Abstract
Background
Primary sclerosing cholangitis (PSC) is a chronic inflammatory condition causing bile duct strictures. Differentiating inflammatory strictures from malignant transformation is challenging. Cholangioscopy allows direct visualization with the option to biopsy. We describe our experience of cholangioscopy in PSC and propose a novel stricture classification system based on cholangioscopic findings.
Methods
All patients with PSC and a dominant stricture referred for cholangioscopy were reviewed. Based on visual characteristics with direct cholangioscopy, we propose a novel classification system for the extrahepatic form of PSC.
Results
The proposed Edmonton Classification system for extrahepatic PSC strictures consists of the following phenotypes: 1) ‘inflammatory type’, with mucosal erythema and active inflammatory exudate, 2) ‘fibro-stenotic type’, with concentric fibrotic scars, and 3) ‘nodular or mass-forming type’, with a mass in the involved segment of extrahepatic bile duct. From 2011–2017, 30 patients with PSC and a dominant stricture (21 M, mean age 46 years) underwent 32 cholangioscopy procedures. Cholangioscopy was technically successful in 29 of 32 procedures (91%). In these 29 stricture cases, inflammatory type was seen in 16 (55%), fibro-stenotic type in seven (24%) and nodular or mass-forming type in five (17%). In one (4%) procedure, there was no stricture or abnormality identified.
Conclusion
Cholangioscopy is effective and safe for the evaluation of dominant biliary strictures in PSC. Based on our experience with cholangioscopy, we propose a novel classification system of extrahepatic PSC phenotypes: the Edmonton Classification.
Keywords: Cholangioscopy, Classification, Primary sclerosing cholangitis, Stricture
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease that leads to the formation of multifocal strictures in the intrahepatic or extrahepatic bile ducts—or both—and progresses to end-stage liver disease (1). There is no effective medical therapy (2–6), and most patients eventually require liver transplantation (7). Patients with PSC frequently develop dominant strictures with or without recurrent cholangitis, and up to 20% of patients develop cholangiocarcinoma during the course of their lifetime (8). Published literature with respect to biliary tract investigation in patients with PSC that have worsening liver biochemistry or identification of a dominant stricture on surveillance imaging, as with magnetic resonance cholangiopancreatography (MRCP), has focused on confirmation or exclusion of cholangiocarcinoma.
Per oral cholangioscopy permits direct visualization of extrahepatic bile duct strictures, and compared to endoscopic retrograde cholangiopancreatography (ERCP), has been shown to improve diagnostic accuracy for malignant biliary disease (9). However, cholangioscopy in patients with PSC and dominant strictures has not been evaluated thoroughly. Protocol screening cholangioscopy with biliary biopsies (similar to yearly colonoscopy in patients with PSC and ulcerative colitis for the early detection of colon cancer) looks like an attractive strategy for PSC patients, especially in patients with a dominant common bile duct (CBD) stricture. However, this has not been properly evaluated. A prospective study of patients with PSC who had an ERCP indicated for dominant strictures demonstrated that cholangioscopy did not improve detection of cholangiocarcinoma more than ERCP alone (10). Other small studies have only shown mild improvement of cholangioscopy in detecting cholangiocarcinoma (11, 12).
However, the focus of cholangioscopy in PSC in published literature appears to have been the confirmation or exclusion of cholangiocarcinoma when there is a biochemical or radiographic suggestion of a ‘new’ or ‘worsening’ dominant stricture in the CBD. There has been no published report of cholangioscopy in the diagnostic and prognostic stratification in patients with PSC or dominant strictures of the CBD. Moreover, it has been shown that the severity of cholangiography scores correlates with prognosis in PSC (13–15) and that endoscopic treatment of strictures may affect the natural history of the disease (13, 15, 16). Given that PSC prognosis seems related to stricture type and extent, it would be useful to evaluate ways of subtyping and surveying PSC to gain useful insights into the natural history and perhaps to help explain the variable response to treatments, in addition to assessing for the risk of cholangiocarcinoma development. With the availability of per oral cholangioscopy, we can now directly visualize the bile duct mucosa and have the ability to perform targeted biopsies in diseases that were previously appreciated only through cholangiographic interpretation. Regardless, it is clear that traditional cholangiography will not address these subtleties, and perhaps per oral cholangioscopy, along with histological assessment, will give further insight into targeted lines of therapy.
The aim of this study is to evaluate the current evidence of cholangioscopy for the diagnostic stratification of dominant strictures in PSC. Importantly, based on our experience with cholangioscopy in dominant strictures in PSC, we would like to propose nomenclature for a classification system to better understand the variations in phenotypic expression of this disease process. To our knowledge, this kind of diagnostic stratification has not been reported to date. Such stratification could be used to potentially gain prospective insight into the natural history of dominant strictures in PSC, assist in developing targeted therapeutic options and also exclude a small proportion of patients with PSC mimickers, such as IgG4-associated cholangitis (17).
METHODS
At our center, a single-operator per oral direct visualization system (SpyGlass™, Boston Scientific, Marlborough, MA) is used for all cholangioscopy procedures, including those for the evaluation of dominant biliary strictures in PSC. Indications for cholangioscopy in this subset of patients include abnormal liver biochemistry or a ductal caliber change noted on surveillance imaging with MRCP. Our centre has been performing single-operator cholangioscopy since 2011 with the original SpyGlass™ Legacy system but later transitioned to the SpyGlass™ Digital system when it became available in 2015.
Per oral cholangioscopy is performed under general anesthesia, but if the services of an anesthetist are not available, conscious sedation is administered by the endoscopist and monitored by the assisting nursing staff.
Most, but not all, patients had a previous sphincterotomy, and wherever possible, free-hand cannulation of the bile duct with the cholangioscope was performed. If not, a 0.035-inch stiff guidewire was utilized to assist in cannulating the bile duct. Our approach is to review any available cross-sectional imaging, especially MRCP, before performing cholangioscopy and to minimize the use of contrast dye before direct visualization. Contrast dye was felt to interfere with optimal visualization, but this is not as much of a factor with the current improvements in digital imaging. Where necessary, a stiff guide-wire was used to access specific areas of the biliary tree, and balloon-dilation of a CBD stricture was performed if there was resistance to the passage of the 10-French cholangioscope. Once the cholangioscope was at the desired location in the bile duct, visualization was optimized by minimal amounts of sterile water irrigation and suctioning. Cholangioscopic features such as erythema, neo-vascular proliferation, ulceration, presence of fibrinous exudate, presence of nodules and scars/rings were described.
All patients with PSC undergoing per oral cholangioscopy at our centre for the previously mentioned indications were reviewed for this study. Cases performed at our centre are not routinely video-recorded. Most procedures (27 of 30 [90%] patients) were performed by an experienced endoscopist (GS), and visual characteristics were described and recorded at the time of cholangioscopy. Once the cholangioscopy was completed, cholangiography and any intervention, such as stricture dilation, were at the discretion of the endoscopist and based on clinical indication.
RESULTS
Cholangioscopy in PSC Patients
A total of 30 patients with PSC were referred to our unit for the investigation of a dominant stricture, and they underwent 32 cholangioscopy procedures (Table 2). There were 21 males, and the mean age was 46 years (range 19–74 years). Twenty-eight patients underwent one cholangioscopy procedure each, whereas two patients underwent cholangioscopy twice, at an interval of two years and six months, respectively. Cholangioscopy was technically successful in 29 of 32 procedures (91%). In three procedures, it was not possible to advance the cholangioscope to the desired segment in question, and the procedure had to be aborted.
Table 2.
Patient demographics and cholangioscopy characteristics
| Patients, n | 30 |
| Age, years ± SD (range, years) | 46 ± 15 (19–74) |
| Gender, M:F (%) | 21:9 (70:30) |
| Total procedures, n | 32 |
| Cholangioscopy findings, n (%) | |
| 1. Unsuccessful procedures | 3/32 (9) |
| 2. Successful procedures | 29/32 (91) |
| a). Normal | 1/29 (4) |
| b). Inflammatory type | 16/29 (55) |
| c). Fibro-stenotic type | 7/29 (24) |
| d). Nodular/mass-forming type | 5/29 (17) |
Phenotype Stratification System—the Edmonton Classification
Based on our experience with cholangioscopy in patients with PSC, there appear to be distinct phenotypes that occur among these dominant strictures. We propose the Edmonton Classification system with three distinct phenotypes of dominant strictures: inflammatory, fibro-stenotic and nodular or mass-forming types. The cholangioscopy-based description of characteristic visual features seen in these sub-types is outlined in Table 1.
Table 1.
The Edmonton Classification of dominant strictures in PSC by cholangioscopic features
| Stricture Type | Cholangioscopy features |
|---|---|
| Inflammatory (Figure 1) |
Acute (Figure 1A) 1. Mucosal erythema 2. Ulceration 2. Fibrinous white exudate Chronic (Figure 1B) 1. Patchy erythema with early scar/ring formation 2. No ulceration 3. No exudate |
| Fibro-stenotic (Figure 2) |
1. Fibrotic scars/rings 2. No erythema, ulcer or exudate |
| Nodular or mass-forming (Figure 3) |
1. Focal nodular mass |
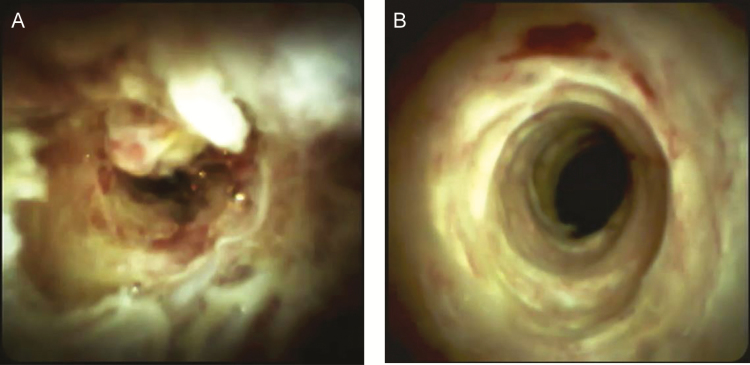
In the ‘inflammatory type’ of stricture, cholangioscopy reveals mucosal erythema and exudate (Figure 1). We have identified a spectrum of disease ranging from acute inflammation (Figure 1A) to chronic, smoldering inflammation with varying degrees of fibrosis (Figure 1B).
Figure 1.
Figure 1 demonstrates the inflammatory type of PSC stricture. Figure 1A demonstrates an ulcerated and erythematous bile duct with a fibrinous exudate (acute inflammation) whereas Figure 1B shows chronic, smoldering inflammation (chronic inflammation). (SpyGlass Digital™ was used in these cases.)
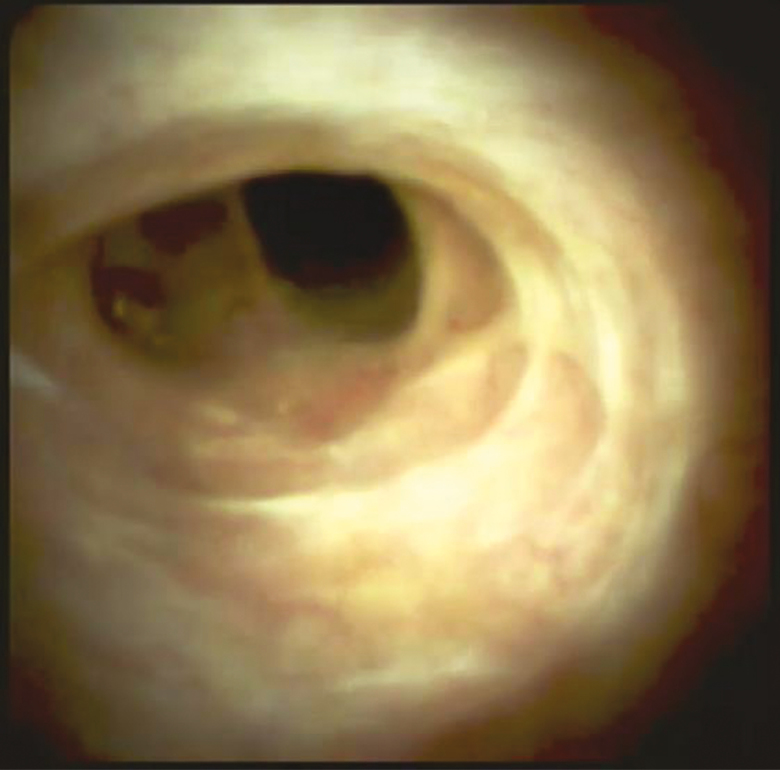
The ‘fibro-stenotic type’ of stricture is typically found in asymptomatic patients. Cholangioscopy shows circumferential rings or asymmetric cicatrization (Figure 2). These strictures are more appropriate for endoscopic therapy if there is evidence of cholestasis (18).
Figure 2.
This demonstrates circumferential fibrotic scars (fibro-stenotic type) with no identifiable feature of inflammation. (SpyGlass Digital™ was used in this case.)
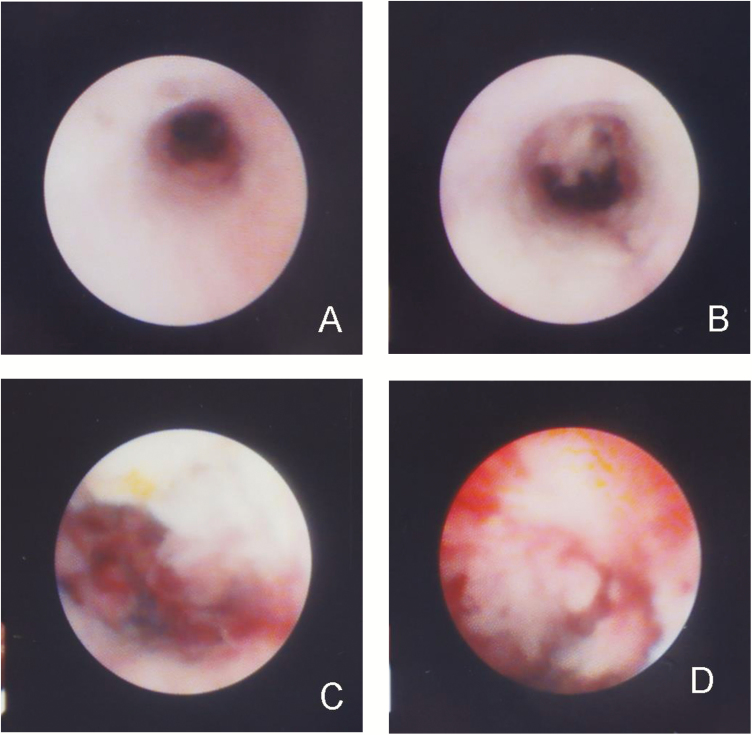
Finally, the ‘nodular or mass-forming type’ of stricture is characterized by a focal nodular growth of tissue within a segment of extrahepatic bile duct (Figure 3A–D). This subtype is most concerning for evolution to cholangiocarcinoma via a dysplasia-to-neoplasia sequence previously suggested (16). With promising results from aggressive neoadjuvant therapy and transplantation (2), it would be ideal to identify these candidates early to optimize outcomes. Since it is challenging to rule out malignancy in these cases, aggressive surveillance cholangioscopy with biopsies are most beneficial here to identify any progression to malignancy (16).
Figure 3.
In the third stricture subtype (nodular or mass-forming), note a normal appearing distal bile duct (3A), followed by a more proximal discrete stricture transitioning to an exophytic lesion (3B-D). (SpyGlass Legacy™ was used in this case.)
Based on the visual characteristics described in Table 1, the remaining 29 procedures were stratified per protocol into inflammatory type in 16 cases (55%), fibro-stenotic type in seven cases (24%) and nodular or mass-forming type in five cases (17%). In one (4%) procedure, there was no stricture or abnormality identified.
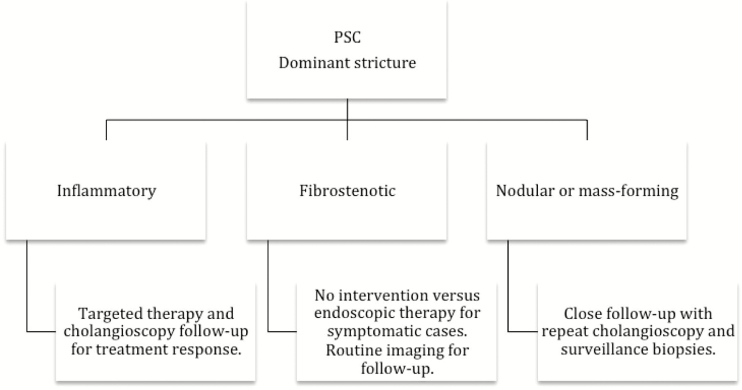
Based on the Edmonton Classification, we also propose an algorithm for the management of dominant extrahepatic biliary strictures in PSC (Figure 4).
Figure 4.
Proposed algorithm for classification and management of dominant strictures in PSC. The Edmonton Classification.
DISCUSSION
In addition to the lack of effective treatment, there are no reliable clinical or biochemical parameters that predict the progression of disease in PSC. The Mayo Clinic Risk Score provides a statistical assessment of the probability of survival but does not predict patterns in progression of disease or development of cholangiocarcinoma (19). Patients come to medical attention either because of jaundice and an increase in cholestatic liver enzymes, specifically alkaline phosphatase or bilirubin, or because of the suggestion of a ‘dominant’ common bile duct (CBD) stricture on surveillance imaging such as MRCP. The finding of a dominant stricture usually warrants further interrogation of the CBD with ERCP and tissue acquisition with brush cytology to differentiate a benign from a malignant etiology (20). It is reasonable to argue that ‘indirect’ imaging with cholangiography—whether with surveillance MRCP or interventional ERCP—tends to view dominant CBD strictures as a single distinct entity with the aim of excluding cholangiocarcinoma. A recent systematic review and meta-analysis of bile duct brushings for cholangiocarcinoma in PSC assessed 747 patients in 11 studies, with a sensitivity and specificity of 43% and 97%, respectively. The obvious limitation in interpretation of this data is the heterogeneity in the patient population and lack of randomized, controlled trials (21). The lifetime incidence of cholangiocarcinoma in patients with PSC is around 20%, with an annual incidence of 1.5%–2% (22–24). Once brush cytology is negative or inconclusive, and it will be for most of these patients, there is no further therapy suggested to intervene for a ‘benign’ inflammatory stricture that has ‘flared’, quite like Crohn’s disease, leading to the luminal narrowing. This may be the reason why, in a recent review article, experts have alluded to issues such as a poor understanding of the pathogenesis, the inability to stratify patients adequately and a lack of therapeutic targets as potential explanations for why there is no effective therapy available in this chronic disease (8).
A review of published literature on the role of cholangioscopy for assessment of dominant CBD strictures in PSC clearly suggests that the focus is either on the performance success of the procedure or the ability to detect cholangiocarcinoma in a localized segment (10–12, 25–27). While some studies make no mention of benign strictures, others have described characteristics of inflammatory strictures; although, there has been no attempt to formally recognize a pattern of phenotypic expression. The ability to differentiate between a dominant benign stricture from a malignant cholangiocarcinoma has been based on visual characteristics, such as stricture length (benign < 1 cm, malignant > 1 cm) and configuration (benign, regular margin; malignant, irregular margin), (12) or simply based on visual abnormalities such as nodularity, ulceration and neo-vascular proliferation (10). However, in our experience, we know that acute inflammatory strictures can be >1 cm long and can appear varyingly irregular depending upon the degree of inflammatory activity (Figure 1A and B). One study evaluated if cholangioscopy was useful in differentiating benign strictures in PSC from IgG4-sclerosing cholangitis (IgG4-SC) (26). Cholangioscopy was performed in 33 patients, including patients with PSC, IgG4-SC and cholangiocarcinoma. The incidence of dilated and tortuous vessels was significantly higher in IgG4-SC patients than in PSC patients (P = 0.02). Scarring and pseudo-diverticula were found significantly more often in PSC patients than in IgG4-SC patients (P = 0.001 and P = 0.0007, respectively). As expected, cholangioscopy after corticosteroid therapy showed resolution of bile duct stenosis; dilated, tortuous or partially enlarged vessels; and resolution of friability in patients with IgG4-SC. Azeem et al. describe the cholangioscopic appearances of a benign PSC stricture as being narrower, more irregular and whiter, when compared with the normal bile duct. They also describe a fibrotic, circular ‘ring’ which was seen in 23 patients either above or below a stricture (11). Published literature has not stratified these visual characteristics of benign inflammatory strictures into phenotypic categories. Such a phenotypic classification system based on cholangioscopy, however, was precisely the focus of our study.
With single operator cholangioscopy, we have the ability to not only directly visualize the area of interest in the CBD but also take targeted biopsies of the mucosal abnormality. Our experience with cholangioscopy in patients with PSC underscores the heterogeneity seen in the phenotypic expression of PSC. Akin to Crohn’s disease of the small and large intestine, we believe there are also distinct phenotypes in the expression of mucosal disease in PSC. Cholangioscopic access is limited to the CBD, common hepatic duct and perhaps the first- and second-degree radicles of the intrahepatic ducts; therefore, cholangioscopic interpretation is limited to dominant strictures seen within these larger caliber ducts. Our results show that a majority of patients (55%) with dominant strictures present with an acute inflammatory component, resulting in biochemical or radiographic abnormalities, whereas fibro-stenotic or mass-forming subtypes occurred at a lower frequency (24% and 17%, respectively). The natural history of these phenotypes and whether there is progression between one subtype to another is not known.
Antibiotics have been shown to improve symptoms and biochemistry in some patients with PSC. In a randomized controlled trial, vancomycin and metronidazole were shown to significantly alleviate symptoms and improve biochemistry in a cohort of 35 patients with PSC (6). Patients had evidence of intrahepatic or extrahepatic manifestation of PSC and were not specifically stratified based on any other parameter. Similarly, corticosteroids were also shown to benefit a subset of patients with PSC, although there seemed to be an overlap of auto-immune hepatitis in this subgroup (5). The explanation for this possible response to antibiotics and steroids may lie in the differences in phenotypic expression of the abnormality seen in the CBD. Cholangioscopy with biopsies has now given us the ability to look at the microscopic architecture of the CBD. It is conceivable that a dominant stricture with acute inflammation (Figure 1A) and a predominantly acute inflammatory infiltrate (comprising neutrophils and plasma cells) on biopsies may respond better to antibiotics, whereas a stricture with chronic inflammation and a lymphocytic infiltrate may respond better to steroids. Similarly, a fibrotic stricture (Figure 2) with little or no inflammatory infiltrate will likely not respond to anti-inflammatories. Balloon dilatation would be more worthwhile if there is a clinical or biochemical indication for intervention. The lack of patient stratification in previous clinical trials may possibly explain the variable response seen with either antibiotics or corticosteroids.
We recognize that there are inherent drawbacks with the design of our study. This is a retrospective review of a single-centre, single-endoscopist experience in a disease process that has not been studied or described in this manner before. At our centre, it is not standard practice to record all procedures, and therefore, a second review was not possible. Moreover, there is no agreed-upon published consensus on the visual characteristics of inflammatory strictures seen in the bile duct. Nonetheless, what we propose is a novel classification scheme to stratify patients with PSC presenting with dominant strictures. Not all PSC patients require cholangioscopic investigation, as a significant proportion are symptomatically and biochemically quiescent. However, although we recognize the inherent difficulty in assessing the entire cohort of PSC patients (as there are variations in their clinical spectrum), a cholangioscopy-based classification scheme can be extremely vital not only in prognostication but also in attempts at directing specific therapeutic intervention, whether it be pharmacologic or endoscopic. For now, cholangioscopy is only indicated for the subset of patients that exhibit a clinical indication for intervention, such as a recent elevation in liver biochemistry or a new change in luminal caliber as seen on surveillance MRCP. Because cholangiocarcinoma is a proven consequence of PSC, however small the incidence may be, cholangioscopy with visual interrogation and biopsies of the dominant stricture is of prime importance in confirming or excluding this complication. But there is more we can do to proactively intervene in a disease process we know little of and to change the natural history—rather than wait for an undesirable sequela to warrant intervention.
CONCLUSION
Even though published literature is limited, cholangioscopy and cholangioscopy-guided sampling can be safely and successfully utilized for the assessment of dominant strictures in patients with PSC. The diagnostic accuracy of cholangioscopy is superior to endoscopic retrograde cholangiography alone for detection of malignancy. Also, cholangioscopy has been shown to be useful in differentiating PSC from IgG4-sclerosing cholangitis.
The Edmonton Classification proposed, based on direct cholangioscopy, aims to stratify patients with dominant extrahepatic biliary strictures in PSC based on differences in phenotypic expression. Validation of this classification in large multicentre cohorts of PSC patients is warranted with the ultimate goal of developing a management algorithm based on a composite of patient and biochemical characteristics, cholangiographic scores, cholangioscopic subtypes and histopathologic grading. We now plan to prospectively enroll patients into this phenotypic stratification and study the histological correlation with the cholangioscopic abnormality, hopefully soliciting multicentre involvement. It is time that a consortium of PSC experts pool resources and actively seek intervention for a disease process that is poorly understood and has suboptimal treatment options.
ACKNOWLEDGEMENTS
GS conceived the idea, co-wrote, reviewed and edited the manuscript. PD co-wrote, reviewed and edited the manuscript. BH reviewed and edited the manuscript. AML co-wrote, reviewed and edited the manuscript.
Conflicts of Interest: GS is a Consultant for Boston Scientific Corporation and has received honoraria for speaking and proctoring engagements. However, no funding was received for the purposes of this study.
PD, BH and AML have no conflict to disclose relevant to this study.
References
- 1. Hirschfield GM, Karlsen TH, Lindor KD et al. Primary sclerosing cholangitis. Lancet 2013;382(9904):1587–99. [DOI] [PubMed] [Google Scholar]
- 2. Rea DJ, Heimbach JK, Rosen CB et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242(3):451–61. [DOI] [PMC free article] [PubMed]
- 3. Olsson R, Boberg KM, de Muckadell OS et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: A 5-year multicenter, randomized, controlled study. Gastroenterology 2005;129(5):1464–72. [DOI] [PubMed] [Google Scholar]
- 4. Lindor KD, Kowdley KV, Luketic VAC et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009;50(3):808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boberg KM, Egeland T, Schrumpf E. Long-term effect of corticosteroid treatment in primary sclerosing cholangitis patients. Scand J Gastroenterol 2003;38(9):991–5. [DOI] [PubMed] [Google Scholar]
- 6. Tabibian JH, Weeding E, Jorgensen RA et al. Randomised clinical trial: Vancomycin or metronidazole in patients with primary sclerosing cholangitis—a pilot study. Aliment Pharmacol Ther 2013;37(6):604–12. [DOI] [PubMed] [Google Scholar]
- 7. Karlsen TH, Folseraas T, Thorburn D et al. Primary sclerosing cholangitis—a comprehensive review. J Hepatol 2017;67(6):1298–323. [DOI] [PubMed] [Google Scholar]
- 8. Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishikawa T, Tsuyuguchi T, Sakai Y et al. Comparison of the diagnostic accuracy of per oral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: A prospective study. Gastrointest Endosc 2013;77(2):219–26. [DOI] [PubMed] [Google Scholar]
- 10. Azeem N, Gostout CJ, Knipschield M et al. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest Endosc 2014;79(5):773. [DOI] [PubMed] [Google Scholar]
- 11. Arnelo U, von Seth E, Bergquist A. Prospective evaluation of the clinical utility of single-operator peroral cholangioscopy in patients with primary sclerosing cholangitis. Endoscopy 2015;47(8):696–702. [DOI] [PubMed] [Google Scholar]
- 12. Tischendorf JJ, Kruger M, Trautwein C et al. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy 2006;38(7):665–9. [DOI] [PubMed] [Google Scholar]
- 13. Baluyut AR, Sherman S, Lehman GA et al. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. YMGE 2001;53(3):308–12. [DOI] [PubMed] [Google Scholar]
- 14. Ponsioen CY, Vrouenraets SME, Prawirodirdjo W et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut 2002;51(4):562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gluck M, Cantone NR, Brandabur JJ et al. A twenty-year experience with endoscopic therapy for symptomatic primary sclerosing cholangitis. J Clin Gastroenterol 2008;42(9):1032–9. [DOI] [PubMed] [Google Scholar]
- 16. Fleming KA, Boberg KM, Glaumann H et al. Biliary dysplasia as a marker of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol 2001;34(3):360–5. [DOI] [PubMed] [Google Scholar]
- 17. Ghazale A, Chari ST, Zhang L et al. Immunoglobulin G4-associated cholangitis: Clinical profile and response to therapy. Gastroenterology 2008;134(3):706–15. [DOI] [PubMed] [Google Scholar]
- 18. European Society of Gastrointestinal Endoscopy, European Association for the Study of the Liver. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J Hepatol 2017;66(6):1265–81. [DOI] [PubMed] [Google Scholar]
- 19. Kim WR, Therneau TM, Weisner RH et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc 2000;75(7):688–94. [DOI] [PubMed] [Google Scholar]
- 20. Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011;54(5):1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trikudanathan G, Navaneethan U, Njei B et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: A systematic review and meta-analysis. Gastrointest Endosc 2014;79(5):783–9. [DOI] [PubMed] [Google Scholar]
- 22. Bergquist A, Ekbom A, Olsson R et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36(3):321–7. [DOI] [PubMed] [Google Scholar]
- 23. Boonstra K, Weersma R, van Erpecum K et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013;58(6):2045–55. [DOI] [PubMed] [Google Scholar]
- 24. Rizvi S, Eaton JE, Gores GJ. Primary sclerosing cholangitis as a premalignant biliary tract disease: Surveillance and management. Clin Gastroenterol Hepatol 2015;13(12):2152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Awadallah NS, Chen YK, Piraka C et al. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis?Am J Gastroenterol 2006;101(2):284–91. [DOI] [PubMed] [Google Scholar]
- 26. Itoi T, Kamisawa T, Igarashi Y et al. The role of peroral video cholangioscopy in patients with IgG4-related sclerosing cholangitis. J Gastroenterol 2013;48(4):504–14. [DOI] [PubMed] [Google Scholar]
- 27. Siiki A, Rinta-Kiikka I, Koivisto T et al. Spyglass single-operator per oral cholangioscopy seems promising in the evaluation of primary sclerosing cholangitis-related biliary strictures. Scand J Gastroenterol 2014;49(11):1385–90. [DOI] [PubMed] [Google Scholar]