Abstract
Background
The purpose of the present study was to investigate the function and mechanism of dihydroartemisinin (DHA) in treating ovalbumin-induced asthma in BALB/c mice.
Material/Methods
Thirty female BALB/c mice were randomly separated into 3 groups: the control group, the asthma model group stimulated by ovalbumin (OVA group), and the DHA treatment group (DHA group). The therapeutic effects and potential pharmacological mechanisms of DHA were specifically clarified by examining its effects on asthma-related phenomena, such as body weight, lung function, cell counts in bronchoalveolar lavage fluid (BALF), and hemotoxin and eosin staining. In addition, the expression of inflammatory factors was checked by enzyme-linked immunosorbent assay kits, and fractions of Th17 cells were detected by FACS analysis. Moreover, the downstream molecular pathway of IL-6/Stat3 (interleukin-6/signal transducer and activator of transcription 3) and expression of miR-183C was investigated by western blot and/or quantitative real-time polymerase chain reaction. Luciferase assay was used to reveal the function of miR-183C on the transcriptional regulation of Foxo1 (forkhead box O).
Results
DHA administration significantly relieved the severity of the asthma through its effect on body weight, survival rate, and airway pressure. DHA was able to ameliorate lung damage in terms of pathological morphology and it reduced the percentage of helper T 17 (Th17) cells and the secretion of cytokines. As a result, the activity of the IL-6/Stat3 pathway was inhibited by DHA. In addition, the adoption of DHA decreased the expression of miR-183C but increased the expression of the transcription factor Foxo1.
Conclusions
Our results suggest that the therapeutic effects of DHA on asthma are partially realized via the regulation of miR-183C and IL-6/Stat3 pathway.
MeSH Keywords: Artemisinins, Asthma, Forkhead Transcription Factors, MicroRNAs, Th17 Cells
Background
Dihydroartemisinin (DHA) is the main active metabolite of the Chinese traditional herb medicine-Artemisinin, which is a chemical compound isolated from the plant of Artemisia annua L. [1,2]. With the typical structure of a sesquiterpenoid lactone, DHA has been studied to develop its biological potential in bench work and clinical trials [3]. Therapies based on artemisinin are the best treatments for malaria [4,5]. Previous reports have shown that artemisinin and its derivatives are promising natural products to induce apoptosis in hepatocarcinoma cells by regulating the Bim-mediated intrinsic signaling pathway [6], in human breast cancer MCF-7 cells by arresting the cell cycle at the G1 phase [7], in human glioblastoma LN-229 cells by enhancing oxidative DNA damage through the ATM/ATR damage response [8], and in non-small cell lung cancer A549 cells by increasing reactive oxygen species (ROS) via caspase 3, caspase-9, and Bcl-xL [9]. In addition, artemisinin is able to mitigate amyloidogenesis and neuroinflammation via inhibition of nuclear factor-κB (NF-κB) and NLR family pyrin domain containing 3 (NLRP3) inflammasome activation [10], and activate natural killer (NK) cells for tumor killing in K562 cells [11]. A derivative of artemisinin, SM933, exhibits anti-inflammatory properties by inhibiting encephalitogenic T cell responses and reducing the production of nitric oxide [12]. Artemisinin relieves the severity of inflammation in caerulein-induced acute pancreatitis [13] and attenuates lipopolysaccharide-induced inflammatory responses by blocking the NF-κB pathway in microglia and in phorbol 12-myristate 13-acetate (PMA)-stimulated THP-1 monocytes [14,15]. The anti-tumor and inflammation function of artemisinin has been enormously investigated, while its function in airway inflammation inhibition is still unclear.
Allergic asthma is one of the most notorious airway inflammatory disorders, which is characterized by reversible and recurrent airway obstruction accompanied with airway inflammation, mucus hypersecretion, and airway hyper-responsiveness (AHR) [16]. Cumulative clinical and experimental evidence reveal that typical inflammatory responses are regulated by the dynamic Th1/Th2 (T helper) and Th17/Treg (T regulatory) cell balance [17]. In some pathogenic systems, the fraction of Th1/Th2 or Th17/Treg may dominate the process, and thus may play a pivotal role in the contribution to the pathogenesis of asthma [18,19]. Recent advances on Th17 cells have shed light on the mechanisms that are important in lowering the immunoreactivity in asthma [20–22]. Upstream expression of interleukin-6 (IL-6) and IL-23 activates the signal transducer and activator of transcription 3 (Stat3), and thus induces the transcription of the steroid receptor type nuclear receptor (RORγt) to promote the differentiation of Th17 cells [23]. Transcriptional factors in the forkhead box O (Foxo) family are involved in multiple cellular responses, especially in the immune system [24]. Foxo transcription factors enhance the expression of the Foxp3 gene and thus promote the induction of Treg cells [25]. Foxo1 inhibits the generation of follicular T helper cells and IL-17A excretion from memory T cells [26,27]. High doses of IL-6 in naïve T cells, together with transforming growth factor beta (TGF-β), reciprocally inhibit the activity of FoxP3 and enhance Th17 differentiation [28]. The activated Th17 cells secrete many inflammatory cytokines, such as IL-17A, IL-17F, IL-21, and IL-22. The combined cytokines result in the excretion of IL-1β, IL-6, IL-8, TNF, IFNγ, granulocyte macrophage colony-stimulating factor (GM-CSF), and several chemokines [29–31].
In addition to the interleukins and transcription factors, a large number of biological molecules have been reported in regulating the differentiation and function of Th cells. MicroRNAs (miRNAs) are a class of small, noncoding and regulatory RNAs that post-transcriptionally downregulate gene expression [32]. Published studies have verified the vital role of certain miRNAs in Th cell differentiation and function and in airway immune responses [33]. The miR183-96-182 cluster (miR-183C) contains 3 members, miR-183, miR-96, and miR-182, which are located in the intergenic region within 4413 bp on human chromosome 7q32.2 and are transcribed in the same direction with independent promoters. The expression of miR-183C is coordinated and involved in physiology and pathology, especially in tumor and syndromic retinal degeneration [34,35]. However, the synergic functions on asthmatic immune responses are still not well elucidated.
Here, we show that DHA ameliorates the pathology in the OVA-induced mouse model and inhibits the fraction of pathogenic Th17 cells. Furthermore, miR-183C may negatively regulate the transcriptional ability of Foxo1. Collectively, our results demonstrate a critical role for DHA and miR-183C in OVA-induced asthma.
Material and Methods
Animals and treatments
Female BALB/c mice aged 5 weeks were purchased from the Laboratory Animal Center of Nantong University. The mice were housed and treated under pathogen free conditions. Animal care and experimental protocols were approved by the Animal Welfare Committee of Nantong University (20180312-001). Mice were randomly separated into 3 groups and received different challenges. In the control group (Control), the mice were immunized and challenged by phosphate-buffered saline (PBS) alone. In the asthmatic model group (Model), the mice were immunized and challenged by ovalbumin (OVA) (Sigma Aldrich, USA). In the dihydroartemisinin treated group (DHA), the mice were immunized and challenged by OVA followed by DHA treatment.
The mice were sensitized with emulsified 200 μL PBS solution containing 20 μg of OVA and 2 mg of aluminum hydroxide (Thermo Fisher Scientific, USA) by intraperitoneal injection on day 1 and day 14. The mice then underwent 5% OVA inhalation for 25 minutes once a day from day 21 to 49.
The mice were euthanized 24 hours after the final challenge, followed by collecting serum and bronchoalveolar lavage fluid (BALF), and the lungs and spleens for subsequent analysis.
DHA administration
DHA (Cat No: D831931, CAS: 71939-50-9, Formula=C15H24O5, MW=235.84, purity over 98%, density=1.24 g/cm3) is the active metabolite of artemisinin compounds and was bought from MACKLIN company. The compound was dissolved in dimethyl sulfoxide (DMSO) and diluted with PBS. DHA (50 mg/kg, intraperitoneal injection) was administrated 1 day before booster immunization. The mice were injected once a day and 2 days in a row, then rested for 1 day. In general, the mice were injected 5 times per week to assess the preventive effects of DHA on asthma.
Histological analysis
The mice were perfused by saline followed by 4% paraformaldehyde (PFA), and the lungs were dissected and soaked in 4% PFA overnight at 4°C. The tissues underwent gradient dehydration with 30%, 50%, 70%, 80%, 90%, 95%,%100, and 100% ethanol for 1 hour each. After immersing in xylene for 30 minutes twice to make the tissues transparent, the tissues were placed in wax for 2 hours twice and then were embedded for preparing 5 μm sections. After dewaxing and rehydration, the slides were stained in Harris hematoxylin solution and eosin solution. Peribronchial inflammatory infiltration was assessed and graded semi-quantitatively using lung inflammatory scores [36]. The pictures were taken by an Olympus DP71 microscope and then processed by ImageJ software.
Lung function evaluation and collection of bronchoalveolar lavage
The mice were anesthetized (pentobarbital sodium, 70 mg/kg intraperitoneal) and fastened on a hot pad for airway hyper-responsiveness (AHR) detection as previously described [37,38]. The mice were tracheostomized with 18G cannula ventilator (RWD Inc., Shenzhen, China) and the tidal volume of 0.2 mL was set with a positive end-expiratory pressure of 5 cmH2O. The ratio of inspiratory time/expiratory time was 1: 2. Mice were then administrated increasing concentrations of methacholine (MCh) (3.125, 6.25, 12.5, 25, and 50 mg/mL) by a body plethysmograph (Buxco Electronics, Inc., Wilmington, NC, USA). Data were presented as airway resistance (RI) and dynamic compliance (Cdyn).
After determining the AHR, the lungs and bronchia were lavaged by gentle flushing with 1 mL PBS containing 1 mM EDTA through syringe for 3 times. BALF cells were isolated by centrifugation at 1200 rpm for 5 minutes, and then were stained with Diff-Quik and Wright Giemsa (Solarbio Inc., Beijing, China). Five views on each slide were counted with a light microscope. In addition, the BALF cell supernatant was harvested and stored at −80°C for subsequent enzyme-linked immunosorbent assay (ELISA) analysis.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of inflammatory cytokines (IL-17, IL-10, IL-1β, and TNFα) and Th17 cytokines (IFN-γ, IL-17F, IL-17A, IL-22, IL-10, Il-21, and GM-CSF) in BALF were measured with ELISA kits based on the instructions of the manufacturers. ELISA kits were supplied by BD Biosciences, R&D Systems, and Abcam.
Flow cytometry analysis
Flow cytometry analysis was used to check the ratio of CD4+IL-17+ cells. Cells were harvested by centrifugation from BALF and then fixed. After staining with an anti-CD4-PE mAb (eBioscience, USA) the cells were permeabilized and stained with anti-IL-17-APC mAb (eBioscience, USA) Data were acquired through BD FACSCalibur and were analyzed with CellQuest.
Western blot analysis
Lungs were dissected out and washed with pre-cooled PBS twice and dried with an absorbent paper quickly, and then the tissues were homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer (Epizyme, Shanghai, China) containing the protein inhibitor cocktail and phosphatase inhibitor cocktail (Epizyme, Shanghai, China). Extracted protein was diluted to 1.5 μg/μL with loading buffer and boiled in a 100°C water bath for 10 minutes. The target proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA) in an ice bath electrophoresis trough. The membranes were blocked with 5% non-fat milk in tris-buffered saline with 1‰ Tween 20 (TBST) for 2 hours at room temperature. Primary antibodies (IL-6, total Stat3, Stat3-Tyr705, Foxo1, and GAPDH; Abcam, MA, USA) were diluted according to the guidance of the manufacturer in 5% bovine serum albumin (BSA) solution and incubated with the membranes overnight at 4°C. The unbundled antibodies were washed with TBST and then incubated with HRP-conjugated secondary anti-rabbit/mouse antibody (1: 100 000, Vazyme, Nanjing, China) for 2 hours at room temperature. The lanes were detected by a Bio-Rad image system with enhanced chemiluminescent western blotting substrate (Vazyme, Nanjing, China). Pictures were analyzed with ImageJ software, and the relative protein expression level was calculated using GAPDH as a control.
Real-time polymerase chain reaction (RT-PCR) analysis
Total RNA from lung tissues was extracted with TRIzol reagent (Vazyme, China) lysis. For mRNA analysis, the cDNAs were synthesized with HiScript II QRT SuperMix for qPCR (+gDN A wiper) (Vazyme, China) from 1 μg RNA and analyzed with Stepone plus (Applied Biosystems, USA). The primers were obtained from Genescript Biotech (Nanjing, China) and checked for the specificity. GAPDH was used as the internal reference control. The following genes were checked with primers listed below:
Gapdh, 5′-ACCACAGTCCATGCCATCAC-3′ and
5′-TCCACCACCCTGTTGCTGTA-3′;
il6, 5′-CCAAGAGATAAGCTGGAGTCACA-3′ and
5′-CGCACTAGGTTTGCCGAGTA-3′;
il-10, 5′-GGCGCTGTCATCGATTTCTC-3′ and
5′-ATGGCCTTGTAGACACCTTGG-3′;
Tnfα, 5′-AGGCACTCCCCCAAAAGATG-3′ and
5′-CCAC TTGGTGGTTTGTGAGTG-3′;
stat3, 5′-GTTGGAGCAGCATCTTCAGG-3′ and
5′-GCATGTCTCCTTGGCTCTTG-3′;
il17a, 5′-CAGCAG CGATCATCCCTCAAAG-3′ and
5′-CAGGACCAGGATCTCTTGCTG-3′;
il17f, 5′-CCCATGGGATTACAACATCACTC-3′ and
5′-CACTGGGCCTCAGCGATC-3′;
il22, 5′-CATGCAGGAGGTGGTACCTT-3′ and
5′CAGACGCAAGCATTTCTCAG-3′;
il1r1, 5′-AAGCTGACCCAGGATCAATG-3′ and
5′-T GGTTGAAGGATGTTCCACA-3′;
Rorc, 5′-ACCTCCACTGCCAGCTGTGTGCTGTC-3′ and
5′-TCATTTCTGCACTTCTGCATGTAGACTGTCCC-3′;
Foxo1, 5′-CGGGCTGGAAG AATTCAATTC-3′, and
5′-AGTTCCTTCATTCTGCACTCGAA-3′.
The relative expression of the genes was normalized using GAPDH.
For miRNA analysis, cDNA was prepared with miRNA 1st Strand cDNA Synthesis Kit (Vazyme, China) and analyzed with 7500 Real-time PCR system and miRNA Universal SYBR qPC R Master Mix (Vazyme, China). The relative expression was normalized to a U6 snRNA control. The primer sequences were as follows:
U6 snRNA, 5′-TGGCCCCTGCGCAAGGATG-3′;
miR-183-5p, 5′-TATGGCACTGGTAGAATTCACT-3′;
miR-182-5p, 5′-TTTGGCAATGG TAGAACTCACACCG-3′;
miR-96-5p, 5′-TTTGGCACTAGCACATTTTTGCT-3′.
Luciferase assay
The BALB/c mice were sensitized with ovalbumin (OVA) and then used for OVA-primed splenocyte preparation according to the protocol as described previously [39]. Briefly, 100 μg OVA and 1 mg aluminum in 0.25 mL saline was administrated by subcutaneous injection in BALB/c mice on day 0 and 14. The spleens of the mice were dissected and digested into single cell suspensions on day 15. The splenocytes were cultured in RPMI 1640 with penicillin and streptomycin and supplied with 5% heat-inactivated fetal bovine serum (FBS) in the incubator (37°C, 5% CO2). Splenocytes (5×106 cells/mL) were seeded on 24-well plates and transfected with various molecules (mimic, miR-183C, 3′-UTR wild-type and mutant; Ribobio, China) with Lipofectamine 3000 (ThermoFisher, USA) based on the manufacture’s instruction, or with DHA at a final concentration of 10 uM. The Firefly and Renilla luciferase activity were measured with a dual-luciferase reporter system (Promega, USA) at 24 hours after treatment. Data were collected and normalized to the activity of Renilla luciferase.
Statistical analysis
All data were processed with Microsoft Excel 2016 and expressed as the mean ± standard error (SEM) for each group and analyzed using SPSS 19.0. The difference between groups were compared via non-parametric tests and one-way analysis of variance. P<0.05 was considered as statistically significant.
Results
The administration of DHA ameliorated the severity of OVA-induced asthma and improved lung function
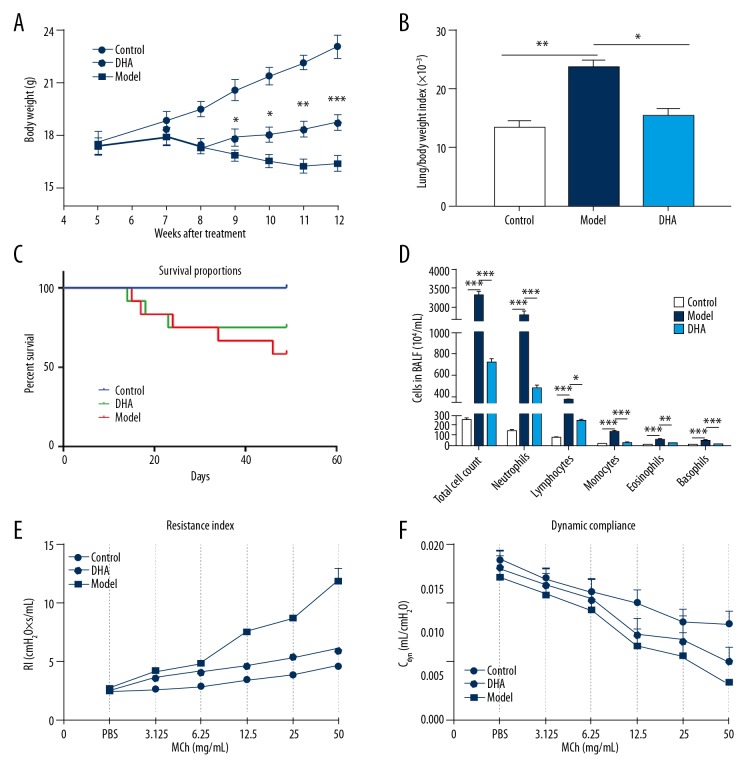
In the present study, an OVA-induced asthmatic BALB/c mouse model was developed by OVA injection and inhalation. The body weight of the mice dropped drastically after the second injection of OVA and kept decreasing after the booster immunization until 11 weeks old. DHA treatment significantly increased body weight when compared with the OVA challenged group from 9 weeks old (Figure 1A). A large difference in body weight was observed between the model group and DHA group mice (Figure 1A). OVA inhalation led to a significant increase in the lung/body weight index compared to the control mice. DHA treatment significantly decreased the lung/body weight index (Figure 1B). OVA challenge significantly decreased the survival rate, but DHA reversed the reduction (Figure 1C). OVA stimulation significantly enhanced basophils, eosinophils, monocytes, neutrophils, lymphocytes, and the total cell counts in the BALF in the model group compared to the control group. While administration of DHA significantly reduced the elevated cell populations in the model group, the levels were still significantly higher when compared with the control group (Figure 1D).
Figure 1.
Administration of DHA improves the systematic conditions of OVA-induced asthma. (A) Body weight of mice in the Control, Model, and DHA groups during the induction and treatment period. (B) The ratio of lung and body weight of mice in the 3 groups (n=10). (C) Survival rate of mice in the 3 groups (n=15). (D) Cell count in BALF (n=4). (E) Resistance index (RI) and (F) dynamic compliance to double multiplication concentration of methacholine (3.125, 6.25, 12.5, 25, and 50 mg/mL) and PBS were measured (n=10). * P<0.05, ** P<0.01, *** P<0.001. DHA – dihydroartemisinin; OVA – ovalbumin; BALF – bronchoalveolar lavage fluid; PBS – phosphate-buffered saline.
To assess the role of DHA on lung function in the OVA-sensitized and challenged asthma model, the airway hyper-responsiveness of mice was checked under the stimulation of methacholine. The anesthetized mice were first challenged with PBS, then with an increasing concentration of methacholine of 3.125, 6.25, 12.5, 25, and 50 mg/mL. Significant differences were recorded in airway resistance (RI) and compliance between the control group and the model group mice (Figure 1E, 1F). In comparison with the model group, the DHA treated group showed marked reduction in the airway resistance at 6.25 to 50 mg/mL concentration of methacholine (Figure 1E), and in dynamic compliance (Cdyn) between 12.5 and 50 mg/mL concentrations (Figure 1F). In line with this observation, DHA alleviated the symptoms of the asthmatic mice and partially restored lung function.
DHA treatment decreased inflammation and Th17 cell response
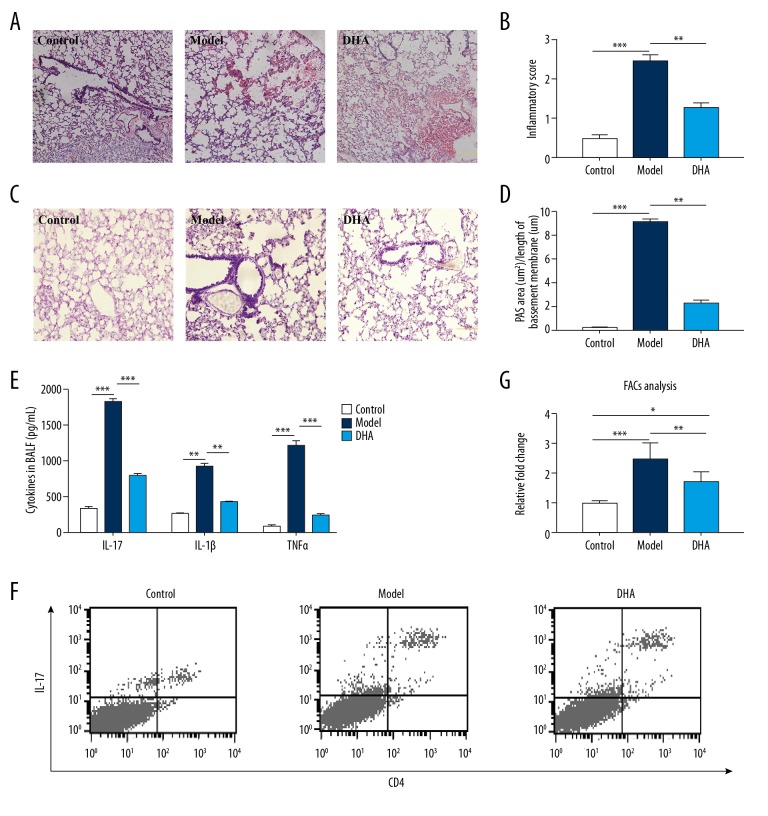
The OVA-induced asthmatic mouse model showed enhanced histological inflammatory scores and exhibited typical pathological features with enormous inflammatory cell infiltration at multiple sites (Figure 2A). DHA treatment attenuated the peribronchial and perivascular inflammation in the lung tissue (Figure 2A, 2B). The mucus production indicated by Periodic acid–Schiff (PAS) staining was also significantly increased in OVA-stimulated mice, while the administration of DHA significantly decreased the PAS positive areas (Figure 2C, 2D). Th17 cells play an important pathogenic role in immune response and disease, local inflammation, and tissue destruction. OVA stimulation boosted the level of IL-17, IL-1β, and TNFα (Figure 2E). In addition, DHA interference significantly diminished the level of IL-17, IL-1β, and TNFα, (Figure 2E). To estimate the proportion of Th17 cells, the CD4 and IL-17 dual positive cells in BALF were checked by FACs and calculated (Figure 2F). OVA sensitization augmented the percentage of pathogenic cells, whereas DHA drastically decreased the ratio (Figure 2G). These results imply that DHA ameliorated the severity of asthma and may function via reduction of the Th17 cell response.
Figure 2.
DHA treatment ameliorates lung function and inflammation by lowering Th17 cytokine secretion and cells in BALF. (A) Micrographs of H&E staining of lung tissue sections (200× magnification, scale bar=50 μm, n=5). (B) Statistics for inflammation score of 2A. (C) Micrographs of PAS staining of lung tissue sections (200× magnification, scale bar=50 μm, n=5). (D) Statistics for degree of mucus accumulation [PAS+ area (μm 2) divided by the length of basement membrane (μm)] of 2C. (E) Inflammatory cytokine (IL-17, IL-10, IL-1β, and TNFα) changes in BALF in the Control, Model, and DHA group mice by ELISA (n=7). (F) Representative density plots of CD4+IL-17+ cells in BALF are shown (n=5). (G) Relative ratio of CD4+IL-17+ cells in 2D.* P<0.05, ** P<0.01, *** P<0.001. BALF – bronchoalveolar lavage fluid; H&E – hemotoxin and eosin; PAS – periodic acid-Schiff; DHA – dihydroartemisinin; OVA – ovalbumin.
DHA might ameliorate asthma via inhibition of the IL-6/Stat3 pathway
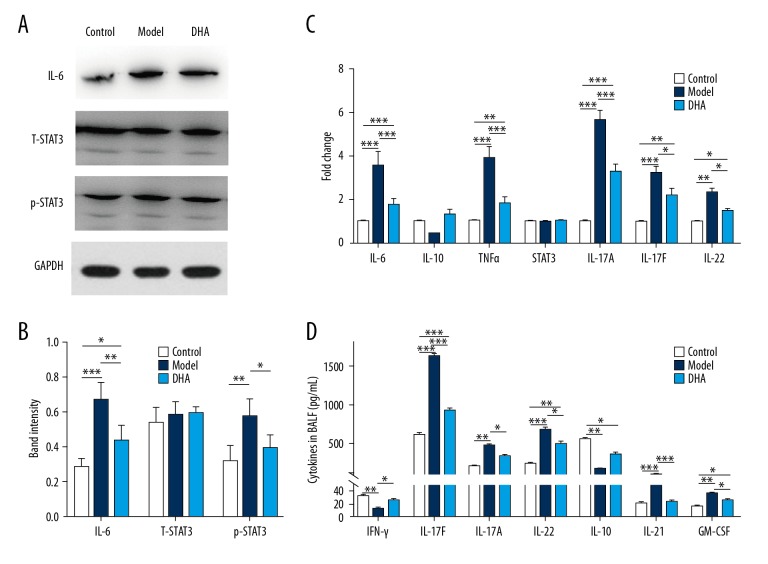
IL-6 stimulation could promote the pathologic effects of the Th17 cells, and Stat3 is an important transcriptional factor that facilitates the production of Th17 cell cytokines. OVA inducement enhanced the expression level of IL-6 protein (Figure 3A), but the level was significantly decreased by DHA treatment (Figure 3A, 3B). While the expression level of total Stat3 did not exhibit statistical difference among the 3 groups, phosphorylated Stat3 (Tyr705) increased in the model group and the augmentation was abolished by the addition of DHA (Figure 3A, 3B). To determine the role of DHA in Th17 cell differentiation and development, the transcriptional level variations were checked and the expression level of il6, Tnfa, il17a, il17f, and il22 were significantly exaggerated under the OVA induction when compared to the control group, while DHA treatment significantly diminished the expression level (Figure 3C). Moreover, the expression level of stat3 showed no statistical significance (Figure 3C). Th17 cells are important for autoimmune diseases, and the cytokines they secrete play critical roles in the pathologic processes of asthmatic lungs. The content of IL-17F, IL-17A, IL-22, IL-21, and GMCSF in BALF were determined by ELISA kits and were significantly improved in the model group mice (Figure 3D). However, the content of IFN-γ and IL-10 was significantly decreased after the use of OVA but was enhanced after the administration of DHA (Figure 3D). These results suggest that DHA may inhibit the Th17 cell response through the IL6/Stat3 pathway.
Figure 3.
DHA inhibits the IL-6/Stat3 pathway in OVA-induced asthmatic mice. (A) Protein expression of IL-6, total Stat3, and p-Stat3 (Tyr705) in lung tissues (n=5). (B) Relative band intensity of IL-6, T-Stat3, and p-Stat3 (Tyr705) in 3A. (C) Expression of inflammatory factors (il6, il10, tnf, il17a, il17f, il22) and transcription factor Stat3 in lung tissues were measured by qRT-PCR (n=7). (D) Cytokines in BALF (IFN-γ, IL-17F, IL-17A, IL-22, IL-10, Il-21, and GM-CSF) were measured by ELISA (n=7). * P<0.05, ** P<0.01, *** P<0.001. DHA – dihydroartemisinin; OVA – ovalbumin; qRT-PCR – quantitative real-time polymerase chain reaction; ELISA – enzyme-linked immunosorbent assay.
DHA inhibited the expression of miR-183C and Foxo1 pathway
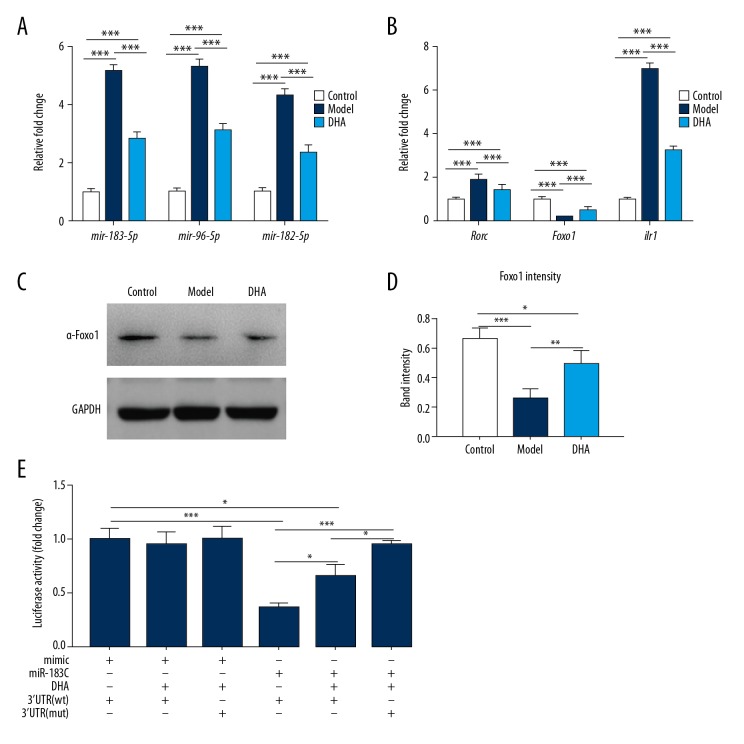
Recent studies have shown that the miR-183C and Foxo1 pathway interact with the IL-6 signaling pathway. The transcriptional levels of miR-183-5p, miR-96-5p, and miR-182-5p were significantly increased in OVA-induced asthmatic mice in our study (Figure 4A), but the expression levels of the miRNAs were significantly decreased after the administration of DHA (Figure 4A). Moreover, OVA exertion boosted the expression of Rorc and il1r1, while DHA injection diminished the difference (Figure 4B). The expression levels of the transcriptional factor Foxo1 were significantly decreased at both the transcriptional level (Figure 4B) and translational level (Figure 4C), and DHA injection enhanced the expression (Figure 4D). The results of luciferase assay revealed that miR-183C inhibited the transcriptional ability of Foxo1 and the function was significantly abolished by DHA (Figure 4E), but miR-183C showed no effect on mutant Foxo1 3′-UTR (Figure 4E). In summary, DHA administration almost inhibited the expression of miR183C and lowered the expression of Rorc and il1r1. In addition, Foxo1 was found to be critical in DHA function.
Figure 4.
DHA inhibits the expression of miR-183C and Foxo1 pathway. (A) The expression of mir-183-5p, mir-96-5p, and mir-182-5p in the lungs of the treatment groups (n=7). (B) The expression of rorc, foxo1 and il1r1in the lungs of treated mice were checked by qRT-PCR (n=7). (C) Protein expression of Foxo1 in lungs was determined by western blot (n=5). (D) Band intensity of Foxo1 in 4C. (E) Luciferase assay for the DHA regulation on Foxo1 transcription by targeting miR-183C (n=7). * P<0.05, ** P<0.01, *** P<0.001. DHA – dihydroartemisinin; qRT-PCR – quantitative real-time polymerase chain reaction.
Discussion
Allergic asthma is a kind of chronic, non-communicable disease in children and adults with high incidence, and the global prevalence in adults is 4.3% which means nearly 334 million people suffer around the world [40]. The highest prevalence is observed in developed countries (21% in Australia), while the lowest is in developing countries (0.2% in China) [41]. The asthma burden tends to worsen the economic situation of asthma patients and public health funding. Corticosteroids are universally used as inhaled medication that lower mortality of patients. Although glucocorticoids have been documented to possess potent anti-inflammation and immunosuppression, the resistance profile and other adverse effects limit its long-term use. Whereas dihydroartemisinin (DHA), a semi-synthetic derivative of artemisinin that is extracted from the traditional Chinese herb Artemisia annua L., has been shown to ameliorate inflammation in experimental autoimmune encephalomyelitis mice by its reciprocal regulation on Th and Treg cell function via attenuating the mTOR pathway [42]. Especially, US Patent (2012/0015922) demonstrated that artemisinin derivatives exhibited a pivotal role in treating asthma and chronic obstructive pulmonary disease (COPD). Another report showed that DHA suppressed ovalbumin (OVA)-induced airway inflammation in an allergic asthma mouse model by inhibiting the phosphorylation level of the extracellular signal-regulated protein kinase (ERK) and p38 mitogen-activated protein kinase, and also prohibited the activity of NF-κB [43]. However, the precise underlying mechanism that is critical for the function of DHA on anti-inflammation in murine models is still unknown.
In the current research, the effectiveness of DHA for control of chronic inflammation in an OVA-induced asthma mouse model and related inflammatory factors was evaluated. OVA injection and inhalation drastically decreased the body weight of mice in the model group when compared to the control group mice. DHA introduction significantly regained the body weight when compared to mice in the model group (Figure 1A). Meanwhile, administration of DHA significantly reversed the morbid elevation of the lung/body weight ratio which was mainly due to the decrease in body weight loss (Figure 1B). Mice treated with DHA survived more than those in the model group, which might be because of a systematic enhancement of their body condition (Figures 1C, 2A). Previous studies revealed that infiltrated inflammatory cells and secreted cytotoxic proteins might account for the severity of allergic asthma [44]. Certain immune cells, such as neutrophils, lymphocytes, monocytes, eosinophils, and basophils, were significantly reduced in BALF with DHA administration, as well as reduced in lung tissue (Figures 1D, 2C). In addition, DHA alleviated the pathology of the OVA-induced lungs and also partially recovered lung function, as shown by decreased airway resistance index (RI) and increased dynamic compliance (Cdyn) (Figure 1E, 1F). The results in our study might hint at an obvious infiltration of inflammatory cells in lung tissue that was attenuated by treatment with DHA.
Naïve CD4+ T cells are activated when challenged by antigens and thus regulate the adaptive immune response [45]. Numerous reports have confirmed a correlation between Th17 cells and asthmatic inflammation. Together with their own secreted cytokines IL-17A, IL-17F, and IL-22, Th17 cells are involved in many biological processes such as autoimmune diseases and host versus extracellular microbiota [46]. Studies have suggested that various induction conditions might be responsible for the difference of pathogenicity of Th17 cells. Many more pathogenic Th17 cells were observed in the differentiation system containing IL-1β, IL-6, and IL-23 when compared to compartment conditions that included TGF-β and IL-6 [47]. In the present study, cytokines related to Th17 cells were significantly increased in the BALF in OVA challenged mice but were reversed by administration of DHA (Figure 2C). The drastically enhanced concentration of cytokine IL-17 indicates there is a special impact of Th17 cells in the process. Since the fraction of Th17 cells was significantly decreased by treatment with DHA, a conclusion can be deduced that these cells might be responsible for the pathogenicity of the lung tissues (Figure 2D, 2E). Our results showed that Th17 cell differentiation was negatively regulated by many transcription factors, for example, the signal transducer and activator of transcription 3 (Stat3). Taken together, the translational level of total and phosphorylated Stat3 (Tyr705) and IL-6 were examined in our study (Figure 3A, 3B). Combined with both the transcriptional variation and cytokine alteration in the BALF, the IL-6 signaling pathway may take part in the Th17 cell differentiation (Figure 3C, 3D).
The synergistic expression pattern of miR-183, miR-96, and miR-182 led us to investigate their function in asthma development and recovery (Figure 4A). There are 4 members in the Foxo family, including Foxo1, Foxo3, Foxo4, and Foxo6, and they have been reported to combine with the same motif sequence but with different biological roles. Foxo1 was identified as a major regulator in Th17 cell differentiation and activation. Reports have suggested that miR-183C targets all Foxo transcription factors in Th17 and Treg cell differentiation [48]. The variation of Foxo1 was checked in this study and the results implied certain functions related to DHA treatment (Figure 4C, 4D) [49]. The ability of miR-183C to inhibit Foxo1 was partially abolished by the addition of DHA, while no obvious difference was recorded with the mutant Foxo1 fragment. Collectively, Foxo1 participates in the functional regulation of the Th17 mediated immune response by miR-183C. Therefore, further assessment on the specific mechanism of the regulation between Foxo1 and miR-183C should be based on genetically modified animal models.
Conclusions
Our data suggests that DHA administration attenuates the immune response of the OVA-induced allergic asthma mouse model. In addition, the demonstrated reversal ability might rely on the regulation of the expression of miR-183C, and might be sequentially influenced by the transcriptional activity of Foxo1. Moreover, additional investigations and clinical studies are still needed to verify these effects.
Footnotes
Source of support: Departmental sources
References
- 1.Woodrow CJ, Haynes RK, Krishna S. Artemisinins. Postgrad Med J. 2005;81:71–78. doi: 10.1136/pgmj.2004.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercereau-Puijalon O, Fandeur T. Antimalarial activity of artemisinins: Identification of a novel target? Lancet. 2003;362:2035–36. doi: 10.1016/S0140-6736(03)15146-X. [DOI] [PubMed] [Google Scholar]
- 3.Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–32. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:1807. doi: 10.1056/NEJMoa0808859. author reply 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bin LI, Hong Z. Advances on the study of the pharmacological effects of artemisinin and its derivatives. Chinese Journal of Clinical Pharmacology & Therapeutics. 2010;5:572–76. [in Chinese] [Google Scholar]
- 6.Qin G, Zhao C, Zhang L, et al. Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis. 2015;20:1072–86. doi: 10.1007/s10495-015-1132-2. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Li G, Yang X, et al. Design, synthesis and biological evaluation of artemisinin derivatives containing fluorine atoms as anticancer agents. Bioorg Med Chem Lett. 2018;28(13):2275–78. doi: 10.1016/j.bmcl.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Bedell N, Nikolova T, Quirks S, et al. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther. 2011;10:2224–33. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 9.Zhao C, Gao W, Chen T. Synergistic induction of apoptosis in A549 cells by dihydroartemisinin and gemcitabine. Apoptosis. 2014;19:668–81. doi: 10.1007/s10495-013-0953-0. [DOI] [PubMed] [Google Scholar]
- 10.Shi JQ, Zhang CC, Sun XL, et al. Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neurosci Ther. 2013;19:262–68. doi: 10.1111/cns.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houh YK, Kim KE, Park S, et al. The effects of artemisinin on the cytolytic activity of natural killer (NK) cells. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071600. pii: E1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Qiu J, Guo TB, et al. Anti-inflammatory properties and regulatory mechanism of a novel derivative of artemisinin in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5958–65. doi: 10.4049/jimmunol.179.9.5958. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Xue DB, Zheng B, et al. Induction of apoptosis by artemisinin relieving the severity of inflammation in caerulein-induced acute pancreatitis. World J Gastroenterol. 2007;13:5612–17. doi: 10.3748/wjg.v13.i42.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu C, Xiong Z, Chen X, et al. Artemisinin attenuates lipopolysaccharide-stimulated proinflammatory responses by inhibiting NF-κB pathway in microglia cells. PLoS One. 2012;7:e35125. doi: 10.1371/journal.pone.0035125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Huang Z, Wang L, et al. The anti-malarial artemisinin inhibits pro-inflammatory cytokines via the NF-κB canonical signaling pathway in PMA-induced THP-1 monocytes. Int J Mol Med. 2011;27:233–41. doi: 10.3892/ijmm.2010.580. [DOI] [PubMed] [Google Scholar]
- 16.Cheng C, Ho W, Goh F, et al. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS One. 2011;6:e20932. doi: 10.1371/journal.pone.0020932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Wang J, Zhu F, et al. HMGB1 regulates T helper 2 and T helper 17 cell differentiation both directly and indirectly in asthmatic mice. Mol Immunol. 2018;97:45–55. doi: 10.1016/j.molimm.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Wang M, Yan Y, et al. OX40L induces helper T cell differentiation during cell immunity of asthma through PI3K/AKT and P38 MAPK signaling pathway. J Transl Med. 2018;16:74. doi: 10.1186/s12967-018-1436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao YP, Shen CC, Huang CH, et al. Iron oxide nanoparticles attenuate T helper 17 cell responses in vitro and in vivo. Int Immunopharmacol. 2018;58:32–39. doi: 10.1016/j.intimp.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Tang H, Shi G, et al. CD39/CD73 and the imbalance of Th17 cells and regulatory T cells in allergic asthma. Mol Med Rep. 2013;8:1432–38. doi: 10.3892/mmr.2013.1692. [DOI] [PubMed] [Google Scholar]
- 21.Joetham A, Schedel M, O’Connor B, et al. Inducible and naturally occurring regulatory T cells enhance lung allergic responses through divergent transcriptional pathways. J Allergy Clin Immunol. 2017;139:1331–42. doi: 10.1016/j.jaci.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Wu C, Sun H, et al. Fungal immunomodulatory protein-fve could modulate airway remodel through by affect IL17 cytokine. J Microbiol Immunol Infect. 2018;51(5):598–607. doi: 10.1016/j.jmii.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Stadhouders R, Lubberts E, Hendriks RW. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J Autoimmun. 2017;87:1–15. doi: 10.1016/j.jaut.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Hedrick SM, Michelini RH, Doedens AL, et al. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–61. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao N, Eto D, Elly C, et al. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol. 2014;15:657–66. doi: 10.1038/ni.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic Th17 cells by inducible salt sensing kinase SGK1. Nature. 2013;496:513–17. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan Q, Kozhaya L, Elhed A, et al. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J Exp Med. 2011;208:1875–87. doi: 10.1084/jem.20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Wei C, Xu H, et al. The Immunoregulation of Th17 in Host against intracellular bacterial infection. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/6587296. 6587296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eizenbergmagar I, Rimer J, Zaretsky I, et al. Diverse continuum of CD4(+) T-cell states is determined by hierarchical additive integration of cytokine signals. Proc Natl Acad Sci USA. 2017;114:E6447–56. doi: 10.1073/pnas.1615590114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666–78. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumayag S, Haldin C, Corbett N, et al. Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc Natl Acad Sci USA. 2013;110:E507–16. doi: 10.1073/pnas.1212655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer A, Jung MH. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–76. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 35.Ma L, Zeng J, Mo B, et al. High mobility group box 1: A novel mediator of Th2-type response-induced airway inflammation of acute allergic asthma. J Thorac Dis. 2015;7:1732–41. doi: 10.3978/j.issn.2072-1439.2015.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zevallos VF, Raker VK, Maxeiner J, et al. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur J Nutr. :2018. doi: 10.1007/s00394-018-1681-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Raker V, Stein J, Montermann E, et al. Regulation of IgE production and airway reactivity by CD4(–)CD8(–) regulatory T cells. Immunobiology. 2015;220:490–99. doi: 10.1016/j.imbio.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Choi MS, Park S, Choi T, et al. Bee venom ameliorates ovalbumin induced allergic asthma via modulating CD4+CD25+ regulatory T cells in mice. Cytokine. 2013;61:256–65. doi: 10.1016/j.cyto.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yii ACA, Tan GL, Tan KL, et al. Fixed airways obstruction among patients with severe asthma: Findings from the Singapore General Hospital-Severe Asthma Phenotype Study. BMC Pulm Med. 2014;14:191. doi: 10.1186/1471-2466-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao YG, Wang Y, Guo Z, et al. Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol. 2012;189:4417–25. doi: 10.4049/jimmunol.1200919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei M, Xie X, Chu X, et al. Dihydroartemisinin suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Immunopharmacol Immunotoxicol. 2013;35:382–89. doi: 10.3109/08923973.2013.785559. [DOI] [PubMed] [Google Scholar]
- 44.Elkashef DH. Nicorandil alleviates ovalbumin-induced airway inflammation in a mouse model of asthma. Environ Toxicol Pharmacol. 2018;59:132–37. doi: 10.1016/j.etap.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 46.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Ann Rev Immunol. 2009;8:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 47.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–6. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eijkelenboom A, Burgering BMT. FOXOs: Signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 49.Ichiyama K, Gonzalezmartin A, Kim BS, et al. The microRNA-183-96-182 cluster promotes T helper 17 cell pathogenicity by negatively regulating transcription factor foxo1 expression. Immunity. 2016;44:1284–98. doi: 10.1016/j.immuni.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]