Abstract
Nutrient-deprivation autophagy factor-1 (NAF-1, miner1; gene cisd2) is part of the [2Fe-2S]-containing protein family which includes mitoNEET (gene cisd1) and MiNT (miner2; gene cisd3). These proteins are redox active and are thought to play an important role in cellular energy homeostasis with NAF-1 playing a critical role in calcium regulation and aging. To date, no studies have investigated potential ligand interaction with NAF-1. Here we show that the thiazolidinediones pioglitazone and rosiglitazone along with the mitoNEET ligand, NL-1, bind to NAF-1 with low micromolar affinities. Further, we show that overexpression of NAF-1 in hepatocellular carcinoma (HepG2) cells reduces inhibition of mitochondrial respiration by pioglitazone. Our findings support the need for further efforts of the rational design of selective NAF-1 ligands.
Keywords: Glitazones, Wolfram syndrome, CDGSH, Zinc-finger, CISD' miner1
Nutrient-deprivation autophagy factor-1 (NAF-1, miner1; gene cisd2) is a [2Fe-2S]-containing protein located on the endoplasmic reticulum (ER), and was recently discovered to play an important role in aging.1–3 NAF-1 belongs to the CDGSH iron sulfur domain (CISD) containing family of proteins, which also includes the outer mitochondrial membrane associated protein mitoNEET (gene cisd1)2 and the mitochondrial matrix protein MiNT (miner2; gene cisd3).4 NAF-1 discovery was motivated by the genetic disease Wolfram syndrome-2 (WFS2), where a mutation in the cisd2 gene causes a premature stop codon resulting in a truncated NAF-1 protein product.5 Patients with this mutation suffer from a range of maladies including deafness, blindness, and diabetes2. Genetic studies with NAF-1 showed a correlation between the overexpression of NAF-1 and increased life span in mice, while knock-out of the protein resulted in a shortening of life, and clinical symptoms similar to those seen in humans during aging.2,3,6,7 Furthermore, NAF-1 was found to play a role in breast cancer proliferation, and the knock-out of NAF-1 was found to significantly reduce tumor size.8,9 Considering the important role of NAF-1 in cell homeostasis and dysregulation of proliferation, it is surprising that therapeutic ligands that selectively interact with NAF-1 are currently lacking.
NAF-1 is located on the ER where it is thought to regulate calcium movement and autophagy via interaction with the pro-survival protein B-cell lymphoma protein 2 (Bcl-2) and mediating Bcl-2 interactions with Beclin-1.10 The crystal structure of NAF-1 revealed that it has similar [2Fe-2S] clusters in the homodimer as the mitochondrial counterpart mitoNEET.11 These iron-sulfur clusters are thought to play an important role in the function(s) of the protein, and may act as redox sensor during oxidative stress.12 Although the cell signaling aspects of NAF-1 were described in several studies, the in vivo interaction partners of this aspect of NAF-1 remain to be identified. Nevertheless, interactions between NAF-1 and synthetic ligands such as the TZD pioglitazone have been described.12 To date, there has been a lack of medicinal chemistry literature describing campaigns for ligand discovery for NAF-1 as drug target. Because the interactions between mitoNEET and NAF-1 and their protein partners most likely diverge, there is an opportunity to make rational efforts at designing ligands selective for mitoNEET or NAF-1. Previously we had designed a new mitoNEET ligand, NL-113, which was found to confer protection in models of neurodegeneration such as stroke and Parkinson’s disease.14,15
Since the anti-diabetic drug pioglitazone was one of the first drugs described to interact with the CISD family of proteins,2,12 we were interested to investigate pioglitazone binding to NAF-1, as well as the analog rosiglitazone (Fig. 1). Additionally, we wanted to evaluate the binding of the mitoNEET ligand NL-1 (Fig. 1), which was developed by us as a first-in-class mitoNEET-specific ligand.13,16 Our binding assays were essentially done as previously reported. In brief, human recombinant NAF-1 containing a N-terminal 6XHIS-tag was expressed in E. coli grown in an auto-induction LB broth, and the protein was isolated via Ni2+-affinity chromatography and imidazole washes on an FPLC system. The 6XHIS-tagged protein was bound to scintillation proximity assay (SPA) beads and incubated in the presence of [3H] rosiglitazone and pioglitazone, the latter as part of an 8-point concentration curve series. The data form the binding assay is shown in Fig. 2 demonstrating that rosiglitazone was the most potent binder, with an IC50 of 2.29 μM, while pioglitazone was found to bind with a somewhat lower affinity of IC50 = 4.80 μM. This difference in affinity for rosiglitazone follows a similar rank that was observed for mitoNEET, with rosiglitazone being the most potent TZD ligand used in the clinic described so far with a PPAR-γEC50 of 0.69 μM for pioglitazone, and rosiglitazone a PPAR-γEC50 of 0.06 μM.17 The mitoNEET ligand NL-1 was previously developed using the crystal structures of mitoNEET and the PPAR-γ crystal18 and designing out the PPAR-y activity, with the NL-1 showing a marked lower affinity for NAF-1 compared to other the tested TZDs with an IC50 of 29.77 μM. NL-1 displayed a higher affinity for mitoNEET binding than for NAF-1 therefore giving a selectivity index of NAF-1/mitoNEET of 33.13,16,19 Interestingly, the data show that the TZDs tested here had lower affinity for NAF-1 than for mitoNEET, which showed IC50 values between 0.9 and 1.1 μM for the three tested TZDs for mitoNEET binding.13
Fig. 1.
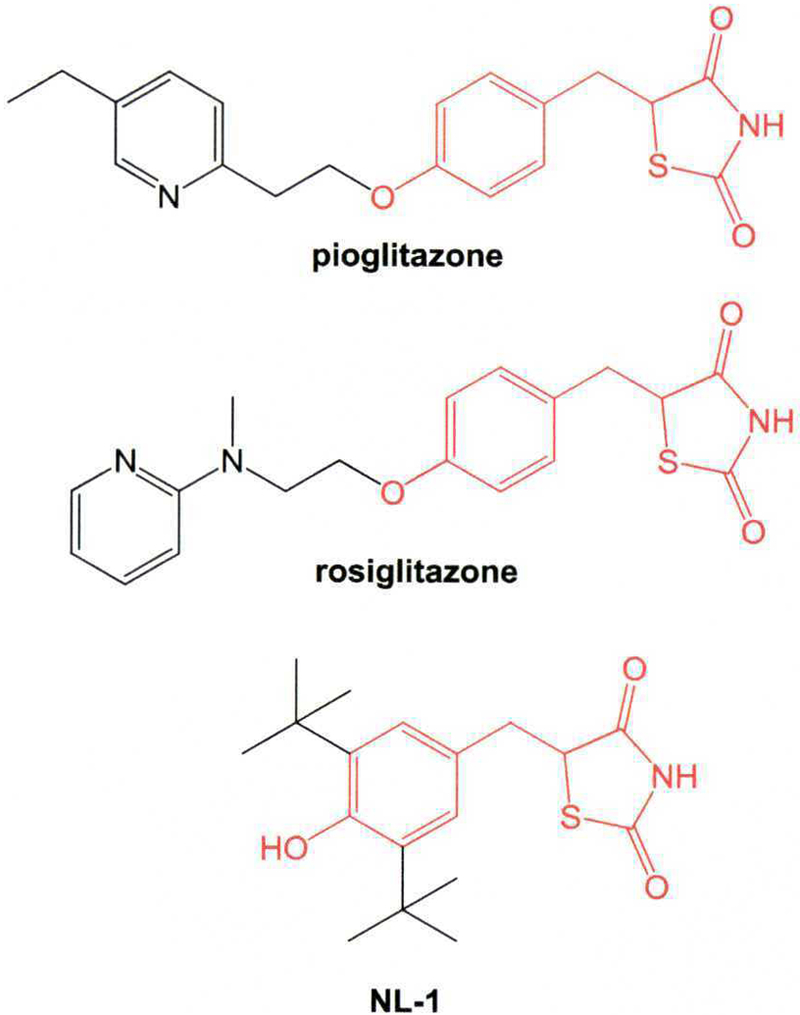
Structures of the three thiazolidinediones (TZDs) screened in this study. The common core scaffold of all three TZDs is shown in red.
Fig. 2.

Inhibitor-binding curves of tritium labeled rosiglitazone with the recombinant protein NAF-1. The IC50 for the three compounds were found to be A) rosiglitazone = 2.29 μM; B) pioglitazone = 4.8 μM and C) NL-1 = 29.77 μM. Data represented as averaged and standard error. N = 2.
NAF-1 has been crystalized and the structure compared with that of mitoNEET.11 Several differences were noted, one of which was the electrostatic potential differences between the two proteins. To investigate the molecular interactions between NAF-1 and the TZDs we docked pioglitazone, rosiglitazone and NL-1 into the published crystal structure of NAF-1 (Protein Data Bank Accession Code: 3FNV).11 Since the precise binding site has not been identified, we used the Site Finder module of MOE 2018, and chose a large binding pocket, which was in a similar region as previously shown for mitoNEET docking with pioglitazone and resveratrol-3-sulfate.13,20 We used an induced-fit docking method as found in the molecular modeling software suite MOE 2018 (www.chemcomp.com). The choice of the induced-fit docking was made based on the previous molecular dynamics studies21 which indicated that there was a degree of flexibility in the protein structure, which could account for the protein’s ability to act a redox sensor as well as play a role in protein-protein interactions.22 The results from the docking study are shown in Fig. 3, showing the ligand interaction diagrams of all three compounds to NAF-1. Binding energy calculations of the top binding pose suggested that the compounds would be able to bind to the protein, with energies of - 6.00 kcal/mol for rosiglitazone, −5.49kcal/mol for pioglitazone and - 5.45 kcal/mol for NL-1. These findings corroborate the binding assay data, following a similar rank order of affinities to NAF-1. For rosiglitazone, the most likely major amino acid interactions were found to be Ser69, Asp68 and Lysll6/ 105, where the latter can interchange depending on the binding flexibility. Pioglitazone interacts with Lysl05, Argl03 and Hisll7, while NL-1 interacts with Argl03 and Ser69. Fig. 3 also shows the spatial location of the binding pocket which accommodates all three TZDs. Closer inspection of the binding environment indicated that rosiglitazone exploits a possible accessory binding pocket chamber deeper into the protein, where the lipophilic N-methyl is able to extend into this pocket for increased affinity. This position still allows for hydrogen bonding with the TZD warhead, but seems to give rosiglitazone an anchor that is not shared by the other two TZDs tested. The structural motif can be exploited in future virtual screening campaigns or in de novo structure-based synthetic lead optimization studies.
Fig. 3.

Docking simulations of the investigated thiazolidinediones using the published crystal structure of NAF-1. Two-dimensional ligand interaction pose from docking studies: A) rosiglitazone, B) pioglitazone, and C) NL-1. The binding pocket of the TZDs to show the spatial relationship in the protein as surface of binding pocket surrounding the TZD docking site D) rosiglitazone, E) pioglitazone, and F) NL-1. A cavity exploited by the N-methyl of rosiglitazone shown in D could be exploited for compound development. The crystal structure of NAF-1 used in the docking studies was Protein Data Bank Access Code: 3FNV.11
NAF-1 is located on the ER playing a role in ER calcium homeostasis, and cross-talk with mitochondria.10 TZDs have been demonstrated to inhibit mitochondrial respiration by affecting complex I of the electron transport system.23 We investigated the relationship between TZD-mediated complex 1 inhibition and overexpression of NAF-1 in an acute treatment paradigm. Human hepatocellular carcinoma (HepG2) cells were engineered to overexpress NAF-1 and complex I activity was assessed in presence or absence of TZDs (30 μM). Pyruvate, malate, and glutamate were titrated into the respirometer and respiration was stimulated by addition of adenosine diphosphate (ADP). As seen in Fig 4, acute TZD treatment inhibits complex I activity as seen previously,23 with pioglitazone most severely inhibiting mitochondrial respiration. Interestingly, the inhibition of complex I activity by the presence of the TZDs was not significantly different between control and NAF-1 overexpressing cells, when treated with rosiglitazone or NL-1. However, in presence of pioglitazone, cells overexpressing NAF-1 had a significant increase of 13% complex I activity compared to the cells treated with pioglitazone with native concentration of endogenous NAF-1. In a report by Brunmair et al23 rosiglitazone showed significant inhibition of complex I, with pioglitazone only moderately showing inhibition. Since pioglitazone showed reduced inhibition of complex I inhibition, and this response was more pronounced than the cells treated with the other two TZDs, it might suggests that either NAF-1 can compensate for complex I inhibition or pioglitazone binding to NAF-1 can modulate mitochondrial performance. Taken together with the previous data, the differences in structure between pioglitazone and rosiglitazone can be used for future drug discovery to exploit the mitochondrial-ER cross-talk, seen on the cellular level.
Fig. 4.

Respiration of HepG2 cells permeabilized with digitonin (10 μg/mL) in absence or presence of different TZDs. Cells were treated with 30 μM of the respective TZD and the rate of maximal stimulated respiration in presence of NADH producing substrates (5 mM pyruvate, 2 mM malate, 10 mM glutamate) and ADP (5 mM) was determined. Overexpression of NAF-1 reduced the extent of complex I inhibition by pioglitazone, but not the inhibitory effects of rosiglitazone or NL-1 (n = 3; mean ±SE). *Indicates statistical significance differences in response to TZD addition between control cells and cells overexpressing NAF-1 (p ≤ 0.05). All respiration rates were significantly lower after addition of any TZD compared to respective untreated cells (p ≤ 0.05).
In conclusion, three TZDs including one FDA-approved drug pioglitazone, rosiglitazone (European approved drug) and the mitoNEET probe NL-1 were found to bind to NAF-1 with low micromolar affinity; and overexpressing NAF-1 were found to ameliorate the mitotoxic effects of pioglitazone in particular. The differences in binding of rosiglitazone to NAF-1 and mitoNEET can be used to start drug discovery campaigns of selective compounds for these two proteins. Further discovery of NAF-1 selective ligands may allow for novel therapeutically approaches to treat several diseases including Wolfram syndrome-2, cancer, aging-associated dysfunctions and neurodegeneration.
Acknowledgements
This work was funded in part by the National Institute of General Medical Sciences, U54GM104942-03, WV INBRE P20GM103434, the National Science Foundation CHE-1806266, and the WVU Stroke CoBRE grant P20 GM109098. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NSF.
References
- 1.Sohn YS, Tamir S, Song L, et al. NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc Natl Acad Sci USA 2013;110:14676–14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colca JR, McDonald WG, Waldon DJ, et al. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab 2004;286:E252–E260. [DOI] [PubMed] [Google Scholar]
- 3.Chen YF, Kao CH, Chen YT, et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev 2009;23:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upper CH, Karmi O, Sohn YS, et al. Structure of the human monomeric NEET protein MiNT and its role in regulating iron and reactive oxygen species in cancer cells. Proc Nad Acad Sci USA 2018;115:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CH, Kao CH, Chen YF, Wei YH, Tsai TF. Cisd2 mediates lifespan: is there an interconnection among Ca(2)(+) homeostasis, autophagy, and lifespan? Free Radic Res 2014;48:1109–1114. [DOI] [PubMed] [Google Scholar]
- 6.Wu CY, Chen YF, Wang CH, et al. A persistent level of Cisd2 extends healthy lifespan and delays aging in mice. Hum Mol Genet 2012;21:3956–3968. [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Wu CY, Kirby R, Kao CH, Tsai TF. A role for the CISD2 gene in lifespan control and human disease. Ann N Y Acad Set 2010;1201:58–64. [DOI] [PubMed] [Google Scholar]
- 8.Sun AG, Meng FG, Wang MG. CISD2 promotes the proliferation of glioma cells via suppressing beclin 1 mediated autophagy and is targeted by microRNA449a. Mol Med Rep 2017;16:7939–7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittler R, Darash-Yahana M, Sohn YS, et al. NEET proteins: a new link between iron metabolism, reactive oxygen species, and cancer. Antioxid Redox Signal 2018. [DOI] [PMC free article] [PubMed]
- 10.Wiley SE, Andreyev AY, Divakaruni AS, et al. Wolfram Syndrome protein, Miner1, regulates sulphydryl redox status, the unfolded protein response, and Ca2+ homeostasis. EMBO Mol Med 2013;5:904–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlan AR, Axelrod HL, Cohen AE, et al. Crystal structure of Miner1: the redox-active 2Fe-2S protein causative in Wolfram Syndrome 2. J Mol Biol 2009;392:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamir S, Zuris JA, Agranat L, et al. Nutrient-deprivation autophagy factor-1 (NAF-1): biochemical properties of a novel cellular target for anti-diabetic drugs. PLoS One 2013;8:e61202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geldenhuys WJ, Funk MO, Barnes KF, Carroll RT. Structure-based design of a thia-zolidinedione which targets the mitochondrial protein mitoNEET. Bioorg Med Chem Lett 2010;20:819–823. [DOI] [PubMed] [Google Scholar]
- 14.Logan SJ, Yin L, Geldenhuys WJ, et al. Novel thiazolidinedione mitoNEET ligand-1 acutely improves cardiac stem cell survival under oxidative stress. Basic Res Cardiol 2015;110:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll RT, Dluzen DE, Stinnett H, Awale PS, Funk MO, Geldenhuys WJ. Structure-activity relationship and docking studies of thiazolidinedione-type compounds with monoamine oxidase B. Bioorg Med Chem Lett 2011;21:4798–4803. [DOI] [PubMed] [Google Scholar]
- 16.Geldenhuys WJ, Yonutas HM, Morris DL, Sullivan PG, Darvesh AS, Leeper TC. Identification of small molecules that bind to the mitochondrial protein mitoNEET. Bioorg Med Chem Lett 2016;26:5350–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willson TM, Cobb JE, Cowan DJ, et al. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the anti-hyperglycemic activity of thiazolidinediones. J Med Chem 1996;39:665–668. [DOI] [PubMed] [Google Scholar]
- 18.Gelin M, Delfosse V, Allemand F, et al. Combining ‘dry’ co-crystallization and in situ diffraction to facilitate ligand screening by X-ray crystallography. Acta Crystallogy D Biol Crystallogr 2015;71:1777–1787. [DOI] [PubMed] [Google Scholar]
- 19.Geldenhuys WJ, Leeper TC, Carroll RT. mitoNEET as a novel drug target for mitochondrial dysfunction. DrugDiscov Today 2014;19:1601–1606. [DOI] [PubMed] [Google Scholar]
- 20.Arif W, Xu S, Isailovic D, Geldenhuys WJ, Carroll RT, Funk MO. Complexes of the outer mitochondrial membrane protein mitoNEET with resveratrol-3-suIfate. Biochemistry 2011;50:5806–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baxter EL, Jennings PA, Onuchic JN. Strand swapping regulates the iron-sulfur cluster in the diabetes drug target mitoNEET. Proc Natl Acad Sci USA 2012;109:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang NC, Nguyen M, Shore GC. BCL2-CISD2: an ER complex at the nexus of autophagy and calcium homeostasis? Autophagy 2012;8:856–857. [DOI] [PubMed] [Google Scholar]
- 23.Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 2004;53:1052–1059. [DOI] [PubMed] [Google Scholar]


