Abstract
INTRODUCTION:
We investigated whether cholinesterase inhibitors (ChEIs) benefit cognitive outcomes in mild cognitive impairment (MCI) due to Alzheimer disease (AD) and in mild AD dementia.
METHODS:
Data from 2,242 individuals, clinically diagnosed with MCI due to AD (MCI-AD, Clinical Dementia Rating [CDR] = 0 or 0.5) or with mild AD dementia (ADdem, CDR= 0.5 or 1), were available from the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set (UDS). General linear mixed models were used to examine the annual change in the CDR Sum Boxes (CDR-SB) and in neuropsychological performance. We compared slopes before and after ChEI initiation among ChEI users, and also compared change in scores of ChEI users versus non-users.
RESULTS:
Thirty-four percent of 944 MCI-AD and 72% of 1,298 ADdem participants were ChEI users. Cognitive decline was greater after ChEI initiation in MCI-AD and ADdem groups (e.g., MCI-AD, CDR-SB: 0.03 points/year before initiation; 0.61 points/year after initiation, p<0.0001). Both MCI-AD and ADdem groups had faster decline after ChEI initiation than non-users (e.g., MCI-AD, CDR-SB: 0.61 points/year, ChEI users; 0.24 points/year, non-users, p<0.0001).
DISCUSSION:
This study suggests that ChEI use may not improve the cognitive course in MCI-AD and mild ADdem.
Keywords: Cholinesterase inhibitor, Cognitive outcome, Mild cognitive impairment, Alzheimer disease, Alzheimer disease dementia
Introduction
Cholinesterase inhibitor (ChEI) drugs are approved for the symptomatic treatment of Alzheimer disease (AD) dementia and these agents (e.g., donepezil; rivastigmine; galantamine) have consistently demonstrated modest improvement in cognitive outcomes1–3. Their utility in individuals with mild cognitive impairment (MCI) due to AD4 is uncertain, however, as efficacy at this initial stage of symptomatic AD has not been convincingly demonstrated. For example, although trials of ChEIs in persons with MCI have failed to demonstrate efficacy in primary outcome measures, some have showed benefit for secondary outcome measures5–7. One trial of donepezil in MCI found an initial slowing of cognitive decline in the early portion of the trial but not at its conclusion8. Another study of galantamine in combination with memantine (a non-ChEI) seemed to show benefit for the combination therapy, although the study was limited by small sample size (N=232)9. Finally, a meta-analysis concluded that there was no therapeutic benefit for ChEI use in MCI10.
The cholinergic hypothesis11,12, which serves as the rationale for ChEI therapy in AD, posits a central cholinergic deficit in AD that contributes to cognitive dysfunction. However, hippocampal and frontal cortical upregulation of choline acetyltransferase (CAT) activity occurs in MCI, which may reduce the efficacy of ChEIs13. In contrast, another study showed protective effects of ChEIs for neurodegeneration14. These minimal and conflicting mechanistic studies of ChEI effects on central nervous systems processes provide an uncertain rationale for the use of ChEIs therapy in MCI.
Nonetheless, use of ChEI in MCI individuals is prevalent in clinical practice15. To determine whether ChEI use benefits individuals with MCI due to AD, and to ascertain what factors are associated with use of ChEIs in such individuals, we conducted an observational study with data from the Uniform Data Set (UDS)16 at the National Alzheimer’s Coordinating Center (NACC).
Methods
Participants
Data from the UDS are collected at annual visits and are supplemented by standardized Neuropathology (NP) Data Set (NDS) for those who had autopsy. These data accrue from approximately 30 actively-funded National Institute on Aging Alzheimer Disease Centers (ADCs) and are maintained at NACC. Data collection at the ADCs occurs for each participant on an annual basis and includes demographics, medication history, clinical characteristics and diagnoses, neuropsychological test performances, and neuropathological characteristics, using standardized UDS and neuropathology forms. Data collected from September 2005 through August 2016 were included in this study. Details of the participant recruitment in NACC sample and data collection procedures have been described previously16–18. Institutional review board of each institutions have reviewed and approved the study protocols. Written informed consent was obtained from all participants and informants of this study.
Participants who were naïve to ChEI use at their initial UDS visit were included if they had three or more annual UDS visits and had a primary etiological diagnosis of MCI due to AD (global Clinical Dementia Rating19 [CDR] of 0 or 0.5) or mild AD dementia (CDR of 0.5 or 1) at baseline; the mild AD dementia group was included as it is recognized that symptomatic AD represents a continuum from its incipient stage (MCI due to AD) through progressively severe stages20. Two groups were compared: ChEI users were participants who reported use of any ChEI at any follow-up visit after the initial UDS visit and who had at least one visit subsequent to the one where ChEI use was reported, and ChEI non-users were participants who never reported ChEI use at any UDS visit.
Demographic information (eg. sex, age, education level), past self-reported medical history (e.g., stroke, diabetes), family history of cognitive impairment (first degree family member), presence of co-existing problems assessed by clinician (eg. behavior, language) and medication use were collected at each UDS visit. All participants were evaluated using the standardized UDS clinical measures including the CDR and neuropsychological test battery18. Coexisting mood disorders were evaluated in both subjective self-report of depression and the participant’s score on the 15-item Geriatric Depression Scale (GDS)21. Apolipoprotein E (APOE) genotype was available for the majority of the sample (86%).
Neuropsychological Tests
The cognitive measures in the UDS neuropsychological battery18 included Mini-Mental State Examination (MMSE) for global cognition, Wechsler Memory Scale Logical Memory - Immediate Recall and Delayed Recall for episodic memory, Wechsler Memory Scale-Revised (WMS-R) Digit Span Forward and Backward for attention, Wechsler Adult Intelligence Scale- Revised Digit Symbol and the Trail Making Test (Part A and B) for measuring processing speed and executive function, Animal and Vegetable list generation for semantic fluency, and Boston Naming Test (30 odd items) for naming. Dementia progression was measured by CDR Sum of Boxes (CDR-SB), which sums the individual scores in the six domains assessed by the CDR: memory, orientation, judgment, home and hobbies, community affairs, and personal care22.
Neuropathology
For participants in this study who died and came to autopsy (N=283), neuropathological data were retrieved from NACC’s NDS as described in its standardized Neuropathology Form and Coding Guidebook23. The NDS has applied the National Institute on Aging-Alzheimer’s Association (NIA-AA) Alzheimer’s disease neuropathological change (ADNC) guidelines24 which rank AD neuropathology changes in three parameters of an “ABC score” - consisting of “A”- immunohistochemistry of amyloid beta plaque score25, “B” - neurofibrillary tangle (NFT) stage of Braak26, and “C” - neuritic plaque score from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)27. However, ADNC scores were not gathered prior to 2014 [because “A” (Thal Phase) was not done]. To permit an adequate sample size, and to provide some consistency with the “B” and “C” scores, we used the criteria of moderate to frequent neuritic plaques from CERAD (“C”) and Braak stage III-VI (“B”) to define AD neuropathology.
Statistical Analyses
Using analyses of variance models or Chi-squared tests, ChEI users and non-users in both MCI due to AD (MCI-AD) and mild Alzheimer disease dementia (ADdem) groups were compared on age, education, sex, race, Hispanic ethnicity, presence of APOE ε4 allele, family history of cognitive impairment, and global CDR at the baseline. We also compared total number of UDS visits and presence of AD neuropathology in individuals whom had autopsy. Additionally, we compared age of onset and duration of cognitive decline as reported by the participants, clinician-assessed affected cognitive domains, presence of behavioral problems, self/proxy-reported history of vascular risk factors, other contributing etiologies, initial GDS scores, and antidepressant and memantine use at initial visit in these groups.
In the MCI-AD group, less than 1% of participants had missing data for education and race but 12% were missing APOE genotypes. In the ADdem group, less than 2% of participants had missing data for education and race but 16% had missing APOE genotypes. The unadjusted logistic regression was used to calculate p-values. Statistical significance was based on alpha level of 0.05.
General linear mixed effects models were used to examine the annual rate of change in the CDR-SB and the neuropsychological test outcomes in both MCI-AD and ADdem groups. Specifically, for the ChEI users, we implemented a piecewise linear growth/decline pattern over time that was linked at the initiation of the ChEI use (treated as time 0). The model then assumed the vector of two slopes (i.e., the annual rates of change prior to and after the initiation of ChEI use) and the performance at the initiation of ChEI use (the intercept) as both random and fixed effects28. For the ChEI non-users, we implemented a simple random intercept and random slope model. Mean slopes were then compared before and after ChEI initiation among ChEI users in the MCI-AD and ADdem groups, and also to the slope of ChEI non-users. Adjusted analyses were performed, controlling for baseline age, sex, education, race, presence of APOE ε4 allele, behavioral problems, and GDS score at baseline. All analyses were done by SAS29.
Results
Demographics and baseline characteristics
A total of 944 MCI-AD participants (322 ChEI users, 622 ChEI non-users) and 1298 mild ADdem participants (932 ChEI users, 366 ChEI non-users) met our eligibility criteria. Baseline characteristics differed in most of the measures between ChEI users and non-users of both MCI-AD and ADdem groups (Table 1). Compared to non-users, ChEI users were more likely to have greater years of education, be of Caucasian race, and have at least 1 APOE ε4 allele. At the initial visit, ChEI users had better global CDR scores compared to the non-users; however, at their first use of ChEI, ChEI users have worse global CDR scores compared to non-users at the initial visit. ChEI users in both groups more often had family history of dementia. In the ADdem group only, ChEI users were more likely to be younger at initial visit than non-users.
Table 1.
Baseline characteristics of sample
| MCI-AD* |
p-value† | Mild AD dementia* |
p-value† | |||
|---|---|---|---|---|---|---|
| Characteristic | ChEI users | Never used ChEI | ChEI users | Never used ChEI | ||
| Sample size, n | 322 | 622 | NA | 932 | 366 | NA |
| Age at Initial Visit, mean (SD) | 74.1 (8.1) | 73.5 (9.1) | 0.37 | 74.7 (8.8) | 77.7 (9.7) | <0.0001 |
| Male, n (%) | 170 (52.8%) | 309 (49.7%) | 0.36 | 411 (44.1%) | 166 (45.4%) | 0.68 |
| Education (years), mean (SD) | 15.8 (2.9) | 15.3 (3.4) | 0.03 | 14.7 (3.6) | 14.2 (4.0) | 0.03 |
| Race, n (%) | ||||||
| White | 288 (90.3%) | 474 (77.0%) | Ref. | 763 (83.5%) | 273 (76.9%) | Ref. |
| African American | 21 (6.6%) | 75 (12.2%) | 0.003 | 94 (10.3%) | 55 (15.5%) | 0.008 |
| Other | 10 (3.1%) | 67 (10.9%) | <0.0001 | 57 (6.2%) | 27 (7.6%) | 0.25 |
| Hispanic ethnicity, n (%) | 16 (5.0%) | 45 (7.2%) | 0.18 | 64 (6.9%) | 31 (8.5%) | 0.32 |
| Number of years of follow-up, mean (SD) | 5.4 (2.2) | 3.9 (1.6) | <0.0001 | 4.8 (2.2) | 3.9 (1.9) | <0.0001 |
| Presence of at least 1 APOE e4 allele, n (%) | 151 (53.0%) | 225 (41.2%) | 0.001 | 460 (57.4%) | 139 (46.6%) | 0.0002 |
| Family history of cognitive impairment, n (%) | 216 (71.5%) | 354 (60.4%) | 0.001 | 557 (66.1%) | 184 (58.4%) | 0.02 |
| Global CDR at Initial Visit, n (%) | ||||||
| 0 | 70 (20.8%) | 38 (6.0%) | <0.0001 | 86 (9.2%) | 0 (0.0%) | <0.0001 |
| 0.5 | 258 (76.8%) | 584 (92.7%) | 567 (60.8%) | 170 (46.5%) | ||
| 1 | 8 (2.4%) | 8 (1.3%) | 279 (30.0%) | 196 (53.6%) | ||
| Global CDR at First ChEI use‡, n (%) | ||||||
| 0 | 5 (1.6%) | 38 (6.1%) | 0.003 | 0 (0.0%) | 0 (0.0%) | 0.02 |
| 0.5 | 317 (98.5%) | 584 (93.9%) | 365 (39.2%) | 170 (46.5%) | ||
| 1 | 0 (0.0%) | 0 (0.0%) | 567 (60.8%) | 196 (53.6%) | ||
| Had neuropathology exam/autopsy, n (%) | 33 (10.2%) | 23 (3.7%) | 0.0002 | 156 (16.7%) | 71 (19.4%) | 0.28 |
| ADNP among those with autopsy, n (%) | 24 (72.7%) | 15 (65.2%) | 0.55 | 135 (86.5%) | 54 (76.1%) | 0.05 |
Abbreviations: MCI-AD = Mild cognitive impairment with primary etiologic diagnosis of Alzheimer’s disease; AD = Alzheimer’s disease; ChEI = cholinesterase inhibitor; CDR = Clinical Dementia Rating; APOE = apolipoprotein E; ADNP = moderate to frequent neuritic plaques and Braak Stage III-VI Missing data: MCI-AD (ChEI users, Never used ChEI): Education (n=1, n=1); Race: (n=3; n=6); Hispanic ethnicity (n=1; n=4); APOE genotype (n=37, n=76); Mild AD dementia (ChEI users, Never used ChEI): Education (n=6, n=2); Race: (n=18; n=11); Hispanic ethnicity (n=2, n=0); APOE genotype (n=130, n=68)
Diagnosis at time of first ChEI use among ChEI users or at Initial Visit for never users
Used unadjusted logistic regression comparing ChEI users and never users
For those who never used ChEI, providing CDR at Initial Visit
Bolded if statistically significant at p<0.05`
In this sample, 283 had a neuropathological examination (Table 1). The availability of neuropathology data was similar between ChEI users and non-users in the ADdem group, but ChEI users in the MCI-AD group more often had neuropathology data than non-users. In the ADdem group, ChEI users more frequently met neuropathological criteria for AD compared with non-users (135 cases, 86.5% in ChEI users; 54 cases, 76.1% in non-users, p-value = 0.05).
Presence of other diagnoses potentially contributing to cognitive impairment were more prominent in the non-users (Table 2); in particular, cerebrovascular disease was more likely to contribute in both MCI-AD and ADdem non-users. Co-morbid cardiovascular risk factors were also more often present in the ADdem non-users. Compared to ChEI users, ChEI non-users more often reported diabetes in the MCI-AD group and more often reported diabetes, stroke and cardiovascular disease in the ADdem group.
Table 2.
Clinical characteristics and symptoms of sample
| Characteristic at Initial Visit | MCI-AD* |
p-value† | Mild AD dementia* |
p-value† | ||
|---|---|---|---|---|---|---|
| ChEI users | Never used ChEIs | ChEI users | Never used ChEIs | |||
| Age of onset of cognitive decline, mean (SD) | 71.8 (8.7) | 70.3 (9.7) | 0.03 | 71.8 (9.3) | 73.1 (9.7) | 0.02 |
| Contributing clinical diagnoses, n (%) | ||||||
| None | 274 (85.1%) | 460 (74.0%) | 0.0001 | 740 (79.4%) | 262 (71.6%) | 0.003 |
| Lewy body disease including PD | 1 (0.3%) | 4 (0.6%) | 0.51 | 12 (1.3%) | 4 (1.1%) | 0.78 |
| FTLD including bvFTD and PPA | 0 (0.0%) | 1 (0.2%) | 0.98 | 13 (1.4%) | 6 (1.6%) | 0.74 |
| Cerebrovascular disease | 6 (1.9%) | 38 (6.1%) | 0.006 | 30 (3.2%) | 36 (9.8%) | <0.0001 |
| Other | 43 (13.4%) | 128 (20.6%) | 0.007 | 152 (16.3%) | 69 (18.9%) | 0.27 |
| History of stroke, n (%) | 9 (2.8%) | 23 (3.7%) | 0.48 | 52 (5.6%) | 44 (12.1%) | <0.0001 |
| History of diabetes, n (%) | 34 (10.6%) | 104 (16.8%) | 0.01 | 118 (12.7%) | 62 (17.0%) | 0.05 |
| History of hypertension, n (%) | 166 (51.6%) | 357 (57.5%) | 0.08 | 503 (54.3%) | 215 (58.7%) | 0.15 |
| History of hypercholesterolemia, n (%) | 175 (55.4%) | 345 (55.7%) | 0.92 | 486 (52.6%) | 179 (49.6%) | 0.33 |
| History of cardiovascular disease, n (%) | 63 (19.6%) | 120 (19.4%) | 0.93 | 168 (18.1%) | 102 (28.0%) | <0.0001 |
| Depression in past 2 years, self-report, n (%) | 75 (23.4%) | 168 (27.2%) | 0.21 | 293 (31.7%) | 128 (35.2%) | 0.24 |
| Depression medication use, n (%) | 64 (20.1%) | 125 (20.2%) | 0.98 | 216 (23.5%) | 81 (22.6%) | 0.75 |
| Geriatric Depression Scale at Initial Visit, mean (SD) | 1.8 (2.0) | 2.1 (2.3) | 0.04 | 2.4 (2.4) | 2.7 (2.7) | 0.08 |
| Cholinesterase inhibitor at first ChEI use, n (%) | ||||||
| Donepezil | 271 (84.2%) | N/A | N/A | 710 (76.2%) | N/A | N/A |
| Rivastigmine | 35 (10.9%) | N/A | N/A | 125 (13.4%) | N/A | N/A |
| Galantamine | 18 (5.6%) | N/A | N/A | 100 (10.7%) | N/A | N/A |
| Memantine use, n (%) | 4 (1.2%) | 11 (1.8%) | 0.54 | 81 (8.7%) | 67 (18.3%) | <0.0001 |
Abbreviations: MCI-AD = Mild cognitive impairment with primary etiologic diagnosis of Alzheimer’s disease; AD = Alzheimer’s disease; ChEI = cholinesterase inhibitor; N/A = Not applicable, not calculated; bvFTD = behavioral variant frontotemporal dementia; PPA = primary progressive aphasia; FTLD = frontotemporal lobar degeneration
Missing data: MCI-AD (ChEI users, Never used ChEI): Family history of cognitive impairment (n=20, n=36); stroke (n=2, n=1); diabetes (n=0, n=1); hypertension (n=0, n=1); hypercholesterolemia (n=6, n=3); cardiovascular disease (n=1, n=3); depression (n=2, n=4); depression medications (n=4, n=3); Age of onset of cognitive decline (n=28, n=102); Mild AD dementia (ChEI users, Never used ChEI): Family history of cognitive impairment (n=89, n=51); stroke (n=3, n=2); diabetes (n=3, n=1); hypertension (n=6, n=0); hypercholesterolemia (n=8, n=5); cardiovascular disease (n=3, n=2); depression (n=9, n=2); depression medications (n=11, n=8); Age of onset of cognitive decline (n=9, n=10)
Diagnosis at time of first ChEI use among ChEI users or at Initial Visit for never users
Used unadjusted logistic regression comparing ChEI users and never users (analysis of normal cognition at Initial Visit used Fisher’s exact chi-square test)
Bolded if statistically significant at p<0.05
Reported symptoms and medication use related to depression did not differ between the groups (Table 2), but GDS scores were higher in the non-user groups. Use of memantine, a N-Methyl-D-aspartic acid-receptor antagonist approved only for moderate to severe AD dementia, was reported in a small subset of the MCI-AD participants (<2%), and was reported more frequently among the ChEI non-users (18.3%) than ChEI users (8.7%) in the ADdem group. Behavioral problems were reported more frequently in ChEI non-users of ADdem group (62.8%) than ChEI users (51.9%) at their initial visit, but upon initation of first ChEI use, both MCI-AD (47.2%) and ADdem (67.3%) group showed increased reports of behavioral problems (see Table, Supplementary Digital content 1, which outlines additional clinical symptoms of sample).
Baseline cognitive test scores significantly differed between ChEI users and ChEI non-users (see Table, Supplementary Digital content 6). At the initial visit, ChEI non-users scored worse than ChEI users on all cognitive measures in the ADdem group; ChEI non-users in the MCI-AD group performed worse than ChEI users on Digit Span Forward and Backward and the Boston Naming Test.
Comparisons in rates of cognitive decline before and after ChEI initiation
When we compared rate of cognitive decline before and after the initiation of ChEI in unadjusted analyses, there was an increase in the rate of decline after the initiation of drug (see Table, Supplementary Digital content 2, which shows results from Unadjusted annual change in scores in MCI-AD and AD dementia participants) in both MCI-AD and ADdem groups. After controlling for demographic differences (e.g., age, sex, education, race, APOE ε4 status), baseline cognition and symptoms (i.e., presence of behavior problems, GDS score), the annual increase in CDR-SB before ChEI initiation was 0.03 (95% CI: −0.03, 0.09) in MCI-AD, which increased to 0.61 (95% CI: 0.52, 0.69) after ChEI initiation (Table 3, Figure 1). Annual decline in MMSE scores before ChEI initiation in MCI-AD was −0.09 (95% CI −0.20, −0.01) which worsened to −0.68 (95% CI: −0.79, −0.58) after ChEI initiation. For ADdem group, the annual increase in CDR-SB before ChEI initiation was 0.30 (95%CI 0.24, 0.36), and increased to 1.26 (95% CI 1.20, 1.33) after initiation. In MMSE, rate of decline before initiation was −0.44 (95% CI −0.54, −0.34) and −1.58 (95% CI −1.68, −1.48) after initiation. This accelerated rate of cognitive decline after ChEI initiation was observed for almost all cognitive measures in the MCI-AD and ADdem groups, with the exception of Digit Span Backward in the MCI-AD group and Logical Memory Immediate, Delayed in the ADdem group
Table 3.
Adjusted annual change in scores in MCI-AD and AD dementia participants before and after begin taking cholinesterase inhibitors
| MCI-AD* | Mild AD dementia* | |||||
|---|---|---|---|---|---|---|
| Measure‡ | Before first ChEI use | After first ChEI use | Before first ChEI use | After first ChEI use | ||
| Annual score change (95% CI)† |
Annual score change (95% CI)† |
p-value | Annual score change (95% CI)† |
Annual score change (95% CI)† |
p-value | |
| CDR-SB |
0.03 (−0.03, 0.09) |
0.61 (0.52, 0.69) |
<0.0001 |
0.30 (0.24, 0.36) |
1.40 (1.31, 1.49) |
<0.0001 |
| Mini Mental State Exam |
−0.09 (−0.19, 0.02) |
−0.68 (−0.79, −0.58) |
<0.0001 |
−0.44 (−0.54, −0.34) |
−1.58 (−1.68, −1.48) |
<0.0001 |
| Logical Memory Immediate |
−0.04 (−0.23, 0.16) |
−0.56 (−0.67, −0.45) |
<0.0001 |
−0.60 (−0.74, −0.47) |
−0.76 (−0.82, −0.71) |
0.06 |
| Logical Memory Delayed |
−0.04 (−0.29, 0.22) |
−0.54 (−0.65, −0.42) |
0.0001 | −0.53 (−0.68, −0.39) |
−0.54 (−0.61, −0.48) |
0.72 |
| Digit Span Forward |
−0.07 (−0.15, 0.01) |
−0.16 (−0.20, −0.12) |
0.03 | −0.05 (−0.11, 0.01) |
−0.23 (−0.26, −0.19) |
<0.0001 |
| Digit Span Backward | −0.13 (−0.19, −0.06) |
−0.18 (−0.23, −0.14) |
0.16 |
−0.15 (−0.22, −0.09) |
−0.34 (−0.37, −0.31) |
<0.0001 |
| Animals |
−0.45 (−0.68, −0.23) |
−0.81 (−0.93, −0.68) |
0.002 |
−0.70 (−0.85, −0.54) |
−1.21 (−1.29, −1.13) |
<0.0001 |
| Vegetables |
−0.26 (−0.40, −0.11) |
−0.67 (−0.77, −0.57) |
<0.0001 |
−0.62 (−0.72, −0.52) |
−0.87 (−0.93, −0.81) |
<0.0001 |
| Boston Naming Test |
−0.12 (−0.25, 0.01) |
−0.66 (−0. 79, −0.52) |
<0.0001 |
−0.62 (−0.77, −0.46) |
−1.46 (−1.59, −1.33) |
<0.0001 |
| Trail Making Part A |
0.09 (−0.55, 0.73) |
2.50 (1.89, 3.11) |
<0.0001 |
2.23 (1.43, 3.04) |
7.72 (7.06, 8.39) |
<0.0001 |
| Trail Making Part B |
1.41 (−1.36, 4.19) |
11.08 (9.20, 12.97) |
<0.0001 |
11.50 (8.70, 14.29) |
17.61 (16.14, 19.07) |
<0.0001 |
| Digit Symbol |
−0.55 (−0.80, −0.29) |
−1.53 (−1.78, −1.29) |
<0.0001 |
−1.66 (−1.98, −1.33) |
−3.08 (−3.29, −2.87) |
<0.0001 |
Abbreviations: MCI-AD = Mild cognitive impairment with primary etiologic diagnosis of Alzheimer’s disease; AD = Alzheimer’s disease; ChEI = cholinesterase inhibitor; CDR-SB = Clinical Dementia Rating Sum of Boxes; CI = Confidence Interval
Diagnosis at time of first ChEI use among ChEI users or at Initial Visit for never users
Controlling for age, sex, education (years), race (White, African American, other), presence of ≥1 APOE e4 allele, behavioral problems, Geriatric Depression Scale score at first visit reported taking ChEI
Higher test score equates to a better score for all tests, except CDR-SB, Trail Making Part A, Trail Making Part B in which lower score is better score
Bolded if statistically significant at p<0.05
Figure1.
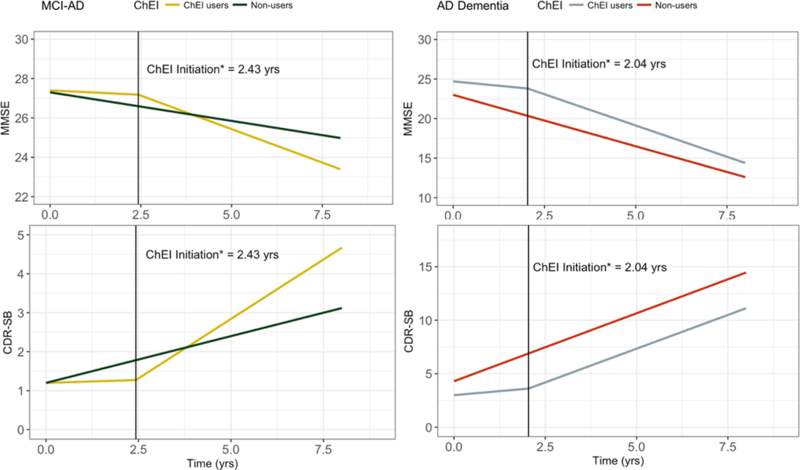
Annual rates of cognitive decline in CDR-SB and MMSE in MCI-AD and AD dementia participants of cholinesterase inhibitor users and non-users.
Vertical lines indicate inflection point of ChEI initiation in ChEI users; intercepts of y-axis reflect average scores at the baseline.
*ChEI initiation refers to average time in years to ChEI initiation from first visit
In a sensitivity analysis (see Table, Supplementary Digital content 3, which shows results from sensitivity analysis in adjusted annual change in CDR-SB in MCI-AD and AD dementia participants before and after begin taking cholinesterase inhibitors, restricting to two years before and after first ChEI use), we focused on the annual change in CDR-SB scores, restricted the analysis to two years before and after first ChEI use, and controlled for demographic differences (e.g., age, sex, education, race, APOE ε4 status), presence of behavior problems and GDS scores. In both MCI-AD and ADdem groups, the rate of cognitive decline increased significantly after ChEI initiation, showing a similar trend compared to the main analysis.
Comparisons in rates of cognitive decline between ChEI users and ChEI non-users
In both the MCI-AD and ADdem groups, compared to non-users, ChEI users after initiation of ChEI had faster decline after controlling for the demographics and baseline cognitive and behavioral symptoms (Table 4). ChEI users showed faster CDR-SB progression than non-users (MCI-AD: 0.24, 95% CI: 0.19, 0.30; ADdem: 1.27, 95% CI: 1.12, 1.43). Similar findings were observed for all of the other measures in both MCI-AD and ADdem groups except for Vegetable naming and Trail making B in ADdem groups. However, when we compared the non-users to ChEI users before initiation, Logical Memory Immediate, Delayed, and Animal naming showed faster decline in ChEI users of MCI-AD groups. In ADdem groups, CDR-SB, MMSE, Boston naming test and Trail Making A showed slower decline than non-users, but faster decline in Logical Memory Immediate and Delayed.
Table 4.
Comparing adjusted annual change in scores over entire follow-up in MCI-AD and AD dementia participants in cholinesterase inhibitor users and non-users
| MCI-AD* | Mild AD dementia* | |||||
|---|---|---|---|---|---|---|
| Measure‡ | Never used ChEI |
Never used ChEI versus Took ChEI – before first ChEI use |
Never used ChEI versus Took ChEI – after first ChEI use |
Never used ChEI |
Never used ChEI versus Took ChEI – before first ChEI use |
Never used ChEI versus Took ChEI – after first ChEI use |
| Annual score change (95% CI) † |
p-value | p-value | Annual score change (95% CI) † |
p-value | p-value | |
| CDR-SB |
0.24 (0.19, 0.30) |
0.28 | <0.0001 |
1.27 (1.12, 1.43) |
<0.0001 | 0.003 |
| Mini Mental State Exam |
−0.29 (−0.37, −0.20) |
0.74 | <0.0001 |
−1.30 (−1.49, −1.11) |
<0.0001 | <0.0001 |
| Logical Memory Immediate |
0.11 (0.00, 0.22) |
0.02 | <0.0001 |
−0.42 (−0.56, −0.29) |
0.02 | 0.003 |
| Logical Memory Delayed |
0.25 (0.13, 0.36) |
0.0003 | <0.0001 |
−0.14 (−0.27, −0.01) |
0.0002 | 0.05 |
| Digit Span Forward | −0.04 (−0.08, 0.00) |
0.42 | 0.05 |
−0.13 (−0.19, −0.06) |
0.10 | 0.04 |
| Digit Span Backward | −0.03 (−0.07, 0.01) |
0.07 | 0.003 |
−0.14 (−0.20, −0.09) |
0.99 | 0.003 |
| Animals |
−0.27 (−0.38, −0.17) |
0.05 | <0.0001 |
−0.79 (−0.96, −0.62) |
0.78 | 0.006 |
| Vegetables |
−0.25 (−0.33, −0.17) |
0.35 | <0.0001 |
−0.66 (−0.79, −0.53) |
0.19 | 0.10 |
| Boston Naming Test | −0.09 (−0.18, 0.01) |
0.18 | <0.0001 |
−1.16 (−1.40, −0.91) |
0.005 | 0.0001 |
| Trail Making Part A |
0.93 (0.41, 1.46) |
0.10 | <0.0001 |
5.80 (4.52, 7.08) |
0.0003 | 0.003 |
| Trail Making Part B |
4.19 (2.54, 5.85) |
0.91 | <0.0001 |
11.66 (8.02, 15.29) |
0.19 | 0.19 |
| Digit Symbol |
−0.42 (−0.67, −0.16) |
0.19 | <0.0001 |
−2.08 (−2.54, −1.62) |
0.01 | 0.0005 |
Abbreviations: MCI-AD = Mild cognitive impairment with primary etiologic diagnosis of Alzheimer’s disease; AD = Alzheimer’s disease; ChEI = cholinesterase inhibitor; CDR-SB = Clinical Dementia Rating Sum of Boxes; CI = Confidence Interval
Diagnosis at time of first ChEI use among ChEI users or at Initial Visit for never users
Controlling for age, sex, education (years), race (White, African American, other), presence of ≥1 APOE e4 allele, behavioral problems, Geriatric Depression Scale score at first visit reported taking ChEI
Higher test score equates to a better score for all tests, except CDR-SB, Trail Making Part A, Trail Making Part B in which lower score is better score
Bolded if statistically significant at p<0.05
In post-hoc analyses, the adjusted analyses for Tables 3 and 4 were repeated additionally controlling for age of onset of cognitive decline and history of diabetes, stroke, and cardiovascular disease, to control for additional factors that may influence differences in cognitive decline between the groups (Supplementary Digital Content 4 and 5). The overall conclusions remained unchanged.
Discussion
Although ChEI drugs are the major symptomatic therapy for AD, their efficacy in MCI-AD is uncertain. Fewer than half (34%) of MCI-AD participants reported taking ChEIs, as would be expected given the lack of specific recommendations for the drug use at the MCI stage. Seventy-two percent of ADdem participants reported taking ChEIs; although we did not assess reasons for not taking ChEIs, these may include personal preferences, costs, or contraindication due to side effects. In this study, we aimed to determine whether use of ChEI drugs benefit MCI-AD individuals. We compared the rate of cognitive decline prior to and after the initiation of ChEIs and also compared the overall course of decline of ChEI users and non-users.
We found that initiation of ChEIs seemed to be associated with aggravated cognitive decline. This unexpected observation was consistent across all cognitive outcome measures used in the study in both MCI-AD and in mild AD dementia. Despite demonstrated efficacy of ChEIs in mild-moderate AD dementia (ie, a broader range of dementia severity)1–3, we found significant acceleration in rate of cognitive decline after the initiation of ChEIs in both MCI-AD and mild AD dementia.
It is possible that our observations reflect the natural course of decline in ADdem and MCI-AD, the earliest symptomatic stages of AD, whereas rate of cognitive decline is known to accelerate with disease progression30–32. The rate of pre-treatment decline in our study is faster in ADdem group than the MCI-AD group, and the rate of decline after ChEI initiation in the MCI-AD group lies between the pre-treatments decline of MCI-AD and ADdem groups, suggesting that the measured rate of cognitive decline rate in these early symptomatic groups may be affected more by inherent disease progression itself rather than by ChEI treatment.
Another possibility is that the decision to initiate ChEI therapy was made at the inflection point where greater cognitive decline was perceived. Although the baseline cognitive test scores were comparable between ChEI users and non-users in the MCI-AD group, and were significantly higher in the ChEI users of ADdem group (see Table, Supplementary Digital content 6, which shows the unadjusted mean scores at initial visit), at the time of ChEI initiation the cognitive scores of ChEI users in both the MCI-AD and ADdem groups were lower than those of non-users at baseline (see Table, Supplementary Digital content 7, which shows the unadjusted mean scores at first visit reporting ChEI use among ChEI users). If this greater decline was recognized by the individual’s physician, it may have prompted initiation of ChEIs in an effort to slow disease progression.
It also is possible that individuals who were not prescribed ChEIs had a perceived slower rate of progression or suspicion of non-AD etiology, hence discouraging the use of ChEIs. There were trends of higher burden of AD pathology in ChEI users in the ADdem group (ADNP: 86.5% in ChEI users, 76.1% in non-users, p =0.05), which implies that non-users of ChEI included more non-AD etiologies. Although non-significant, this trend was also seen in the MCI-AD group (ADNP: 72.7% in ChEI users, 65.2% in non-users, p =0.55). Although the neuropathological data were drawn from small sample sizes, this suggest that non-users more often had non-AD dementing disorders, which also may have factored into the non-use of ChEIs. In this regard, ChEI non-users in the MCI-AD group showed improved longitudinal performance on the Logical Memory immediate and delayed measures, an unlikely result for the Logical Memory test33 when the underlying disorder is AD.
However, the immediate change from the two years before ChEI initiation showed an average increase of 0.03 per year on CDR-SB (see Table, Supplementary Digital content 3), yet ChEI was prescribed to participants. Among factors that may have played a role in the decision to initiate ChEIs, informants may have reported perceived greater memory decline or presence of behavioral problems between initial visit and at the first ChEI use (see Table, Supplementary Digital content 1). Whether this or other factors influenced treatment decisions warrant further investigation.
We noted some use of memantine in the MCI-AD and ADdem participants despite a lack of efficacy for this drug at these early symptomatic stages. We also noted a lower rate of ChEI use in African American participants in our sample compared with Caucasians, consistent with recent Medicare data34 showing health disparities for therapeutic interventions for African-Americans.
We observed overall slower cognitive decline of the study cohort, both in treatment and non-treatment groups compared to prior untreated cohorts. Previous cohorts from pre-ChEI era in 1990s have shown average rate of decline of 3 points per year on the MMSE35, whereas observations from recent studies align with our study, showing slow decline in ChEI treated patients36,37. It may be possible that previous untreated cohorts were mixed population of AD dementia and non-AD dementia, of which AD dementia patients were treated, and for those who did not decline remained as the untreated cohort.
Alternatively, the patient population may have changed over the past few decades, and the change in rate of decline may not be related to use of ChEIs. For example, the most recent cohort from Framingham studies showed decreased incidence of dementia38 regardless of decreased incidence of stroke, atrial fibrillation and heart failure. This indicates the relevance of cardiovascular risk factors with dementia; although results from multi-modality intervention trials have mixed findings39,40, improved control of major cardiovascular risk factors such as hypertension, diabetes mellitus, and hyperlipidemia may have affected overall course of cognitive decline in general population.
Although our results appear to contradict the symptomatic benefit for ChEIs demonstrated in previous clinical trials, trials1–3 had a treatment duration of less than 52 weeks. One trial with a treatment duration of 3 years36 showed no clear efficacy of the ChEI drug compared with the placebo arm.
A major limitation of our findings is that they derive from a retrospective cohort study. Interpretation of results are limited to possible correlations and associations. We tried to overcome this restriction by implementing models that show drug effects prior and after ChEI initiation; however, the factors leading to a physician’s decision regarding drug therapy were not available for analysis and thus it is unknown whether perceived underlying disease progression triggered the initiation of ChEIs versus ChEI drugs themselves contributing to worsening cognitive decline. In addition, neuropathology data were missing for a large majority of our sample, so that we could not restrict the study to participants with confirmed AD neuropathology nor could we evaluate the possible effect of co-pathologies.
Although our study does not support the use of ChEI drugs in MCI-AD or mild ADdem, we were unable to control for various factors that may have influenced our findings. Randomized clinical trials are needed to evaluate the efficacy of ChEI use in the prodromal stage of symptomatic AD (MCI due to AD and mild AD dementia).
Supplementary Material
Acknowledgements:
Lena McCue assisted in statistical analysis consultation.
The NACC database is funded by NIA/NIH Grant U01 AG016976 (PI Walter Kukull, PhD). NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John C. Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD,PhD).
Dr Morris reports receiving research support from grants P50AG005681, P01AG003991, P01AG026276, and UF01AG032438 from the NIH. Dr. Kukull reports receiving grants U01 AG016976 from the NIH.
Footnotes
Conflict of Interests
No other disclosures were reported.
References
- 1.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50(1):136–145. http://www.ncbi.nlm.nih.gov/pubmed/9443470. [DOI] [PubMed] [Google Scholar]
- 2.Farlow M, Anand R, Messina J Jr, Hartman R, Veach J. A 52-Week Study of the Efficacy of Rivastigmine in Patients with Mild to Moderately Severe Alzheimer’s Disease. Eur Neurol. 2000;44(4):236–241. doi: 10.1159/000008243. [DOI] [PubMed] [Google Scholar]
- 3.Tariot PN, Solomon PR, Morris JC, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology. 2000;54(12):2269–2276. doi: 10.1212/WNL.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 4.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. doi: 10.1212/01.WNL.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 6.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 7.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70(22):2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 9.Peters O, Lorenz D, Fesche A, et al. A combination of galantamine and memantine modifies cognitive function in subjects with amnestic MCI. J Nutr Health Aging. 2012;16(6):544–548. http://www.ncbi.nlm.nih.gov/pubmed/22659994. Accessed September 6, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9(9):CD009132. doi: 10.1002/14651858.CD009132.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies P, Maloney AJF. Selective Loss of Central Cholinergic Neurons in Alzheimer’s Disease. Lancet. 1976;308(8000):1403. doi: 10.1016/S0140-6736(76)91936-X. [DOI] [PubMed] [Google Scholar]
- 12.Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. J Neurol Sci. 1977;34(2):247–265. doi: 10.1016/0022-510X(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 13.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51(2):145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Chupin M, Hampel H, et al. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer’s disease. Alzheimer’s Dement. 2015;11(9):1041–1049. doi: 10.1016/j.jalz.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JS, Karlawish JH, Uhlmann WR, Petersen RC, Green RC. Mild cognitive impairment in clinical care: A survey of American Academy of Neurology members. Neurology. 2010;75(5):425–431. doi: 10.1212/WNL.0b013e3181eb5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data From Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 17.Beekley DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) Database: The Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 20.Aisen PS, Cummings J, Jack CR, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. 2017;9(1):60. doi: 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1–2):165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 22.Berg L, Miller JP, Storandt M, et al. Mild senile dementia of the alzheimer type: 2. Longitudinal assessment. Ann Neurol. 1988;23(5):477–484. doi: 10.1002/ana.410230509. [DOI] [PubMed] [Google Scholar]
- 23.National Alzheimer’s Coordinating Center. Coding Guidebook. Neuropathol Data Set Coding Guid. 2014;(January). [Google Scholar]
- 24.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thal DR, Rub U, Orantes M, Braak H. Phases of Abeta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Intergovernmental Panel on Climate Change, ed. Neurology. 1991;41(4):479–486. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Laird NM, Ware JH. Random-Effects Models for Longitudinal Data. Biometrics. 1982;38(4):963–974. doi: 10.2307/2529876. [DOI] [PubMed] [Google Scholar]
- 29.Inc SI. SAS/STAT® 9.2 User’s Guide, Second Edition. 2009.
- 30.Morris JC, Edland S, Clark C, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43(12):2457–2465. doi: 10.1212/WNL.43.12.2457. [DOI] [PubMed] [Google Scholar]
- 31.Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer’s disease: Measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994;151:390–396. [DOI] [PubMed] [Google Scholar]
- 32.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59(7):1034–1041. doi: 10.1212/WNL.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 34.Koller D, Hua T, Bynum JPW. Treatment Patterns with Antidementia Drugs in the United States: Medicare Cohort Study. J Am Geriatr Soc. 2016;64(8):1540–1548. doi: 10.1111/jgs.14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA. Modelling mini mental state examination changes in Alzheimer’s disease. Stat Med. 2000;19(11–12):1607–1616. http://www.ncbi.nlm.nih.gov/pubmed/10844722. Accessed March 26, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet (London, England). 2004;363(9427):2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 37.Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R. Cognitive performance in Alzheimer’s disease patients receiving rivastigmine for up to 5 years. Int J Clin Pract. 2005;59(4):473–477. doi: 10.1111/j.1368-5031.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 38.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 40.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


