Abstract
Background
Pelvic organ prolapse (POP) is due to age-related atrophy and the weakening of the tissues of the pelvic floor, with degradation of collagen and extracellular matrix (ECM) by metalloproteinases (MMPs). This study aimed to investigates the role of the age-related enzyme klotho, encoded by the KL gene, in cultured fibroblasts obtained from patients with POP and the levels of reactive oxygen species (ROS), interleukin-6 (IL-6), and MMPs.
Material/Methods
Pelvic floor fibroblasts were obtained from connective tissue from three patients with POP and three normal subjects. Cell proliferation and ROS production were measured using a cell counting kit-8 (CCK-8) assay and flow cytometry. Levels of interleukin-6 (IL-6), klotho, metalloproteinase-1 (MMP-1), MMP-3, extracellular signal-regulated kinases 1/2 (ERK1/2), and p-ERK1/2 were measured by enzyme-linked immunoassay (ELISA), quantitative real-time polymerase chain reaction (qRT-PCR), and Western blot.
Results
In cultured pelvic floor fibroblasts from patients with POP, the expression of klotho protein and klotho mRNA were significantly down-regulated in fibroblasts from patients with POP compared with normal fibroblasts. Klotho supplementation in cultured fibroblasts for patients with POP included increased cell growth, reduced expression of ROS reduction, and reduced the secretion of IL-6. Using qRT-PCR and Western blot, klotho supplementation of fibroblasts from patients with POP increased cell growth and reduced the levels of IL-6 and ROS in a dose-dependent way.
Conclusions
Klotho protein reduced the expression of MMP-1 and MMP-3 in fibroblasts from patients with POP by down-regulating the phosphorylation of ERK1/2.
MeSH Keywords: MAP Kinase Signaling System, Matrix Metalloproteinases, Pelvic Organ Prolapse
Background
Pelvic organ prolapse (POP) affects between 10–40% of post-menopausal women [1,2]. POP is a disorder that is characterized by the loss of normal pelvic muscle support, resulting in the prolapse of the pelvic organs into the vaginal canal [3]. The etiology of POP is multifactorial [4]. It has been proposed that POP is due to the abnormal structure or organization of pelvic connective tissue, including type I collagen, which is a protein that has an essential role in supporting the pelvic floor [2]. Collagen can be degraded by matrix metalloproteinases (MMPs), which includes 24 different enzymes [5]. MMP-1 is an enzyme that can be activated by MMP-3, and MMP-1 also has a significant role in the degradation of collagen I [6,7]. Women who are affected by POP have been reported to have reduced levels collagen-I and increased levels of MMP-1 [4].
Recent attention has turned to the age-related enzyme, klotho, encoded by the KL gene, which is an age-suppressing gene [8]. Klotho is a membrane-bound protein that is mainly expressed in the kidneys [9]. The extracellular component of klotho contains two homologous domains [10,11], and cleavage at different sites results in the release of soluble extracellular klotho proteins [12,13]. Several important roles have been proposed for klotho [14], with the effect mainly mediated by soluble klotho [15]. However, there have been no previously reported studies that have investigated the potential role for klotho in women affected by POP.
However, it is possible that a relationship exists between the expression of klotho protein and POP as both are associated with the aging process [16–19]. Older women are at an increased risk from weakening of the muscles of the pelvic floor [16]. Recent preclinical studies using KL (−/−) knockout mice demonstrated muscle and connective tissue atrophy [9]. Also, klotho has been recognized to be a key regulator of oxidative stress, which is closely linked to the pathophysiology of POP [20–22]. Based on these findings, the role of klotho in the pathogenesis of POP deserves investigation.
Therefore, this study aimed to investigates the role of the age-related enzyme, klotho, in cultured fibroblasts obtained from patients with POP and the levels of reactive oxygen species (ROS), interleukin-6 (IL-6), MMP-1, and MMP-3.
Material and Methods
Chemicals and antibodies
The extracellular signal-regulated kinase (ERK) inhibitor, U0126, was purchased from Selleck Chemicals (Houston, TX, USA) and the epidermal growth factor (EGF) activator was purchased from PeproTech Inc. (Rocky Hill, NJ, USA). The protease inhibitor cocktail and the phosphatase inhibitor cocktail were obtained from Sigma-Aldrich (St. Louis MO, USA). All other reagents were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA), and included the BCA protein assay kit, the Lipofectamine 2000 transfection reagent, TRIzol reagent, and the RevertAid first strand cDNA synthesis kit. The Maxima SYBR Green/ROX quantitative polymerase chain reaction (qPCR) Master Mix (2X) was used (Thermofisher Scientific, Waltham, MA, USA), and human recombinant klotho protein was purchased from PeproTech Inc. (Rocky Hill, NJ, USA). Antibodies to klotho, MMP-1, and MMP-3 were purchased from Abcam (Cambridge, MA, USA) and antibodies to extracellular signal-regulated kinase 1 (ERK1), ERK2, pERK1, pERK2, and GAPDH were obtained from CST (Beverly, MA, USA).
Cell culture
Pelvic floor fibroblasts were obtained from connective tissue from three patients with POP and three normal subjects from The International Peace Maternity and Child Health Hospital of Shanghai Jiaotong University. All subjects signed an informed consent to participate in the study, as well as consent for surgery. The study was approved by the Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No, GKLW 2015-54).
The clinical and demographic characteristics of the study participants are summarized in Table 1. Details of the methods used for the isolation and culture of the pelvic fibroblasts has been previously reported [23,24]. Briefly, fresh pelvic floor specimens were obtained from the surgical margin in the most distal portion of the anterior (POP-Q point Ba) and/or posterior (POP-Q point Bp) vaginal wall. The cells were then washed with phosphate-buffered saline (PBS) at 4°C, followed by a 30 min digestion with PBS containing 2% collagenase II (1 mg/ml) (Thermofisher Scientific, Waltham, MA, USA) at 37°C. After cell isolation, the human pelvic floor fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS) (Thermofisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin solution (Solarbio Science & Technology Co., Ltd., Beijing, China) at 37°C. The medium was replaced every 2 days. A low passage number of POP cells (<6) was used in this study. Intracellular expression of CK19 (negative) and vimentin (positive) was measured with immunohistochemistry to identify fibroblasts (Supplementary Figure 1).
Table 1.
Patient’s characteristics for both POP and control groups.
| Group | Age | Menopausal status | Bump | Hormonotherapy | Smoking status | Delivery times | Complications | Surgical approach | POP-Q |
|---|---|---|---|---|---|---|---|---|---|
| POP1 | 64 | Postmenopause | Uterine prolapse II degree; Anterior vaginal prolapse III degree | No | No | 1 | Uterine fibroids; Uterine adenomyosis; Chronic cervicitis; Hypertension | Complete vaginal hysterectomy; anterior vaginal repair; transvaginal vaginal sacral ligament fixation | Aa: +2; Ba: +3; C: 0; gh: 5; pb: 3; Tvl: 7; Ap: −1.5; Bp: −1.5; D: −2 |
| POP2 | 68 | Postmenopause | Uterine prolapse III degree; Anterior vaginal prolapse III degree | Yes | No | 2 | Chronic cervicitis; Hypertension; Diabetes | Complete vaginal hysterectomy; anterior vaginal repair; transvaginal vaginal sacral ligament fixation | Aa: +2; Ba: +2.5; C: +2.5; gh: 5; pb: 3; Tvl: 7; Ap: −1; Bp: −1; D: 0 |
| POP3 | 45 | Premenopause | Stress incontinence; Anterior vaginal prolapse II degree | Yes | No | 2 | No | transseptal transvaginal tensionless urethral suspension; anterior vaginal repair | Aa: 0; Ba: −1; C: −3; gh: 4; pb: 2; Tvl: 7; Ap: −2; Bp: −3; D: −4 |
| Control1 | 62 | Postmenopause | No pelvic organ prolapse | No | No | 1 | Endometrial malignancy IA phase | Laparoscopic total hysterectomy; laparoscopic pelvic lymphadenectomy; laparoscopic complex pelvic adhesions; laparoscopic bilateral salpingo-oophorectomy | No |
| Control2 | 64 | Postmenopause | No pelvic organ prolapse | Yes | No | 1 | Endometrial malignancy IA phase | Laparoscopic total hysterectomy; laparoscopic pelvic lymphadenectomy; laparoscopic bilateral salpingo-oophorectomy | No |
| Control3 | 41 | Premeno|pause | No pelvic organ prolapse | No | No | 1 | Vaginal cysts; Vaginal neoplasms | Vaginal tumor resection | No |
Cell proliferation assay
Cell proliferation was measured using the colorimetric water-soluble tetrazolium salt cell counting kit-8 (CCK8) assay, according to the manufacturer’s instructions. Cells were seeded into 96-well plates (2×103 cells/well), and cell proliferation was evaluated every 24 h. The number of viable cells was assessed by measuring the absorbance at 450 nm using a DNM-9602 microplate reader (Pulang New Technology Corp. Beijing, China).
Enzyme-linked immunosorbent assay (ELISA)
The cell culture supernatants were collected. Levels of IL-6 in the culture supernatant were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit (Jrdun Biotechnology Co., Ltd., Shanghai, China), according to the manufacturer’s instructions. For each well, the absorbance at 450 nm was measured with a microplate reader. Concentrations of IL-6 were determined by comparing the optical density (OD) with the standard curve.
Reactive oxygen species (ROS) assay
Intracellular ROS was detected using a dichloro-dihydro-fluorescein diacetate (DCFH-DA) fluorescence probe (Beyotime, Shanghai, China). The cells were treated with DCFH-DA (10 μM) and incubated for 20 min at 37°C in a dark and humidified environment. The analysis was performed via flow cytometry.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the pelvic floor fibroblasts (3×105 cells/well), which were isolated from patients with POP and normal controls, with TRIzol reagent. RNA was reverse transcribed using a RevertAid First Strand cDNA Synthesis kit (Thermofisher Scientific, Waltham, MA, USA) before quantification with spectrophotometry. The GAPDH primers (accession number: NM_001256799.1) were as follows:
Forward primer: 5′-CACCCACTCCTCCACCTTTG-3′;
Reverse primer: 5′-CCACCACCCTGTTGCTGTAG-3′.
The primers for klotho (KL gene) (accession number: NM_004795.3) were as follows:
Forward primer: 5′-GTGCGTCCATCTGGGATACG-3′;
Reverse primer: 5′-TGTCGCGGAAGACGTTGTT-3′.
Quantitative real-time PCR (qRT-PCR) was performed using an Applied Biosystems Prism 7300 (Applied Biosystems, Foster City, CA, USA) sequence detection system with Maxima SYBR Green/ROX qPCR Master Mix, according to the manufacturer’s instructions. GAPDH was used as the internal control.
Western blot
Western blot was performed as previously described [25]. Briefly, fibroblasts (3×105 cells/well) that were isolated from patients with POP and normal controls were harvested and lysed and then treated with recombinant klotho protein at doses of 50 ng/mL and 500 ng/mL in the absence or presence of epidermal growth factor (EGF) (20 ng/ml) for 24 h, or with U0126 [27], a selective inhibitor of ERK1/2 (1 μM), for 24 h. The supernatants were collected by centrifuging the cell suspensions at 12000 rpm at a temperature of 4°C. After being denatured and fractionated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins were then transferred to polyvinylidene fluoride (PVDF) membranes and probed with antibodies to klotho, ERK1/2, phospho-ERK1/2, MMP-1, and MMP-3. GAPDH was used as an internal control. The results were analyzed by enhanced chemiluminescence (ECL).
Statistical analysis
All experiments were performed in triplicate. The results were expressed the mean ± standard deviation (SD). Data were analyzed with SPSS software (Chicago, IL, USA). Comparison between groups was performed using Student’s t-test and one-way analysis of variance (ANOVA). Differences were accepted as statistically significant with a p-value <0.05.
Results
Klotho protein expression was reduced in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP)
The expression of klotho in pelvic floor fibroblasts was compared between both patients with pelvic organ prolapse (POP) and normal subjects. Klotho expression was significantly reduced at both mRNA (p<0.001) and protein levels (p<0.01) in the fibroblasts from patients with POP. Specifically, klotho expression in fibroblasts from patients with POP decreased by more than 60% at the mRNA level and 40% at the protein level when compared with fibroblasts from normal controls (Figure 1A–1C).
Figure 1.
Reduced expression of klotho mRNA and protein in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. (A) Quantitative real-time polymerase chain reaction (qRT-PCR) for klotho in fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. (B) Western blot analysis for klotho protein in fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. The expression of GAPDH served as an internal control. (C) Quantitative results of Western blot analysis of fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from the normal control group. The asterisks indicate the significance of the differences (** p<0.01, *** p<0.001).
Levels of reactive oxygen species (ROS) and interleukin-6 (IL-6) were increased in pelvic floor fibroblasts from patients with POP
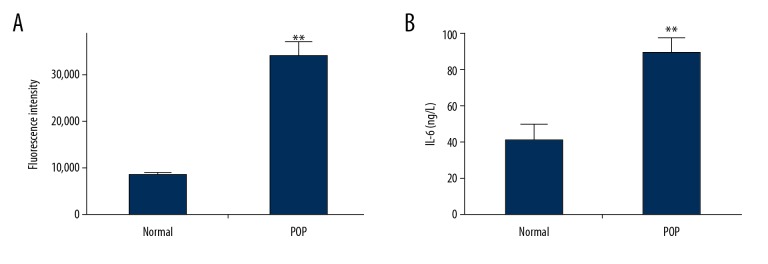
Levels of reactive oxygen species (ROS) was measured in pelvic floor fibroblasts. As shown in Figure 2A, patients with POP had more than three times the amount of ROS when compared with fibroblasts from normal controls, which indicated an increased level of oxidative stress in patients with POP in this study. As shown in Figure 2B, the secretion of IL-6 by fibroblasts from patients with POP was more than twice the amount of that found in control fibroblasts.
Figure 2.
Flow cytometry for the measurement of reactive oxygen species (ROS) and interleukin-6 (IL-6) in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. (A) Examination ROS levels in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) and normal control cells using flow cytometry using dichloro-dihydro-fluorescein diacetate (DCFH-DA). (B) IL-6 production increased in supernatants of cultured pelvic floor fibroblasts from patients with POP when compared with fibroblasts from the normal control group. The asterisks indicate the significance of differences (** p<0.01, *** p<0.001).
Klotho treatment of pelvic floor fibroblasts from patients with POP promoted cell growth, and down-regulated the expression of ROS and IL-6
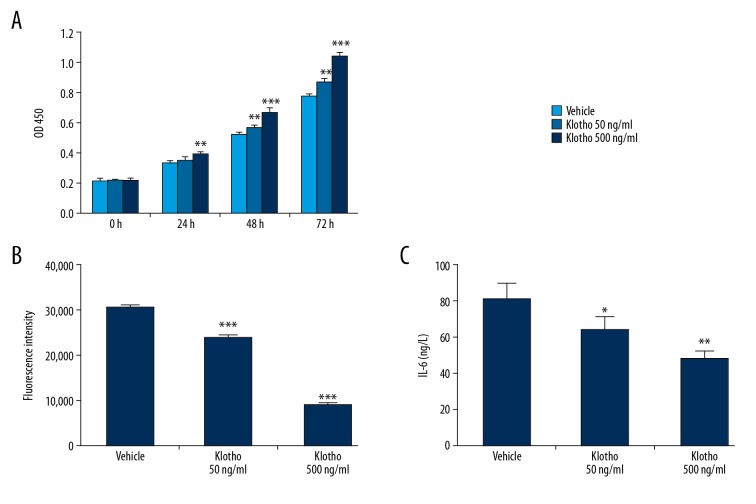
Pelvic floor fibroblasts in patients with POP were treated with recombinant klotho protein [15]. The effects of klotho on cell growth, ROS production, and IL-6 secretion were assessed. As shown in Figure 3A, klotho treatment significantly promoted cell growth in a dose-dependent and time-dependent manner during 72 h. Also, the administration of the klotho protein for 48 h significantly down-regulated levels of ROS (p<0.001) (Figure 3B) and IL-6 (p<0.05) (Figure 3C) in a dose-dependent manner.
Figure 3.
The effects of klotho supplementation on pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. (A) Supplementation with klotho protein increased fibroblast growth in a dose-dependent and time-dependent way for 72 hrs. (B) Treatment with klotho (50 ng/mL and 500 ng/mL) for 48 h significantly decreased the levels of reactive oxygen species (ROS). (C) Interleukin-6 (IL-6) production in supernatants was reduced following klotho administration (50 ng/ml and 500 ng/ml) for 24 h. The asterisks indicate the significance of the differences (* p<0.05, ** p<0.01, *** p<0.001).
Klotho treatment of pelvic floor fibroblasts from patients with POP reduced the expression of MMP-1 and MMP-3
As shown in Figure 4A and 4B, MMP-1 and MMP-3 expression were significantly increased in cells from patients with POP compared with those from normal controls. As shown in Figure 4C and 4D, the increased doses of klotho reduced the expression of MMP-1 and reduced the expression of MMP-3 protein, which is the activator of MMP-1 (Figure 4C, 4D). Both changes were significant and dose-dependent (p<0.001 and p<0.01).
Figure 4.
Western blot for the measurement of matrix metalloproteinase- (MMP-1) and MMP-3 in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. (A) The expression of MMP-1 and MMP-3 in pelvic floor fibroblasts in patients with or without pelvic organ prolapse (POP) measured by Western blot. (B) Quantitative results of the Western blot analysis in A. (C) Supplementation with klotho protein decreased the expression of MMP-1 and MMP-3 in pelvic floor fibroblasts in patients with POP. (D) Quantitative results of Western blot analysis in C. The asterisks indicate the significance of differences (** p<0.01, *** p<0.001).
Klotho treatment of pelvic floor fibroblasts from patients with POP reduced the phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2)
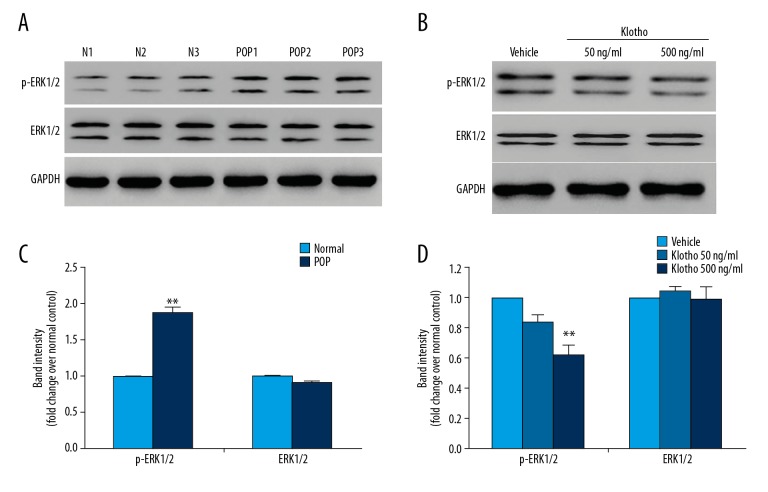
According to the findings from previous studies, ERK1/2 are known to be upstream signaling proteins and their activities are believed to be necessary for the induction of the MMP-1 protein expression [6,26]. As shown in Figure 5A and 5B, the phosphorylation of ERK1/2 at Thr202/Tyr204 was significantly increased in fibroblasts from patients with POP when compared with fibroblasts from controls (p<0.01). The total amounts of ERK1/2 were unchanged.
Figure 5.
Western blot for the measurement of phosphorylated ERK1/2 in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls. (A) The expression and phosphorylation of ERK1/2 in pelvic floor fibroblasts from patients with or without pelvic organ prolapse (POP) were measured using Western blot. (B) Quantitative results of the Western blot analysis from A. (C) Supplementation with klotho down-regulated the phosphorylation but not the expression of ERK1/2 in pelvic floor fibroblasts in fibroblasts from patients with POP. (D) Quantitative results of the Western blot analysis from C. The asterisks indicate the significance of differences (**p<0.01).
The effects of klotho treatment on ERK1/2 phosphorylation were investigated. As shown in Figure 5C and 5D, klotho supplementation significantly decreased the phosphorylation of ERK1/2 at Thr202/Tyr204, with a dose-dependent relationship (p<0.01) but did not significantly change the total amount of protein.
ERK1/2 mediated klotho regulation of MMP-1 expression in pelvic floor fibroblasts in patients with POP
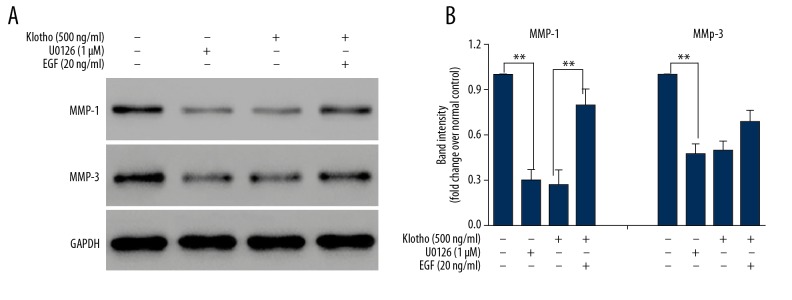
To confirm the intermediary role of ERK1/2 for the effect of klotho on MMPs, inhibition of ERK1/2 kinase activity was performed using the specific inhibitor, U0126 [27], and the activator, epidermal growth factor (EGF) [28]. When the effects of the inhibitor and activator on pelvic floor fibroblasts from patients with POP, compared with vehicle treatment (column A, Figure 6), incubation with U0126 [27], at a concentration of 1 μM for 24 h significantly reduced the amount of MMP-1 and MMP-3 proteins (column B, Figure 6), supporting the direct control of MMP expression by ERK. Treatment of the fibroblasts with 500 ng/ml of klotho (column C, Figure 6) produced similar results to treatment with U0126, which indicated that ERK mediated the effect of klotho. Also, the addition of EGF (column D, Figure 6) at a concentration of 20 ng/mL significantly inhibited the effects of klotho treatment (500 ng/mL) by increasing the expression of MMP-1, which indicated that klotho regulated MMP-1 expression, which was mediated by ERK1/2.
Figure 6.
Western blot for the measurement of matrix metalloproteinase- (MMP-1) and MMP-3 in pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) compared with fibroblasts from normal controls treated with klotho, U0126, and epidermal growth factor (EGF). (A) The effects of administration of klotho, U0126, and epidermal growth factor (EGF) on the expressions of MMP-1 and MMP-3 in pelvic floor fibroblasts in patients with pelvic organ prolapse (POP) were measured using Western blot. (B) Quantitative results of the Western blot analysis of A. The asterisks indicate the significance of differences (**p<0.01).
Discussion
The findings of this study showed that the expression of klotho was significantly down-regulated in the pelvic tissue fibroblasts collected from patients with pelvic organ prolapse (POP), at both the mRNA and protein expression levels. There is increasing evidence for the relationship between the expression of klotho proteins and POP, as both of them are associated with the aging process [16–19]. POP is more common in elderly women [16]. Klotho protein expression has been shown to have a role in the suppression of several aging phenotypes, including muscle atrophy [9].
In the present study, the levels of reactive oxygen species (ROS) and interleukin-6 (IL-6) were significantly increased in pelvic floor fibroblasts from patients with POP. Recent studies have shown that oxidative stress may be involved in the pathophysiology of POP by contributing to the degradation of collagen by regulating matrix metalloproteinases (MMPs), while the klotho protein increases resistance to oxidative stress [20–22]. These previous studies support the findings of the present study.
In this study, klotho treatment of fibroblasts from patients with POP that were grown in vitro showed significantly increased cell growth, reduction in ROS, and decreased IL-6 secretion. These findings suggest that klotho might increase resistance to oxidative stress and the inflammatory response in pelvic floor fibroblasts from patients with POP.
According to previous studies, as women age, the increases in intracellular levels of ROS constitute oxidative stress and cause cell and tissue injury [29]. Also, it has previously been reported that oxidative stress may play a key role in the development of POP [22,30]. Also, previous studies have shown that mRNA expression levels of IL-6, which is as an important inflammatory mediator, was significantly increased in patients with POP [31,32]. In the present study, fibroblasts from patients with POP treated with klotho resulted in cell resistance to oxidative stress and inflammation, which means that the findings were consistent with previous studies that have investigated the effects of klotho on other types of cells [20,21].
To explore the molecular basis of the underlying functions of klotho, this study focused on the protein family of matrix metalloproteinases (MMPs), which are known to be important to the pathogenesis of POP [6,7]. Among the MMPs, MMP-1 is known to be essential to the degradation of type I collagen, which is the most abundant protein in connective tissue [33]. The increasing production of MMP-1 could lead to a weakened pelvic floor and increase the susceptibility to POP. Given the significance of MMP-1 in the development of POP, the possible relationship between klotho and MMP-1 was investigated. The findings showed that klotho controlled the expression of MMP-1 and MMP-3. Specifically, treatment with soluble klotho protein significantly decreased the protein expression of MMP-1 and its activator MMP-3, which established the molecular connection between the pathophysiology of POP pathophysiology and the klotho protein. This finding was also consistent with the previous study by Vulic et al. [4], which showed an association between POP and MMP-1.
The results of the present study indicated that treatment of cultured fibroblasts from patients with POP with klotho protein resulted in the significant inhibition of extracellular signal-regulated kinases 1/2 (ERK1/2). However, the inhibition of ERK1/2 activity with a small-molecule inhibitor at a concentration of 1 μM for 24 hsignificantly reduced the amount of MMP-1 and MMP-3 proteins. Consistent with these results, previous molecular studies have shown that klotho regulates the ERK/MMP pathway [6,26]. In the present study, the addition of 20 ng/mL of epidermal growth factor (EGF) significantly counteracted the effects of treatment of fibroblasts with 500 ng/mL of klotho by significantly increasing the expression of MMP-1. These findings supported that the regulation of MMP-1 by the klotho protein could be mediated by ERK1/2 and that klotho treatment of fibroblasts in vitro protected fibroblasts from growth inhibition, oxidative stress, and inflammation. Also, klotho protein treatment down-regulated the expression of MMP-1 by inhibiting ERK1/2 activity in fibroblasts cultured in vitro. Although the mechanisms involved in the effects of klotho on ERK1/2 inhibition remain unknown, these findings provided new insights into the possibility of future therapeutic approaches for the treatment of POP.
Conclusions
The findings from this study compared pelvic floor fibroblasts from patients with pelvic organ prolapse (POP) and fibroblasts from normal controls without POP. Decreased expression of klotho mRNA was found in fibroblasts from patients with POP with significantly increased levels of metalloproteinase-1 (MMP-1) and MMP-3 and increased phosphorylation of ERK1/2. Treatment of fibroblasts with the age-suppressing protein, klotho, reduced the expression of MMP-1 and MMP-3 in fibroblasts from patients with POP by down-regulating the phosphorylation of ERK1/2.
Supplementary Figure
Intracellular expression of cytoskeleton proteins CK19 and vimentin using immunohistochemistry. Photomicrographs show representative images of the light microscopy of the immunohistochemistry for CK19 and vimentin. Intracellular expression of the CK19 was negative (A, B), and vimentin was positive (C, D), which highlights that these fibroblasts are interstitial cells. Scale bars: 100 μm.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China General Program (81571419) and the Interdisciplinary Program of Shanghai Jiao Tong University (YG2015MS40)
Conflict of interest
None.
References
- 1.Samuelsson EC, Victor FT, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180:299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- 2.Skorupski P, Jankiewicz K, Miotla P, et al. The polymorphisms of the MMP-1 and the MMP-3 genes and the risk of pelvic organ prolapse. Int Urogynecol J. 2013;24:1033–38. doi: 10.1007/s00192-012-1970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow D, Rodriguez LV. Epidemiology and prevalence of pelvic organ prolapse. Curr Opin Urol. 2013;23:293–98. doi: 10.1097/MOU.0b013e3283619ed0. [DOI] [PubMed] [Google Scholar]
- 4.Vulic M, Strinic T, Tomic S, et al. Difference in expression of collagen type I and matrix metalloproteinase-1 in uterosacral ligaments of women with and without pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;155:225–28. doi: 10.1016/j.ejogrb.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Piperi C, Papavassiliou AG. Molecular mechanisms regulating matrix metalloproteinases. Curr Top Med Chem. 2012;12:1095–112. doi: 10.2174/1568026611208011095. [DOI] [PubMed] [Google Scholar]
- 6.Alge-Priglinger CS, Kreutzer T, Obholzer K, et al. Oxidative stress-mediated induction of MMP-1 and MMP-3 in human RPE cells. Invest Ophthalmol Vis Sci. 2009;50:5495–503. doi: 10.1167/iovs.08-3193. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169–75. doi: 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 10.Shiraki-Iida T, Aizawa H, Matsumura Y, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 12.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–47. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 14.Erben RG. Update on FGF23 and Klotho signaling. Mol Cell Endocrinol. 2016;432:56–65. doi: 10.1016/j.mce.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Mencke R, Olauson H, Hillebrands JL. Effects of klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2017;121:85–100. doi: 10.1016/j.addr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Kinman CL, Lemieux CA, Agrawal A, et al. The relationship between age and pelvic organ prolapse. Int Urogynecol J. 2017;28:751–55. doi: 10.1007/s00192-016-3175-5. [DOI] [PubMed] [Google Scholar]
- 17.Dietz HP. Prolapse worsens with age, doesn’t it? Aust NZJ Obstet Gynaecol. 2008;48:587–91. doi: 10.1111/j.1479-828X.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113–22. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee EJ, Oh KW, Lee WY, et al. The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism. 2006;55:1344–51. doi: 10.1016/j.metabol.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389(3):233–41. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong S, Hong L, Li B, et al. The role of GPX1 in the pathogenesis of female pelvic organ prolapse. PLoS One. 2017;12:e0181896. doi: 10.1371/journal.pone.0181896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Chen HY, Wang XQ, Wang JX. Correlations between mitofusin 2 expression in fibroblasts and pelvic organ prolapse: An in vitro study. Chinese Med J. 2017;130:2951–59. doi: 10.4103/0366-6999.220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YS, Wang XJ, Feng W, Hua KQ. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-kappa B pathways. Int J Mol Med. 2017;40:987–98. doi: 10.3892/ijmm.2017.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 26.Westermarck J, Holmstrom T, Ahonen M, et al. Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promoter okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38. Matrix Biol. 1998;17(8–9):547–57. doi: 10.1016/s0945-053x(98)90107-x. [DOI] [PubMed] [Google Scholar]
- 27.Marampon F, Bossi G, Ciccarelli C, et al. MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol Cancer Ther. 2009;8:543–51. doi: 10.1158/1535-7163.MCT-08-0570. [DOI] [PubMed] [Google Scholar]
- 28.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 29.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Yang Q, Fang G, et al. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol Med Rep. 2016;13(4):2999–3008. doi: 10.3892/mmr.2016.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brizzolara SS, Killeen J, Urschitz J. Gene expression profile in pelvic organ prolapse. Mol Hum Reprod. 2009;15:59–67. doi: 10.1093/molehr/gan074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–77. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Ewies AA, Al-Azzawi F, Thompson J. Changes in extracellular matrix proteins in the cardinal ligaments of post-menopausal women with or without prolapse: A computerized immunohistomorphometric analysis. Hum Reprod. 2003;18:2189–95. doi: 10.1093/humrep/deg420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracellular expression of cytoskeleton proteins CK19 and vimentin using immunohistochemistry. Photomicrographs show representative images of the light microscopy of the immunohistochemistry for CK19 and vimentin. Intracellular expression of the CK19 was negative (A, B), and vimentin was positive (C, D), which highlights that these fibroblasts are interstitial cells. Scale bars: 100 μm.