INTRODUCTION
The three clinical parameters used to evaluate the uterine cervix in today’s obstetrical practice are its length, softness, and dilatation. These parameters are usually assessed by similar methods that were used during the time of Hippocrates, namely, via digital examination, and they provide about as much information about timing of delivery as they did 2000 years ago.1 The major problem with these three parameters are measurement imprecision and subjectivity. For instance, although practitioners consider cervical softness a critical parameter,2 they use their face as a frame of reference to assess cervical softness: a soft cervix feels like a cheek, a medium cervix like a nose, and a firm cervix like a forehead. Dilatation seems less subjective because it is measured in centimeters, but in a study using soft simulation training models (to replicate the in vivo situation), only 19% of experienced clinicians’ measurements of cervical diameter were correct.3 Another study employing a position-tracking system to verify the practitioner’s measurement of cervical dilatation in nearly 200 women during labor showed a mean error of 10.2±8.4 mm4, an error that seems alarmingly large considering that a 1 cm difference informs clinical decision-making about interventions such as cesarean section. Cervical length is the only objectively quantifiable parameter in clinical use for cervical evaluation, but even the gold standard transvaginal cervical length measurement has significant limitations with respect to predicting timing of delivery.1 Fortunately, at present there is effort by many groups to objectively quantify cervical parameters to improve understanding of cervical remodeling in pregnancy.
QUANTIFYING CERVICAL REMODELING
The cervix is not a simple structure. It is comprised of cells (e.g. smooth muscle cells, fibroblasts, glandular cells, vascular cells and immune cells) embedded in an extracellular matrix (ECM). The ECM contains proteins (mostly collagen) and proteoglycans that are arranged in a scaffold, or matrix, that define cervical mechanical properties. The ECM actively changes throughout gestation, allowing the cervix to transform from a stiff, long and closed structure to one that is soft, short and dilated to allow delivery. Understanding the process of cervical remodeling is crucial to understanding both normal and abnormal pregnancy, and therefore is a subject of active research. Current attempts to quantify cervical remodeling can be separated into two general categories: those that address tissue deformability and those that address the presence, organization and/or concentration of extracellular matrix components.
Quantifying Tissue Deformability
Softer tissue is more compressible than stiffer tissue; in other words, it deforms more under compression. This is the principle behind techniques that evaluate tissue stiffness/softness via measuring tissue response to a stimulation, such as stretching or compressing (pushing).
Aspiration
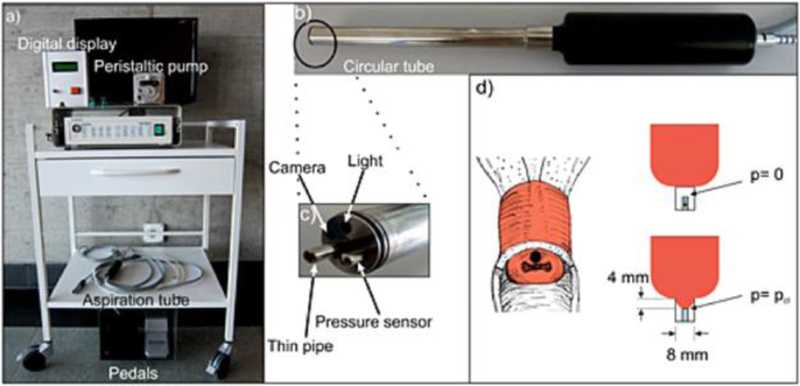
The aspiration technique focuses on stretching, as opposed to compressing, cervical tissue to measure distensibility. The aspiration device consists of a thin tube (into which cervical tissue is sucked), a minicamera to observe cervical distensibility, a light, and a pressure sensor and a vacuum. Figure 1 shows an image of the aspiration device. With the vacuum running, the tube is placed against the distal end of the anterior cervix (at 12:00) and cervical tissue is sucked into the cylinder a preset amount (4mm), after which the pressure is reversed, and the tube automatically detaches from the cervix when atmospheric pressure is reached.5 More tension is required to reach 4mm of deformation in stiffer tissues as compared to softer tissues, and therefore the closing pressure value (pcl, millibar) can directly measures stiffness. In a longitudinal study of 42 pregnant women, the aspiration technique showed a statistically significant difference between the 1st and 2nd, but not the 2nd and 3rd, trimesters, with postpartum values returning to nonpregnant stiffness values.5
Figure 1.
The aspiration device. (Reprinted with permission from reference #5)
Cervical Consistency Index
The cervical consistency index (CCI) is a ratio of the anteroposterior (AP) diameter of the cervix under maximal compression with the transducer compared to the AP diameter prior to compression.6,7 Figure 1 shows a typical transvaginal cervical image on the left, and on the right is the same cervix under maximal compression from the transducer. For the CCI, the AP diameter is measured from images such as these. A ratio of 1 corresponds to a cervix that does not compress at all (stiff) and a ratio of 0.5 to a cervix in which the diameter is reduced by half (soft). A significant correlation was found between CCI and gestational age (r2=0.66, p <0.001) in a cross-sectional study of more than 1000 women undergoing ultrasound at 5–36 weeks of gestation; specifically, a lower CCI was seen in women in late, as compared to early, pregnancy.6 The area under the receiver-operator curve (AUROC) for prediction of preterm birth (PTB) at <32 weeks, <34 weeks and <37 weeks was 0.947, 0.943, and 0.907, respectively, although sensitivities were low (67%, 64%, and 45%, respectively). A recent prospective study evaluated low risk women (n=532) with both CCI and transvaginal cervical length (TVU CL) at 19 to 24+6 weeks of gestation. For the primary outcome of spontaneous PTB <37 weeks, CCI outperformed TVU CL [AUROC 0.84 (95% CI 0.75–0.93) versus 0.68 (95% CI 0.56–0.81], and the authors have called for corroborative studies in other centers.7
Static (Strain) Elastography
Strain imaging (static elastography) is another method that can quantitate tissue deformability. A contact force (stress) is induced, and the displacement field between two points tracked to determine strain. The relationship between the contact force and strain value depends upon tissue deformability, with greater strain seen in softer tissues. Cervical strain elastography involves deforming the cervix extrinsically (manual compression with the transducer) or intrinsically (holding the transducer still while the cervix moves against it due to vascular pulsation). The relative strain is the rate of change in tissue displacement in a region of interest, computed from ultrasound signals acquired before and after the deformation. This is often depicted in a color map (elastogram). An important point is that an elastogram is qualitative, not quantitative, because strain is a relative measurement of stiffness and its value changes based on amount of pressure applied. Also, strain image interpretation is complicated in all but the most trivial conditions because it makes assumptions such as that the tissue under investigation is homogeneous.8–10
Although the cervix is a complex, heterogeneous tissue, many studies have explored strain elastography approaches for its evaluation. In an early feasibility study, 12 women were evaluated at 15 to 33 weeks.11 No specific trends were noted with respect to gestational age, and the authors expressed uncertainty about the value of the technique because they noted the largest deformations were consistently nearest the transducer, leading them to question whether the deformation was due to actual tissue properties. In a study of 29 women presenting for term induction of labor, the internal os area of the cervix was significantly softer in the 13 patients with successful induction compared to those with failed induction (n=16).12 The analysis was based on calculation of an elasticity index (EI), a composite score of points given for colors in various regions of the cervix: purple=0 (hardest), blue=1, green=2, yellow=3 and red=4 (softest). The authors articulated that, because of the inability to standardize the force on the transducer, it was impossible to meaningfully compare scores between women. A subsequent cross-sectional study of 112 women at 12 to 40 weeks attempted to standardize the transducer force by advancing the probe into the anterior lip of the cervix by a reproducible amount (1 cm).13 Using the EI, they reported that tissue closest to the probe was always softest, regardless of gestational age, leading them to conclude that elastography “may be merely a reflection of the force applied by the transducer to different parts of the cervix.”
Another study of women at 35 to 42 weeks (n=66) evaluated elastographic score (at the internal os) as well as cervical length and angle of progression (a measure of cervical position) for predicting success of labor induction.14 They found cervical length, but not angle or elastographic score, to be a significant predictor of vaginal delivery. Two subsequent small studies using the EI found significant differences in patients with failed induction as compared to successful vaginal delivery.15,16 In another two studies comprising nearly 700 prenant women examined in the 1st and 2nd trimesters (11 – 28 weeks of gestation), strain measurements at the internal os were associated with PTB, but there was no correlation between strain and gestational age.17,18 Assignment to one of four color groups based on elastogram [red (soft), yellow (medium soft), blue (medium hard) and purple (hard)] was used to evaluate risk of PTB in low risk women (n=333) in the 2nd trimester.19 Statistically significantly higher rates of preterm births in the red and yellow groups with a sensitivity, specificity, PPV and NPV of 85.7%, 97.6%, 98.3%, and 81.1%, respectively.
In all of the above studies, the investigators noted that inability to standardize of transducer force was problematic. One group’s answer to this was a semi-quantitative approach by maintaining a consistent value on a ‘pressure bar’ displayed on the ultrasound system’s monitor. In a cross-sectional study (n=262) of patients across the spectrum of gestation (8 to 40 weeks) strain was evaluated at the endocervical canal, the external os and the internal os.20 They reported that the cervix was significantly softer proximally as compared to distally, and that strain varied by previous preterm delivery and cervical length. However, simulations and modeling experiments suggest that the pressure bar, a common feature on commercially available ultrasound systems, only indicates whether the contact pressure is adequate for a good quality strain image, and provides no information about actual applied force by the transducer.9,10
In another attempt to circumvent the problem of transducer force and relative strain, one group designed a reference cap to fit on the end of a transvaginal transducer because interposition of a material with known stiffness reference values can facilitate calculation of actual tissue softness (Young’s modulus or elastic modulus).22 In 49 women presenting for induction of labor at term, strain elastography with the cap performed better than Bishop score or cervical length for predicting induction success. Results must be interpreted with caution, however, because calculation of Young’s modulus assumes an homogeneous stress distribution, but simulations and modeling experiments suggest an inhomogeneous deformation in both the reference cap and the cervix, which would violate assumptions and lead to inaccurate calculations.10
Yet another method combines features of both maximum compressibility (like CCI) and strain elastography. This method uses tissue Doppler imaging (Toshiba Medical Systems) to calculate values of compressive strain in the area of maximum deformation by the transducer.23,24 This deformation is expressed as Lagrangian strain (deformation of tissue from its original to its current length) or natural strain (like Lagrangian strain, but also accounts for instantaneous tissue deformation).23 For this technique, pressure is applied with the transducer perpendicular to the AP axis until the anterior cervical lip reaches maximal deformation, and strain values are recorded during the subsequent relaxation phase.23 In a cross-sectional study of 74 women at 12 to 42 weeks of gestation, significant correlations of strain with gestational age [ρ=0.82 (95% CI 0.73 -- 0.88)] and cervical length [ρ=−0.59 (95% CI −0.72–0.4)] were found.24 Another group found significantly higher strain ratios in the 2nd trimester cervix of 17 (of 182) women who ultimately delivered preterm (n=17) compared to those who delivered at term (n=165), but the sensitivity and specificity were only 57% and 50%, respectively.25
In summary, regardless of approach, it seems there is no way around the central problem of strain elastography: because the applied force cannot be known, quantification of absolute softness (Young’s modulus or elastic modulus) is impossible.9,10
Dynamic elastography (shear wave elasticity imaging)
Dynamic elastography involves displacing tissue with a high-frequency ultrasound pulse (excitation) and observing its reaction. The degree of tissue displacement is related to its stiffness because softer tissue will displace more than stiffer tissue. While this method is much less affected by transducer force than strain elastography, a caution is that excessive pressure by the transducer may itself cause tissue stiffening. Also, in highly attenuating (i.e. ultrasound-wave absorbing) tissues, absorption of the pushing pulse may result in limited penetration, and the frame rate may need to be reduced because of limitations on thermal and mechanical indices required to produce high frequency pulses, either of which could yield poor results.26–27
Shear wave elasticity imaging (SWEI) is the most common form of dynamic elastography applied to the cervix. The shear wave created by the excitation pulse can be tracked at several positions as it radiates outward, which allows estimation of its speed (shear wave speed, SWS).28–29 Because shear waves travel faster in stiffer, and slower in softer, tissues, SWS can be used to quantify tissue stiffness/softness. The most common measure of SWS is shear wave group velocity. An important feature of SWEI is that, unlike strain elastography, Young’s modulus and shear modulus can be directly calculated (under simple conditions). For SWEI, it is important to choose a relatively homogenous region in the tissue away from boundaries and structures that may disrupt wave propagation (e.g. blood vessels or Nabothian cysts). This is because wave generation is unpredictable near boundaries. This includes the boundary of the tissue which is in contact with the transducer, which means that it is best to evaluate tissue at least a few mm away from the transducer surface. A related issue is that measurements maybe unreliable in thin tissues (less than 12–15 mm).
Recently, SWEI has been used to evaluate the pregnant cervix30–34 although producing adequate shear waves in the cervix is not trivial. As discussed above, thin, heterogeneous tissue violates some assumptions needed to quantify elastic moduli. In addition, cervical tissue is highly attenuating (wave energy is rapidly lost) because of its microstructural complexity. These characteristics, including that imaging is typically performed nearly at the transducer surface, cause difficulties. For these reasons, a typical curvilinear transvaginal probe is less than ideal for SWEI because its large acoustic elements limit how close to the transducer reliable shear waves can be produced.
In a cross-sectional study of women at 24–35 weeks of gestation, a “slight” decrease in SWS was demonstrated in women hospitalized for preterm labor (n=81) compared to a control group (n=27).33 Limitations of the technique noted by the authors included high variance in estimates. This may be due to the use of a standard transvaginal transducer. An additional factor is that these investigators evaluated the cervix only near the external os. Another group evaluated SWS in multiple areas of the cervix, and found that SWS decreased with increasing gestational age only at the internal os.34 An feasibility study using a prototype linear transducer (128 elements, 3mm diameter, 14mm aperture) affixed to the clinician’s finger to evaluate SWS before and after prostaglandin ripening prior to term induction of labor in 20 women demonstrated a significant difference (mean SWS estimate 2.53 ± 0.75 before and 1.54 ± 0.31 m/s 4 hours after prostaglandin application, p > 0.001).32 Figure 3 shows a B-mode image (acquired with a prototype linear transducer) of a 1st trimester cervix with superimposed shear wave speed (SWS) estimates. The same approach was used to compare subjects in the 1st trimester (5–14 weeks of gestation, n=15) to those in the 3rd trimester (37–41 weeks, n=18) Average SWS estimates for 1st trimester were 4.42±0.32 m/s (n=12) and 2.13±0.66 m/s (n = 18) for 3rd trimester (p<0.0001). The AUROC was 0.95 (95% CI: 0.82–0.99) with a sensitivity and specificity of 83% at the operating point of maximum combined sensitivity and specificity.
Figure 3.
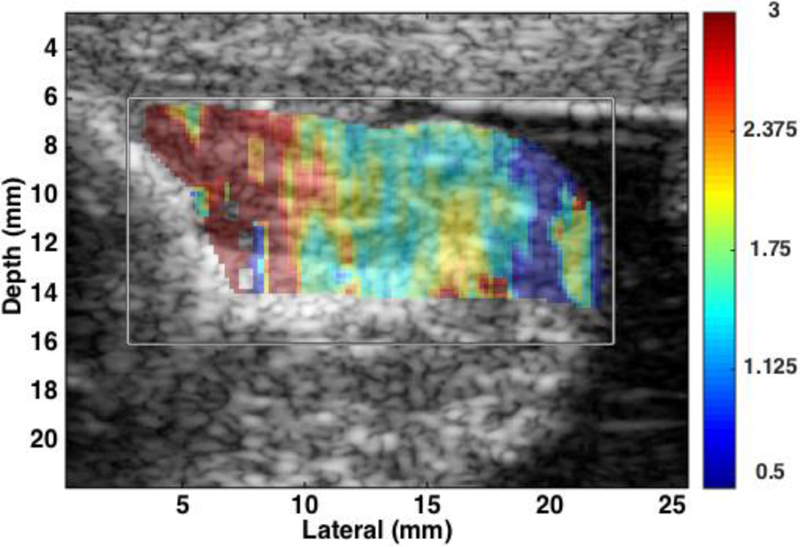
B-mode image of 1st trimester cervix with superimposed shear wave speed (SWS) estimates. SWS are represented by color (red is faster and blue slower). The gray box indicates region of interest. SWS are higher near the proximal end (left) and slower near the distal end (right).
Quantifying the Presence, Orientation and/or Concentration of Extracellular Matrix Components
Cervical evaluation techniques directed at assessing ECM components include attempts to quantify hydration and microstructural collagen organization/alignment because these properties change (specifically, hydration increases and collagen microstructure disorganizes) as gestation progresses.
Backscattered Power Difference
Measuring the backscattered power difference (BSPD) between a tissue and a reference phantom with isotropic scatterers is a means to investigate tissue microstructure. When an ultrasound wave encounters a scatterer (e.g. collagen), its pattern of backscatter (reflection) is affected by both the shape of the scatterer and the angle of the acoustic beam. For instance, if a beam encounters an isotropic (looks the same on all sides) scatterer such as a sphere, the backscatter will be affected only by the angle at which the beam hits the sphere. If, on the other hand, a beam encounters an anisotropic (looks different from different sides) scatterer like a rod, the backscatter will be affected by both where the beam hits the rod (its end looks different than its length) and the angle of the beam. Because of that, measuring the difference in backscattered power as a function of the beam angle provides information about the shape and organization of scattering sources. When a microstructure is highly organized (such as is found in the early pregnant cervix, with an organized collagen scaffolding that is highly aligned and anisotropic), backscatter should be high. The reverse should be true in late pregnancy (when the structure is broken down and more isotropic). Backscatter is measured and reported in dB, but the principle of how it is affected by tissue microstructure is seen in Figure 4. The figure shows 3 consecutive B-mode images of an ex vivo cervix with the ultrasound beams angled to −28, 0, and 28 degrees with respect to the transducer face. As expected, in a study comparing backscatter parameter anisotropy of the first trimester cervix (n=13) to that of the third trimester cervix (n=20), mean backscatter power differences (mBSPD) estimates were 4.99 ± 2.03 dB in the 1st trimester compared to 2.83 ± 1.17 dB in the 3rd trimesters (p < 0.003), indicating that the first trimester cervix is significantly more anisotropic (aligned and organized scattering sources, presumably collagen) than the third trimester cervix.36,37
Figure 4.
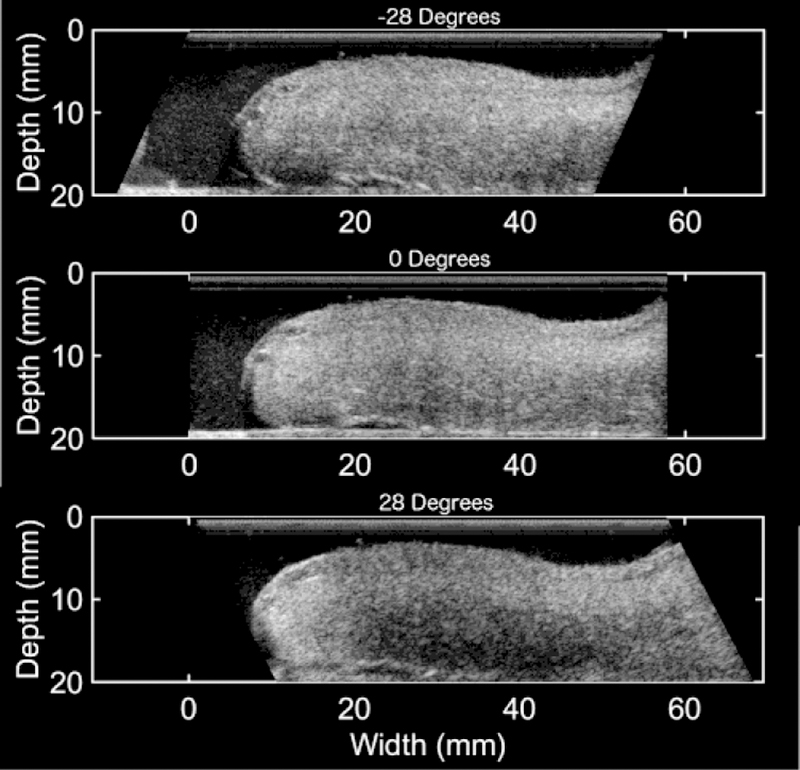
B-mode images at −28, 0, and 28 degrees with respect to the transducer face of an ex vivo anterior cervix (proximal end on the right and distal end on the left). The brightness of the image is visibly different for each angle. The decreasing brightness from −28 degrees to 28 degrees corresponds to an increase in BSPD.
Attenuation
Attenuation describes the phenomenon of ultrasound signal amplitude decrease with distance from the transducer. Attenuation depends upon properties such as hydration and microstructural organization. Because cervical remodeling involves ECM changes such as increasing hydration and decreasing microstructural organization, measuring attenuation could theoretically quantitate remodeling. In 67 pregnant women, attenuation was lower at 17–21 weeks of gestation in women who delivered preterm (1.02 dB/cm-MHz versus 1.34 dB/cm-MHz, p=0.04), but not at 22–26 weeks. Cervical length was significantly lower in women with sPTB, 3.16 cm versus 3.68 cm, p=0.004.38 The finding that attenuation did not predict sPTB as well as cervical length was unexpected, but may be explained by the fact that tissue anisotropy was ignored. Attenuation values, like BSPD discussed above, are known to vary with angle of ultrasound beam and with organization/alignment of microstructure. In fact, in the cervix, attenuation has been shown to be affected by angle of interrogation and location (distal versus proximal cervix).39 Therefore, measuring attenuation without accounting for sources of potential variability such as beam angle could contribute to estimate bias and variance.
Sources of potential variability were addressed in a study of women (n=20) at 37–41 weeks of gestation undergoing prostaglandin ripening in preparation for induction of labor.40 Mean attenuation before prostaglandin administration was higher in women who labored spontaneously after 1 dose of misoprostol compared to those who required further ripening (2.38±0.33 versus 1.37±0.10 dB/cm-MHz (mean±SE), respectively, p<0.01) but attenuation dependence on beam angle was not observed. These results may suggest that the term “ripe” cervix has greater hydration than the “unripe” cervix but the collagen microstructure has already disorganized so much by late pregnancy that it appears isotropic.
Although attenuation and backscatter techniques are promising, they will require extensive validation and verification of correct interpretation of results before they are clinically viable. For instance, differentiating between rod-like scatterers oriented parallel to the transducer face and spherical scatterers requires additional assessment because, although the former is anisotropic and the latter isotropic, they will appear the same to a beam at incident angle, which could lead to inaccurate interpretation. To address this, backscatter parameters to evaluate the presence, orientation, and the magnitude of anisotropy in tissue have been recently developed but to date have only been rigorously tested in phantoms and a simple human tissue (bicep muscle).41
Electrical Impedance
Flow of electrical current is affected by water, and greater hydration reduces electrical impedance (resistance to flow). Impedance can be measured by placing electrodes on the cervix. A study of electrical impedance in 50 nonpregnant and 90 pregnant women found impedance to be decreased in the first trimester cervix as compared to the nonpregnant cervix, but, surprisingly, impedance in late pregnancy were larger than in early pregnancy or the nonpregnant state42 The authors speculated that perhaps this was due to increased cellular and collagen content in late pregnancy. A subsequent study impedance for prediction of successful labor induction in 200 women presenting for induction at term found that the measurements were only weakly correlated. The authors concluded that the device was not “sufficiently accurate to justify clinical use”,43 although impedance is currently being investigated longitudinally with a novel device that can be worn like a pessary on the cervix.44
Raman Spectroscopy
Raman spectroscopy exploits the energy exchange between photons and scattering molecules because molecules will scatter photons in a specific way based on their unique properties. Raman spectroscopy is sensitive to tissue hydration, collagen content and structure, and density of certain cells, lipids, and proteins. Raman spectroscopy, used longitudinally during pregnancy in mice, detected decreased lipids and increased solubilization of the collagen matrix, consistent with biomechanical testing on the same tissue which demonstrated decreasing tensile strength and increasing distensibility in late, as compared to early, pregnancy.45
The Future
Finite element modeling of the pregnant uterus, cervix, and fetal membranes shows that the mechanics of these tissues, which is based on their individual ECM properties, are extremely complex and interrelated.46 These and other theoretical modeling experiments suggest that a minimalist approach in which a single characteristic is measured (e.g. softness or microstructural organization) in a single tissue (e.g. cervix alone) is too limiting.
Current approaches to SWEI in the cervix exemplifies this principle. They all report estimates of SWS group velocity. Such measurement assumes that differences between tissues are defined primarily by purely elastic stiffness, which in turn presumes both homogeneity and purely elastic behavior.46,48 The cervix, however, is decidedly viscoelastic; specifically, it behaves like a viscoelastic solid in which magnitude of the response depends on not only its elasticity but also the rate of the applied strain.48 Further, even small changes in collagen fiber morphology (waviness and diameter) and orientation markedly affect shear wave propagation speed and dispersion (separation of the sound wave as it passes through a material).48 Viscosity, as well as microstructural organization, might be expected to cause dispersion in the cervix.49 The point is that the elastic modulus of the cervix, the parameter that could both explain and predict behavior of a particular cervix, cannot be directly deduced from simple shear wave group velocity.46 This principle, namely that a single parameter cannot describe the extremely complex cervix, applies to all of the techniques described above.
As stated by one of the groups researching cervical SWEI, “only a sound knowledge of microstructure will allow correct interpretation of results.”47 This is not easy, but, with careful analysis, it is possible. For instance, in the case of SWEI, measurement accuracy can be improved by testing for microstructural homogeneity in the region of interest and assessing viscosity.49 A recently developed approach tests for reliability of individual measurements (and rejects spurious data), for significant and uniform shear wave displacement in a region of interest (to confirm it is sufficiently homogeneous for accurate assessment), and assesses the viscous component of cervix (via quantification of shear wave dispersion). These tests have been applied to analysis of SWEI in the Rhesus macaque cervix. Simple shear wave group velocity showed no difference between the prostaglandin-ripened and unripened cervix, but applying these tests to the same data revealed a difference. In other words, as suggested by theoretical modeling, taking into account multiple properties such as local microstructure and viscosity seems to improve the accuracy of cervical evaluation.
Conclusion
While today’s clinical practice involves subjective digital examination of the cervix, tomorrow’s will likely involve integration of various quantitative parameters to directly and robustly evaluate characteristics of an individual cervix.
Figure 2.
A typical transvaginal cervical image on the left, and on the right the same cervix under maximal compression from the transducer. (Reprinted with permission from reference #7)
Acknowledgments
Supported in part by Intermountain Research & Medical Foundation, and NIH grants R01HD072077, R21HD061896, R21HD063031 and T32CA009206 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure: The corresponding author’s research group receives technical and engineering support from Siemens Ultrasound USA. She holds intellectual property in cervical quantitative imaging.
References
- 1.Feltovich H Cervical evaluation: from ancient medicine to precision medicine. Obstet Gynecol 2017; in press. [DOI] [PMC free article] [PubMed]
- 2.Papillon-Smith J, Abenhaim HA. The role of sonographic cervical length in labor induction at term. J Clin Ultrasound 2015; 43(1): 7–16. [DOI] [PubMed] [Google Scholar]
- 3.Huhn KA, Brost BC. Accuracy of simulated cervical dilation and effacement measurements among practitioners. Am J Obstet Gynecol 2004; 191(5): 1797–1799. [DOI] [PubMed] [Google Scholar]
- 4.Nizard J, Haberman S, Paltieli Y, Gonen R, Ohel G, Nicholson D, Ville Y. How reliable is the determination of cervical dilation? Comparison of vaginal examination with spatial position-tracking ruler. Am J Obstet Gynecol 2009; 200(4): 402.e1–e4. [DOI] [PubMed] [Google Scholar]
- 5.Badir S, Mazza E, Zimmermann R, Bajka M. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat Diagn 2013; 33(8): 737–41 [DOI] [PubMed] [Google Scholar]
- 6.Parra-Saavedra M, Gomez L, Barrero A, Parra G, Vergara F, Navarro E. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet Gynecol 2011; 38(1): 44–51. [DOI] [PubMed] [Google Scholar]
- 7.Baños N, Murillo-Bravo C, Julià C, Migliorelli F, Perez-Moreno A, Ríos J, Gratacos E, Valentin L, Palacio M. Mid-trimester sonographic Cervical Consistency Index to predict spontaneous preterm birth in a low-risk population. Ultrasound Obstet Gynecol 2017; Epub ahead of print. [DOI] [PubMed]
- 8.Barbone PE, Bamber JC. Quantitative elasticity imaging: what can and cannot be inferred from strain images. Phys Med Biol 2002; 47(12): 2147–64. [DOI] [PubMed] [Google Scholar]
- 9.Mazza E, Parra-Saavedra M, Bajka M, Gratacos E, Nicolaides K, Deprest J. In vivo assessment of the biomechanical properties of the uterine cervix in pregnancy. Prenat Diagn 2014; 34(1): 33–41. [DOI] [PubMed] [Google Scholar]
- 10.Maurer MM, Badir S, Pensalfini M, Bajka M, Abitabile P, Zimmermann R, Mazza E. Challenging the in-vivo assessment of biomechanical properties of the uterine cervix: A critical analysis of ultrasound based quasi-static procedures. J Biomech 2015; 48(9): 1541–8.Fruscalzo A, Mazza E, Feltovich H, Schmitz R. Cervical elastography during pregnancy: a critical review of current approaches with a focus on controversies and limitations. J Med Ultrason 2016; 43(4): 493–504. [DOI] [PubMed] [Google Scholar]
- 11.Khalil MR, Thorsen P, Uldbjerg N. Cervical ultrasound elastography may hold potential to predict risk of preterm birth. Dan Med J 2013; 60(1): A4570. [PubMed] [Google Scholar]
- 12.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol 2011; 38(1): 52–56. [DOI] [PubMed] [Google Scholar]
- 13.Molina FS, Gómez LF, Florido J, Padilla MC, Nicolaides KH. Quantification of cervical elastography: a reproducibility study. Ultrasound Obstet Gynecol 2012; 39(6): 685–689. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs T, Woyton R, Pomorski M, Wiatrowski A, Slejman N, Tomialowicz M, Florjanski J, Milnerowicz-Nabzdyk E, Zimmer M. Sonoelastography of the uterine cervix as a new diagnostic tool of cervical assessment in pregnant women – preliminary report. Ginekol Pol 2013; 84(1): 12–16. [DOI] [PubMed] [Google Scholar]
- 15.Pereira S, Frick AP, Poon LC, Zamprakou A, Nicolaides KH. Successful induction of labor: prediction by preinduction cervical length, angle of progression and cervical elastography. Ultrasound Obstet Gynecol 2014; 44(4): 468–475. [DOI] [PubMed] [Google Scholar]
- 16.Hwang HS, Sohn IS, Kwon HS. Imaging analysis of cervical elastography for prediction of successful induction of labor at term. J Ultrasound Med 2013; 32(6): 937–946. [DOI] [PubMed] [Google Scholar]
- 17.Muscatello A, Di Nicola M, Accurti V, Mastrocola N, Franchi V, Colagrande I, Patacchiola F, Carta G. Sonoelastography as method for preliminary evaluation of uterine cervix to predict success of induction of labor. Fetal Diagn Ther 2014; 35(1): 57–61. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Andrade E, Romero R, Korzeniewski SJ, Ahn H, Aurioles-Garibay A, Garcia M, Schwartz AG, Yeo L, Chaiworapongsa T, Hassan SS. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J Perinat Med 2014; 42(2): 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-Andrade E, Garcia M, Ahn H, Korzeniewski SJ, Saker H, Yeo L, Chaiworapongsa T, Hassan SS, Romero R. Strain at the internal cervical os assessed with quasi-static elastography is associated with the risk of spontaneous preterm delivery ≤34 weeks of gestation. J Perinat Med 2015; 43(6): 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozniak S, Czuczwar P, Szkodziak P, Milart P, Wozniakowska E, Paszkowski T. Elastography in predicting preterm delivery in asymptomatic, low-risk women: a prospective observational study. BME Pregnancy Childbirth 2014; 14: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Andrade E, Hassan SS, Ahn H, Korzeniewski SJ, Yeo L, Chaiworapongsa T, Romero R. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet Gynecol 2013; 41(2): 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hee L, Rasmussen CK, Schlütter JM, Sandager P, Uldbjerg N. Quantitative sonoelastography of the uterine cervix prior to induction of labor as a predictor of cervical dilation time. Acta Obstet Gynecol Scand 2014; 93(7): 684–690. [DOI] [PubMed] [Google Scholar]
- 23.Fruscalzo A, Schmitz R, Klockenbusch W, Steinhard J. Reliability of cervical elastography as a new ultrasound tool for cervical stiffness assessment in pregnancy. Ultraschall Med 2012; 33(7): E101–7. [DOI] [PubMed] [Google Scholar]
- 24.Fruscalzo A, Londero AP, Fröhlich C, Möllmann U, Schmitz R. Quantitative elastography for cervical stiffness assessment during pregnancy. Biomed Res Int 2014; 2014: 836535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köbbing K, Fruscalzo A, Hammer K, Möllers M, Falkenberg M, Kwiecien R, Klockenbusch W, Schmitz R. Quantitative elastography of the uterine cervix as a predictor of preterm delivery. J Perinatol 2014; 34(1): 774–80. [DOI] [PubMed] [Google Scholar]
- 26.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic Priniciples and technology. Ultraschall Med 2013; 34(2): 169–184. [DOI] [PubMed] [Google Scholar]
- 27.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015; 41(5):1126–1147. [DOI] [PubMed] [Google Scholar]
- 28.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol 1998; 24(9): 1419–1435. [DOI] [PubMed] [Google Scholar]
- 29.Nightingale K, Palmeri M, Trahey G. Analysis of contrast in images with transient acoustic radiation force. Ultrasoud Med Biol 2006; 32(1): 61–72. [DOI] [PubMed] [Google Scholar]
- 30.Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, del Rio A, Hall TJ. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet Gynecol 2014;43(4):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson LC, Feltovich H, Palmeri ML, Munoz del Rio A, Hall TJ. Statistical Analysis of Shear Wave Speed in the Uterine Cervix. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61(10):1651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson LC, Romero ST, Palmeri ML, Rio DEL, Esplin SM, Rotemberg VM, Hall TJ, Feltovich H. Changes in shear wave speed pre- and post-induction of labor : a feasibility study. Ultrasound Obstet Gynecol 2015;46(1):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller M, Aït-Belkacem D, Hessabi M, Gennisson J-L, Grangé G, Goffinet F, Lecarpentier E, Cabrol D, Tanter M, Tsatsaris V. Assessment of the Cervix in Pregnant Women Using Shear Wave Elastography: A Feasibility Study. Ultrasound Med Biol 2015;41(11):2789–2797. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Andrade E, Aurioles-Garibay A, Garcia M, Korzeniewski SJ, Schwartz AG, Ahn H, Martinez-Varea A, Yeo L, Chaiworapongsa T, Hassan SS, Romero R. Effect of depth on shear-wave elastography estimated in the internal and external cervical os during pregnancy. J Pernat Med 2014; 42(5): 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drehfal LC, Hall TJ, Rosado-Mendez I, Palmeri M and Feltovich H. Detection of changes in cervical softness using Shear Wave Speed in early vs. late pregnancy: an initial in vivo application (submitted) [DOI] [PMC free article] [PubMed]
- 36.Guerrero QW, Drehfal LC, Rosado-Mendez IM, Feltovich H, Hall TJ. Monitoring collagen remodeling in the cervix with quantitative ultrasound. Reprod Sci 2017; 24(S1): 242A–243A.27324432 [Google Scholar]
- 37.Guerrero QW, Drehfal LC, Santoso AP, Rosado-Mendez IM, Feltovich H, Hall TJ. Quantitative ultrasound anisotropy of in vivo uterine cervix: early vs. late stage pregnancy. Ultrason Imaging 2017. (under).
- 38.McFarlin BL, Kumar V, Bigelow TA, Simpson DG, White-Traut RC, Abramowicz JS, O’Brien WD Jr. Beyond cervical length: a pilot study of ultrasonic attenuation for early detection of preterm birth risk. Ultrasound Med Biol 2015; 41(11): 3023–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerrero QW, Carlson LC, Feltovich H, Hall TJ. Quantitative ultrasound backscatter parameters in the human cervix. IEEE Int Ultrason Symp 2014; 2014: 224–227. [Google Scholar]
- 40.Guerrero QW, Drehfal LC, Rosado-Mendez I, Feltovich H, Hall TJ. EP22.03: Quantitative ultrasound suggests collagen disorganization in the human cervix in late pregnancy. Ultrasound Obstet Gynecol 2016; 48: 364. [Google Scholar]
- 41.Guerrero QW, Rosado-Mendez I, Drehfal LC, Feltovich H, Hall TJ. Quantifying backscatter anisotropy using the reference phantom method. IEEE Trans Ultrason Ferroelectr Freq Control 2017; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 42.Gandhi SV, Walker DC, Brown BH, Anumba DO. Comparison of human uterine cervical electrical impedance measurements derived using two tetrapolar probes of different sizes. Biomed Eng Online 2006; 5: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jokhi RP, Brown BH, Anumba DO. The role of cervical Electrial Impedance Spectroscopy in the prediction of the course and outcome of induced labour. BME Pregnancy Childbirth 2009; 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etemadi M, Chung P, Heller JA, Liu J, Grossman-Kahn R, Rand L, Roy S. Novel device to trend impedance and fluorescence of the cervix for preterm birth detection. Conf Proc IEEE Eng Med Biol Soc 2013; 2013: 176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien CM, Vargis E, Paria BC, Bennett KA, Mahadevan-Jansen A, Reese J. Raman spectroscopy provides a noninvasive approach for determining biochemical composition of the pregnant cervix in vivo. Acta Paediatr 2014; 103(7): 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez M, House M, Jambawalikar S, Zork N, Vink J, Wapner R, Myers K. Investigating the mechanical function of the cervix during pregnancy using finite element models derived from high-resolution 3D MRI. Comput Methods Biomech Biomed Engin 2016; 19(4): 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peralta L, Rus G, Bochud N, Molina FS. Mechanical assessment of cervical remodeling in pregnancy: insight from a synthetic model. J Biomech 2015; 48(9): 1557–1565. [DOI] [PubMed] [Google Scholar]
- 48.Myers KM, Paskaleva AP, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater 2008; 4(1):104–116. [DOI] [PubMed] [Google Scholar]
- 49.Rosado-Mendez IM, Palmeri ML, Drehfal LC, Guerrero QW, Simmons H, Feltovich H, Hall TJ. Assessment of structural heterogeneity and viscosity in the cervix using shear wave elasticity imaging: initial results from a Rhesus macaque model. Ultrasound Med Bio 2017; 43(4):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]