Abstract
Context: Parkinson’s disease (PD) is a neurodegenerative disorder due to gradual loss of dopaminergic nerves in the substantia nigra (SN) in the midbrain. PD leads to certain motor disorders including resting tremor, muscle stiffness and slow movement. Medicinal plants have shown positive pharmacological effects in treating different models of PD.
Objective: Tendency to use natural products, especially plants, for the treatment of PD has been growing. This article reviews the basic aspects of medicinal plants and their bioactive compounds that could be used to treat PD.
Methods: Reliable articles indexed in databases ISI, SID, PubMed, PubMed Central, Scopus and Web of Science were used. A total of 12 plant-derived active ingredients and 18 herbal extracts were included. Different compounds have so far been isolated from plants that affect PD especially by targeting pathways associated with the pathogenesis of the disease.
Results: Although some herbal extracts such as Hibiscus asper Hook. f. (Malvaceae), Ginkgo biloba L. (Ginkgoaceae), Carthamus tinctorius L (Asteraceae) and certain active ingredients, such as berberine and curcumin, have shown positive effects in animal models of PD, potential active ingredients and mechanisms of action should be investigated in additional studies.
Discussion and conclusions: Despite the wide variety of plants in the world, a limited number of them have been studied for anti-Parkinsonian activity, and therefore, there are numerous perspectives in this field for future studies on plants and their bioactive compounds.
Keywords: Dopaminergic receptors, L-DOPA, Ginkgo biloba, curcumin
Introduction
Parkinson’s disease (PD) was first described by James Parkinson in 1817. The prevalence of PD is about 1% in people aged over 65 years. It begins between the ages of 40 and 70 and is very rare under the age of 20. If PD begins under the age of 20 years, it is referred to as young onset PD, which has a different pathology than other types of PD, is usually inherited, and is due to Wilson’s disease or Huntington’s disease. PD is more prevalent in men than in women by a ratio of 3:2 (Postuma et al. 2015).
The prevalence of PD is nearly 160 people per 100,000 population and its incidence is about 20 people per 100,000 population. The prevalence and incidence of the disease increase with age, so that at the age of 70 years, the prevalence is nearly 550 people per 100,000 and the incidence is 120 people per 100,000. Factors such as trauma, overwork, exposure to coldness, inflexible personality and stress are considered to be the predisposing factors; however, this has not yet been definitively proven (Tysnes and Storstein 2017).
Etiology
The aetiology of PD is unknown. The disease, however, seems to be due to the dopamine depletion in the nigrostriatal pathway. Typically, intra-cytoplasmic inclusions, called Lewy bodies of dopamine neurons, are detected in PD. The degeneration of dopaminergic neurons in the substantia nigra (SN) compacta, followed by impaired release of dopamine in striatum, is a major cause of the disease; however, genetic factors have also been shown to be effective in the development of this disease. In 23% of cases, no genetic factor is identified. Several hypotheses have been raised for the death of dopaminergic cells in the SN compacta, such as mitochondrial complex defect associated with the electron transport chain, iron accumulation, protein accumulation, inflammatory immune responses along with environmental factors such as physical trauma and infection, liver cytochrome p450 dysfunction and increased formation of free radicals (Wang et al. 2015).
Pathogenesis
PD is a neurodegenerative and progressive disease that is associated with various motor and debilitating disorders, including bradykinesia, muscle stiffness, resting tremor and imbalance. PD is pathologically characterized by slow and gradual degeneration of dopaminergic neurons in the SN compacta that leads to a decrease in the level of dopamine in the striatum, tailed nuclei and the putamen (Lees 2012). The progressive loss of dopaminergic neurons in the basal complexes is the most important pathological finding in the brain of patients with PD. Destruction of these neurons results in the reduction of dopamine neurotransmitter in this area. After 50–60% of dopaminergic neurons are degraded and dopamine levels in the striatum decrease by around 80–85%, the symptoms of the disease appear. The exact molecular mechanism of the degradation of dopaminergic neurons and the incidence of PD is unclear; however, studies have shown that oxidative stress and mitochondrial dysfunction probably play a key role in the pathogenesis of PD; the loss of nigrostriatal dopaminergic neurons and the presence of intracellular cytoplasmic proteins, i.e., Lewy bodies, are also involved. The cells are located in the nigrostriatal neurons in the SN pars compacta (SNpc) and are sent to putamen. The absence of these neurons, which typically contain small amounts of melanin, leads to depigmentation of SNpc (Lees 2012).
Abnormal mitochondrial function and oxidative stress
Accumulating evidence suggests that in PD, the function of mitochondrial complex I partially decreases. Approximately 100% of molecular oxygen is consumed by the mitochondria during cellular respiration, and powerful oxidants, including hydrogen peroxide and superoxide radicals, are produced as a by-product. Reactive oxygen species (ROS) production increases by inhibiting mitochondrial complex I, which can produce toxic hydroxyl radicals or react with nitric oxide and produce peroxynitrites. These molecules can damage the nucleic acids, proteins and lipids by reacting with nucleic acids. One of these injuries can occur in the electron transport chain, which can lead to mitochondrial damage and the formation of ROS that, in turn, can increase the inappropriate folding of proteins (Gaki and Papavassiliou 2014).
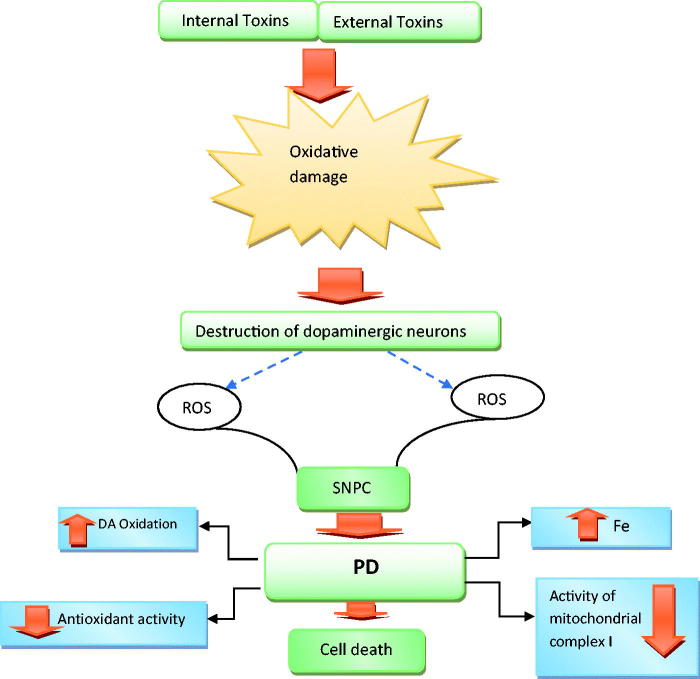
Much research has also suggested that ROS plays a role in the degeneration of dopaminergic neurons in the brain tissues of PD patients (Figure 1). High levels of lipid peroxidation, glutathione depletion and increase in protein oxidation are observed in the brain tissues of PD patients. Oxidation of dopamine leads to the formation of dopamine quinone that can directly alter proteins. In healthy people, there are mechanisms that prevent the cells from inappropriate folding and accumulation. For example, these proteins are harvested by lysosomal and ubiquitin-proteasome system, as well as certain chaperones can correct these foldings. It has been suggested that defective mitochondrial function causes the death of dopaminergic neurons at advanced ages. Oxidation of RNA and DNA of membrane proteins and lipids is one of the important factors for defective mitochondrial function. Oxidation changes the structure of many enzymes and thereby reduces their tendency to substrates or coenzymes, and thus their activity (Hwang 2013).
Figure 1.
Factors that contribute to oxidative stress and ultimately neuronal cell death in PD.
So far, many studies have been done on PD and the effects of various factors on the symptoms of the disease, the rate of its progression, and its treatment. Different methods have been used to induce PD in animal models and then the effects of various factors on the improvement of its symptoms have been evaluated. Animal models greatly help investigate pathogenic mechanisms and design therapeutic strategies in human diseases. Given that the pathogenesis of PD has not yet been well understood, it is important to use animal models to better understand the cause of PD and design the approaches to treat it. To induce PD in laboratory animals, certain compounds such as reserpine, methamphetamine, 6-hydroxydopamine, paraquat, maneb, rotenone and 3-nitrotyrosine also transgenic mice are used (Xu et al. 2002).
The most important approach to treat this disease is the use of precursors and other analogues of dopamine. There is a limitation in prescribing levodopa. Its chronic use is frequently associated with long-term motor impairment and decreased efficacy, requiring the increased dose of levodopa to reduce the complications of the disease. The function of levodopa depends on its enzymatic conversion to dopamine. Therefore, levodopa is usually prescribed in combination with carbidopa to be converted to dopamine by the DOPA decarboxylase (Antonini et al. 2010).
The general tendency to use herbal drugs and, in general, natural products in the world can be due to the side effects of chemical drugs on the one hand and environmental pollution on the other hand. Due to increasing prevalence of the nervous system disorders (Hasanpour-Dehkordi and Solati 2016; Hasanpour-Dehkordi et al. 2016; Solati and Lo’Bat Ja’Farzadeh 2016; Solati 2016, 2017) there is a growing tendency to the use of these plants and the revival of traditional medicine. Recent research has also reported promising results regarding the effects of herbal remedies in the treatment or prevention of various diseases, including Alzheimer’s disease (Rabiei et al. 2015; Rabiei and Setorki 2018), stroke (Rabiei et al. 2012; Rabiei and Rafieian-Kopaei 2014), depression (Rabiei et al. 2017, 2018) addiction (Rabiei et al. 2016) and many other conditions. The effects of medicinal plants and their active ingredients on the treatment of PD are presented in Tables 1 and 2.
Table 1.
Plant active ingredients effective on Parkinson’s disease.
| Name compound | Used model | Concentration | Effects | Chemical structures | References |
|---|---|---|---|---|---|
| Astragaloside IV | Cultures of primary nigral cells (PNCs) | 50, 100 and 200 µM | 1. Increased the level of TH and nitrite oxide synthase (NOS) immune reactivities 2. Protect dopaminergic neurons degeneration 3. Promoted neurite outgrowth and increased TH and NOS immune reactive of dopaminergic neurons |
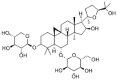 |
Chan et al. (2009) |
| Berberine | 1. 6-OHDA induced cytotoxicity in PC12 cells 2. Unilateral 6-OHDA-lesioned rats |
5, 10 and 30 µM | 1. Depleted tyrosine hydroxylase-immuno positive cells in the substantia nigra 2. Decreased dopamine and norepinephrine levels in striatal regions |
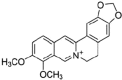 |
Kwon et al. (2010) |
| Baicalein | 6-OHDA induced in vitro and in vivo | 0.5, 5 μg/mL | 1. Ameliorate SH-SY5Y cell apoptosis 2. Promote neurite outgrowth of PC12 cell 3. Attenuate muscle tremor 4. Increase tyrosine hydroxylase (TH)-positive neurons |
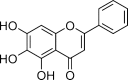 |
Mu et al. (2009) |
| Caffeic acid phenethyl ester | In vitro (cerebellar granule neurons) | 10 µm | 1. Modulate the Ca2+-induced release of cyctochrome c 2. Inhibit caspase-3 activation 3. Blocks cell death |
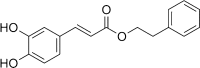 |
Noelker et al. (2005) |
| Carnosic acid | Rat induced by 6-OHDA | 20 mg/kg | 1. Improved the locomotor activity 2. Reduced the apomorphine-caused rotation 3. Reduced lipid peroxidation 4. GSH reduction 5. Increased the protein expression of c-glutamate-cysteine ligase catalytic subunit, superoxide dismutase, and glutathione reductase 6. Reduction of the Bcl-2/Bax ratio 7. Induction of caspase 3 cleavage 8. Induction of poly (ADP ribose) polymerase (PARP) cleavage |
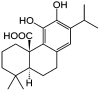 |
Wu et al. (2015) |
| Curcumin | Rat induced by 6-OHDA | 80 mg/kg pretreatment | 1. Decreased MDA, 2. Increased glutathione, GPx, glutathione reductase, SOD, catalase, tyrosine hydroxylase and D2 receptor binding in brain tissue |
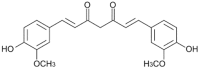 |
Khuwaja et al. (2011) |
| Gallic acid | Rat induced by 6-OHDA | 50, 100 and 200 mg/kg | 1. Increased the passive avoidance memory 2. Increased total thiol in brain tissue 3. Increased GPx 4. Decreased MDA levels in brain tissue |
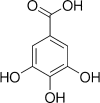 |
Mansouri et al. (2013) |
| Ellagic acid | Rat induced by 6-OHDA | 50 mg/kg | 1. Increased of stride-length 2. Decreased the contralateral rotations 3. Decreased TNF-α and IL-1β levels in brain tissue |
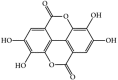 |
Farbood et al. (2015) |
| Ginsenoside Rg1 | (6-OHDA) induced neurotoxicity in human neuroblastoma SK-N-SH cells | 0.01 µM | 1. Increased survival 2. Rescue occurred on cell viability 3. Restore the up-regulation of Bax and down-regulation of Bcl-2 mRNA and protein expression 4. Attenuate 6-OHDA-induced apoptosis |
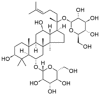 |
Gao et al. (2009) |
| Quercetin | Rat induced by 6- OHDA | 50 mg/kg | 1. Increased dopamine 2. Decreased protein carbonyl content |
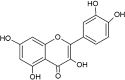 |
Haleagrahara et al. (2013) |
| Desferrioxamine | Rat induced by 6-OHDA | 50 mg/kg | 1. Decreased protein carbonyl content 2. Increased dopamine, GSH and SOD levels |
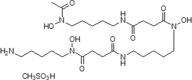 |
Haleagrahara et al. (2013) |
| Thymoquinone | Rat induced by 6- OHDA | 5, 10 mg/kg | 1. Improved turning behaviour 2. Decreased MDA levels 3. Increased activity of superoxide dismutase 4. Prevented loss of SNC (substantia nigra pars compacta) neurons |
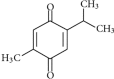 |
Sedaghat et al. (2014) |
| Tripchlorolide | (MPTP)-lesioned C57BL/6 mice | 1 µg/kg | 1. Increased level of dopamine in the substantia nigra and striatum | 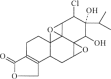 |
Hong et al. (2007) |
| Sulforaphane | mice induced by 6-OHDA | 5 mg/kg | 1. Improved motor coordination 2. Blocking DNA fragmentation and caspase-3 activation 3. Increased glutathione levels |
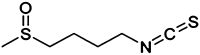 |
Morroni et al. (2013) |
Table 2.
Medicinal plants effective on Parkinson’s disease.
| Plant | Used model | Concentration | Effects | References |
|---|---|---|---|---|
| Tinospora cordifolia | Rat induced by 6-OHDA | 200, 400 mg/kg | 1. Increased the dopamine levels 2. Decreased iron asymmetry ratio 3. Decreased MDA levels 4. Increased mitochondrial complex I activity 5. Improved locomotor activity |
Kosaraju et al. (2014) |
| Sesame seed oil (SO) | Mice induced by 6-OHDA | SO mix diet | 1. Increased glutathione reductase (GR), glutathione-S-transferase (GST), glutathione peroxidase (GPx), catalase (CAT) and content of glutathione (GSH) and thiobarbituric acid reactive substance (TBARS) 2. Inhibit the activation of Nox2 and Cox2 3. Restored MnSOD expression |
Ahmad S et al. (2012) |
| Carthamus tinctorius L. | (MPTP)-lesioned rat | 70, 35 mg/kg | 1. Improve behavioural performances 2. Suppression of α-synuclein overexpression or aggregation 3. Suppression of reactive astrogliosis |
Ren et al. (2016) |
| Chaenomeles speciosa | In vitro and in vivo assays, Chinese hamster ovary (CHO) cells, rat induced by 6-OHDA (MPTP)-lesioned mice | 250, 500, 1000 mg/kg | 1. Increased tyrosine hydroxylase-positive neurons in the substantia nigra 2. Increased D8 cell viability 3. Time-dependently reduced abnormal turns in apomorphine-induced rotational turning |
Zhao et al. (2008) |
| Portulaca oleracea | Rat induced by 6-OHDA | 200, 400 mg/kg | 1. Increase in crossings and rearing in open field test | Martins et al. (2016) |
| Paeonia suffruticosa | (MPTP)-lesioned mice | 25, 50 mg/kg | 1. Increased movement distance in the open field test 2. Increased total striatal dopamine 3. Attenuated the loss of dopaminergic neurons 4. Reversed down regulation Akt and the mitochondrial OXPHOS subunits |
Kim et al. (2014) |
| Mucuna pruriens | Rat induced by 6-OHDA | 40, 80, 120 mg/kg | 1. Reduced risk for drug-induced dyskinesias 2. Increased nigrostriatal catecholamine content |
Lieu et al. (2010) |
| Hyoscyamus niger seeds | Unilateral intrastriatal injection of rotenone in rat | 125, 250, 500 mg/kg | 1. Attenuated motor disabilities 2. Increased level of GSH content and GPX, SOD and CAT activities |
Khatri and Juvekar (2015) |
| Hibiscus asper leaves | Rat induced by 6-OHDA | 50, 100 mg/kg | 1. Increased SOD, GPX and CAT activities, total GSH content 2. Reduced MDA level |
Hritcu et al. (2011) |
| Gynostemma pentaphyllum | Rat induced by 6-OHDA | 10, 30 mg/kg | 1. Recovered the levels of dopamine, 3,4 dihydroxyphenylacetic acid, homovanillic acid and norepinephrine in striatum 2. Ameliorated the loss of TH-immunopositive neurons in substantia nigra |
Choi et al. (2010) |
| Ginkgo biloba | Rat induced by 6-OHDA | 50, 100, 150 mg/kg | 1. Decreased rotation 2. Improved locomotor activity and muscular coordination 3. Increased GSH content 4. Decreased generation of TBARS 5. Increased SOD, CAT activities 6. Increase in the number of dopaminergic D2 receptors in striatum |
Ahmad M et al. (2005) |
| Fructus Alpiniaoxyphylla | Zebrafish and PC12 cell models | 20% solution | 1. Restored dopaminergic (DA) neuron degeneration 2. Attenuated a deficit of locomotor activity 3. Increased the viability of 6-OHDA-treated PC12 cells 4. Attenuating cellular apoptosis |
Zhang et al. (2012) |
| Delphinium denudatum | Rat induced by 6-OHDA | 200, 400, 600 mg/kg | 1. Decreased MDA levels 2. Increased GSH content 3. Increased SOD, CAT activities 4. Increased levels of dopamine |
Ahmad M et al. (2006) |
| Bacopa monniera Linn | Rat induced by 6-OHDA | 20, 40 mg/kg | 1. Decreased MDA levels 2. Increased GSH content 3. Increased SOD, CAT activities |
Shobana et al. 2012 |
| Althaea officinalis L. | Rat induced by 6-OHDA | 10 mg/kg | 1. Attenuated rotational behaviour 2. Decreased MDA levels |
Rezaei and Alirezaei (2014) |
| Albizia adianthifolia | Rat induced by 6-OHDA | 150, 300 mg/kg | 1. Improved working memory and reference memory 2. Attenuated the contralateral rotational asymmetry |
Beppe et al. (2014) |
| Valeriana officinalis | Rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells | 0.049, 0.098 and 0.195 mg/mL | 1. Increase in cell viability | de Oliveria et al. (2009) |
| Black tea | Rat induced by 6-OHDA | 1.5% | 1. Recovery in d-amphetamine induced circling behaviour and spontaneous locomotor activity 2. Recovery indopamine (DA)-D2 receptor binding, striatal DA and 3-4-dihydroxy phenyl acetic acid (DOPAC) level 3. Decreased MDA levels 4. Increased GSH content 5. Increased SOD and CAT activities 6. Increased TH protein level and TH mRNA expression in substantianigra |
Chaturvedi et al. (2006) |
| Panax ginseng | Rat received β-sitosterol β-D-glucoside | 100 mg/kg/d | 1. Reduced dopaminergic cell loss, microgliosis, and accumulation of α-synuclein aggregates | Van Kampen et al. (2014) |
| Safflower | Mouse induced with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- | 35, 70 mg/kg/d | 1. Reversed the decreased protein expression of tyrosine hydroxylase, dopamine transporter and DJ-1 2. Increased dopamine levels 3. Decreased acetylcholine levels |
Ablat et al. (2016) |
| Hypericum perforatum | Rat induced by 6-OHDA | 200 mg/kg/d | 1. Attenuated apomorphine-induced rotational behaviour, 2. Decreased the latency to initiate and the total time on the narrow beam task 3. Decreased MDA levels 4. Increased catalase activity |
Kiasalari et al. (2016) |
| Oxalis corniculata l. | C57 male mice MPTP administration | 250, 500 mg/kg | 1. Decreased SOD activity 2. Increased catalase activity |
Aruna et al. (2016) |
Discussion
Medicinal plants have been discovered by different nations across the world thousands of years ago. These plants are used in many communities and countries for centuries due to their safety, efficiency, acceptability and comparably fewer side effects than chemical drugs (Alok et al. 2013). Medicinal plants have long been known throughout the world for their unique and valuable benefits. Research has also shown that these plants have a special status in the health and well-being of various communities due to the antioxidant effect of phenolic compounds identified in them, in addition to their economic value. Therefore, the global approach is to identify new plant species and their active ingredients. Today, plants play an important role in the therapeutic approaches and new drugs used for diseases. Therefore, the demand for new oral medicines without side effects continues. In this study, the therapeutic effects of medicinal plants and their compounds on the treatment of PD observed in vivo and in vitro were reviewed. Although most of the herbal extracts and their active ingredients have been studied in PD models in vivo, some of them have so far been investigated only in cell models (Noelker et al. 2005; Chan et al. 2009; Gao et al. 2009).
To date, a large number of medicinal plants and their active ingredients have been reported to prevent and treat PD. Most studies have focused on antioxidant (Ahmad M et al. 2005, 2006; Chaturvedi et al. 2006; Hritcu et al. 2011; Khuwaja et al. 2011; Ahmad S et al. 2012; Shobana et al. 2012; Mansouri et al. 2013; Khatri and Juvekar 2015; Wu et al. 2015), anti-inflammatory (Farbood et al. 2015) and antiapoptotic (Gao et al. 2009; Morroni et al. 2013; Kim et al. 2014) properties of these plants.
A number of studies have demonstrated the protective effects of tea polyphenols on brain damage in different animal models of PD. Studies have used either a single compound such as EGCG, or a combination of extracts from tea. Individual EGCG and black tea polyphenol extracts were observed to mitigate striatal dopamine depletion and SN dopaminergic neurons loss after being administered for the long term to rats or mice receiving the parkinsonism-inducing neurotoxins, e.g., 6-OHDA and 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) (Chaturvedi et al. 2006).
The aqueous extract of Albizia adianthifolia W. Wight (Leguminosae) leaves has antioxidant potential and can help manage neurological abnormalities due to PD. The HPLC analysis showed that apigenin is the main compound among flavones, and therefore the cause of the cognitive-enhancing impacts in the 6-OHDA-lesion rodent model of PD. Apigenin and similar compounds stimulate neurogenesis in adults both in vivo and in vitro via promoting neuronal differentiation, and also promote learning and memory according to Morris water task (Beppe et al. 2014).
At present, the pathogenesis of PD is attributed to the formation of ROS and the onset of oxidative stress, leading to damage to the SN compacta, particularly changes in the brain’s iron content, mitochondrial dysfunction, changes in antioxidant defence system (especially reduction in superoxide dismutase and glutathione [GSH]) and oxidative damage to fats, proteins and DNA. The loss of GSH is related to Lewy body disease (Figure 1). GSH may be the most important biochemical marker for loss of nigral cells. The depletion of GSH may not be the only reason for damage to nigral neurons, but may increase the susceptibility to exposure to toxic or free radicals (Hwang 2013). Most of the medicinal plants and active ingredients reported in this paper increase the levels of glutathione, superoxide dismutase and catalase in the brain, and thus exert neuroprotective effects. Evidence indicates the role of inflammation in the pathogenesis of PD. In patients with PD, the active microglia increase in the striatum and the SN. Microglial cells, which are macrophages in the brain, respond under numerous unfavourable conditions through fast hypertrophic proliferation and expression of a number of cytokines. Active microglia upregulate cell surface markers, such as macrophage antigen complex 1, produce a variety of pro-inflammatory cytokines. A number of cytokines, including IL-1, IL-6 and TNF-α, contribute to the inflammation process (Niranjan 2014).
Certain active ingredients of plants, such as ellagic acid, reduce rotation in rats with 6-OHDA-induced PD due to reduced inflammation by lowering the levels of IL-1β and TNF-α in the brain tissue (Farbood et al. 2015). Since a plant extract contains a wide range of compounds, the effects of synergistic reaction of two or more compounds is higher than those of a single substance (Williamson 2001). Synergistic effects may be responsible for the therapeutic effects of many plant-based products. Therefore, herbal extracts can be used to treat PD due to various bioactive ingredients (Wang et al. 2008).
Conclusions
We sought to present a summary of current experimental evidence on anti-Parkinsonian activities of medicinal plants and their active ingredients. We found that different natural compounds and herbal extracts exhibit various anti-Parkinsonian activities. Taken together, various PD neurotoxic models are a good basis for discovering anti-Parkinsonian drugs, and herbal drugs can be used to develop new drugs for PD. However, the efficacy of plant extracts and their active ingredients in PD models should be further investigated in future empirical studies. In addition, the active ingredients and action mechanisms of herbal extracts remain to be adequately explained.
Funding Statement
This study was funded by the Research and Technology Deputy of Shahrekord University of Medical Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ablat N, Lv D, Ren R, Xiaokaiti Y, Ma X, Zhao X, Sun Y, Lei H, Xu J, Ma Y, et al. 2016. Neuroprotective effects of a standardized flavonoid extract from safflower against a rotenone-induced rat model of Parkinson’s disease. Molecules. 21:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Chaturvedi RK, Agrawal AK, Islam F. 2005. Ginkgo biloba affords dose‐dependent protection against 6‐hydroxydopamine‐induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J Neurochem. 1:94–104. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Yousuf S, Khan MB, Ahmad AS, Saleem S, Hoda MN, Islam F. 2006. Protective effects of ethanolic extract of Delphinium denudatum in a rat model of Parkinson’s disease. Hum Exp Toxicol. 7:361–368. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Khan MB, Hoda MN, Bhatia K, Haque R, Fazili IS, Jamal A, Khan JS, Katare DP. 2012. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochem Res. 3:516–526. [DOI] [PubMed] [Google Scholar]
- Alok S, Jain SK, Verma A, Kumar M, Sabharwal M. 2013. Pathophysiology of kidney, gallbladder and urinary stones treatment with herbal and allopathic medicine: a review. Asian Pac J Trop Dis. 6:496–504. [Google Scholar]
- Antonini A, Barone P, Ceravolo R, Fabbrini G, Tinazzi M, Abbruzzese G. 2010. Role of pramipexole in the management of Parkinson’s disease. CNS Drugs. 10:829–841. [DOI] [PubMed] [Google Scholar]
- Aruna K, Rajeswari PDR, Sankar SR. 2016. The effects of Oxalis corniculata L. extract against MPTP induced oxidative stress in mouse model of Parkinson’s disease. J Pharm Sci Res. 8:1136. [Google Scholar]
- Beppe GJ, Dongmo AB, Foyet HS, Tsabang N, Olteanu Z, Cioanca O, Hancianu M, Dimo T, Hritcu L. 2014. Memory-enhancing activities of the aqueous extract of Albizia adianthifolia leaves in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. BMC Complement Altern Med. 14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WS, Durairajan SSK, Lu JH, Wang Y, Xie LX, Kum WF, Koo I, Yung KK, Li M. 2009. Neuroprotective effects of astragaloside IV in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem Int. 6:414–422. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Shukla S, Seth K, Chauhan S, Sinha C, Shukla Y, Agrawal AK. 2006. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiol Dis. 2:421–434. [DOI] [PubMed] [Google Scholar]
- Choi HS, Park MS, Kim SH, Hwang BY, Lee CK, Lee MK. 2010. Neuroprotective effects of herbal ethanol extracts from Gynostemma pentaphyllum in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Molecules. 4:2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveria DM, Barreto G, De Andrade DVG, Saraceno E, Aon-Bertolino L, Capani F, Dos Santos El Bachá R, Giraldez LD. 2009. Cytoprotective effect of Valeriana officinalis extract on an in vitro experimental model of Parkinson disease. Neurochem Res. 2:215–220. [DOI] [PubMed] [Google Scholar]
- Farbood Y, Sarkaki A, Dolatshahi M, Mansouri SMT, Khodadadi A. 2015. Ellagic acid protects the brain against 6-hydroxydopamine induced neuroinflammation in a rat model of Parkinson’s disease. Basic Clin Neurosci. 2:83. [PMC free article] [PubMed] [Google Scholar]
- Gaki GS, Papavassiliou AG. 2014. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2:217–230. [DOI] [PubMed] [Google Scholar]
- Gao QG, Chen WF, Xie JX, Wong MS. 2009. Ginsenoside Rg1 protects against 6‐OHDA‐induced neurotoxicity in neuroblastoma SK‐N‐SH cells via IGF‐I receptor and estrogen receptor pathways. J Neurochem. 5:1338–1347. [DOI] [PubMed] [Google Scholar]
- Haleagrahara N, Siew CJ, Ponnusamy K. 2013. Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J Toxicol Sci. 1:25–33. [DOI] [PubMed] [Google Scholar]
- Hasanpour-Dehkordi A, Solati K. 2016. The efficacy of three learning methods collaborative, context-based learning and traditional, on learning, attitude and behaviour of undergraduate nursing students: integrating theory and practice. J Clin Diagn Res. 4:VC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanpour-Dehkordi A, Jivad N, Solati K. 2016. Effects of yoga on physiological indices, anxiety and social functioning in multiple sclerosis patients: a randomized trial. J Clin Diagn Res. 6:VC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Wang G, Gu J, Pan J, Bai L, Zhang S, Chen SD. 2007. Tripchlorolide protects against MPTP‐induced neurotoxicity in C57BL/6 mice. Eur J Neurosci. 6:1500–1508. [DOI] [PubMed] [Google Scholar]
- Hritcu L, Foyet HS, Stefan M, Mihasan M, Asongalem AE, Kamtchouing P. 2011. Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J Ethnopharmacol. 137:585–591. [DOI] [PubMed] [Google Scholar]
- Hwang O. 2013. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. 1:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri DK, Juvekar AR. 2015. Propensity of Hyoscyamus niger seeds methanolic extract to allay stereotaxically rotenone-induced Parkinson’s disease symptoms in rats. Orient Pharm Expe Med. 4:327–339. [Google Scholar]
- Khuwaja G, Khan MM, Ishrat T, Ahmad A, Raza SS, Ashafaq M, Javed H, Khan MB, Khan A, Vaibhav K, et al. 2011. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: behavioral, neurochemical and immunohistochemical studies. Brain Res. 1368:254–263. [DOI] [PubMed] [Google Scholar]
- Kiasalari Z, Baluchnejadmojarad T, Roghani M. 2016. Hypericum perforatum hydroalcoholic extract mitigates motor dysfunction and is neuroprotective in intrastriatal 6-Hydroxydopamine rat model of Parkinson's disease. Cell Mol Neurobiol. 36:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Park G, Piao Y, Kang MS, Pak YK, Hong SP, Oh MS. 2014. Effects of the root bark of Paeonia suffruticosa on mitochondria-mediated neuroprotection in an MPTP-induced model of Parkinson’s disease. Food Chem Toxicol. 65:293–300. [DOI] [PubMed] [Google Scholar]
- Kosaraju J, Chinni S, Roy PD, Kannan E, Antony AS, Kumar MS. 2014. Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced Parkinsonism. Indian J Pharmacol. 2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon IH, Choi HS, Shin KS, Lee BK, Lee CK, Hwang BY, Lim SC, Lee MK. 2010. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci Lett. 1:29–33. [DOI] [PubMed] [Google Scholar]
- Lees AJ. 2012. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease: accuracy of clinical diagnosis of idiopathic Parkinson’s disease. Vol. 2. London: BMJ Publishing Group Ltd.; p. 34–39. [Google Scholar]
- Lieu CA, Kunselman AR, Manyam BV, Venkiteswaran K, Subramanian T. 2010. A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. Parkinsonism Relat Disord. 7:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri MT, Farbood Y, Sameri MJ, Sarkaki A, Naghizadeh B, Rafeirad M. 2013. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2:1028–1033. [DOI] [PubMed] [Google Scholar]
- Martins WB, Rodrigues SA, Silva HK, Dantas CG, Junior W, Cardoso JC, Filho LX, Gomes MZ. 2016. Neuroprotective effect of Portulaca oleracea extracts against 6-hydroxydopamine-induced lesion of dopaminergic neurons. Acad Bras Cienc. 3:150–439. [DOI] [PubMed] [Google Scholar]
- Morroni F, Tarozzi A, Sita G, Bolondi C, Zolezzi Moraga JM, Cantelli-Forti G, Hrelia P. 2013. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicol. 36:63–71. [DOI] [PubMed] [Google Scholar]
- Mu X, He G, Cheng Y, Li X, Xu B, Du G. 2009. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 4:642–648. [DOI] [PubMed] [Google Scholar]
- Niranjan R. 2014. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol. 1:28–38. [DOI] [PubMed] [Google Scholar]
- Noelker C, Bacher M, Gocke P, Wei X, Klockgether T, Du Y, Dodel R. 2005. The flavanoide caffeic acid phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity. Neurosci Lett. 1:39–43. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, et al. 2015. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 12:1591–1601. [DOI] [PubMed] [Google Scholar]
- Rabiei Z, Bigdeli MR, Rasoulian B, Ghassempour A, Mirzajani F. 2012. The neuroprotection effect of pretreatment with olive leaf extract on brain lipidomics in rat stroke model. Phytomedicine. 10:940–946. [DOI] [PubMed] [Google Scholar]
- Rabiei Z, Jahanbazi S, Alibabaei Z, Rafieian-kopaei M. 2018. Antidepressant effects of oleuropein in male mice by forced swim test and tail suspension test. Middle East J Fam Med. 10:132. [Google Scholar]
- Rabiei Z, Lorigooini Z, Kopaei MR. 2016. Effects of hydroalcoholic extract of Borago officinalis on naloxone-precipitated withdrawal syndrome in morphine-dependent mice. Bangladesh J Pharmacol. 4:824–829. [Google Scholar]
- Rabiei Z, Mokhtari S, Asgharzade S, Gholami M, Rahnama S, Rafieian-kopaei M. 2015. Inhibitory effect of Thymus vulgaris extract on memory impairment induced by scopolamine in rat. Asian Pac J Trop Biomed. 10:845–851. [Google Scholar]
- Rabiei Z, Naderi S, Rafieian-Kopaei M. 2017. Study of antidepressant effects of grape seed oil in male mice using tail suspension and forced swim tests. Bangladesh J Pharmacol. 4:397–402. [Google Scholar]
- Rabiei Z, Rafieian-Kopaei M. 2014. Neuroprotective effect of pretreatment with Lavandula officinalis ethanolic extract on blood-brain barrier permeability in a rat stroke model. Asian Pac J Trop Med. 7:S421–S6. [DOI] [PubMed] [Google Scholar]
- Rabiei Z, Setorki M. 2018. Effect of hydroalcoholic Echium amoenum extract on scopolamine-induced learning and memory impairment in rats. Pharm Biol. 56:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Shi C, Cao J, Sun Y, Zhao X, Guo Y, Wang C, Lei H, Jiang H, Ablat N, et al. 2016. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of Parkinson’s disease. Sci Rep. 6:221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei M, Alirezaei M. 2014. Protective effects of Althaea officinalis L. extract in 6-hydroxydopamine-induced hemi-Parkinsonism model: behavioral, biochemical and histochemical evidence. J Physiol Sci. 3:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat R, Roghani M, Khalili M. 2014. Neuroprotective effect of thymoquinone, the Nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-Parkinsonian rat model. Iran J Pharm Res. 1:227. [PMC free article] [PubMed] [Google Scholar]
- Shobana C, Kumar RR, Sumathi T. 2012. Alcoholic extract of Bacopa monniera Linn. protects against 6-hydroxydopamine-induced changes in behavioral and biochemical aspects: a pilot study. Cell Mol Neurobiol. 7:1099–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati K, Lo’Bat Ja’Farzadeh AH. 2016. The effect of stress management based on group cognitive-behavioural therapy on marital satisfaction in infertile women. J Clin Diagn Res. 7:VC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati K. 2016. Effectiveness of cognitive-behavior group therapy, psycho-education family, and drug therapy in reducing and preventing recurrence of symptoms in patients with major depressive disorder. J Chem Pharm Res. 4:3414–3418. [Google Scholar]
- Solati K. 2017. The efficacy of mindfulness-based cognitive therapy on resilience among the wives of patients with schizophrenia. J Clin Diagn Res. 4:VC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes OB, Storstein A. 2017. Epidemiology of Parkinson’s disease. J Neural Transm. 8:901–905. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Baranowski DB, Shaw CA, Kay DG. 2014. Panax ginseng is neuroprotective in a novel progressive model of Parkinson’s disease. Exp Gerontol. 50:95–105. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu Y, Zhou J. 2015. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liang XB, Li FQ, Zhou HF, Liu XY, Wang JJ, Wang XM. 2008. Therapeutic strategies for Parkinson’s disease: the ancient meets the future—traditional Chinese herbal medicine, electroacupuncture, gene therapy and stem cells. Neurochem Res. 10:1956–1963. [DOI] [PubMed] [Google Scholar]
- Williamson EM. 2001. Synergy and other interactions in phytomedicines. Phytomedicine. 5:401–409. [DOI] [PubMed] [Google Scholar]
- Wu CR, Tsai CW, Chang SW, Lin CY, Huang LC, Tsai CW. 2015. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: involvement of antioxidative enzymes induction. Che-Biol Interact. 225:40–46. [DOI] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. 2002. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 6:600–606. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Cheang LC, Wang MW, Li GH, Chu IK, Lin ZX. 2012. Ethanolic extract of fructus Alpinia oxyphylla protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Cell Mol Neurobiol. 1:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Jiang Z-H, Zheng X-w, Zang S-Y, Guo L-H. 2008. Dopamine transporter inhibitory and antiparkinsonian effect of common flowering quince extract. Pharmacol Biochem Behav. 90:363–371. [DOI] [PubMed] [Google Scholar]