 1976). 2010;35:E25–E26. doi:10.1097/BRS.0b013e3181b9fc45. [DOI] [PubMed] [Google Scholar]
- 25. Blackburn SL, Smyth MD. Hydrogel-induced cervicomedullary compression after posterior fossa decompression for Chiari malformation. Case report J Neurosurg. 2007;106(4 suppl):302–304. doi:10.3171/ped.2007.106.4.302. [DOI] [PubMed] [Google Scholar]
- 26. Rustagi T, Lavelle WF. Spontaneous resolution of cervical cord compression induced by hydrogel (Duraseal). Spine J. 2014;14:2511–2512. doi:10.1016/j.spinee.2014.05.008. [DOI] [PubMed] [Google Scholar]