Abstract
Current theory supports that the numerous functional areas of the cerebral cortex are organized and function as a network. Using connectional databases and computational approaches, the cerebral network has been demonstrated to exhibit a hierarchical structure composed of areas, clusters and, ultimately, hubs. Hubs are highly connected, higher-order regions that also facilitate communication between different sensory modalities. One region computationally identified network hub is the visual area of the Anterior Ectosylvian Sulcal cortex (AESc) of the cat. The Anterior Ectosylvian Visual area (AEV) is but one component of the AESc that also includes the auditory (Field of the Anterior Ectosylvian Sulcus - FAES) and somatosensory (Fourth somatosensory representation - SIV). To better understand the nature of cortical network hubs, the present report reviews the biological features of the AESc. Within the AESc, each area has extensive external cortical connections as well as among one another. Each of these core representations is separated by a transition zone characterized by bimodal neurons that share sensory properties of both adjoining core areas. Finally, core and transition zones are underlain by a continuous sheet of layer 5 neurons that project to common output structures. Altogether, these shared properties suggest that the collective AESc region represents a multiple sensory/multisensory cortical network hub. Ultimately, such an interconnected, composite structure adds complexity and biological detail to the understanding of cortical network hubs and their function in cortical processing.
Keywords: Multisensory, areal connectivity, cortical hierarchy, brain networks, visual, auditory, somatosensory cortical representations, superior colliculus
Introduction
For the brain to generate a unified perception of the environment, it must combine multiple streams of information originating from the different sensory organs. For that to occur, projections of different sensory systems must converge within the central nervous system. Specifically, multisensory convergence occurs when inputs from two (or more) different sensory modalities target the same neuron. This connectional convergence onto individual neurons functionally results in multisensory integration. These multisensory principles were initially described for neurons in the deep layers of the superior colliculus (SC; for review, see Stein and Meredith, 1993; see also Multisensory Nomenclature and Definitions, below). Since that monograph, multisensory neurons have also been identified in numerous cortical regions (e.g., Benevento et al., 1977; Berman, 1961; Bruce et al., 1981; Hikosaka et al., 1988; Wallace et al.,1992; Jiang et al., 1994a,b; Duhamel et al., 1998; Graziano et al.,1999; Bremmer et al., 2002; Yaka et al., 2002; Schlack et al., 2005; Russ et al., 2006; Avillac et al., 2007; Clemo et al., 2007; Romanski, 2007; Cohen 2009; Foxworthy et al., 2013a,b). However, unlike the spatially overlapping representations of different sensory modalities within the superior colliculus, the functional regions of cortex are largely segregated by sensory modality (e.g., the occipital lobe is mostly visual, etc.). How the connectivity of cortical regions contributes to sensory processing that ultimately underlies perception has been the subject of a vast number of studies, the overwhelming majority of which examined a specific sensory system (e.g., vision). Among those findings are the observations that the complexity of processing (for example: receptive field organization) tends to progressively increase as one ascends through the processing hierarchy. Also, the laminar distribution of connected elements is a structural indicator of regional hierarchical relationships (for review, see Felleman & Van Essen, 1991). These (and many other) organizational features of cortex have been critical for revealing the linkage of multiple cortical regions into large-scale, functionally specialized networks (Borra and Luppino, 2016; Catani et al., 2012).
The connectional features of cortical networks have been evaluated using several large studies of cat and primate cortical organization and connectivity (Scannell and Young, 1993; Scannell et al., 1995; Hilgetag et al., 2000; Hilgetag and Kaiser, 2004). Computational evaluation of these data sets has revealed that many cortical areas are interconnected and are grouped into clusters largely by dominant sensory modality (depicted schematically in Figure 1). In addition, these studies identified other cortical areas which were designated as network hubs that were distinguished by their location high in the cortical hierarchy, their high levels of connectivity (up to 60% of the entire network), and their ability to facilitate communication between clusters representing different sensory modalities (Zemanová et al., 2006; Sporns et al., 2007; Zhou et al., 2006; Hagmann et al., 2008; Zamora-Lopez et al., 2009; 2010). In a computational study using corticocortical data from the cat (from Scannell and Young, 1993; Scannell et al., 1995), Zamora-Lopez et al., (2010) specifically identified 11 multisensory cortical hubs (see Figure 6B, Zamora-Lopez et al., 2010) that formed a highly interconnected module of their own.
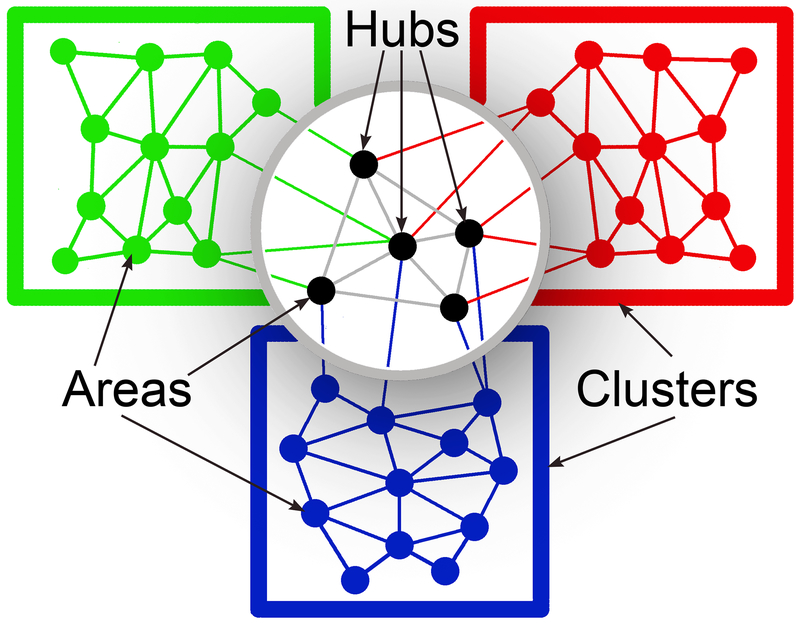
Figure 1.
In the cerebral cortex, functional regions or areas (small, filled circles) interconnect with one another to form clusters (colored rectangles), usually of representations of the same sensory modality. Areas that that are high in the cortical hierarchy (highlighted above the cast shadow) and provide communication between areas representing different sensory modalities (converging lines of different colors) are regarded as hubs (black). Redrawn and adapted from Figure 9C, Zamora-Lopez et al., 2010.
One such network hub was identified as the “AES” that is hierarchically located at the top of the cluster of visual cortical areas (Zamora-Lopez et al., 2010). This term (“AES”), however, is a misnomer because numerous publications that explicitly examined the region identified it as the Anterior Ectosylvian Visual area (AEV or EVA) (Mucke et al., 1982; Norita et al., 1986; Olson & Graybiel, 1987; Grant & Shipp, 1991; Carriere et al., 2007; Meredith et al., 2017). In addition, since the establishment of the cat cortico-cortical data base (from Scannell and Young, 1993; Scannell et al., 1995) used in the computational analyses, a considerable amount of new information has been uncovered that provides a more comprehensive understanding of not just the AEV, but the entire cortical region of the Anterior Ectosylvian Sulcal cortex (AESc) and its connections and complex neurological features, as detailed below. First, however, because a requisite property of a cortical hub is for it to facilitate multisensory processing (Zamora-Lopez et al., 2010), it is important to define the multisensory terminology that will be used herein.
Multisensory nomenclature and definitions
The first neurons identified as “multisensory” (Horn & Hill, 1966) were activated (i.e., generated action potentials) by stimuli from more than one sensory modality, and were described as “bimodal” (activated by two different sensory modalities) or “trimodal” (activated by three sensory modalities). With further study, other neurons were identified that were activated by only one sensory modality, but these spiking responses were significantly modulated by stimuli from a different sensory modality. Such neurons met the definition for being multisensory, since they could be influenced by stimulation in more than one sensory modality. These neurons, first recognized in the AESc, were designated “subthreshold” multisensory neurons (Dehner et al., 2004) and neurons showing similar modulatory characteristics have now been identified in many other cortical regions as well (e.g., Barraclough et al. 2005; Meredith et al., 2006; Sugihara et al. 2006; Allman and Meredith, 2007; Bizley et al. 2007; Carriere et al. 2007; Clemo et al., 2007; Clemo et al. 2008; Meredith and Allman, 2009; Iurilli et al., 2012; Foxworthy et al., 2013a,b; Oclese et al., 2013; Meredith and Allman, 2015). Significant biophysical differences have been reported between bimodal and subthreshold forms of multisensory neurons (Foxworthy et al., 2013a; Meredith and Allman, 2015), and cortical areas have been quantitatively described as containing differing proportions of unisensory, bimodal and subthreshold multisensory neurons (Allman et al., 2009; Meredith et al., 2011). Although association cortices have traditionally been regarded as “multisensory (or polysensory)” regions, numerous recent studies have identified multisensory neurons in lower level cortices (e.g., Bizley et al., 2007; Ghanzafar and Schroeder, 2006). However, a cortical area that exhibits some multisensory properties is not necessarily the same as a cortex designated as multisensory. Also, some cortices show evidence of parallel processing streams for multisensory and unisensory signals (Foxworthy et al., 2013b). Thus, a robust definition of a “multisensory cortex” is not currently available and such terminology will be avoided here.
For all multisensory neurons identified thus far, synaptic inputs from different sensory modalities must meet on the same neuronal membrane for their post-synaptic effects to have the potential to influence one another. This fundamental connectional feature, defined as multisensory convergence, is the necessary prerequisite for the multisensory processing that results. Numerous techniques have been used to measure and quantify multisensory processing, but one method for evaluating the multisensory performance of single neurons (e.g., see Meredith & Stein, 1983) involves the comparison of spike counts of responses evoked by different sensory conditions. When a neuronal response to combined-sensory stimulation (multisensory) is significantly changed from its response to the most effective individual stimulus (unisensory), the response is defined as representing multisensory integration. Integrated responses that represent increases in activity are termed “enhanced” while those representing decreases are described as “depressed.” Ultimately, these different integrative effects, which can occur on the same neuron, are governed by the location, relative timing and effectiveness of the stimuli (Meredith and Stein, 1986; Meredith et al. 1987; Perrault et al., 2005). For further details about quantifying multisensory integration, see Stevenson et al., (2014).
Sensory areas of the AESc
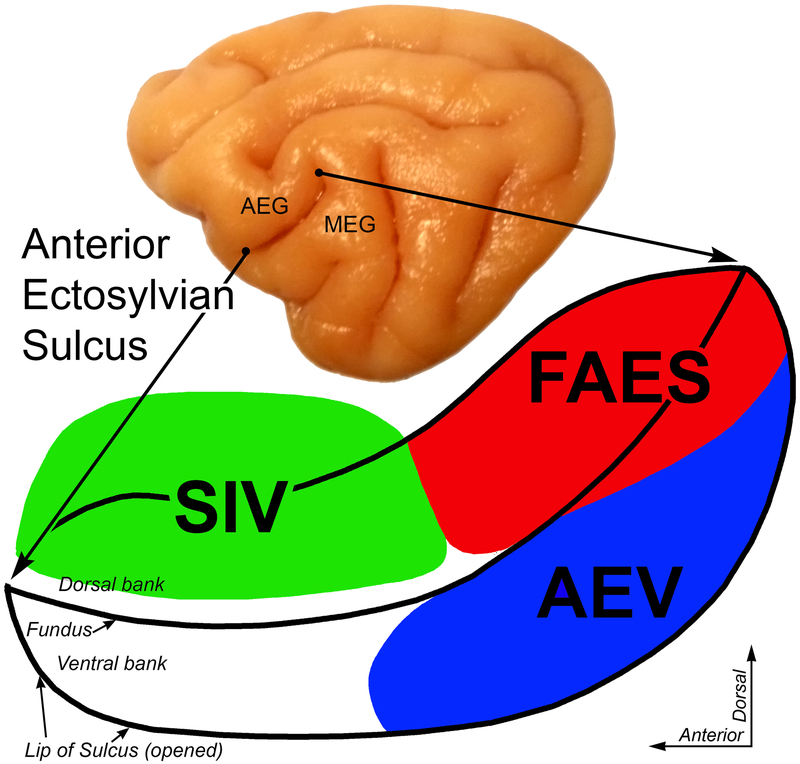
On a lateral view of the cat cerebral hemisphere (Figure 2-top), the Anterior Ectosylvian Sulcus separates the Anterior and Middle Ectosylvian Gyri. This sulcus also occurs at the junction of the feline temporal (inferiorly) and parietal (posterior-superior) lobes. When the lips of the sulcus are separated (Figure 2-bottom), the upper bank of the sulcus contains the Fourth Somatosensory representation (SIV) and the auditory Field of the Anterior Ectosylvian Sulcus (FAES). On the ventral bank of the AES, the posterior two-thirds contains the visual representation known as the anterior ectosylvian visual area, or AEV (also referred to by some authors as AESv or EVA).
Figure 2.
Location of the Anterior Ectosylvian Sulcal cortex (AESc) and its multiple core sensory representations. On the lateral view of the cat cerebral hemisphere (top), the Anterior Ectosylvian Gyrus (AEG) and Middle Ectosylvian Gyrus (MED) are separated by the Anterior Ectosylvian Sulcus. This sulcus is expanded and opened (bottom) to reveal, on the dorsal bank, the fourth somatosensory representation (SIV-colored green) and the auditory field of the AES (FAES-colored red); on the opened ventral bank is located the anterior ectosylvian visual area (AEV-colored blue).
Core visual area AEV
Although no global, retinotopic representation has been identified in AEV, visually responsive neurons are binocular, movement selective, and sensitive to large moving contrasts (Minciacchi et al., 1987). Visual receptive fields are relatively large, with average diameters of >60°, with the greatest representation devoted to the central visual field. Visual responses in AEV are tuned to detection of pattern motion, such as the direction of drifting gratings (Nagy et al., 2003) or plaid patterns made by superimposing two differently oriented gratings (Scannell et al., 1995). Encoding of such complex feature selectivity is indicative of higher-order motion processing similar to that observed for the primate area MT (Movshon et al., 1985; Rodman and Albright, 1989). Other studies have suggested a role for the AEV in the initiation of centering eye movements (Tamai et al., 1989; Tamai and Kimura, 1996). However, although the AEV is strongly connected with the superior colliculus, and deactivation of the AEV significantly affects visual activity in the SC (Wallace et al., 1993), deactivation of AEV failed to the produce deficits in visual localization and orienting behaviors (Lomber and Payne, 2004).
The AEV is a typical, six-layered neocortex that exhibits a modest layer 4 in which stellate (granule) cells and sublamination have not been observed. The laminar distribution of inhibitory neuronal cell types is consistent with that of neocortex in general (Clemo et al. 2003). Cortical inputs to the AEV, as summarized in Figure 3A, largely arise from the ipsilateral lateral suprasylvian visual areas (Meredith et al., 2017) although other reports also include Area 20 and Area 21 (Mucke et al., 1982; Miceli et al., 1985; Reinoso-Suarez and Roda, 1985; Norita et al., 1986; Olson and Graybiel, 1987; Grant and Shipp, 1991). In addition, ipsilateral projections from auditory cortices A2, IN, FAES and A1/AAF represent 20.1% of total inputs to AEV while those from ipsilateral somatosensory areas SII and SIV constitute another 15.7% (Meredith et al., 2017). Therefore, although AEV functionally is a visually dominant area, over 35% of its cortical inputs are derived from non-visual sources. In addition to these cortical inputs, thalamic inputs to the AEV arrive from non-primary and multisensory nuclei such as the LP-Pulvinar, Po and LM-Sg (Heath and Jones, 1971; Graybiel, 1973; Graybiel and Berson, 1981; Bentivoglio et al., 1983; Roda and Reinsos-Suarez, 1983; Meredith et al., 2017). Output targets of the AEV connect with motor (McNair and Avendano, 1980), prefrontal (Cavada and Reinoso-Suarez, 1981) granular insular (Olson and Graybiel, 1987), and perirhinal cortex (Heath and Jones, 1971; Reinoso-Suarez and Roda, 1985; Olson and Graybiel, 1987) as well as the deep layers of the superior colliculus (Casagrande et al., 1972; Tortelly et al., 1980; Segal and Beckstead, 1984; 1989). On the basis of this connectivity and its functional properties, the AEV has been implicated in roles relating visual motion to motor and limbic system activity to effect orientation and alertness behaviors or to direct visual attention (Norita et al., 1986), although direct involvement with visual orienting behavior was not demonstrated (Lomber and Payne, 2004).
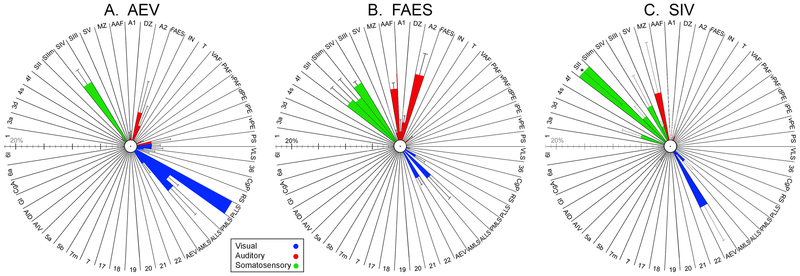
Figure 3.
Ipsilateral cortical projection sources to AESc core areas AEV (A), FAES (B) and SIV (C). These radial plots depict the percent of the total ipsilateral corticocortical projections to the target area where each bar represents mean (± se) percentage of projection proportion from the area designated at the edge of the column (see Table 1 for Abbreviations). Colors are indicative of modality of input source (green=somatosensory; blue=auditory; red=visual). Note that each area receives a unique pattern of inputs, but all receive inputs from multiple sensory modalities. Part (A) replotted from Meredith et al., (2017); Part (B) replotted from Meredith et al., (2016); Part (C) generated from unpublished analysis of tissue from Dehner et al., (2004). Asterisk (*) indicates off-scale value (37.5% ± 8.8).
Multisensory involvements of AEV
As stated above, the AEV receives considerable ipsilateral projections (~35%) from non-visual cortical areas as well as from the multisensory thalamic nuclei. Of those non-visual afferents to AEV, neighboring AESc regions of SIV and FAES contribute 13.1% and 3.7%, respectively (Meredith et al., 2017). Accordingly, a large proportion of AEV neurons functionally exhibit non-visual properties, of which 13% demonstrate bimodal/ trimodal multisensory properties while another 14% show subthreshold multisensory effects (Wallace et al., 2006; Carriere et al., 2007). In addition, studies of adjoining areas (e.g, FAES; Meredith and Allman, 2009) whose recording tracks passed into AEV, show evidence for bimodal neurons (usually visual-auditory) at the border between the two regions, (see Figure 1 of Meredith and Allman, 2009). Furthermore, restricted sensory experience during development has profound effects on AEV multisensory processing. Animals reared in darkness were found to have AEV neurons that showed significantly increased proportions of multisensory response depression (Carriere et al., 2007), while those raised with binocular lid suture became crossmodally reorganized to respond to auditory cues (Rauschecker and Korte, 1993). As also mentioned above, the AEV has extensive output projections to motor and multisensory areas, including the superior colliculus. These projections appear to play an important role in gating the integration of SC neurons, as deactivation of AEV eliminates multisensory response enhancement in superior colliculus neurons (Alvarado et al., 2007; 2009).
Core auditory area FAES
Auditory field AES (FAES), located within the dorsal-posterior portion of AES (see Figure 2), is situated posterior to the fourth somatosensory area (SIV) and dorsal to anterior ectosylvian visual area (AEV). The physical location of FAES, which is lateral to the anterior auditory field (AAF), as well as its physiological response properties are consistent with the FAES constituting part of the belt of auditory association cortices. Much of the FAES lies submerged deep to the middle ectosylvian gyrus where its grey matter surrounds the posterior remnant of the sulcus on its medial, dorsal and lateral aspects like an inverted “U” shape. The more anterior portions of the FAES are apparent in the dorsal bank of the AES as it emerges from its position deep to the middle ectosylvian gyrus and this portion shares a border with the antero-ventral aspects of the AAF. The FAES exhibits the 6-layer pattern typical of neocortex (Mellott et al., 2010), but layer 4 can be quite compressed (Meredith and Clemo, 1989). Within the FAES, the laminar distribution of inhibitory neuronal cell types is consistent with other areas of neocortex (Clemo et al. 2003).
Unlike the well-known tonotopic arrangement of AAF and A1, no such organization has been identified for the FAES (but see Las et al., 2008). Approximately 65% of FAES neurons respond exclusively to acoustic stimuli (Meredith and Allman, 2009) and usually prefer broadband noise, but can also respond to pure tones and generally exhibit broad tuning curves (Clarey and Irvine, 1986; 1990a). The majority of FAES neurons can be activated by monaural stimulation of either ear (Clarey and Irvine, 1986; Meredith and Clemo 1989; Jiang et al., 2000). When tested for spatial tuning acuity, FAES neurons have been reported as varying from having fairly narrow spatial tuning (Meredith and Clemo, 1989; Korte and Rauschecker, 1993) to responding to stimuli at all spatial locations (i.e., omnidirectional; Clarey and Irvine, 1986). Behaviorally, deactivation of FAES blocks orienting responses to contralateral acoustic stimuli (Malhotra et al., 2004; Malhotra and Lomber, 2007; Meredith et al., 2011), suggesting a role for FAES in auditory spatial localization.
The FAES receives strong ipsilateral corticocortical projections from other auditory cortical areas, predominantly areas A2 and AAF with smaller contributions from areas A1, DZ, PAF, IN and T. As illustrated in Figure 3B, the FAES has also been shown to receive inputs from non-auditory cortical regions, such as the ventral bank of the suprasylvian sulcus (corresponding with PMLS/PLLS visual regions), insular cortex, posterior rhinal sulcus, as well as somatosensory regions SIV and para-SIV (Reinoso-Suarez and Roda, 1984; Clarey and Irvine, 1990b; Meredith 2004; Meredith et al., 2016). In fact, of the total ipsilateral cortical projections to FAES, 18% arise from visual sources while 41% arise from somatosensory sources (Meredith et al., 2016). Thalamic inputs to the FAES arise from non-specific (i.e., non-lemniscal) nuclei including the suprageniculate nucleus, the posterior nuclear group, the pulvinar complex, and the principle division of the ventromedial nucleus (Roda and Reinoso-Suarez, 1983; Clarey and Irvine, 1990b; Meredith et al., 2016).
Multisensory involvements of FAES
As stated above, nearly 60% of cortical inputs to FAES arise from non-auditory sources, of which 13% of total connections arise from the adjoining SIV region and another 6.4% from AEV (Meredith et al., 2016). Such an input architecture provides the opportunity for substantial multisensory convergence, and indeed 17% of FAES neurons (Meredith et al., 2006; Meredith and Allman, 2009) are excited by stimulation from more than one sensory modality. These neurons include both auditory-visual and auditory-tactile types of bimodal neurons (Meredith et al., 2009). Another 14% of FAES neurons appear to be excited only by auditory cues, but those responses can be influenced by the presence of non-auditory stimuli (either visual or somatosensory) that are ineffective when presented alone and are termed ‘subthreshold’ multisensory neurons (Meredith et al., 2006; Meredith and Allman, 2009). For these subthreshold neurons, multisensory interactions were found to be modality dependent: whereas auditory responses were significantly facilitated by visual cues in some neurons, in others auditory responses were suppressed by somatosensory cues (Meredith et al., 2006). In summary, the FAES is composed of a mixed population of unisensory auditory neurons (~65%), bimodal multisensory neurons (~17%) and subthreshold multisensory neurons (~14%) (Meredith and Allman, 2009) and, in addition to processing auditory information, also shapes information flow through multisensory processing.
Core somatosensory Area SIV
The major somatosensory component of the AESc is the fourth somatosensory representation, area SIV, (Clemo and Stein, 1982; Clemo and Stein, 1983) as depicted in Figure 2. Activity in SIV is overwhelmingly driven by hair receptors on the contralateral body surface with only rare instances of inputs from skin and deep receptors. The receptive fields of SIV neurons are somatotopically organized such that the head is represented anteriorly and the tail/hindlimb regions are represented posteriorly, with forepaw/hindpaw representations extending dorsally onto the anterior ectosylvian gyrus, while the trunk and dorsal aspects of the body are represented ventrally, deep within the wall of the sulcus (Clemo and Stein, 1982; 1983). Unlike receptive fields in the primary somatosensory area, those of SIV neurons are consistently larger and generally include multiple vibrissae or multiple digits, or extend across a joint or multiple joints (Clemo and Stein, 1983). Somatosensory Area SIV is characterized by lamination typical of neocortex with a narrow, non-sublaminated layer 4 (Clemo and Stein, 1983). SIV has been cytoarchtectonically described (Clemo et al., 2003) and is easily identified by the row of large pyramidal neurons found in layer 5 (Clemo and Stein, 1983; Clemo et al., 2003). As summarized in Figure 3C, somatosensory activity in SIV is largely driven by inputs from ipsilateral somatosensory cortical areas SII (37%); SIII (8%) and SV (3%; calculated from tissue from Dehner et al., 2004) and from the suprageniculate (SG) and posterior (PO) thalamic regions (Burton and Kopf, 1984; McHaffie et al., 1988; Reinoso-Suarez and Roda, 1985). Inputs to SIV from non-somatosensory areas of cortex include visual areas AEV (12%) and ALLS (3.6%), auditory FAES (1.7%) as well as the auditory-somatosensory MS (9.5%; from Dehner et al., 2004). Inputs from non-somatosensory areas (see Fig. 3C) total ~30% of cortical inputs to SIV. Outputs from SIV have not been extensively examined, but include those to the somatosensory region SV (Clemo and Meredith, 2004), the multisensory areas of the rostral suprasylvian sulcus (Clemo et al., 2007) and to the auditory FAES (Burton & Kopf, 1984; Reinoso-Suarez and Roda, 1985; Dehner et al., 2004). The best characterized projections of SIV are to the deep layers of the superior colliculus (SC) (Clemo and Stein, 1984, 1986; McHaffie et al., 1988; Wallace et al., 1993). This corticotectal projection is topographic, such that neurons in SIV that connected with neurons in the SC exhibited receptive fields that spatially overlapped (Clemo and Stein, 1984). Furthermore, tactile responses in SC neurons were reduced or eliminated by deactivation of SIV, indicating that the SIV provides a robust excitatory input to SC neurons (Clemo and Stein, 1986). Given this strong relationship to the SC, it has been proposed that SIV plays an important role in controlling or modifying orienting behaviors to somatosensory stimulation (Clemo and Stein, 1986). Consistent with this notion, a preliminary study in awake cats showed that stimulation of this region of the AES elicited coordinated gaze shifts and contralateral reaching movements of the forepaw (Jiang and Guitton, 1995).
Multisensory involvements of SIV
As detailed above, nearly 30% of inputs to SIV arise from non-somatosensory cortical areas. Of those, over 13% originate in AESc areas of AEV (12%) and FAES (1.6%). However, numerous studies of SIV have documented the largely unisensory nature of neuronal responses to somatosensory stimulation, since bimodal multisensory neurons were rarely encountered (Clemo and Stein, 1982, 1983, 1984; Jiang et al., 1994a, b). Instead, when combined with auditory activation, approximately 66% of SIV neurons showed suppression of concurrent somatosensory responses (Dehner et al., 2004), falling into the “subthreshold” category of multisensory neuron described earlier.
Interconnections among core representations in AESc
Unlike many of the substrates underlying multisensory convergence and that involve long projections from distant cortical or subcortical regions, some of the multisensory properties of the AESc regions described above are the result of short connections between immediately adjacent sensory representations. Specifically, and as depicted in Figure 4, neurons in auditory FAES project to somatosensory SIV (Burton and Kopf, 1984; Reinoso-Suarez and Roda, 1985; Dehner et al., 2004). If these neurons were classic pyramidal projection neurons, which largely express the excitatory neurotransmitter glutamate, this crossmodal projection would be expected to be excitatory. However, auditory stimulation on its own was ineffective in inducing responses in SIV neurons. Instead, when combined tactile and auditory stimulation was used, significant suppression of the tactile responses was seen in 66% of the neurons (Dehner et al., 2004). This observation of crossmodal suppression suggested that a signal reversal must occur, possibly via local inhibitory interneurons within SIV (Clemo et al., 2003). Confirming this, when a GABA antagonist was applied to SIV, the suppressive effect of auditory stimulation on somatosensory responses was blocked. That crossmodal projections from auditory FAES connect with inhibitory interneurons in SIV was anatomically confirmed using confocal microscopy and immunostaining techniques (Keniston et al., 2010). Collectively, these observations support a short-distance crossmodal circuit whereby activation by one sensory modality (auditory) suppresses activity in another (somatosensory), as schematically illustrated in Figure 4. Likewise, a similar circuit has been identified in the reciprocal direction (also illustrated in Figure 4), whereby SIV or somatosensory activity suppresses ongoing auditory responses in FAES (Meredith et al., 2006). In this manner, SIV and FAES mutually suppress one another. Furthermore, although not yet demonstrated, putative inhibitory connections to output neurons (Fig. 3, dotted lines) could provide additional crossmodal effects that suppresses crossmodal input to the active area. Such short-distance connectivity has not been examined in AEV, although AEV receives considerable inputs from neighboring SIV (13.1%) and FAES (3.1%; Meredith et al., 2017) and subthreshold crossmodal suppression has been demonstrated in this AESc region (Carriere et al., 2007).
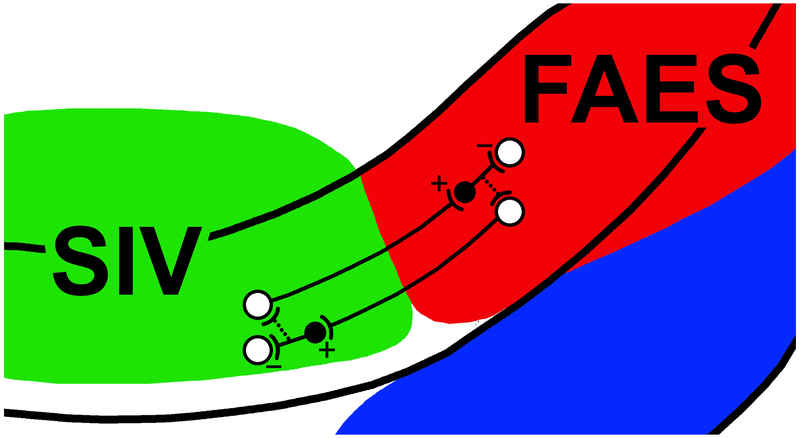
Figure 4.
Cross-modal suppression circuits between areas SIV and FAES. This schematic enlarges the dorsal bank of the AESc where somatosensory area SIV (green) and auditory FAES (red) reside. Within each area, some excitatory neurons (white circles, ‘+’) project to the other area to terminate on inhibitory interneurons (black circle). The inhibitory interneuron, in turn, synapses locally to inhibit (‘-’) activity. In this manner, stimulation of one modality/region can cross-modally suppress activity of another modality/region. Furthermore, putative inhibitory connections (dotted lines) with output neurons could provide an additional mechanism for activity in one area to suppress that of another. After Dehner et al., 2004; Meredith et al., 2006.
Summary of core representations in AESc:
From the brief review above, it is clear that the different sensory representations of the AESc show dominant modality-specific properties that are consistent with their identification and naming. Area AEV is predominantly visual in function, and shows higher-order features such as lack of visuotopy, large visual receptive fields and sensitivity to pattern motion. The area FAES is predominantly auditory in function, exhibits higher-order features such as a lack of tonotopy and broad frequency sensitivity, and is critical for orienting behaviors toward acoustic stimuli. Area SIV is essentially somatosensory in function and exhibits higher-order features such as large receptive fields that include multiple digits or body regions. However, each region also receives a high proportion of cross-modal inputs (AEV inputs from nonvisual areas = 35.7%; FAES inputs from non-auditory areas = 59%; SIV inputs from non-somatosensory areas = 30%), including those from other AESc regions, as well as from multisensory thalamic nuclei. Therefore, if the definition of a network hub is an area that is highly interconnected and provides communication between areal clusters representing different sensory modalities (Zemanová et al., 2006; Sporns et al., 2007; Zhou et al., 2006; Hagmann et al., 2008; Zampora-Lopez et al. 2009; 2010), then not just AEV but also FAES and SIV seem to meet that definition. Regarding the omission of FAES and SIV as network hubs from the cited cortical network studies, it seems likely that insufficient data was available for the modeling analysis to include them. Indeed, area FAES is not even listed as part of the cat cortical connectional data base (Scannell and Young, 1993; Scannell et al., 1995) used in the Zampora-Lopez et al. (2010) analysis. Alternatively, the concept of a cortical network hub may not be sufficiently inclusive of the current understanding of not just brain connectivity, but also of multisensory organization, as proposed in the following discussion.
Transitions between core representations: Borders or zones?
One open question regarding cortical multisensory organization is how one cortical representation transitions into another at the border between them. It is known that borders of representations within a given sensory area can be quite sharp. For example, the border between the representations of the forepaw and the lower jaw within rat S1 is very abrupt and has been functionally measured to be about 50-75um wide (Hickmott and Merzenich, 1998). The border between visual Area 17 and Area 18 is characterized by distinct cytoarchitectonic changes whose transition occurs within ~250 μm in cats (Payne, 1990) and ~500 μm in the opossum (Volchan et al., 1988). On the other hand, functional assessments of the core AESc areas indicate that transitions from one to another take place over an expanse, or ‘zone,’ as the dominance of one modality is gradually replaced by that of another. Perhaps best studied is the transition between somatosensory SIV (on the dorsal bank) and visual AEV (on the ventral bank of the AES). This transition occurs broadly across several millimeters of the fundus of the sulcus and has been named “Para-SIV” (Clemo and Stein, 1983; 1984). This fundic region is distinct from its somatotopically-organized neighbor (SIV) because Para-SIV lacks a somatotopic organization, its somatic receptive fields are very large and often bilateral, and can even represent discontinuous segments of the body. Moreover, neurons in Para-SIV often respond to both somatic and to visual stimulation (e.g., are bimodal) (Clemo and Stein, 1983; 1984). Likewise, evidence for a transition zone between auditory FAES and visual AEV is apparent in Figure 5. Here, unisensory responses clearly dominate within the core of each region, while bimodal neurons are increasingly prevalent in the intervening transition. This transition could be described as the Audiovisual zone (AVZ) of the AESc. The transition between SIV to FAES areas has not been systematically examined. Given its cortical location, the transition between SIV and FAES may represent a continuation of a Multisensory Zone (MZ) described between somatosensory SII and the anterior auditory field, where neurons with whole-body receptive fields were found in accompaniment with auditory responses (Burton et al., 1982). Ultimately for each of the transition regions (Para-SIV, AVZ and MZ), there seems to be a consistent trend that bimodal neurons occur in higher proportions at the transitions between all adjoining sensory representations in the AESc, as documented in Figure 6 (from Meredith, 2004).
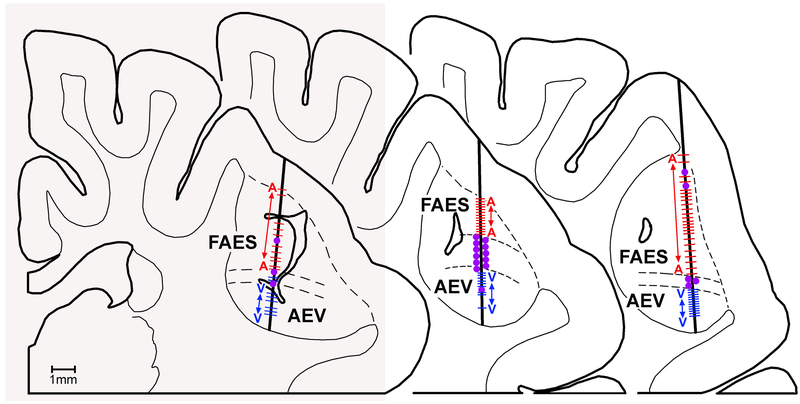
Figure 5.
These three coronal sections through the AESc show histologically reconstructed recording penetrations (thick vertical lines) where single-unit activity was sampled approximately every 100-200 μm in depth. In each example unisensory auditory responses (A-red dashes) predominate in the FAES, while unisensory visual responses (V-blue dashes) predominate in the AEV. The large purple dots indicate the location of bimodal (AV) neurons which primarily occur at the transition zone (between parallel dashed lines) between the FAES and AEV. Redrawn from Meredith and Allman, 2009.
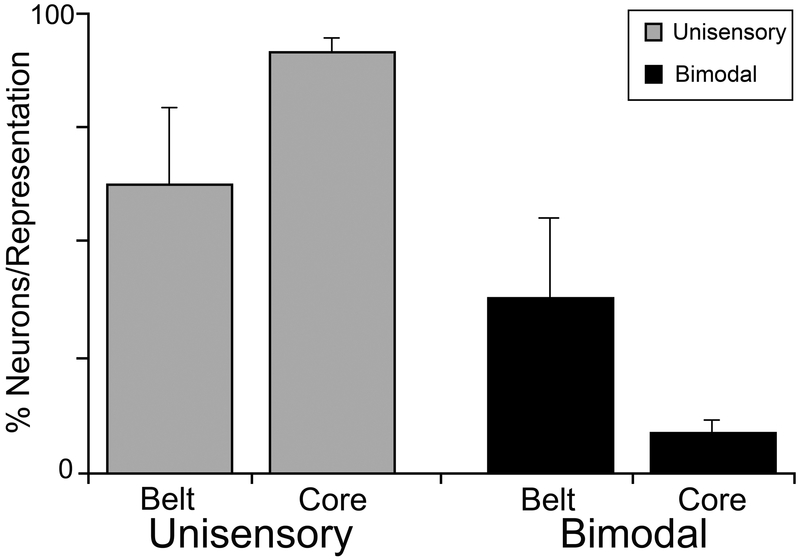
Figure 6.
Bimodal neurons primarily occur at the transition zones between the core modality-specific representations in the AESc. Data (mean ± standard deviation) are derived from histological reconstructions of recording penetrations through AESc with the location of each neuron (unisensory or bimodal) categorized as being either inside (core) or between (transition zone) the three different regions of the AESc. Significant differences (X2, p<0.01) were observed for the distribution of unisensory and bimodal neurons across the core/transition zone arrangement of the AESc. Unisensory values sum >100% because values for core and for transition zones were calculated separately. From Meredith, 2004.
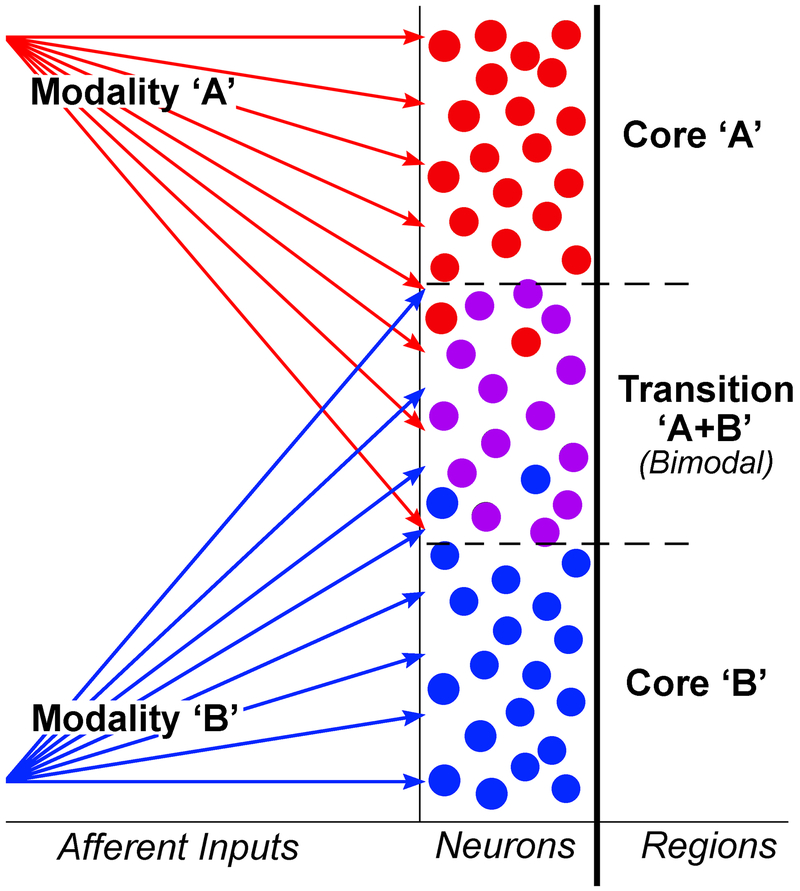
A schematic depicting a magnified view of this core/transition zone organization is depicted in Figure 7. The termination of inputs originating from one sensory region (e.g., red arrows) overlap with those that originate from a different sensory region (blue arrows) at the intersection between those two representations. Where such overlap occurs, neurons are responsive to both inputs (bimodal). Therefore, given the dimensions and the different neuronal functional composition, it might be better to regard regions between the AESc core representations not as sharp borders but as transition zones. Such a pattern of sensory cores and bimodal transition zones could be accounted for by a connectional model like that shown in Figure 7. Furthermore, such a pattern is not unique to the AESc and may represent a general principle of how cortical sensory representations of different sensory modalities are organized and separated. For example, in rat cortex, transition zones enriched in multisensory neurons are found between occipital and temporal cortex, and between temporal and parietal cortex (Wallace et al., 2004; Schormans et al., 2017).
Figure 7.
Between core areas representing different sensory modalities, the intervening transition zones can result from overlap of afferent inputs from two different sources. For the top core area (dominated by inputs from modality ‘A’; red arrows), a preponderance of neurons are driven by modality ‘A.’ In the lower core area, dominant inputs from another modality (‘B’; blue arrows) activate the majority of neurons there. However, neurons between area ‘A’ and ‘B’ receive shared and overlapping projections from both sources and can be activated by both modalities “A+B” (i.e., as bimodal multisensory neurons).
Core areas and transition zones: common output targets?
While inputs to a transition zone seem likely to show the same connectional patterns as both the adjoining core areas combined, it is not known if the core areas and transition zones exhibit similarities in their output connectivity. Numerous studies have demonstrated that the different AESc core representations share a major, common output target: the superior colliculus (Hollander, 1974; Stein et al., 1983; Meredith and Clemo, 1989; Harting et al., 1992; 1997; Chabot et al., 2013; Butler et al., 2016). In each of the AESc areas, corticotectal projections originate from large, layer 5 pyramidal neurons. Coronal sections containing areas SIV, FAES and AEV are shown in Figure 8, in which labeled corticotectal neurons form a continuous chain within lamina 5 across the entire AESc region (see also Figs 8 and 12 Butler et al., 2016). Moreover, this chain of corticotectal neurons remains continuous where the transition zones between FAES and AEV might occur. This same continuous pattern of corticocortical neurons also occurs across the SIV, Para-SIV and AEV representations (see also Figures 8 and 12 from Butler et al., 2016). These observations indicate that corticotectal projections originate in both core area and transition zones of the AESc and do not appear to differentially distribute according to the sensory differences between the regions. Collectively, these observations suggest that the core/transition zone system in the AESc operates as a functional unit with at least one common output target – the superior colliculus.
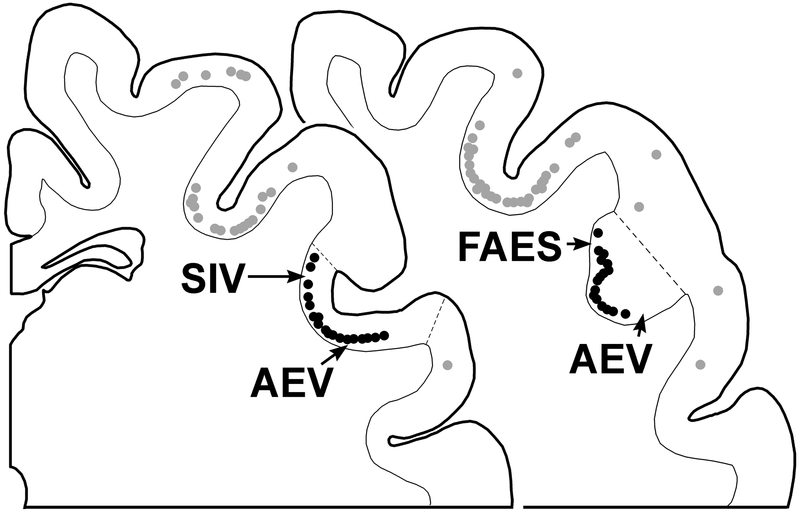
Figure 8.
AESc corticotectal projection neurons. On a series of coronal sections (anterior = left) through the AESc are plotted neurons (dark circles) labeled from tracer injection into the ipsilateral superior colliculus. Note that labeled neurons in the AESc form a continuous band that crosses un-interrupted from the banks into the fundic region of the sulcus and is present in the named regions (FAES, AEV, SIV) as well as in the transitional zones between those different regions. Redrawn and plotted from Butler et al., 2016.
It is important to recognize that corticotectal neurons are but one component of a coordinated output system that is arguably the major pathway by which cortex affects behavior (reviewed in Sherman and Guillery, 2013; Sherman, 2016). In layer 5 are the largest pyramidal neurons in cortex whose apical dendrites typically extend through the cortical column to reach layer 1, thereby sampling inputs across an entire column. Outputs from these layer 5 neurons exhibit thickly myelinated axons that branch repeatedly to simultaneously innervate multiple targets in thalamus, basal ganglia, brainstem and even spinal cord. Functionally, layer 5 neurons that project subcortcially provide driving (as opposed to metabotropic modulation) inputs to their targets (Sherman and Guillery, 2013; Sherman, 2016). Therefore, layer 5 neurons in the AESc are expected to exhibit simultaneous and potent excitatory control of their targets not just in the superior colliculus, but thalamus, basal ganglia and other brainstem regions as well.
Core areas and transition zones: differential activation patterns.
To this point, it is established that the modality-dominant core areas of the AESc receive extensive inputs from their related, lower-level input clusters as well as receive considerable crossmodal inputs from each other. Within the AESc, these modality-dominant core areas transition gradually from one to another over a span of several millimeters. Such transition zones contain a high proportion of neurons that share features of both adjoining core regions and, as such, cannot be assigned to one core or another. Underlying this entire core/transition zone organization is a continuous sheet of layer 5 neurons that project to common subcortical targets, especially the superior colliculus. This organization of the AESc is schematically rendered in Figure 9A (generated using standard graphics software), which depicts the core AEV, FAES and SIV areas as well as the transition zones of Para-SIV, AVZ and MZ.
Figure 9.
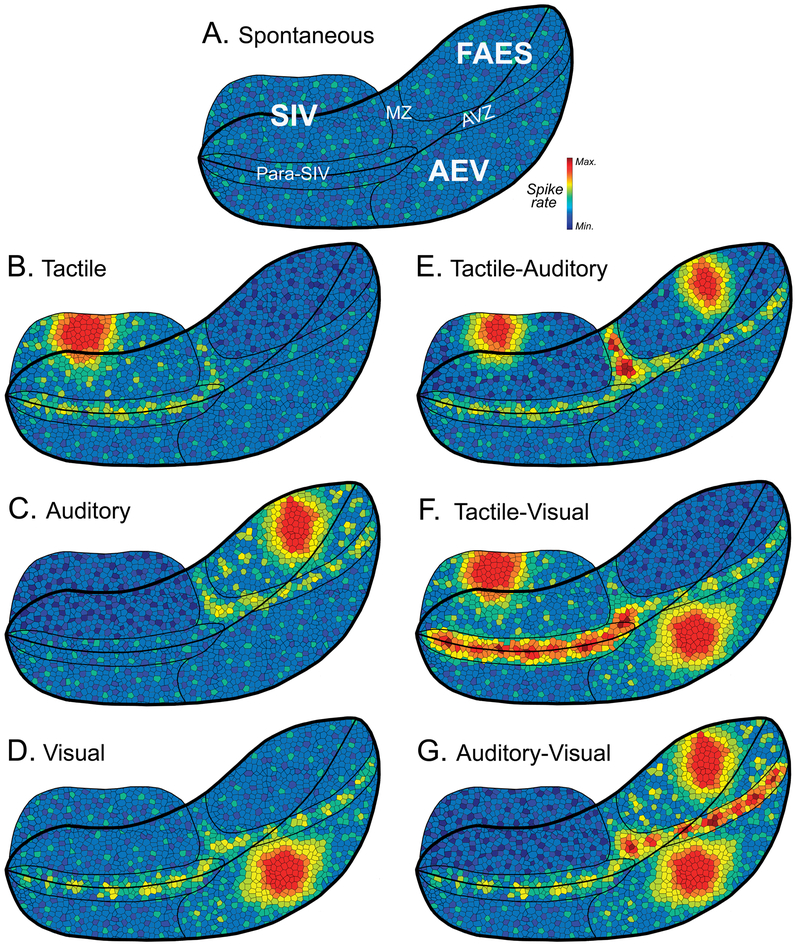
Hypothetical depictions of spontaneous and evoked activity patters for the sheet of layer 5 corticotectal neurons of the AESc. Each schematic is a repeated depiction of the opened AES to reveal all three core representations (SIV, FAES and AEV) and the transition zones (para-SIV, MZ, AVZ) between them where color represents hypothetical spike rate of layer 5 corticotectal output neurons. Part (A) depicts spontaneous levels in the different core (SIV, FAES, AEV) and transition (Para-SIV, MZ, AVZ) regions of the AESc. Hypothetical activation patterns evoked by individual, unisensory stimulation are depicted for tactile (part B), auditory (part C) and visual cues (part D). Likewise, hypothetical activation patterns are illustrated for combined tactile-auditory (part E), tactile-visual (Part F) and auditory-visual (part G) stimulation. When comparing the responses to unisensory (parts B-D) to multisensory (parts E-G) stimulation, note the prominent roles provided by the transition zones revealing the highest activity levels. See text for further description.
Also depicted in figure 9A is a tessellated representation of hypothetical activity levels (color heat-map) across the two-dimensional sheet of corticotectal neurons within the AESc. In this hypothetical example, spontaneous (non-driven) activity levels are represented as similar across the different core areas and transition zones of the AESc. Subsequent figures show hypothetical changes and differences in levels of evoked activity that occur in response to unisensory or multisensory stimulation, as described next.
One of the simplest permutations of proposed AESc activation is depicted in Figure 9B, where a punctate tactile stimulus distinctly activates the portion of the somatotopic map in SIV core area representing the stimulation site (bright red/orange colors). In addition, this stimulation broadly activates Para-SIV neurons whose large somatosensory receptive fields also include the stimulation location. Also, because of the projection from SIV to auditory FAES, somatosensory stimulation should have a suppressive effect on activity levels within the FAES (decreased proportion of bright colors). In Figure 9C, the reciprocal pattern is depicted, where broadband acoustic stimulation would activate the FAES core area as well as auditory neurons in the AVZ and MZ transition zones while suppressing SIV. For Figure 9D, visual stimulation would elicit a strong response from neurons in AEV core area as well as from those visually-responsive neurons located in the transition zones of Para-SIV and AVZ. Unisensory visual suppression of SIV and FAES is not depicted because this effect has not been empirically demonstrated, although the requisite short-distance connections are known to be present. Collectively, these different unisensory activity scenarios depict a hypothetical pattern of AESc activation and suppression that is modality-dependent and would be relayed to the superior colliculus (and other subcortical regions) as a target of the sheet of layer 5 output neurons.
In comparison to unisensory stimulation, AESc activation patterns elicited by multisensory stimulation would preferentially activate the transition zones due to their higher proportion of bimodal neurons, as illustrated in Figures 9E-G. In Figure 9E, combined tactile-auditory stimulation would activate components of both SIV and FAES, but the size and possibly magnitude of the activation would be reduced (from unisensory response levels) by the reciprocal suppressive circuit between these two regions. In contrast, high levels of activation (deep red color) would be expected from bimodal tactile-auditory neurons in the MZ transition between SIV and FAES. The hypothesized effect of combined tactile-visual stimulation on the AESc is depicted in Figure 9F, where the expected loci within SIV and AEV are activated by the tactile or visual components of the stimulus combination. Under these stimulus conditions, however, the highest level of activation (deep red color) would be predicted to occur within the Para-SIV, where bimodal tactile-visual neurons would generate enhanced responses to the combined cues. Likewise, as rendered in Figure 9G, combined visual-auditory stimulation would be expected to elicit core activity where visual or auditory unisensory responses are represented (AEV, FAES) while the highest response levels would occur in the transition zone AVZ between the two regions where bimodal visual-auditory neurons predominate and generate multisensory enhancement. Thus, in each multisensory condition, unisensory responses would be evoked within the core areas, while stronger multisensory responses (a result of response enhancement in bimodal neurons) would predominantly occur within the transition zones of the AESc. Collectively, these hypothetical renditions suggest that stimulation (and combined stimulation) produces concurrent and predictable patterns of activity within the multiple components of the AESc.
AESc as a unit and cortical network hub:
Based on known connectivity and function, the observations provided to this point have demonstrated that the core sensory representations within the AESc are higher-order sensory areas that project to one another and influence each other’s activity. Furthermore, each core area receives inputs not just from its dominant sensory modality, but also from numerous cross-modal areas. These core regions are separated by broad transition zones. Each transition zone exhibits sensory properties in common with their adjoining core regions. The likely functional interactions between these core and transition zones are summarized in Figure 9. Both core as well as transition zones share a common output target: the superior colliculus, and corticotectal neurons are part of the larger, layer 5 output system that simultaneously drives numerous subcortical regions. Therefore, given their multisensory properties, their mutual effect on one another and their shared output targets, it is logical to propose that the AESc region acts as a functional unit, as depicted in Figure 10. In addition, given that the AEV is identified as a cortical network hub (Zamora-Lopez et al., 2009; 2010), and AEV shares connections, properties and activity with the other components of the AESc, it also is logical to propose that the AESc region itself serves as a cortical network hub. Furthermore, the AESc has connections with other regions designated as hubs, which is consistent with findings from computational modeling (Zamora-Lopez et al., 2009; 2010). Of the eleven cortical network hubs identified in cat cortex, area AEV receives inputs from hubs 35/36 (2.4% of total ipsilateral cortical projections), CgP (1.6%), area 5 (0.2%) and Area 7 (0.2%; Meredith et al., 2017) while FAES is targeted by hubs 35/36 (1.5%), CgP (0.4%), area 5 (0.3%) and area 7 (0.3%; Meredith et al., 2016). Unpublished evaluation of projections to SIV indicates that it receives inputs from hub area 5 (2.7%). These connectional patterns of AEV, FAES and SIV with other identified hubs further support the notion that the AESc region collectively represents a cortical network hub.
Figure 10.
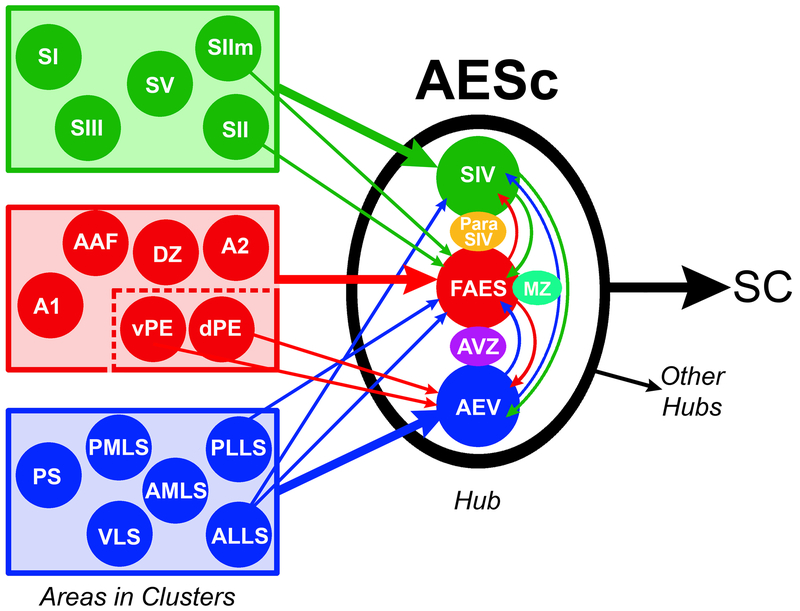
Summary. Cortical areas (filled circles) representing somatosensory (green), auditory (red) and visual (blue) modalities form clusters (rectangles). Some cortical clusters project (large colored arrows) to their corresponding core areas of the AESc as well as send crossmodal connections (small colored arrows) to representations of different sensory modalities in the AESc. Within the AESc, crossmodal connections (curved colored arrows) interconnect each core area (connectional data for transition zones is not available). Each of the components of the AESc project to the SC (superior colliculus) and to other specific cortical hubs. These observations suggest that the sensory representations in the AESc (SIV, FAES and AEV) and transition zones (Para-SIV, MZ and AVZ) collectively act as a multiple sensory/multisensory unit and together represent a multisensory cortical network hub. Dashed lines inside auditory cluster indicate that specific auditory areas project to FAES while others connect with AEV. Connectional data derived from Figure 3 and its sources.
Limitations:
The present text provides a review of the biological data regarding the organization and function of a well-examined region of cat cerebral cortex. Correspondingly, the cited computational studies of cortical network organization and function are based on a data-set derived from cat cortex. However, the proposal that the collective core/transition zone structure of the AESc represents a hub within the cat cortical network is based on the extrapolation of the biological data and remains to be computationally demonstrated using a contemporary connectional data-set. Nonetheless, for such a computational re-evaluation of cat cortical network organization and function, the present review provides several brain-based features (e.g., core/transition zone structure, regional interconnectivity, dynamic population response patterns based on neuronal properties) that would add complexity and power to such future models.
A “hub” is a network feature and concept, and the criteria for hub status is largely computational, not biological. As stated earlier, a network hub is computationally defined as an area that is high in the cortical hierarchy, is “highly interconnected and provides communication between clusters representing different sensory modalities” (Zemanová et al., 2006; Sporns et al., 2007; Zhou et al., 2006; Hagmann et al., 2008; Zampora-Lopez et al., 2009; 2010). However, this computational definition requires some adjustment and nuance based on recent biological findings. Specifically, multisensory properties are not the sole domain of higher-order, association cortices. Indeed, many lower level cortices in gyrencephalic species (all do in lissencephalic species; for review, see Meredith and Lomber, 2017), including primary sensory cortices (Bizley et al., 2007; Bizley and King, 2009; Meredith and Allman, 2015), exhibit some degree of multisensory properties (e.g., Ghanzafar and Schroeder, 2006).
Functional role of AESc cortical hub:
The functional organization of the AESc into a network hub, as supported by the presented evidence, raises the question as to the behavioral and perceptual advantages of such an organization and the role that it plays in sensory information processing and transfer. One clue to this may come from the output architecture of the AESc. As described above, through its pyramidal neurons in layer 5, the AESc has strong connectivity with the superior colliculus – a subcortical site well known for its central role in the control of gaze (Wurtz and Albano, 1980; Sparks, 1986; Stein, 1988). As one of the major cortical inputs to the SC, the AESc is a major player in generating the convergence of visual, auditory and somatosensory inputs that makes the SC a key node for multisensory processing (Wallace et al., 1993). Indeed, this convergence and the consequent integration that takes place at the neuronal level are the likely substrates for the striking behavioral benefits seen in target detection and localization (Stein et al., 1989; Wilkinson et al., 1996; Jiang et al., 2002). Furthermore, these behavioral involvements of the SC are dependent on cortical input, especially from the AESc (Wilkinson et al., 1996; Jiang et al., 2002; Malhotra and Lomber, 2007; Meredith et al., 2011). Ultimately, layer 5 neurons in the AESc not only reach the superior colliculus, but also are likely to have branches that simultaneously drive thalamic, basal ganglia and other brainstem sites (Sherman and Guillery, 2013; Sherman, 2016). Hence, as a cortical multisensory hub, the AESc can be seen as orchestrating numerous behavioral benefits by nature of its output organization.
In addition to its subcortical connectivity, the intrinsic (multi)sensory processing and cortical connections of AESc are likely to be important for multisensory perceptual “binding,” which entails the active integration of perceptual features from the different senses that belong to or are derived from the same object or event. Future work using manipulations such as optogenetic stimulation or deactivation should strive to assess the functional role of the AESc, not from the perspective of its component unisensory representations, but rather from the view of the AESc as a multisensory network hub.
Conclusions:
The present review describes the organization and function of the core representations within the AESc. Each of the core areas (AEV, FAES, SIV) exhibit higher-order receptive field properties, connectivity and organization. In addition, each of these core areas is separated by a transition zone that contain a high proportion of bimodal neurons which exhibit sensory properties of both of the adjoining core areas. Furthermore, the entire AESc is characterized by a nearly continuous sheet of layer 5 neurons which have a common output target: the superior colliculus (and other subcortical regions). Given that the AEV has been demonstrated to represent a cortical network hub (Zamora-Lopez et al., 2010), that areas FAES and SIV also exhibit the same hub-like characteristics, and because each of the core areas are linked to and demonstrate functional influence over one another, these observations indicate that the collective AESc region, acting as a dynamic multiple sensory/multisensory unit, could be regarded as a cortical network hub.
Table 1.
List of Abbreviations
| 1 | Primary somatosensory cortex |
| 3 | Primary somatosensory cortex |
| 4 | Motor cortex |
| 5 | Parietal cortex |
| 6 | Premotor cortex |
| 7 | Parietal cortex |
| 17 | Primary visual cortex |
| 18 | Secondary visual cortex |
| 19 | Third visual cortex |
| 20 | Extrastriate visual cortex |
| 21 | Extrastriate visual cortex |
| 22 | Extrastriate visual cortex |
| 35 | Perirhinal cortex |
| 36 | Perirhinal cortex |
| A1 | Primary auditory cortex |
| A2 | Second auditory cortex |
| AAF | Anterior Auditory Field |
| AES | Anterior Ectosylvian Sulcus |
| AESc | Anterior Ectosylvian Sulcal cortex |
| AEV | Anterior Ectosylvian Visual area |
| AID | Agranular Insular-dorsal |
| AIV | Agranular Insular-ventral |
| ALG | Anterior Lateral gyrus visual area |
| ALLS | Anterolateral Lateral Suprasylvian visual area |
| AMLS | Anteromedial Lateral Suprasylvian visual área |
| AVZ | Auditory-Visual Zone of the AES |
| CgA | Cingulate gyrus, anterior |
| CgP | Cingulate gyrus, posterior |
| dPE | Dorsal Posterior Ectosylvian auditory area |
| DZ | Dorsal Zone of auditory cortex |
| EVA | Ectosylvian Visual Area (see AEV) |
| FAES | Auditory field of the Anterior Ectosylvian sulcus |
| GI | Granular insular area |
| IN | Insular auditory area |
| iPE | Intermediate Posterior Ectosylvian auditory area |
| LM-Sg | Lateral medial suprageniculate thalamic nucleus |
| LP-Pulvinar | Lateral posterior pulvinar thalamic nucleus |
| MT | Middle Temporal visual area |
| MZ | Multisensory zone |
| Para-SIV | Somatosensory zone in fundus of AES; deep to Area SIV |
| PAF | Posterior auditory field |
| PLLS | Posterolateral Lateral Suprasylvian visual area |
| PMLS | Posteromedial Lateral Suprasylvian visual area |
| Po | Posterior nucleus of the thalamus |
| PS | Posterior Suprasylvian visual area |
| RS | Retrosplenial area |
| SII | Second somatosensory cortex |
| SIIm | Second somatosensory cortex, medial |
| SIII | Third somatosensory cortex |
| SIV | Fourth somatosensory cortex |
| SV | Fifth somatosensory cortex |
| SC | Superior Colliculus |
| T | Temporal auditory area |
| VAF | Ventral Auditory Field |
| VLS | Ventral Lateral Suprasylvian visual area |
| vPAF | Ventral Posterior auditory field |
| vPE | Ventral Posterior Ectosylvian auditory field |
Acknowledgements:
We thank Dr. Brian L. Allman for his review of this manuscript. Supported by NIH grant NS 039460 (MAM).
Reference List:
- Allman BL and Meredith MA (2007). Multisensory processing in ‘unimodal’ neurons: cross-modal subthreshold auditory effects in cat extrastriate visual cortex. J. Neurophysiol. 98, 545–549. [DOI] [PubMed] [Google Scholar]
- Allman BL, Keniston LP and Meredith MA (2009). Not just for bimodal neurons anymore: The contribution of unimodal neurons to cortical multisensory processing. Brain Topogr. 21,157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW and Stein BE (2009). Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J. Neurosci. 29, 6580–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW and Stein BE (2007). Cortex mediates multisensory but not unisensory integration in superior colliculus. J. Neurosci. 27, 12775–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avillac M, Ben Hamed S and Duhamel JR (2007). Multisensory integration in the ventral intraparietal area of the macaque monkey. J. Neurosci. 27, 1922–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough NE, Xiao D, Baker CI, Oram MW and Perrett DI (2005). Integration of visual and auditory information by Superior Temporal Sulcus neurons responsive to the sight of actions. J. Cognitive Neurosci. 17, 377–391. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Fallon J, Davis BJ and Rezak M (1977). Auditory-visual interaction in single cells in the cortex of the superior temporal sulcus and the orbital frontal cortex of the macaque monkey. Exp. Neurol. 57, 849–872. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Molinari M, Minciacchi D and Macchi G (1983). Projections of the posterior-complex and intralaminar nuclei of the thalamus as studied by means of retrograde tracers, in: Somatosensory Integration of the Thalamus. Macchi G, Rustioni A and Spreaficio R (Eds.), Elsevier, Amsterdam, pp. 337–363. [Google Scholar]
- Berman AL (1961). Interaction of cortical responses to somatic and auditory stimuli in anterior ectosylvian gyrus of cat. J. Neurophysiol. 24, 608–620. [DOI] [PubMed] [Google Scholar]
- Bizley JK and King AJ (2009). Visual influences on ferret auditory cortex. Hear. Res. 258, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Bajo VM, Nelken I and King AJ (2007). Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb. Cortex 17, 2172–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra E and Luppino G (2016). Functional anatomy of the macaque temporo-parieto-frontal connectivity. Cortex doi: 10.1016/j.cortex.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Klam F, Duhamel JR, Ben Hamed S and Graf W (2002). Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur. J. Neurosci. 16, 1569–1586. [DOI] [PubMed] [Google Scholar]
- Bruce C, Desimone R and Gross CG (1981). Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J. Neurophysiol. 46, 369–384. [DOI] [PubMed] [Google Scholar]
- Burton H and Kopf EM (1984). Connections between the thalamus and the somatosensory areas of the anterior ectosylvian gyrus in the cat. J. Comp. Neurol. 224, 173–205. [DOI] [PubMed] [Google Scholar]
- Burton H, Mitchell G, and Brent D (1982). Second somatic sensory area in the cerebral cortex of cats: Somatotopic organization and cytoarchitecture. J. Comp. Neurol. 210, 109–135. [DOI] [PubMed] [Google Scholar]
- Butler BE, Chabot N and Lomber SG (2016). A quantitative comparison of the hemispheric, areal, and laminar origins of sensory and motor cortical projections to the superior colliculus of the cat. J. Comp. Neurol. 524, 2623–42. [DOI] [PubMed] [Google Scholar]
- Carriere BN, Royal DW, Perrault TJ Jr., Morrison SP, Vaughan JW, Stein BE and Wallace MT (2007). Visual deprivation alters the development of cortical multisensory integration. J. Neurophysiol. 98, 2858–2867. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT and Martin GF (1972). Superior colliculus of the tree shrew: A structural and functional subdivision into superficial and deep layers. Science 177, 444–447. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, Murphy DG and Thiebaut de Schotten M (2012). Beyond cortical localization in clinico-anatomical correlation. Cortex 48, e1262–1287. [DOI] [PubMed] [Google Scholar]
- Cavada C and Reinoso-Suarez F (1981). Intrahemispheric cortico-cortical afferent connections of the prefrontal cortex in the cat. Brain Res. 223, 128–133. [DOI] [PubMed] [Google Scholar]
- Chabot N, Mellott JG, Hall AJ, Tichenoff EL and Lomber SG (2013). Cerebral origins of the auditory projection to the superior colliculus of the cat. Hear. Res. 300, 33–45. [DOI] [PubMed] [Google Scholar]
- Clarey JC and Irvine DR (1986). Auditory response properties of neurons in the anterior ectosylvian sulcus of the cat. Brain Res. 386, 12–19. [DOI] [PubMed] [Google Scholar]
- Clarey JC and Irvine DR (1990a). The anterior ectosylvian sulcal auditory field in the cat: I. An electrophysiological study of its relationship to surrounding auditory cortical fields. J. Comp. Neurol. 301, 289–303. [DOI] [PubMed] [Google Scholar]
- Clarey JC and Irvine DR (1990b). The anterior ectosylvian sulcal auditory field in the cat: II. A horseradish peroxidase study of its thalamic and cortical connections. J. Comp. Neurol. 301, 304–324. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Allman BL, Donlan MA and Meredith MA 2007. Sensory and multisensory representations within the cat rostral suprasylvian cortex. J. Comp. Neurol. 503, 110–127. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Keniston L and Meredith MA (2003). A comparison of the distribution of GABA-ergic neurons in cortices representing different sensory modalities. J. Chem. Neuroanat. 26, 51–63. [DOI] [PubMed] [Google Scholar]
- Clemo HR and Stein BE (1982). Somatosensory cortex: A “new” somatotopic representation. Brain Res. 235, 162–168. [DOI] [PubMed] [Google Scholar]
- Clemo HR and Stein BE (1983). Organization of a fourth somatosensory area of cortex in cat. J. Neurophysiol. 50, 910–925. [DOI] [PubMed] [Google Scholar]
- Clemo HR and Stein BE (1984). Topographic organization of somatosensory corticotectal influences in cat. J. Neurophysiol. 51, 843–858. [DOI] [PubMed] [Google Scholar]
- Clemo HR and Stein BE (1986). Effects of cooling somatosensory cortex on response properties of tactile cells in the superior colliculus. J. Neurophysiol. 55, 1352–1368. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Keniston L,P and Meredith MA (2003). GABAergic neurons show similar distribution patterns within cortical areas representing different sensory modalities. J. Chem. Neuroanat. 26, 51–63. [DOI] [PubMed] [Google Scholar]
- Clemo HR and Meredith MA (2004). Cortico-ortical Relations of Cat Somatosensory Areas SIV and SV. Somat. Motor Res. 21, 199–209. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Sharma GK, Allman BL and Meredith MA (2008). Auditory projections to extrastriate visual cortex: connectional basis for multisensory processing in ‘unimodal’ visual neurons. Exp. Brain Res. 191, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE (2009). Multimodal activity in the parietal cortex. Hear. Res. 258, 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehner LR, Keniston LP, Clemo HR and Meredith MA (2004). Cross-Modal circuitry between auditory and somatosensory areas of the cat Anterior Ectosylvian sulcal cortex: A “new” form of multisensory convergence. Cereb. Cortex 14, 387–401. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL and Goldberg ME (1998). Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J. Neurophysiol. 79, 126–136. [DOI] [PubMed] [Google Scholar]
- Felleman DJ and Van Essen DC (1991). Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47. [DOI] [PubMed] [Google Scholar]
- Foxworthy WA, Allman BL, Keniston LP and Meredith MA (2013a). Multisensory and unisensory neurons exhibit distinct functional properties. Eur. J. Neurosci. 37, 910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxworthy WA, Clemo HR and Meredith MA (2013b). Laminar and connectional organization of a multisensory cortex. J Comp Neurol. 521:1867–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, and Schroeder CE (2006). Is neocortex essentially multisensory? Trends Cogn. Sci. 10, 278–85. [DOI] [PubMed] [Google Scholar]
- Grant S and Shipp S (1991). Visuotopic organization of the lateral suprasylvian area and of an adjacent area of the ectosylvian gyrus of cat cortex: a physiological and connectional study. Vis. Neurosci. 6, 315–38. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (1973). The thalamo-cortical projection of the so-called posterior nuclear group: a study with anterograde degeneration methods in the cat. Brain Res. 49, 229–244. [DOI] [PubMed] [Google Scholar]
- Graybiel AM and Berson DM (1981). On the relation between transthalamic and transcortical pathways in the visual system, in: The organization of the cerebral cortex, Schmitt FO, Worden FG and Dennis F (Eds.), pp. 286–319, MIT Press, Cambridge MA. [Google Scholar]
- Graziano MS, Reiss LA and Gross CG (1999). A neuronal representation of the location of nearby sounds. Nature 397, 428–430. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ and Sporns O (2008). Mapping the structural core of human cerebral cortex. PLoS Biol. 6, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Feig S and Van Lieshout DP (1997). Cortical somatosensory and trigeminal inputs to the cat superior colliculus: light and electron microscopic analyses. J. Comp. Neurol. 388, 313–26. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV and Van Lieshout DP (1992). Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J. Comp. Neurol. 324, 379–414. [DOI] [PubMed] [Google Scholar]
- Heath CJ and Jones EG (1971). An experimental study of ascending connections from the posterior group of thalamic nuclei in the cat. J. Comp. Neurol 141, 397–426. [DOI] [PubMed] [Google Scholar]
- Hickmott PW and Merzenich MM (1998). Single-cell correlates of a representational boundary in rat somatosensory cortex. J. Neurosci. 18, 4403–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Iwai E, Saito H and Tanaka K (1988). Polysensory properties of neurons in the anterior bank of the caudal superior temporal sulcus of the macaque monkey. J. Neurophysiol. 60, 1615–1637. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Burns GA, O’neill MA, Scannell JW and Young MP (2000). Anatomical connectivity defines the organization of clusters of cortical areas in the macaque monkey and the cat. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 355, 91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC and Kaiser M (2004). Clustered organisation of cortical connectivity. Neuroinformatics. 2, 353–360. [DOI] [PubMed] [Google Scholar]
- Holländer H (1974). On the origin of the corticotectal projections in the cat. Exp. Brain Res. 21, 433–9. [DOI] [PubMed] [Google Scholar]
- Horn G and Hill RM (1966). Responsiveness to sensory stimulation of units in the superior colliculus and subjacent tectotegmental regions of the rabbit. Exp. Neurol. 14, 199–223 [DOI] [PubMed] [Google Scholar]
- Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F and Medini P (2012). Sound-driven synaptic inhibition in primary visual cortex. Neuron 73, 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H and Guitton D (1995). Eye, head, body and forelimb movements evoked from the anterior ectosylvian cortex of the unrestrained cat. Soc. Neuorsci. Abstr. 21, 1899. [Google Scholar]
- Jiang H, Lepore F, Poirier P and Guillemot JP (2000). Responses of cells to stationary and moving sound stimuli in the anterior ectosylvian cortex of cats. Hear. Res 139, 69–85. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lepore F, Ptito M and Guillemot JP (1994a). Sensory interactions in the anterior ectosylvian cortex of cats. Exp. Brain Res. 101, 385–396. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lepore F, Ptito M and Guillermot JP (1994b). Sensory modality distribution in the anterior ectosylvian cortex (AEC) of cats. Exp. Brain Res. 97, 404–14. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H and Stein BE (2002). Two corticotectal areas facilitate multisensory orientation behavior. J. Cogn. Neurosci. 14, 1240–55 [DOI] [PubMed] [Google Scholar]
- Keniston LP, Henderson SC and Meredith MA (2010). Neuroanatomical identification of crossmodal auditory inputs to interneurons in somatosensory cortex. Exp. Brain Res. 202, 725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M and Rauschecker JP (1993). Auditory spatial tuning of cortical neurons is sharpened in cats with early blindness. J. Neurophysiol. 70, 1717–1721. [DOI] [PubMed] [Google Scholar]
- Las L, Shapira AH and Nelken I (2008). Functional gradients of auditory sensitivity along the anterior ectosylvian sulcus of the cat. J. Neurosci. 28, 3657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. (2004). Cerebral areas mediating visual redirection of gaze: cooling deactivation of 15 loci in the cat. J. Comp. Neurol. 474, 190–208. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ and Lomber SG (2004). Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J. Neurophysiol. 92, 1625–1643. [DOI] [PubMed] [Google Scholar]
- Malhotra S and Lomber SG (2007). Sound localization during homotopic and heterotopic bilateral cooling deactivation of primary and non-primary auditory cortical areas in the cat. J. Neurophysiol. 97, 26–43. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Kruger L, Clemo HR and Stein BE (1988). Corticothalamic and corticotectal somatosensory projections from the anterior ectosylvian sulcus (SIV cortex) in neonatal cats: an anatomical demonstration with HRP and 3H-leucine. J. Comp. Neurol. 274, 115–26. [DOI] [PubMed] [Google Scholar]
- McNair JL and Avendano C (1980). Cortico-cortical afferents of the motor cortex in the cat. Neurosci. Lett. 5, 110. [DOI] [PubMed] [Google Scholar]
- Mellott JG, Van der Gucht E, Lee CC, Carrasco A, Winer JA and Lomber SG (2010). Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32. Hear. Res. 267, 119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA (2004). Cortico-cortical connectivity and the architecture of cross-modal circuits, in: Handbook of Multisensory Processes, Spence C, Calvert G and Stein B (Eds.), pp. 343–355, MIT Press, Cambridge MA. [Google Scholar]
- Meredith MA and Allman BL (2009). Subthreshold multisensory processing in cat auditory cortex. NeuroReport 20,126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA and Allman BL (2015). Single-unit analysis of somatosensory processing in core auditory cortex of hearing ferrets. Eur. J. Neurosci. 41, 686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Allman BL, Keniston LP, Clemo HR. (2011). Are bimodal neurons the same throughout the brain? in: The Neural Bases of Multisensory Processing, Murray MM and Wallace MT (Eds.), pp. 51–64, CRC Press, Boca Raton FL. [Google Scholar]
- Meredith MA and Clemo HR (1989). Auditory cortical projection from the anterior ectosylvian sulcus (Field AES) to the superior colliculus in the cat: an anatomical and electrophysiological study. J. Comp. Neurol. 289, 687–707. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR, Corley SB, Chabot N and Lomber SG (2016). Cortical and thalamic connectivity of the auditory anterior ectosylvian cortex of early-deaf cats: Implications for neural mechanisms of crossmodal plasticity. Hear. Res. 333, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR and Lomber SG (2017). Is territorial expansion a mechanism for crossmodal plasticity? Eur. J. Neurosci. 45, 1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Keniston LP, Dehner LR and Clemo HR (2006). Cross-modal projections from somatosensory Area SIV to the auditory field of the Anterior Ectosylvian Sulcus (FAES) in cat: Further evidence for subthreshold forms of multisensory processing. Exp. Brain Res. 72, 472–84. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Kryklywy J, McMillan AJ, Malhotra S, Lum-Tai R and Lomber SG (2011). Crossmodal reorganization in the early-deaf switches sensory, but not behavioral roles of auditory cortex. Proc. Natl. Acad. Sci. USA. 108, 8856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA and Lomber SG (2017). Species-dependent role of crossmodal connectivity among the primary sensory cortices. Hear. Res. 343, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Nemitz JW and Stein BE (1987). Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J. Neurosci. 7, 3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA and Stein BE (1983). Interactions among converging sensory inputs in the superior colliculus. Science. 221, 389–391, 1983. [DOI] [PubMed] [Google Scholar]
- Meredith MA and Stein BE (1986). Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 265, 350–354. [DOI] [PubMed] [Google Scholar]
- Miceli D, Reperant J and Ptito M (1985). Intracortical connections of the anterior ectosylvian and lateral suprasylvian visual areas in the cat. Brain Res. 347, 291–298. [DOI] [PubMed] [Google Scholar]
- Minciacchi D, Tassinari G and Antonini A (1987). Visual and somatosensory integration in the anterior ectosylvian cortex of the cat. Brain Res. 410, 21–31. [DOI] [PubMed] [Google Scholar]
- Movshon JA,, Adelson EH,, Gizzi MS and Newsome WT (1985). The analysis of moving visual patterns. Exp. Brain Res. Suppl. 11, 117–151. [Google Scholar]
- Mucke L, Norita M, Benedek G and Creutzfeldt O (1982). Physiologic and anatomic investigation of a visual cortical area situated in the ventral bank of the anterior ectosylvian sulcus of the cat. Exp. Brain Res. 46, 1–11. [DOI] [PubMed] [Google Scholar]
- Nagy A, Eordegh G and Benedek G (2003). Spatial and temporal visual properties of single neurons in the feline anterior ectosylvian visual area. Exp. Brain Res. 151, 108–114. [DOI] [PubMed] [Google Scholar]
- Norita M, Mucke L, Benedek G, Albowitz B, Katoh Y and Creutzfeldt OD (1986). Connections of the anterior ectosylvian visual area (AEV). Exp. Brain Res. 62, 225–240. [DOI] [PubMed] [Google Scholar]
- Olcese U, Iurilli G and Medini P (2013). Cellular and synaptic architecture of multisensory integration in the mouse neocortex. Neuron 79, 579–593. [DOI] [PubMed] [Google Scholar]
- Olson CR and Graybiel AM (1987). Ectosylvian visual area of the cat: location, retinotopic organization and connections. J. Comp. Neurol. 261, 277–294. [DOI] [PubMed] [Google Scholar]
- Payne BR (1990). Representation of the ipsilateral visual field in the transition zone between areas 17 and 18 of the cat’s cerebral cortex. Vis. Neurosci. 4:445–474. [DOI] [PubMed] [Google Scholar]
- Perrault TJ Jr., Vaughan JW, Stein BE and Wallace MT (2005). Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J. Neurophysiol. 93, 2575–86. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP and Korte M (1993). Auditory compensation for early blindness in cat cerebral cortex. J. Neurosci. 13, 4538–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso-Suárez F and Roda JM (1985). Topographical organization of the cortical afferent connections to the cortex of the anterior ectosylvian sulcus in the cat. Exp. Brain Res. 59, 313–324. [DOI] [PubMed] [Google Scholar]
- Roda JM and Reinoso-Suarez F (1983). Topographical organization of the thalamic projections to the cortex of the anterior ectosylvian sulcus in the cat. Exp. Brain Res. 49, 131–139. [DOI] [PubMed] [Google Scholar]
- Rodman HR and Albright TD (1989). Single-unit analysis of pattern-motion selective properties in the middle temporal visual area (MT). Exp. Brain Res. 75, 53–64. [DOI] [PubMed] [Google Scholar]
- Romanski LM (2007). Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cereb. Cortex 17(Suppl 1), i61–i69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ BE, Kim AM, Abrahamsen KL, Kiringoda R and Cohen YE (2006). Responses of neurons in the lateral intraparietal area to central visual cues. Exp. Brain Res. 174, 712–727. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Blakemore C and Young MP (1995). Analysis of connectivity in the cat cerebral cortex. J. Neurosci. 15, 1463–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell J and Young MP (1993). The connectional organization of neural systems in the cat cerebral cortex. Curr. Biol. 3, 191–200. [DOI] [PubMed] [Google Scholar]
- Schlack A, Sterbing-D’Angelo SJ, Hartung K, Hoffmann KP and Bremmer F (2005). Multisensory space representations in the macaque ventral intraparietal area. J. Neurosci. 25, 4616–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schormans AL, Typlt M, Allman BL (2017). Crossmodal plasticity in auditory, visual and multisensory cortical areas following noise-induced hearing loss in adulthood. Hear. Res. 343, 92–107. [DOI] [PubMed] [Google Scholar]
- Segal RL and Beckstead RM (1984). The lateral suprasylvian corticotectal projection in cats. J. Comp. Neurol. 225, 259–275. [DOI] [PubMed] [Google Scholar]
- Segal RL and Beckstead RM (1989). Distribution of corticotectal axons from the caudal part of the anterior ectosylvian sulcus in the cat. Neurosci. Lett. 102, 173–178. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2016). Thalamus plays a central role in ongoing cortical functioning. Nat. Neurosci. 19, 533–541. [DOI] [PubMed] [Google Scholar]
- Sherman SM and Guillery RW (2013). Thalamocortical Processing: Understanding the Messages that Link the Cortex to the World. MIT Press, Cambridge, MA. [Google Scholar]
- Sparks DL (1986). Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol. Rev. 66, 118–171. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ and Kötter R (2007). Identification and classification of hubs in brain networks. PLoS One 10, e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE (1988). Superior colliculus-mediated visual behaviors in cat and the concept of two corticotectal systems. Prog. Brain Res. 75, 37–53. [DOI] [PubMed] [Google Scholar]
- Stein BE and Meredith MA (1993). The Merging of the Senses, MIT Press, Cambridge MA. [Google Scholar]
- Stein BE, Meredith MA, Huneycutt WS and McDade L (1989). Behavioral indices of multisensory integration: Orientation to visual cues is affected by auditory stimuli. J. Cogn. Neurosci. 1, 12–24. [DOI] [PubMed] [Google Scholar]
- Stein BE, Spencer RF and Edwards SB (1983). Corticotectal and corticothalamic efferent projections of SIV somatosensory cortex in cat. J. Neurophysiol. 50:896–909. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Ghose D, Fister JK, Sarko DK, Altieri NA, Nidiffer AR, Kurela LR, Siemann JK, James TW and Wallace MT (2014). Identifying and quantifying multisensory integration: a tutorial review. Brain Topogr. 27, 707–30. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Diltz MD, Averbeck BB and Romanski LM (2006) Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J. Neurosci. 26:11138–11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai Y and Kimura A (1996). Multiple integration of input and output in the cortex. Neuroreport 7, 2401–2405. [DOI] [PubMed] [Google Scholar]
- Tamai Y, Miyashita E and Nakai M (1989). Eye movements following cortical stimulation in the ventral bank of the anterior ectosylvian sulcus of the cat. Neurosci. Res. 7, 159–163. [DOI] [PubMed] [Google Scholar]
- Tortelly A, Reinoso-Suarez F and Llamas A (1980). Projections from non-visual cortical areas to the superior colliculus demonstrated by retrograde transport of HRP in the cat. Brain Res. 188, 543–549. [DOI] [PubMed] [Google Scholar]
- Volchan E, Bernardes RF, Rocha-Miranda CE, Gleiser L and Gawryszewski LG (1988). The ipsilateral field representation in the striate cortex of the opossum. Exp. Brain Res. 73, 297–304. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Carriere BN, Perrault TJ Jr., Vaughan JW and Stein BE (2006). The development of cortical multisensory integration. J. Neurosci. 26, 11844–11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA and Stein BE (1993). Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J. Neurophysiol. 69, 1797–1809. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA and Stein BE (1992). The integration of multiple sensory inputs in cat cortex. Exp. Brain Res. 91, 484–488. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Ramachandran R and Stein BE (2004). A revised view of sensory cortical parcellation. Proc Natl Acad Sci U S A. 101, 2167–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LK, Meredith MA and Stein BE (1996). The role of anterior ectosylvian cortex in cross-modality orientation and approach behavior. Exp. Brain Res. 112, 1–10. [DOI] [PubMed] [Google Scholar]
- Wurtz RH and Albano JE (1980). Visual-motor function of the primate superior colliculus. Annu. Rev. Neurosci. 3, 189–226. [DOI] [PubMed] [Google Scholar]
- Yaka R, Notkin N, Yinon U and Wollberg Z (2002). Visual, auditory and bimodal activity in the banks of the lateral suprasylvian sulcus in the cat. Neurosci. Behav. Physiol. 32, 103–108. [DOI] [PubMed] [Google Scholar]
- Zamora-López G, Zhou C and Kurths J (2009). Graph analysis of cortical networks reveals complex anatomical communication substrate. Chaos. 19, 015117. [DOI] [PubMed] [Google Scholar]
- Zamora-López G, Zhou C and Kurths J (2010). Cortical hubs form a module for multisensory integration on top of the hierarchy of cortical networks. Front. Neuroinformat. 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanová L, Zhou CS and Kurths J (2006). Structural and functional clusters of complex brain networks. Phy. D. 224, 202–212. [Google Scholar]
- Zhou CS, Zemanová L, Zamora-López G, Hilgetag CC and Kurths J (2006). Hierarchical organization unveiled by functional connectivity in complex brain networks. Phys. Rev. Lett. 97, 238103. [DOI] [PubMed] [Google Scholar]