Abstract
Introduction:
Cabozantinib is a receptor tyrosine kinases inhibitor that targets MET (c-MET), VEGF receptor 2 (VEGFR2), RET, AXL, KIT, FLT-3, and TIE-2 and previously showed promising single agent activity in recurrent ovarian cancer.
Methods:
This was an open label, 1:1 randomized study of cabozantinib 60 mg orally (PO) daily versus weekly paclitaxel 80 mg/m2 given 3 out of 4 weeks (NCT01716715); 111 patients were enrolled. Eligibility included persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma and at least one but no more than 3 prior chemotherapy regimens.
Results:
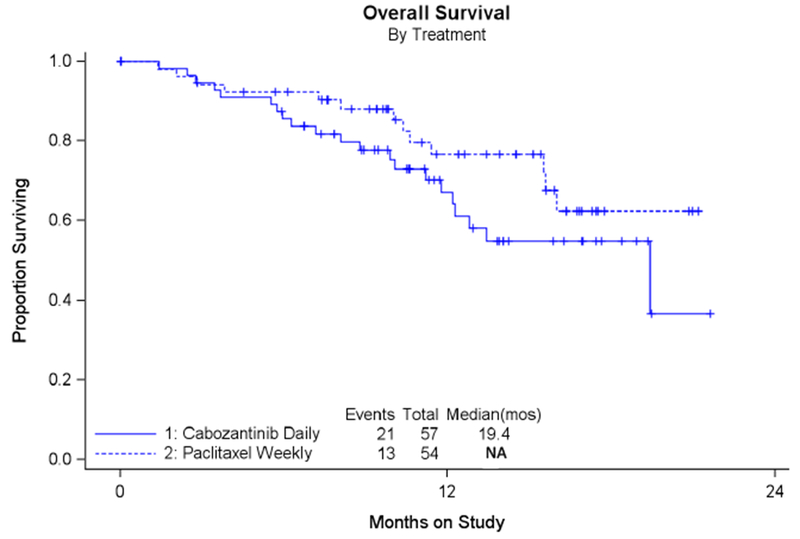
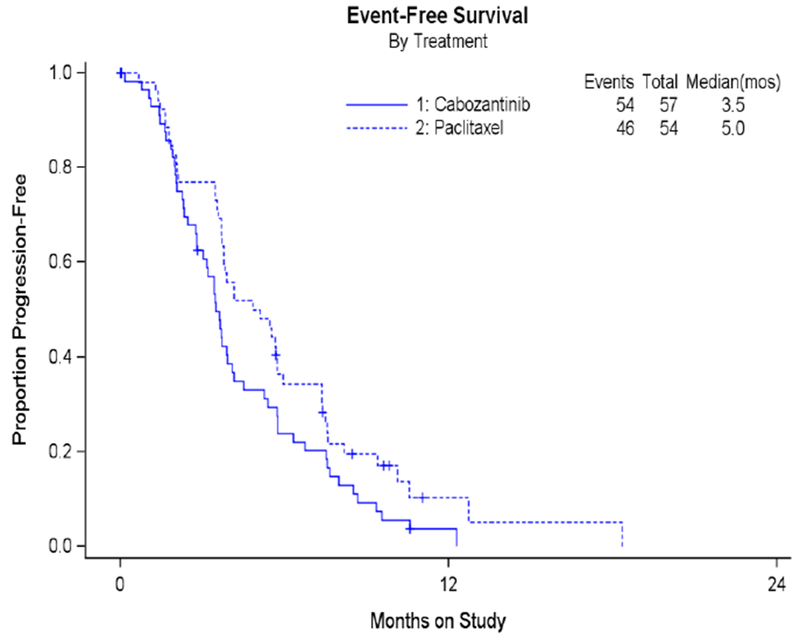
Median PFS was similar for both treatment groups and was 5.3 months for cabozantinib and 5.5 months for weekly paclitaxel (HR 1.11 (90% CI 0.77-1.61, p=0.64)). Secondary analyses of overall survival (OS) and event free survival (EFS) showed that cabozantinib did not perform as well as weekly paclitaxel. Median OS for cabozantinib was 19.4 months and was not reached for weekly paclitaxel (HR 2.27 (90% CI 1.17-4.41, p=0.04). EFS was also worse in the cabozantinib arm, 3.5 months, compared to weekly paclitaxel at 5.0 months (HR 1.81 (90% CI 1.24-2.63, p=0.01). Overall response rate (ORR) was less for cabozantinib compared to weekly paclitaxel (7% versus 24.1%). Gastrointestinal toxicities, specifically nausea, diarrhea, and abdominal pain were worse in the cabozantinib arm.
Conclusions:
Median PFS was similar for cabozantinib and weekly paclitaxel. However, OS, EFS, and ORR were worse for cabozantinib compared to weekly paclitaxel. Cabozantinib given at this dose and schedule cannot be recommended as a treatment for recurrent ovarian cancer.
Keywords: Ovarian cancer, anti-vascular, cabozantinib, paclitaxel
INTRODUCTION
Epithelial ovarian cancer is the most lethal of the gynecologic malignancies, with an estimated 22,240 new cases and 14,070 deaths in the United States in 2018 (1). Despite a combination of cytoreductive surgery and platinum and taxane-based chemotherapy which leads to a complete clinical remission in the majority of patients, most women with advanced ovarian cancer will relapse and eventually die of disease due to the persistence and eventual growth of chemotherapy resistant cancer cells (2, 3). Thus, new treatments for recurrent ovarian cancer are needed.
The study and use of anti-vascular agents has permeated both upfront as well as recurrent strategies and has led to improved outcomes for women with ovarian cancer. Bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), is approved both in the United States and Europe for use when combined with chemotherapy for both recurrent platinum sensitive (platinum free interval (PFI) of >6 months) and platinum resistant (PFI < 6 months) ovarian cancer (4–6). Other anti-vascular agents have been tested in ovarian cancer for the management of recurrence; additionally, the absence of significant overlapping toxicities has enabled anti-vascular agents to be combined with chemotherapy, tyrosine kinase inhibitors and other small molecule inhibitors such as poly (ADP ribose) polymerase inhibitors, as well as immuno-oncology agents.
Cabozantinib inhibits multiple receptor tyrosine kinases implicated in cancer growth, metastasis, and angiogenesis (7), and the primary targets are MET (c-MET) and VEGF receptor 2 (VEGFR2) with additional targets including RET, AXL, KIT, FLT-3, and TIE-2 (7). Both c-MET and VEGFR2 regulate tumor growth and angiogenesis, and preclinical studies of c-MET inhibition have demonstrated anti-ovarian cancer activity (8, 9). Preliminary testing of cabozantinib identified anti-ovarian cancer activity in addition to other cancers such as renal cell cancer and medullary thyroid cancer (10–16).
The primary objective of this study was to compare the efficacy of cabozantinib against weekly paclitaxel for recurrent ovarian cancer by comparing the PFS of these 2 regimens in a 1:1 randomized, open label study. Secondary objectives included toxicity assessment of both agents and comparisons of overall proportion responding by RECIST 1.1, OS, and EFS. Additional exploratory translational objectives included correlating c-MET overexpression by immunohistochemical staining (IHC) and MET amplification by fluorescent in situ hybridization (FISH) analysis with prognosis and responsiveness to either weekly paclitaxel or cabozantinib.
PATIENTS AND METHODS
Study design and eligibility:
Patients were enrolled from member institutions of the NRG Oncology Group, and all patients were required to sign an informed consent that was approved by the National Cancer Institute and each Institution’s Institutional Review Board. Eligibility included the following: persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma, performance status (PS) ≤2, and at least one but no more than 3 prior chemotherapy regimens, measurable via RECIST 1.1 or non-measurable disease. Patients with non-measurable disease had to have had at least one of the following: ascites and/or pleural effusion attributed to tumor or solid and/or cystic abnormalities on radiographic imaging that did not meet RECIST 1.1 criteria). Patients could not have received more than 1 non-platinum, non-taxane regimen for recurrent disease treatment, and prior treatment with weekly paclitaxel for recurrent or persistent disease was not allowed. Patients could have either platinum resistant or platinum sensitive recurrence.
This open-labeled trial randomized eligible patients 1:1 to either cabozantinib 60 mg PO daily continuously or paclitaxel 80 mg/m2 weekly on days 1, 8 and 15 via a 1-hour IV infusion. The randomization was stratified by the following 3 characteristics: Platinum-free interval (PFI) (those with a PFI ≤ 182 day versus those with a PFI of >182 days), measurable disease status (measurable versus non-measurable disease), and prior use of bevacizumab therapy (no use versus prior use of drug). Dose reductions were allowed to 40 mg and then 20 mg for cabozantinib, and paclitaxel dose reductions to either 60 mg/m2 or 40 mg/m2 based on predefined toxicities. Assigned treatment was continued until disease progression or adverse effects prohibited further treatment. One cycle was 28 days. Patients were assessed radiographically by RECIST 1.1 using CT or MRI every 8 weeks from cycle 1, day 1 (regardless of delays and/or changes in treatment schedule) for the first 8 months; then every 12 weeks thereafter until disease progression was confirmed. Toxicities were measured by CTCAE version 4.0.
Statistics:
The primary endpoint of this study was to assess the activity of cabozantinib relative to weekly paclitaxel with a Cochran-Mantel-Haenszel (CMH) statistic (Z) using PFS evaluated at 3.68 months (approximately pre-cycle 5/week 16) and 7.36 months (approximately pre-cycle 9/week 32) (17); if Z < −1.28, then the cabozantinib regimen would be declared worthy of further investigation; more details are described in the Supplementary information. Where appropriate, statistical analyses were stratified by the same factors used in the randomization. If the true PFS endpoint hazard ratio of the experimental to reference regimen was 0.57, the probability of detecting this difference was 85% (with 102 patients accrued; based on simulations with exponentially distributed survival times) when testing at the level of significance of 10%.
RESULTS
The study accrual opened on November 6, 2012 and was closed on May 5, 2014; 111 patients were enrolled at an average accrual rate of 74.4 patients per year. Data for the final analysis were retrieved on March 16, 2015 and April 1, 2015. Fifty-seven patients were randomized to the cabozantinib arm, and 2 were never treated; 54 patients were randomized to weekly paclitaxel, and 4 patients in this group were never treated. All enrolled patients are included in the PFS and OS analyses. The toxicity analyses only included patients who received at least one dose of treatment. The median follow-up time for patients receiving cabozantinib was 13.9 months and 14.5 months for weekly paclitaxel, which was not significantly different.
Patient demographics are listed in Table 1; the arms were balanced for key prognostic factors and prior treatments. Most patients had a performance status of 0 and had serous histology. Most patients had received either 1 or 2 prior regimens for treatment and had not received prior bevacizumab (>75% had no prior bevacizumab in both groups). Approximately 50% of patients had platinum resistant recurrent ovarian cancer, and the remaining patients had platinum sensitive cancer.
Table 1:
Patient characteristics and demographics
| Characteristic | Cabozantinib | Weekly Paclitaxel | Total number | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age (years) | 30-39 | 1 | 1.8 | 1 | 1.9 | 2 |
| 40-49 | 5 | 8.8 | 4 | 7.4 | 9 | |
| 50-59 | 21 | 36.8 | 19 | 35.2 | 40 | |
| 60-69 | 20 | 35.1 | 24 | 44.4 | 44 | |
| 70-79 | 9 | 15.8 | 6 | 11.1 | 15 | |
| ≥ | 1 | 1.8 | 0 | 0 | 1 | |
| Race | Asian | 1 | 1.8 | 3 | 5.6 | 4 |
| Black | 4 | 7 | 2 | 3.7 | 6 | |
| American Indian | 0 | 0 | 1 | 94.4 | 1 | |
| Pacific Islander | 1 | 1.8 | 0 | 1.9 | 1 | |
| White | 50 | 87.7 | 48 | 88.9 | 98 | |
| Unknown | 1 | 1.8 | 0 | 0 | 1 | |
| Performance Status | 0 | 40 | 70.2 | 40 | 74.1 | 80 |
| 1 | 17 | 29.8 | 14 | 25.9 | 31 | |
| Cell Type/Grade | Endometrioid Grade 2 or 3 | 1 | 1.8 | 2 | 3.8 | 3 |
| Serous | 46 | 80.7 | 46 | 85.2 | 92 | |
| Clear cell | 4 | 7.0 | 1 | 1.9 | 5 | |
| Mixed Epithelial | 3 | 5.3 | 1 | 1.9 | 4 | |
| Adenocarcinoma NOS | 2 | 3.5 | 2 | 3.7 | 4 | |
| Mucinous | 1 | 1.8 | 1 | 1.9 | 2 | |
| Transitional cell | 0 | 0 | 1 | 1.9 | 1 | |
| Number of Prior regimens | 1 | 22 | 38.6 | 23 | 42.6 | 45 |
| 2 | 26 | 45.6 | 22 | 40.7 | 48 | |
| 3 | 9 | 15.8 | 9 | 16.7 | 18 | |
| Prior Radiation | No | 55 | 96.5 | 52 | 96.3 | 107 |
| Yes | 2 | 3.5 | 2 | 3.7 | 4 | |
| Prior Immunotherapy | No | 57 | 100 | 53 | 98.1 | 110 |
| Yes | 0 | 0 | 1 | 1.9 | 1 | |
| Prior Surgery | No | 1 | 1.8 | 0 | 0 | 1 |
| Yes | 56 | 98.2 | 54 | 100.0 | 110 | |
| Prior Bevacizumab | No | 46 | 80.7 | 41 | 75.9 | 87 |
| Yes | 11 | 19.3 | 13 | 24.1 | 24 | |
| Measurable Disease | No | 9 | 15.8 | 8 | 14.8 | 17 |
| Yes | 48 | 84.2 | 46 | 85.2 | 94 | |
| Platinum sensitivity | Platinum resistant <6 mo PFI | 28 | 49.1 | 27 | 50.0 | 55 |
| Platinum sensitive 6-12 mo PFI | 17 | 29.8 | 12 | 22.2 | 29 | |
| Platinum sensitive >12 mo PFI | 12 | 21.1 | 15 | 27.8 | 27 | |
PFI = platinum free interval
The CMH test statistic was calculated to be 1.88 (Z=1.88; p=0.97), so cabozantinib was deemed not worthy of further investigation as a single agent. Cabozantinib and weekly paclitaxel appeared to have a similar median PFS. Figure 1A shows PFS by treatment; median PFS analyzed was 5.3 months for cabozantinib and was 5.5 months for weekly paclitaxel (HR 1.11 (0.77-1.61, p=0.64)).
Figure 1: PFS, OS, and EFS results.
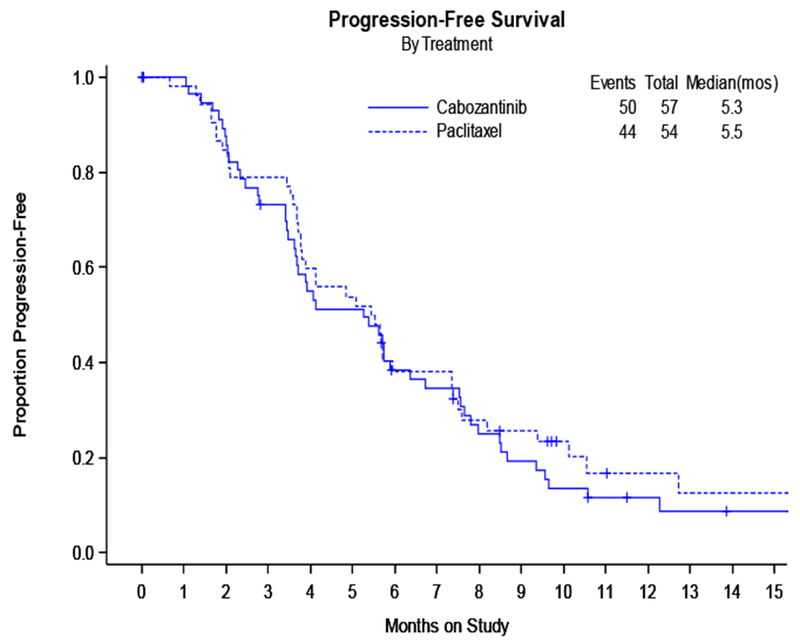
A: Progression Free Survival by Treatment:
B: Analysis of Overall Survival in All Enrolled Patients
C: Event free survival
Secondary analyses of OS and EFS showed that cabozantinib did not perform as well as weekly paclitaxel. Figure 1B shows OS; median OS for cabozantinib was 19.4 months and was not reached for weekly paclitaxel at this final study analysis. EFS which measures death, progression or subsequent therapy was also worse in the cabozantinib arm: 3.5 months compared to 5.0 months in weekly paclitaxel (see Figure 1C). These analyses indicated a poorer performance via a two-sided alternative hypothesis for the CMH test (2-sided p=0.06), a stratified OS analysis (HR 2.27; 90% CI 1.17-4.41), and the hazard ratio for EFS (HR 1.81; 90% CI 1.24-2.63).
Table 2 shows reasons for discontinuation of therapy, best overall response by RECIST 1.1, and patient outcomes. Fewer patients discontinued cabozantinib for disease progression compared to those receiving paclitaxel (49% versus 70%), but more patients discontinued cabozantinib for toxicity compared to weekly paclitaxel (30% versus 6%). A log-rank test suggests that patients on the cabozantinib arm came off therapy sooner for disease progression or toxicity and had fewer numbers of cycles of therapy compared to those receiving weekly paclitaxel. The proportion of patients having either a complete response (CR) or partial response (PR) was less on the cabozantinib arm which was 7% compared to 24.1% (Table 2) for the weekly paclitaxel arm. This translated into an odds ratio for responding on cabozantinib to paclitaxel of 0.23 (90% Exact CI 0.06 ~ 0.72); another analysis, the asymptotic relative probability, also showed that the chances of responding to cabozantinib was about one-quarter to two-thirds the ORR of weekly paclitaxel (0.29 (90% CI 0.12 ~ 0.71)).
Table 2:
Reasons for study treatment discontinuation, overall response to treatment, and patient outcomes
| Characteristic | Category | Cabozantinib (n=57) | Weekly Paclitaxel (n=54) | Total | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Reason off therapy | On study/unspecified | 2 | 3.5 | 2 | 3.7 | 4 |
| Disease progression | 28 | 49.1 | 38 | 70.4 | 66 | |
| Refusal | 5 | 8.8 | 7 | 13.0 | 12 | |
| Adverse Events | 17 | 29.8 | 3 | 5.6 | 20 | |
| Other disease | 1 | 1.8 | 0 | 0 | 1 | |
| Other reason | 4 | 7.0 | 4 | 7.4 | 8 | |
| Response | Complete Response | 0 | 0 | 2 | 3.7 | 2 |
| Partial Response | 4 | 7.0 | 11 | 20.4 | 15 | |
| Stable Disease | 27 | 47.4 | 21 | 38.9 | 48 | |
| Increasing disease | 8 | 14.0 | 8 | 14.8 | 16 | |
| Indeterminate | 9 | 15.8 | 4 | 7.4 | 13 | |
| Non-measureable | 9 | 15.8 | 8 | 14.8 | 17 | |
| Cycles of Treatment | 0 | 2 | 3.5 | 4 | 7.4 | 6 |
| 1 | 10 | 17.5 | 1 | 1.9 | 11 | |
| 2 | 13 | 22.8 | 10 | 18.5 | 23 | |
| 3 | 8 | 14.0 | 3 | 5.6 | 11 | |
| 4 | 8 | 14.0 | 12 | 22.2 | 20 | |
| 5 | 2 | 3.5 | 2 | 3.7 | 4 | |
| 6 | 3 | 5.3 | 9 | 16.7 | 12 | |
| 7 | 1 | 1.8 | 0 | 0 | 1 | |
| 8 | 3 | 5.3 | 4 | 7.4 | 7 | |
| 9+ | 7 | 12.3 | 9 | 16.7 | 16 | |
| Alive/Cause of Death | Alive | 36 | 63.2 | 41 | 75.9 | 77 |
| Dead – disease related | 20 | 35.1 | 13 | 24.1 | 33 | |
| Dead- Neither drug- nor disease related | 1 | 1.8 | 0 | 0 | 1 | |
Other analyses included the impact of the level of platinum sensitivity on several endpoints; however, because of the small numbers of patients in these subgroups and the small number of survival events, definitive statements cannot be made. Additionally, for these same reasons, the impact of treatments and level of platinum resistance on OS could also not be determined. However, when PFS was compared for patients with platinum resistant versus platinum sensitive recurrence, regardless of treatment, there was a suggestion that PFS was worse in the platinum resistant patients compared to the platinum sensitive patients (log-rank p=0.06; HR 1.4 (90% CI 0.97-2.05). When examining the effect of platinum resistance within each treatment group (subset analyses), for the cabozantinib-treated patients, the platinum resistant group had about 2 times higher risk of progression compared to the platinum sensitive patients (HR 1.79 (90% CI 1.082-2.95); this was not observed in the weekly paclitaxel arm where the effects on PFS were similar regardless of platinum sensitivity (HR 1.04 (90% CI 0.59-1.83). For the overall proportion showing response to treatment, there were no suggested differences with regards to platinum sensitivity with either cabozantinib or weekly paclitaxel.
Treatment emergent toxicities are listed in Table 3, and all toxicities are listed in the supplementary section (Supplementary Table 5). Of note, there were no deaths related to toxicities in either arm. The two regimens were comparable by toxicity except for gastrointestinal (GI) toxicities; cabozantinib had a higher rate of grade 3 or higher GI toxicities compared to weekly paclitaxel. No single GI toxicity accounted for this difference, but nausea, abdominal pain and diarrhea were more common on the cabozantinib arm. GI perforations (GIP) have been reported previously with cabozantinib; in this trial, one patient in the cabozantinib arm had a grade 4 GIP and no incidences of GIP occurred in the weekly paclitaxel arm. Other notable toxicities that were more frequent on the cabozantinib arm were hypothyroidism and palmar-plantar erythrodysesthesia (PPE) (supplementary Table 5). The hypothyroidism associated with cabozantinib was all ≤ grade 2, but of the 18 patients on cabozantinib with PPE, 8 were grade 1, 8 were grade 2 and 2 patients had grade 3 PPE. Hypertension (HTN) and vascular events were more frequently reported in the cabozantinib arm. Twenty-five patients reported HTN in the cabozantinib arm (7 patients each having grade 1,10 with grade 2 and 8 with grade 3), and 5 patients had HTN with weekly paclitaxel (1 grade 1, 3 grade 2 and 1 grade 3 HTN). Grade 2 and 3 thromboembolic events were reported in 7 versus 3 patients, cabozantinib versus weekly paclitaxel; one patient on weekly paclitaxel had a grade 4 thromboembolic event. Approximately 50.9% of patients receiving cabozantinib required dose reduction while receiving treatment, while 26% of patients on the paclitaxel arm had dose reductions.
Table 3:
Treatment emergent Adverse events for cabozantinib and weekly paclitaxel
| Site | No of events for Cabozantinib Arm | No. of events for Weekly Paciltaxel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gr 2 | Gr 3 | Gr 4 | Gr 5 | Gr 2 | Gr 3 | Gr 4 | Gr 5 | ||
| Thrombocytopenia | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Neutropenia | 10 | 2 | 0 | 0 | 14 | 4 | 0 | 0 | |
| Anemia | 5 | 3 | 1 | 0 | 12 | 0 | 0 | 0 | |
| Gastrointestinal | 20 | 14 | 1 | 0 | 10 | 2 | 0 | 0 | |
| Endocrine | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metabolism/nutrition | 10 | 8 | 2 | 0 | 7 | 3 | 0 | 0 | |
| Musculoskeletal/Connective Tissue | 8 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | |
| Nervous system | 7 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | |
| Renal/urinary | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Respiratory/Thoracic/Mediastinal | 12 | 3 | 0 | 0 | 8 | 1 | 0 | 0 | |
| Skin/subcutaneous | 12 | 2 | 0 | 0 | 20 | 0 | 0 | 0 | |
| Vascular Disorders | 10 | 12 | 0 | 0 | 6 | 4 | 1 | 0 | |
Additionally, c-Met expression and c-Met copy number were compared to clinical outcome, specifically OS, PFS and RR; neither c-Met expression nor copy number were predictive of any clinical outcomes (data not shown).
DISCUSSION
In this open-label, randomized phase II study of cabozantinib versus weekly paclitaxel for treatment of recurrent ovarian cancer, the primary objective was to compare the PFS between the 2 arms using a CMH statistic, and cabozantinib was deemed clinically uninteresting and not worthy of further investigation. Treatment with cabozantinib had a similar median PFS compared to weekly paclitaxel. However, secondary endpoints including OS, median EFS, and overall RR were all numerically worse for the cabozantinib arm compared to weekly paclitaxel. Additionally, more patients were removed from study for toxicities on the cabozantinib arm; the GI toxicities of nausea, diarrhea and abdominal pain were higher with cabozantinib arm compared to weekly paclitaxel.
Cabozantinib was previously studied in a 70-patient ovarian cancer cohort as part of a larger randomized discontinuation study, and positive results in the ovarian cancer arm from this study served as preliminary data supporting the development of this study (10, 16). In the ovarian cancer cohort, all patients enrolled were started on cabozantinib 100 mg PO daily and those exhibiting stable disease at 12 weeks were randomized to either continued cabozantinib or placebo (10, 16). In the ovarian cancer patient cohort, 50% of patients had platinum refractory or resistant cancer; median PFS was 5.5 months for all ovarian cancer patients, and ORR at week 12 was 21%, which was similar for platinum resistant/refractory and for platinum sensitive patients. The most common grade 3 or 4 toxicities were diarrhea, PPE, asthenia, HTN, and neutropenia; dose reductions were required in 37% of patients. As a consequence of higher toxicities associated with higher doses of cabozantinib, such as 100 mg in Vergote et al as well as other non-ovarian cancer studies of cabozantinib (10), both Exelixis and the study sponsor, the NCI, agreed to start at a lower dose of 60 mg which is also the approved dose for renal cell cancer. Though toxicities associated with cabozantinib appeared less in our current study compared to Vergote et al (10), the overall RR of 7% in this study was less in this study compared to the ORR of 21% in Vergote et al (10). Though eligibility requirements such as PS and number of prior lines of treatment appeared similar for both studies, a possible explanation for the overall RR differences could be the dosing differences.
Most other secondary endpoints including OS and EFS were also worse in the cabozantinib arm in our study compared to weekly paclitaxel; certain GI toxicities were worse in the cabozantinib such as nausea, diarrhea and abdominal pain. Exact reasons why OS was worse with cabozantinib compared to weekly paclitaxel are not known. The cabozantinib regimen may have impacted the ability of patients in this arm to tolerate further treatments off study coupled with lowered overall RR, thus rendering patients more symptomatic from their cancer.
Other anti-vascular therapies have been tested as single agents in the recurrent setting such as bevacizumab, cediranib, ENMD2076, pazopanib, and sunitinib (18–23). Single agent RR of these agents vary from ~8% for sunitinib and ENMD2076, up to 20% for bevacizumab, and up to 17-18% with cediranib and pazopanib (18–23). However, single agent anti-vascular agents-especially the tyrosine kinase inhibitors (TKIs)—for ovarian cancer treatment do not appear to have convincingly viable futures given their limited single agent activity and their toxicity profile as evidenced by our study and others. In certain circumstances, combination strategies have been quite successful especially for combined weekly paclitaxel and bevacizumab yielding impressive overall RR and PFS results (6). Yet, other TKI and weekly paclitaxel strategies have led to mixed results such as the combination of pazopanib and weekly paclitaxel (24, 25).
As a single agent, cabozantinib is currently FDA approved for both medullary thyroid cancer (approved dose is 140 mg using the capsule formation) as well as metastatic renal cell cancer (approved dose is 60 mg using the tablet formation). In medullary thyroid cancer, better outcomes with cabozantinib may be related to underlying RET anomalies although anti-tumor activity was seen both in patients with and without RET mutations. However, in the case of renal cell cancer, in the METEOR study, which studied cabozantinib versus everolimus in metastatic renal cell cancer (12), cabozantinib was shown to have superior PFS, OS and RR independent of tumor MET expression. In our study, c-Met expression and copy number were not predictive of clinical outcomes.
There does not appear to be a justification for pursuing cabozantinib for the treatment of recurrent ovarian cancer as a single agent because the 60 mg dose tested in our study was shown to be inferior to weekly paclitaxel. Weekly paclitaxel had an ORR of 24.1% in our study, which is comparable to other single agent studies of weekly paclitaxel at this dose (26–28). Pursuing testing using a higher dose of cabozantinib of 100 mg dose as was done in Vergote et al (10) would likely incur higher toxicity without PFS benefit, which were similar in both studies despite dosage. Additionally, Konstantinopoulos et al tested cabozantinib in patients with clear cell ovarian cancer and showed disappointing results with no responses in 13 patients (29). Currently, cabozantinib is undergoing further investigation in ovarian cancer combined with atezolizumab, a PD-L1 inhibitor (NCT03170960), as well as for other cancer types including triple negative breast cancer, renal cell cancer, and endometrial cancer with some trials combining cabozantinib with immune-oncology agents (NCT03316586, NCT03149822, NCT03367741).
Supplementary Material
RESEARCH HIGHLIGHTS.
Cabozantinib is a targeted kinase inhibitor with anti-ovarian cancer activity
Cabozantinib compared to weekly paclitaxel had similar PFS but had worse OS and ORR
Cabozantinib had worse gastrointestinal toxicities compared to paclitaxel
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), NRG Oncology (1 U10 CA180822), NRG Operations (U10CA180868) and UG1CA189867 (NCORP). Dr. Matulonis is supported by the Breast Cancer Research Foundation. Drs. Aghajanian and Makker are supported in part by the MSK Cancer Center Support Grant P30 CA008748. The following NRG Oncology/Gynecologic Oncology Group member institutions participated in this study: Memorial Sloan Kettering Cancer Center, Washington University School of Medicine, Cancer Research for the Ozarks NCORP, Women and Infants Hospital, University of Oklahoma Health Sciences Center, University of Iowa Hospitals and Clinics, Carolinas Medical Center/Levine Cancer Institute, Rush University Medical Center, Fox Chase Cancer Center, University of Wisconsin Hospital and Clinics, Fred Hutchinson Cancer Research Center, University of North Carolina at Chapel Hill, Wake Forest University Health Sciences, University of Massachusetts Memorial Health Care, University of Hawaii, University of Alabama at Birmingham, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Cleveland Clinic Foundation, University of Chicago, Case Western Reserve University, Maine Medical Center – Scarborough Campus, Wichita CCOP, Abington Memorial Hospital, University of Colorado Cancer Center – Anschutz Cancer Pavilion, University of California Medical Center at Irvine-Orange Campus, MD Anderson Cancer Center, Women’s Cancer Center of Nevada, University of Virginia, Mayo Clinic, The Hospital of Central Connecticut, Dana-Farber Cancer Institute, University of Southern California, Hartford Hospital, Delaware/Christiana Care CCOP, Virginia Commonwealth University, Iowa-Wide Oncology Research Coalition NCORP, Sanford NCI Community Oncology Research Program of the North Central Plains, Columbus NCI Community Oncology Research Program and Stony Brook University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was originally presented in part at the 2016 Society of Gynecologic Oncology meeting.
CONFLICTS OF INTEREST
Dr. Ursula Matulonis received monies from Clearity Foundation and Ovarian Cancer Research Foundation for Board membership. She also served as a consultant and received monies from Merck, Fujifilm, Mersana, Geneos and Immunogen. She also served as a consultant for 2X Oncology, receiving no monies paid to herself. She has grants/grants pending from Merck for an investigator-initiated clinical trial. She received payment for development of education presentations from i3 Health.
Dr. Vicki Makker received monies paid to her for consultancy from Eisai Pharmaceuticals, Merck Pharmaceuticals and Karyopharm.
Dr. David Mutch received monies paid to him for consultancy from Tesaro.
Dr. Robert Mannel served on the Advisory Board as a consultancy for Clovis and Tesaro, receiving monies paid to his institution.
Dr. Carol Aghajanian served as Honorarium, Ad Boards for Tesaro, Mateon Therapeutics, Cerulean and ImmunoGen. She also served as Honorarium for Steering Committee Meeting for Mateon Therapeutics and Clovis.
All other co-authors have no conflicts of interest to declare.
REFERENCES
- 1.Siegal RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996; 334(1):1–6. [DOI] [PubMed] [Google Scholar]
- 3.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009. March 20; 27(9):1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012. June 10; 30(17):2039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017; 18:779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014. May 1; 32(13):1302–1308. [DOI] [PubMed] [Google Scholar]
- 7. Yakes FM, Chen J, Tan J, Yamagucchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011; 10:2298–2308. [DOI] [PubMed] [Google Scholar]
- 8.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12:89–103. [DOI] [PubMed] [Google Scholar]
- 9.Zillhardt M, Christensen JG, Lengyel E. An orally available small molecule inhibitor of c-Met, PF-2341066, reduces tumor burden and metastasis in a preclinical model of ovarian cancer metastasis. Neoplasia 2010; 12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergote IB, Smith DC, Berger R, Kurzrock R, Vogelzang MH, Sella A, et al. A phase 2 randomised discontinuation trial of cabozantinib in patients with ovarian carcinoma. Eur J Cancer. 2017; 83:229–236. [DOI] [PubMed] [Google Scholar]
- 11.Schoffski P, Gordon M, Smith DC, Kurzrock R, Daud A, Vogelzang MJ, et al. Phase II randomized discontinuation trial of cabozantinib in patients with solid tumors. Eur Journal of Cancer. 2017;86:296–304. [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomized, open label phase 3 trial. Lancet Oncol. 2016; 17:917–927. [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN trial. J Clin Oncol 2017; 35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bersanelli M, Buti S. Cabozantinib in metastatic renal cell carcinoma: latest findings and clinical potential. Ther Adv Med Oncol 2017; 9:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013; 31:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoffski P, Gordon M, Smith DC, Kurzrock R, Daud A, Vogelzang NJ, et al. Phase II randomised discontinuation trial of cabozantinib in patients with advanced solid tumors. Eur J Cancer 2017; 86:296–304. [DOI] [PubMed] [Google Scholar]
- 17.Freidlin B, Korn EL, George SL, Gray R. Randomized clinical trial design for assessing noninferiority when superiority is expected. J Clin Oncol. 2007; 25:5019–5023. [DOI] [PubMed] [Google Scholar]
- 18.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007; 25:5180–5186. [DOI] [PubMed] [Google Scholar]
- 19.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2007; 25:5165–5171. [DOI] [PubMed] [Google Scholar]
- 20.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009; 27:5601–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedlander M, Hancock KC, Rischin D, Messing MJ, Stringer CA, Matthys GM, et al. A phase II open labeled, study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010; 119:32–37. [DOI] [PubMed] [Google Scholar]
- 22.Matulonis UA, Lee J, Lasonde B, Tew WP, Yehwalashet A, Matei D, et al. ENMD-2076, an oral inhibitor of angiogenic and proliferation kinases, has activity in recurrent, platinum resistant ovarian cancer. Eur J Cancer. 2013; 49:121–131. [DOI] [PubMed] [Google Scholar]
- 23.Campos SM, Penson RT, Matulonis U, Horowitz NS, Whalen C, Pereira L, et al. A phase II trial of sunitinib malate in recurrent and refractory ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2013; 128:215–220. [DOI] [PubMed] [Google Scholar]
- 24.Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum resistant-refractory advanced ovarian cancer (MITO 11): a randomized, open-label, phase 2 trial. Lancet Oncol. 2015; 16:561–568. [DOI] [PubMed] [Google Scholar]
- 25.Richardson DL, Sill MW, Coleman RL, Sood AK, Pearl ML, Kehoe SM, et al. Paclitaxel with and without pazopanib for persistent or recurrent ovarian cancer. JAMA Oncol. 2018; 4:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. Phase II weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 2006; 101:436–440. [DOI] [PubMed] [Google Scholar]
- 27.Ghamande S, Lele S, Marchetti D, Baker T, Odunsi K. Weekly paclitaxel in patients with recurrent or persistent ovarian cancer. Int J Gynecol Cancer. 2003; 13:142–147 [DOI] [PubMed] [Google Scholar]
- 28.Kita T, Kikuchi Y, Takano M, Suzuki M, Oowada M, Konno R, et al. The effect of single agent weekly paclitaxel in heavily pretreated patients with recurrent or persistent advanced ovarian cancer. Gynecol Oncol. 2004; 92:813–818. [DOI] [PubMed] [Google Scholar]
- 29.Konstantinopoulos P, Brady WE, Farley J, Armstrong A, Uyar DS, Gershenson DM. Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001). Gynecol Oncol 2018. July;150(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


