Highlights
-
•
History and nystagmus profile paramount to diagnosis of benign positional vertigo.
-
•
Randomised controlled trials demonstrate effectiveness of repositioning manoeuvres.
-
•
Atypical positional nystagmus warrants vestibular function assessment and imaging.
Keywords: Benign paroxysmal positional vertigo, Canalithiasis, Cupulolithiasis, Positional nystagmus
Abstract
The diagnosis of benign positional vertigo (BPV) relies on a history of episodic positional vertigo and a distinctive pattern of nystagmus during provocative positional testing. The direction of the induced nystagmus is specific to the affected canal and the velocity profile reflects the underlying mechanism of canalithiasis (free-floating otoconia within the canal duct) or cupulolithiasis (otoconia adherent to the cupula). We review current theories on the pathophysiology of BPV, the clinical history and examination underlying its diagnosis, and recommended repositioning manoeuvres for each of the BPV subtypes. Disorders other than BPV which may present with a similar history and/or positional nystagmus are discussed.
1. Introduction
Benign positional vertigo (BPV) is a common and treatable peripheral vestibular disorder in which one or more of the semicircular canals are abnormally stimulated by otoconia displaced from the otolith organs. As the head moves with respect to gravity, the otoconia also move, activating semicircular canal afferents and producing a false sense of head rotation and nystagmus. Patients will present with episodic positional vertigo, which is idiopathic in the majority of cases but may be preceded by head trauma or other insult to the inner ear (Karlberg et al., 2000). The diagnosis relies upon the distinctive pattern of nystagmus observed during provocative manoeuvres in the plane of the affected canal. While BPV is self-limiting in many cases, unresolved BPV can limit daily activities and contribute to the risk of falls in elderly patients (von Brevern et al., 2007). Treatment by repositioning manoeuvres specific to the affected canal can offer patients relief from symptoms and allow them to return to normal activities. Although less common, central and peripheral disorders can mimic the presentation of BPV and are an important differential diagnosis for episodic positional vertigo.
2. Pathophysiology
The semicircular canals are the sensors of angular head acceleration. Each canal is filled with endolymphatic fluid, which passes in a loop through the utricle and is sealed by the cupula, a flexible gelatinous mass which is attached to the ampulla. The sensory hair cells are embedded within the cupula. As the head moves in the plane of a semicircular canal, the flow of endolymph within the canal deflects the cupula and bends the stereocilia of the hair cells. Hair cells are directionally polarised therefore the direction of endolymph flow and subsequent deflection of the stereocilia, determines whether there is an excitatory or inhibitory response from the canal afferents. In the vertical canals, endolymph flow away from the ampulla is excitatory and endolymph flow towards the ampulla is inhibitory. The reverse is true for the lateral canals. Each semicircular canal has a contralateral partner, which responds reciprocally to the same plane of stimulation. Movements in a given plane will excite one canal while simultaneously inhibiting its partner. Canal stimulation during natural head movements drives the extraocular muscles to produce an equal and opposite eye movement, thus maintaining gaze stability as we move (Epley, 2001). Canal stimulation by otoconia produces an illusion of head movement, a compensatory (slow phase) eye movement and oppositely directed excitatory nystagmus (fast phase) in the plane of that canal. Search coil studies of benign positional nystagmus confirm that positional vertigo arising from each canal is accompanied by nystagmus with an axis orthogonal to the canal plane (Aw et al., 2005).
The canalithiasis theory of BPV describes calcium carbonate crystals (otoconia) from the otolithic membrane of the utricle, becoming detached and entering the endolymph of one or more of the semicircular canals (Hall et al., 1979). If a critical mass of otoconia is reached within a given canal, when the head changes position, the gravitational movement of the otoconia will cause abnormal endolymph flow, giving a false sensation of head movement and producing nystagmus in the plane of the affected canal (House and Honrubia, 2003). The cupulolithiasis theory describes displaced otoconia attaching to the cupula of the semicircular canals (Schuknecht, 1969). Ordinarily, the cupula is equal in density to the surrounding endolymph and does not exert a force on the hair cells when the head is stationary. The attached otoconia produce a density difference which causes gravity-dependent movement of the cupula. In both canalithiasis and cupulolithiasis, the abnormal stimulation of the canals brought on by changes in head position results in vertigo and nystagmus. In canalithiasis, the response is brief with a delayed onset as the otoconia fall to the new lowest gravitational point. In cupulolithiasis, the response is persistent as the heavy cupula continues to deflect while the head remains in the provoking position but may gradually decay due to central vestibular adaptation (Nuti et al., 2016).
The cause of dislodged otoconia is usually unknown but in some cases may be attributed to head trauma or inner ear diseases such as Meniere’s disease and vestibular neuritis (Karlberg et al., 2000, von Brevern et al., 2007). There is a similar association between BPV and migraine and it has been postulated that the vasospasms known to occur in migraine could cause ischaemic damage to the inner ear and thereby promote detachment of otoconia (Ishiyama et al., 2000). More women are affected by BPV than men, and this may in part or whole reflect the association between BPV and migraine (von Brevern et al., 2007). The incidence of BPV increases amongst adults over 35 years old with a mean onset age of 49 years (von Brevern et al., 2007). This suggests that a degenerative process may play a role. BPV has also been reported after periods of bed rest, which may facilitate the otoconia forming an adequate agglomeration (Gyo, 1988).
Due to the anatomical orientation of the canals and the otoliths, the lowermost posterior canal is most commonly affected by BPV (∼90% of cases), while displaced otoconia in the lateral and anterior canals are more likely to fall back into the utricle spontaneously through natural head movements (Korres et al., 2002). In a hospital study of 108 patients with untreated BPV, the average time taken for BPV to spontaneously remit was just over two weeks for the lateral canal and just over a month for the posterior canal (Imai et al., 2005).
3. Clinical history
Rotational vertigo is the most common complaint of patients with BPV, which is expected given the involvement of the semicircular canals. Most patients will report brief, discrete episodes of vertigo lasting seconds to minutes upon lying down, turning over or getting out of bed (von Brevern et al., 2007). In some cases, the vertigo is associated with nausea and vomiting. Patients with lateral canal BPV typically experience more severe symptoms. Many patients will report restricting head movements to prevent attacks and may develop secondary neck pain (von Brevern et al., 2007).
Often patients will describe imbalance in between episodes of vertigo, and this may persist even after the BPV has resolved (von Brevern et al., 2007). Residual imbalance after vertigo episodes may lead some patients to overestimate the duration of episodes (von Brevern et al., 2015). Not all patients with BPV will report rotatory vertigo and may instead report dizziness, light-headedness or falls (Oghalai et al., 2000, von Brevern et al., 2007). There should be no associated hearing changes or neurological symptoms unless a comorbid condition is present.
4. Clinical examination
The diagnosis of BPV relies on a typical history and provocative positional testing to elicit nystagmus in the plane of the affected canal. Further audio-vestibular testing or imaging is only necessary when the patient shows additional signs or symptoms which may indicate a comorbid condition (Bhattacharyya et al., 2017).
Benign positional nystagmus can often be observed with the naked eye however it is most reliably assessed using video Frenzel goggles with vision-denied. The nystagmus direction, onset, intensity pattern and duration should be consistent with that expected of BPV (Table 1). Throughout this review, the direction of nystagmus is described by the fast phase from the patient’s perspective. Torsion is described according to the movement of the upper pole of the eye. The terms ‘geotropic’ and ‘apogeotropic’ refer to whether the nystagmus beats towards the ground or away from the ground, respectively.
Table 1.
Nystagmus patterns of BPV variants (Baloh et al., 1987, Lopez-Escamez et al., 2006, Vannucchi et al., 2015, von Brevern et al., 2015).
| BPV variant | Location of otoconia in upright position | Provocative manoeuvre | Nystagmus direction* | Nystagmus latency** | Nystagmus duration |
|---|---|---|---|---|---|
| Posterior canalithiasis | Near cupula of posterior canal | Dix-Hallpike test | Upbeating and torsional towards affected ear | Brief latency (rarely up to 40 s) | <1 min |
| Apogeotropic posterior canalithiasis | Near common crus of posterior canal | Dix-Hallpike test (with either ear down) or supine head-hanging test | Downbeating and torsional away from affected ear | No latency | >2 min |
| Posterior cupulolithiasis | Adherent to cupula of posterior canal | Half Dix-Hallpike test | Upbeating and torsional towards affected ear | Brief or no latency | >1 min |
| Anterior canalithiasis | Near cupula of anterior canal | Dix-Hallpike test (with either ear down) or supine head-hanging test | Downbeating with/without visible torsion towards affected ear | Brief or no latency (rarely up to 30 s) | <1 min |
| Lateral canalithiasis | Posterior portion of lateral canal | Supine roll test | Horizontal geotropic | Brief or no latency | <1 min (rarely up to 2 min) |
| Apogeotropic lateral canalithiasis | Anterior portion of lateral canal | Supine roll test | Horizontal apogeotropic | Brief or no latency | <1 min |
| Lateral cupulolithiasis | Adherent to cupula of lateral canal | Supine roll test | Horizontal apogeotropic | Brief or no latency | >1 min |
Nystagmus direction is defined by the fast phase from the patient’s perspective. The torsional component is described according to the movement of the upper pole of the eye.
Nystagmus latency refers to the time between reaching the provocative position and the onset of positional nystagmus.
A typical history of BPV and vertigo on positional testing may sometimes be encountered without nystagmus. This entity has been termed ‘subjective BPV’ and has been shown to be responsive to positional manoeuvres similar to classic BPV (Balatsouras and Korres, 2012, Haynes et al., 2002, Tirelli et al., 2001). It has been explained by a smaller quantity of otoconia, insufficient to produce the characteristic nystagmus (Tirelli et al., 2001). In this case, the intensity of symptoms during positional testing must be used to lateralise the involved canal. The resolution of symptoms following repositioning confirms the diagnosis.
4.1. Posterior canal BPV
The underlying mechanism of posterior canal BPV (PC-BPV) is usually canalithiasis, with the otoconia being trapped near the ampulla, as this is the lowest gravitational point in the upright position. The hallmark nystagmus of PC-BPV is induced by the Dix-Hallpike test in which the patient’s head is turned towards the affected ear and lowered into a head-hanging position so that the posterior canal is in the sagittal plane with the ampulla at the highest gravitational point. The head-hanging position can be more easily achieved if the bed has an adjustable head, or alternatively by placing a pillow behind the patient’s back. In this head position, otoconia will gravitate away from the ampulla towards the common crus, causing excitation of the posterior canal afferents. With the affected ear down, upbeating torsional geotropic nystagmus is observed. Consistent with the canalithiasis theory, the nystagmus should have a brief onset latency of one or a few seconds after reaching the provocative position due to the inertia of the otoconia and resistance of the endolymph. A crescendo-decrescendo pattern of intensity and a short duration of less than one minute should be observed (Fig. 1) as the otoconia settle to the new lowest point in the canal (Parnes et al., 2003). The nystagmus reverses direction when the patient is returned to the upright position and the otoconia fall back towards the ampulla. It is fatigable as otoconia disperse within the canal. In unilateral PC-BPV, the Dix-Hallpike test is negative on the contralateral side. A positive Dix-Hallpike on both sides is consistent with bilateral PC-BPV and is more common in cases of trauma (Katsarkas, 1999). Clinicians should take care to ensure appropriate head alignment during the Dix-Hallpike test as inappropriate positioning may lead to unilateral PC-BPV being mistaken for bilateral PC-BPV (Steddin and Brandt, 1994).
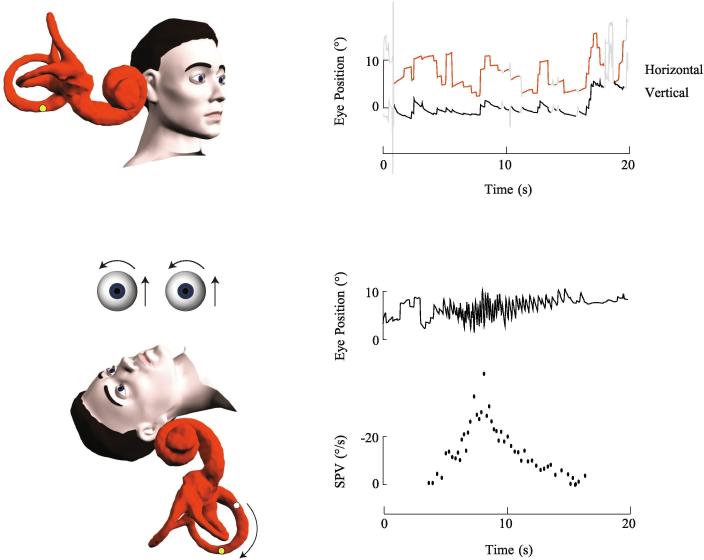
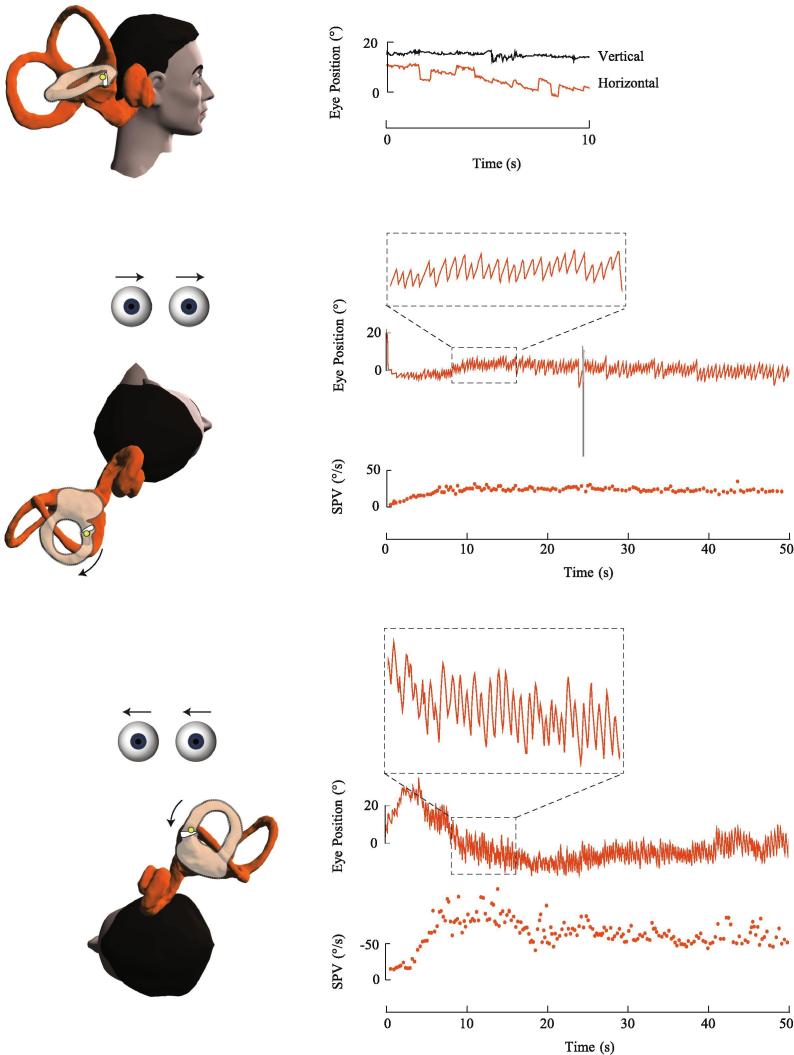
Fig. 1.
Right posterior canalithiasis. In the upright position, the otoconia are positioned close to the ampulla of the right posterior canal. Only subtle spontaneous upbeat nystagmus was seen in this subject. During the right Dix-Hallpike test, the ampulla is brought to a higher gravitational point causing otoconia to gravitate away from the ampulla and generate a brief excitatory nystagmus which is upbeating with a rightward torsion. The nystagmus slow phase velocity (SPV) profile shows a brief onset latency, crescendo-decrescendo intensity pattern and short duration.
A rare apogeotropic variant of PC-BPV has been described in which the otoconia are near the common crus of the canal, thus the Dix-Hallpike test results in the movement of the otoconia towards the ampulla and an inhibitory response (Vannucchi et al., 2012). On Dix-Hallpike testing, the nystagmus is downbeating torsional apogeotropic nystagmus, similarly seen in BPV of the contralateral anterior canal. Unlike typical PC-BPV, the nystagmus may be provoked with either ear down, it has no latency, a longer duration (typically >2 min), a less intense paroxysm, returning to upright position rarely causes the nystagmus to reverse direction, and it is non-fatiguing (Vannucchi et al., 2015).
Another rare variant is cupulolithiasis of the posterior canal, which is distinguished from canalithiasis of the posterior canal by a longer duration nystagmus, exceeding one minute (Fig. 2) (Imai et al., 2009). The nystagmus is maximal in a half Dix-Hallpike position which orientates the cupula horizontally such that the force of gravity is greatest (Nuti et al., 2016).
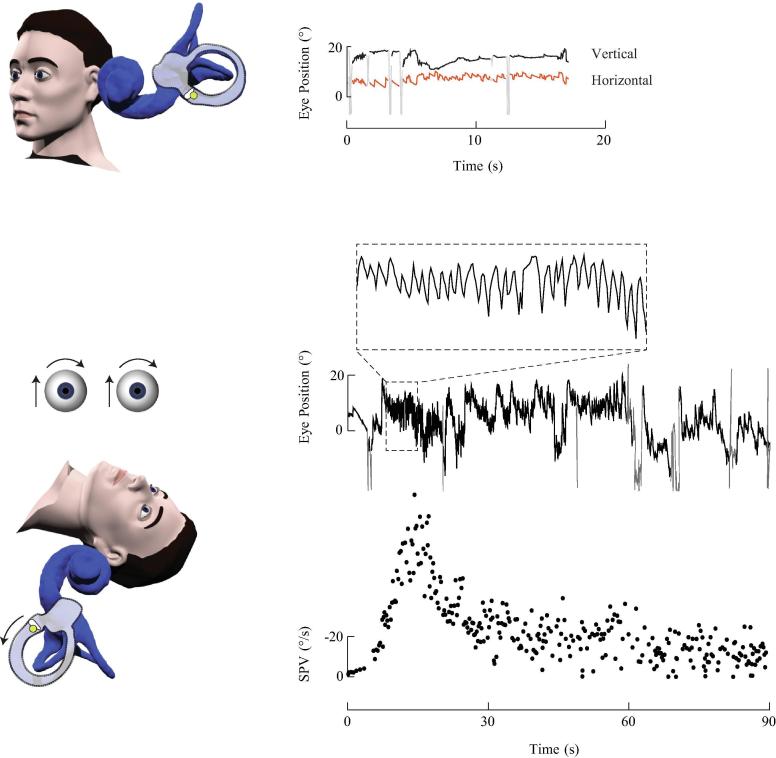
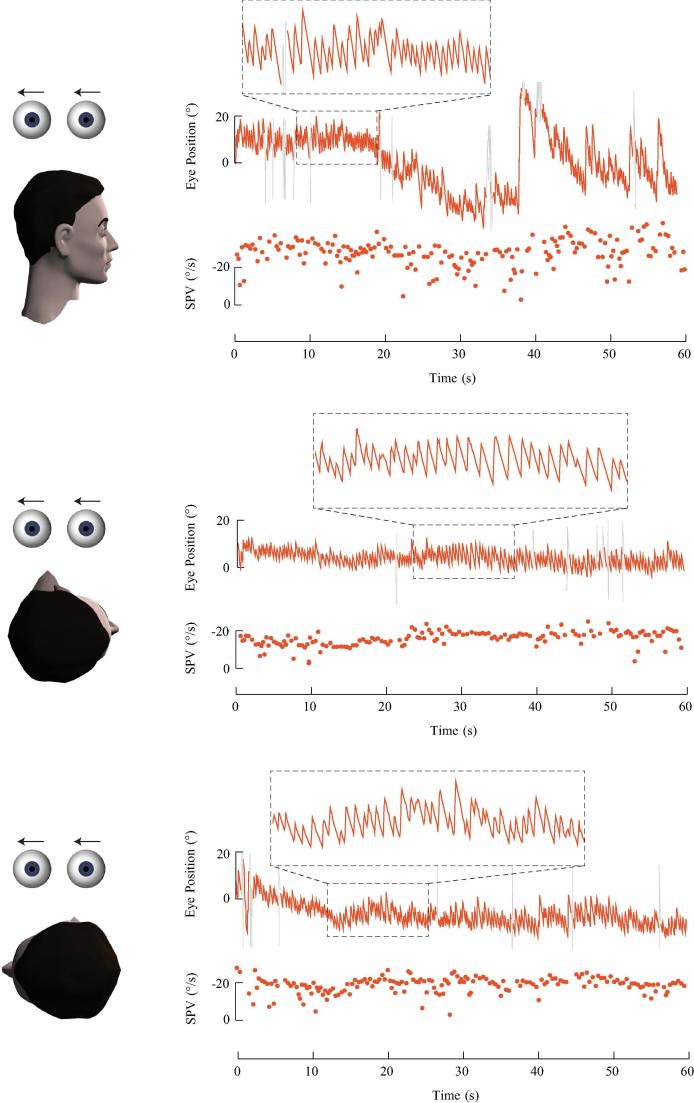
Fig. 2.
Left posterior cupulolithiasis. The otoconia adhere to the cupula of the left posterior canal creating a heavy cupula. This patient had an acute vestibular syndrome and secondary BPV. In the upright position, low velocity right-beating nystgamus is seen, consistent with the left unilateral vestibular loss. During the left Dix-Hallpike test, the cupula is deflected away from the ampulla due to its increased mass, causing excitation of the left posterior canal and generating an upbeating leftward torsional nystagmus. The nystagmus slow phase velocity (SPV) profile shows an immediate onset of nystagmus that rises to a peak, gradually decays but persists for more than one minute.
While the Dix-Hallpike is the most widely used test for PC-BPV, a side-lying test in which the patient’s head is turned 45 degrees away from the symptomatic ear and lies down on their symptomatic side should evoke the same pattern of nystagmus if PC-BPV is present. It offers a useful alternative for patients who cannot hyperextend the neck (Cohen, 2004).
4.2. Anterior canal BPV
Anterior canal BPV (AC-BPV) is rare, accounting for 1–2 percent of cases (Korres et al., 2002). It can be elicited by a Dix-Hallpike test to either side or alternatively by the straight head-hanging position where the head is lowered at least 30 degrees below horizontal. In these positions, otoconia in the anterior canal should gravitate away from the ampulla producing an excitatory response (Bertholon et al., 2002). The nystagmus is downbeat torsional nystagmus towards the affected ear (Fig. 3). The torsional component enables lateralisation of AC-BPV however it is often small and can be absent due to the proximity of the anterior canals to the sagittal plane (Balatsouras et al., 2011). This can make lateralisation impossible. Similar to typical PC-BPV, canalithiasis is the underlying mechanism thus a brief latency, short duration (<1 min) nystagmus is expected (von Brevern et al., 2015). As paroxysmal downbeat nystagmus is also seen in central pathologies, the clinician should look for other signs to support the diagnosis of AC-BPV, including resolution or reduction of the nystagmus with the appropriate repositioning manoeuvre or conversion to another common BPV variant (von Brevern et al., 2015).
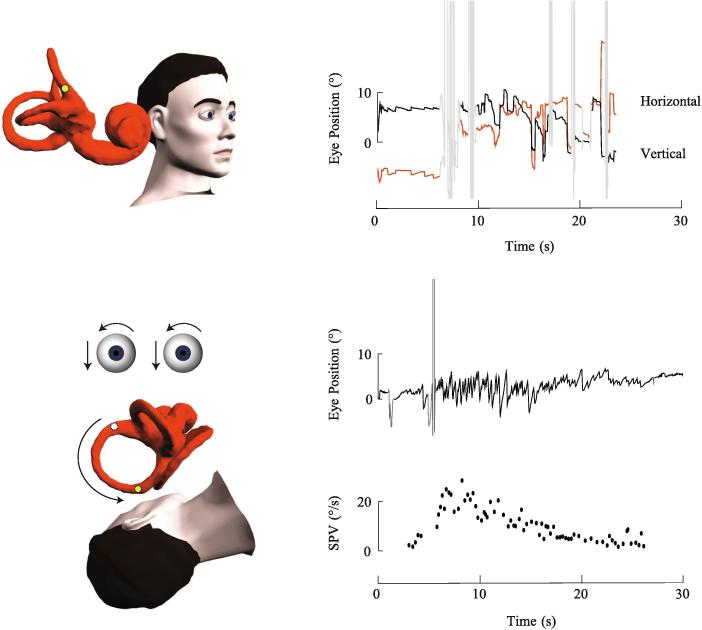
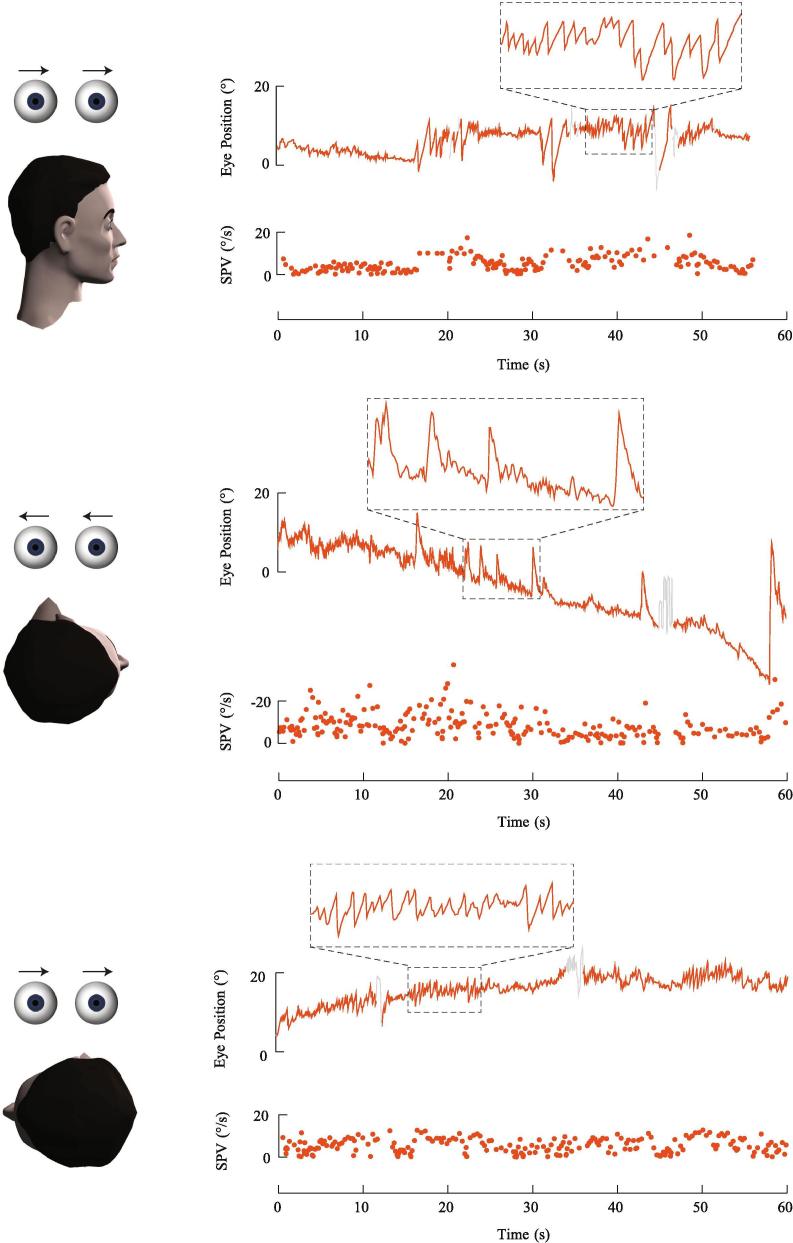
Fig. 3.
Right anterior canalithiasis. In the upright position, the otoconia are positioned near the cupula of the right anterior canal. There is no spontaneous nystagmus. The Dix-Hallpike position brings the ampullary end of the anterior canal to a higher gravitational point. There is an excitatory downbeat rightward torsional nystagmus as the otoconia fall away from the ampulla. The slow phase velocity (SPV) profile illustrates the paroxysmal nature of the nystagmus, the quick rise to a peak velocity, and brief duration of less than 30 s.
4.3. Lateral canal BPV
The characteristic nystagmus of lateral canal BPV (LC-BPV) is brought on by the supine roll test. LC-BPV is characterised by direction-changing horizontal nystagmus which can be geotropic or apogeotropic. Geotropic nystagmus due to canalithiasis is the most common form. Upon lying down and rolling to the affected side, otoconia in the posterior portion of the lateral canal will move towards the ampulla creating an intense excitatory response, while rolling to the unaffected side will produce a less intense inhibitory response (Fig. 4). The nystagmus onset will be immediate or brief, show a rapid increase and then a slower decline in intensity with a short overall duration (<1 min) (von Brevern et al., 2015). The affected ear is usually identified as the side with the more intense nystagmus and subjective symptoms.
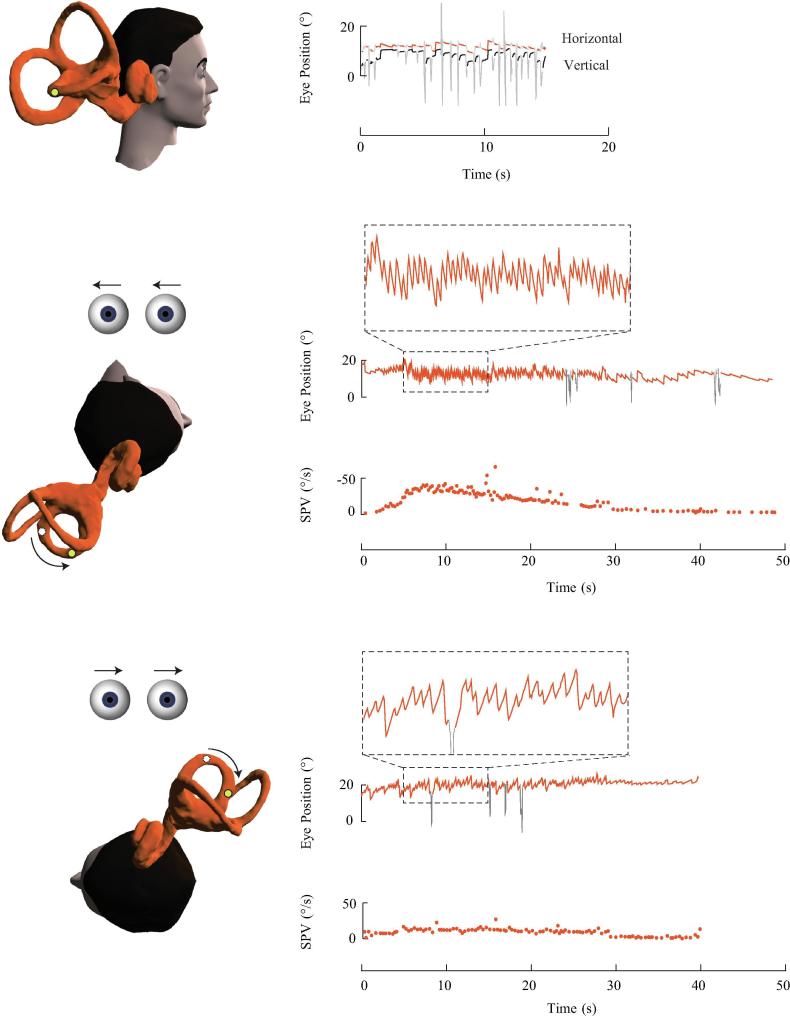
Fig. 4.
Right lateral canalithiasis. In the upright position, the otoconia are located in the long arm of the right lateral canal at a distance from the ampulla. There was no spontaneous nystagmus in this subject. During the right roll test, otoconia descend towards the ampulla creating an intense excitatory right-beating nystagmus. During the left roll test, otoconia gravitate away from the ampulla producing a less intense inhibitory left-beating nystagmus. The slow phase velocity (SPV) profiles for both roll tests show a brief duration nystagmus with a higher peak velocity reached during the right roll test.
As the lateral canals are orientated with the ampullary end elevated at 30 degrees above horizontal, when the head is in the upright position there can be a slow movement of otoconia away from the cupula (Asprella-Libonati, 2005). The resulting “pseudospontaneous nystagmus” beats towards the healthy ear. It can be distinguished from true spontaneous nystagmus by pitching the head forward 30 degrees at which point the canal is horizontal with no incline and the nystagmus disappears (Asprella-Libonati, 2008). Bowing the head forward 90 degrees should produce a nystagmus towards the affected ear while leaning the head back 45 degrees should produce a nystagmus towards the healthy ear (Choung et al., 2006). By the same principle, moving the patient from a seated position to supine will cause an inhibitory nystagmus beating away from the affected ear (Table 2) (Nuti et al., 1996).
Table 2.
Localising the affected ear in lateral canal BPV.
| Geotropic variant | Apogeotropic variant | |
|---|---|---|
| Supine roll test (Nuti et al., 1996) | Excitatory nystagmus when affected ear is undermost – Affected ear down shows the strongest nystagmus | Inhibitory nystagmus when affected ear is undermost – Unaffected ear down shows the strongest nystagmus |
| Bow and Lean Test/Head Pitch Test (Asprella-Libonati, 2008, Choung et al., 2006) | Face up: Inhibitory nystagmus – beats towards unaffected ear Face down: Excitatory nystagmus - beats towards affected ear |
Face up: Excitatory nystagmus – beats towards affected ear Face down: Inhibitory nystagmus – beats towards unaffected ear |
| Sitting to supine (Nuti et al., 1996) | Inhibitory nystagmus – beats towards unaffected ear | Excitatory nystagmus – beats towards affected ear |
| Pseudospontaneous nystagmus (Asprella-Libonati, 2005) | Inhibitory nystagmus – beats towards unaffected ear | Excitatory nystagmus – beats towards affected ear |
Apogeotropic nystagmus is the hallmark of lateral cupulolithiasis. In rare cases it may be attributed to canalithiasis in which the otoconia are located in the anterior portion of the lateral canal, close to the ampulla (Balatsouras et al., 2011). In the apogeotropic form, rolling onto the affected side causes the cupula to deflect away from the ampulla resulting in an inhibitory response while rolling onto the unaffected side will produce a more intense excitatory response. For cupulolithiasis, the nystagmus onset will still be immediate or brief, however the duration will be longer with a gradual decline due to adaptation (Fig. 5).
Fig. 5.
Right lateral cupulolithiasis. The otoconia adhere to the cupula of the right lateral canal. There was no spontaneous nystagmus in this subject. During the right roll test, the cupula is deflected away from the ampulla creating an inhibitory left-beating nystagmus. During the left roll test, the cupula is deflected towards the ampulla generating an excitatory right-beating nystagmus. The slow phase velocity (SPV) profiles during both roll tests reveal persistent nystagmus, with a higher peak velocity reached during the left roll test.
The principles of pseudospontaneous nystagmus and the bow and lean test can, in theory, be applied to lateral cupulolithiasis however the nystagmus is said to be in the opposite direction to the geotropic variant (Asprella-Libonati, 2005, Choung et al., 2006). Pseudospontaneous nystagmus will be excitatory and should beat towards the affected ear. Bowing should elicit nystagmus towards the unaffected ear while leaning backwards or lying down should elicit nystagmus towards the pathological ear. Additionally, a null point for nystagmus may be identified when lying supine with the head turned 10–20 degrees towards the affected side – in this position the cupula is thought to be aligned with the force of gravity and does not deflect despite the abnormal density (Bisdorff and Debatisse, 2001). These theories of lateralisation are based on the expectation that the human lateral canal cupula has its apex oriented posterolaterally, a condition which may not always be fulfilled.
5. Treatment
The treatment of BPV involves positional manoeuvres to direct the displaced otoconia back into the utricle. For patients with the cupulolithiasis variant this will involve first converting to canalithiasis. The nystagmus observed during the manoeuvre can be a useful indicator of the ampullofugal flow of otoconia towards the utricle.
Patients should be warned that experiencing vertigo and nausea during treatment is to be expected. For patients who are likely to be excessively symptomatic (migraine sufferers with motion sensitivity), it may be wise to advise the patient to fast 2–4 h prior to treatment and pre-medicate them with an antiemetic and vestibular suppressant to minimise distress. If the patient has limited mobility or is obese, it is wise to obtain the assistance of a second clinician and to use an adjustable bed that can be tilted into the Trendelenburg position. Some clinicians use a mechanical vibrator on the mastoid as originally proposed by Epley (1992) to help free otoconia, however randomised controlled trials suggest no significant additive effect of mastoid vibration during the Epley manoeuvre (Macias et al., 2004, Motamed et al., 2004).
5.1. Posterior canal BPV
The two validated treatment manoeuvres for PC-BPV are the Epley manoeuvre and Semont manoeuvre (Epley, 1992, Semont et al., 1988). Both can be performed at the bedside with comparable outcomes seen in randomised controlled trials (Hilton and Pinder, 2014).
Epley manoeuvre (Canalith Repositioning Procedure) (Bhattacharyya et al., 2017):
-
1.
The patient is seated on the bed with the head turned 45 degrees towards the affected side.
-
2.
The patient is briskly lowered onto the bed with the neck hyper extended and remains in this position for 20–30 s (this may be achieved with a pillow under the patient’s back or by lowering the head of the bed).
-
3.
The patient’s head is turned 90 degrees towards the healthy side and remains in this position for 20 s.
-
4.
The patient rolls onto their healthy side, turning their head (without lifting it) until their nose is facing downwards and remains in this position for 20–30 s.
-
5.
The patient returns to an upright position.
Epley (2001) originally proposed that the length of time each position in the manoeuvre is held should be such that the particles are kept in a constant state of motion, guided by the duration of the nystagmus. Other authors have advocated for longer intervals in between position changes (Parnes and Price-Jones, 1993).
During the Epley manoeuvre, it is reassuring to see nystagmus continue to beat upwards with a torsional component towards the affected ear, indicating that the otoconia are migrating in the desired direction towards the utricle (Parnes and Price-Jones, 1993). If during step 3 of the manoeuvre, downbeat nystagmus with torsion towards the contralateral ear is seen, it is likely the manoeuvre was unsuccessful and the treatment should be repeated (Oh et al., 2007). After successful completion of the Epley manoeuvre (step 4), a brief downbeat torsional nystagmus towards the treated side may be seen when the patient is returned to the upright position.
Semont manoeuvre (Liberatory manoeuvre) (Mandalà et al., 2012, Nuti et al., 2000, Semont et al., 1988):
-
1.
The patient is seated on the edge of the bed with the head rotated 45 degrees towards the healthy side (aligning the affected posterior canal with the coronal plane).
-
2.
The patient is briskly lowered onto their affected side (in the coronal plane) with the head facing upwards and remains there for 2 min.
-
3.
Without turning the head, the patient is briskly moved to lie on the opposite side in a nose-down position and remains there for 2 min.
-
4.
The patient returns to the upright position.
The Semont manoeuvre requires rapid acceleration of the head in a 180 degrees swing in the plane of the posterior canal to enable otoconia to fall back into the utricle (Faldon and Bronstein, 2008). The same nystagmus as seen in a positive Dix-Hallpike test should be seen at step 3 of the Semont manoeuvre to confirm the desired flow of otoconia away from the cupula (Nuti et al., 2000).
There are differing opinions regarding the number of manoeuvres performed in a single treatment session. Epley (2001) recommended the manoeuvre be repeated until the nystagmus has ceased and that the patient returns for follow up sessions on different days until they are symptom free. Parnes et al. (2003) point out that abolition of the nystagmus with repeat manoeuvres could be due to the fatigability of the response. Immediately repeating the Dix-Hallpike after treatment has been found to increase the risk (16%) of otoconia falling back into the posterior canal or entering the horizontal canal and requiring further manoeuvres (Foster et al., 2012). For these reasons, it may be wise to avoid repeat manoeuvres in quick succession unless the nystagmus pattern is indicative of failed repositioning.
5.2. Anterior canal BPV
Both lateralising and non-lateralising manoeuvres have been proposed for the treatment of AC-BPV. If the affected ear can be identified, a reverse Epley manoeuvre may be performed, which simply involves starting the Epley manoeuvre from the side of the healthy ear (Honrubia et al., 1999). Kim et al. (2005) described a similar technique in which the patient’s head is turned 45 degrees towards the healthy side and lowered into a head-hanging position to enable ampullofugal flow of otoconia into the superior portion of the anterior canal. After two minutes, the patient’s head is lifted to the supine position but remains turned towards the healthy side. After one minute in the supine position, the patient sits up and tucks the chin in to facilitate the movement of otoconia through the common crus into the utricle. Yacovino et al. (2009) described a side-independent treatment for AC-BPV, in which the patient remains in the supine head-hanging position followed by the “chin to chest” position for 30 s each before being brought back to the seated position (Yacovino et al., 2009). Due to the small incidence of AC-BPV, the evidence for treatment efficacy is limited. Uncontrolled case series demonstrate success rates of over 75 percent for the aforementioned manoeuvres (Anagnostou et al., 2015).
5.3. Lateral canal BPV
The Lempert manoeuvre or barbecue rotation may be used to treat both geotropic and apogeotropic variants of LC-BPV. In this manoeuvre, the patient’s head is rotated in 90-degree steps towards the healthy side in intervals of 30–60 s, beginning in the supine position and completing a total head rotation of 270 degrees (Lempert and Tiel-Wilck, 1996). Baloh (1994) proposed a similar manoeuvre with a 360-degree angle of rotation. During the rotation, nystagmus beating towards the healthy ear confirms the flow of otoconia away from the ampulla, towards the utricle.
The Vannucchi-Asprella manoeuvre also uses angular acceleration to shift otoconia ampullofugally and may be used to treat both geotropic and apogeotropic variants. In this manoeuvre the patient lies supine, the head is quickly turned towards the healthy side and the patient returns to an upright position with the head still turned (Asprella-Libonati, 2005). Once upright, the patient slowly turns the head to face forward again. The manoeuvre is repeated several times until the nystagmus beating towards the unaffected ear is no longer elicited.
Alternatively, the Gufoni manoeuvre is a widely-used technique which utilises inertia and linear acceleration. In the Gufoni manoeuvre for geotropic LC-BPV, the patient is quickly moved from a seated position onto their healthy side for two minutes and then turns their face down for two minutes before returning to the upright position (Gufoni et al., 1998). Both the barbecue rotation and Gufoni manoeuvre have been shown to be equally effective treatments for geotropic LC-BPV in a randomised controlled trial (Kim et al., 2012b).
The Gufoni manoeuvre performed on the affected side rather than the healthy side, may be used for lateral cupulolithiasis. Once lying on the affected side, the patient’s head is turned either up or down 45 degrees depending on the position of the otoconia on the cupula (Bhattacharyya et al., 2017). If the otoconia are attached on the canal side of the cupula, quickly turning the nose up 45 degrees should shift the otoconia posteriorly towards the utricle (Appiani et al., 2005). If on the other hand, the otoconia are attached to the utricular side of the cupula, turning the nose down will allow otoconia to return to the utricle. Horizontal head-shaking may help detach otoconia from the cupula, regardless of which side they are adhering to (Kim et al., 2012a). The effectiveness of head-shaking and the Gufoni manoeuvre for apogeotropic LC-BPV have been confirmed in a randomised controlled trial (Kim et al., 2012a).
For less mobile patients with lateral canalithiasis, a simple treatment option is Forced Prolonged Positioning (Vannucchi et al., 1997). This involves the patient lying on their healthy side for approximately 12 h to enable otoconia to gravitate back to the vestibule (Vannucchi et al., 1997).
5.4. Mechanical rotators
The Dix-Hallpike test requires hyperextension of the neck, which may be contraindicated in cases of recent neck trauma, cervical instability, severe rheumatoid arthritis, carotid sinus syncope, Chiari malformation and/or vascular dissection (Humphriss et al., 2003). Cardiac and respiratory problems could also make conventional repositioning movements challenging (Humphriss et al., 2003). Mechanical repositioning chairs such as the Epley Omniax rotator (Vesticon, Portland, USA) and the TRV chair (Interacoustics, France) can rotate the patient’s body to align with the plane of any of the semicircular canals while simultaneously enabling the clinician to observe nystagmus through infra-red video goggles. They provide an alternative for testing and treating patients with mobility limitations and for more challenging BPV variants including cupulolithiasis, bilateral and multicanal BPV. Treatment on mechanical repositioning chairs may be contraindicated in patients with retinal detachment, pseudoexfoliation syndrome with intraocular lenses, severe claustrophobia and patients whose weight exceeds the tested safety limits of the chair. Some patients with a history of severe motion sensitivity and migraine may report an increase in headache frequency after treatment of BPV on a mechanical repositioning chair.
5.5. Post-treatment management
After treatment it is recommended that patients return to their normal lifestyle, although they may experience prolonged residual imbalance (Pérez et al., 2012). In particular, free movement of the head should be advocated despite head motion intolerance. There is mixed evidence regarding the benefit of post-treatment postural instructions including lying on the unaffected side or sleeping upright. While some authors have concluded that there is a lack of evidence to support any benefits and advise against their use (Bhattacharyya et al., 2017, Mostafa et al., 2013), a Cochrane review based on nine randomised controlled studies concluded that there is a small additional benefit from postural restrictions in PC-BPV (Hunt et al., 2012).
Typically, BPV will resolve within one to three treatments (Prokopakis et al., 2013, Song et al., 2015). The American Academy of Otolaryngology recommends reassessment within one month to ensure symptoms have resolved (Bhattacharyya et al., 2017). In patients with ongoing symptoms after repositioning manoeuvres, it is important to consider the possibility of a coexisting inner ear disorder or a central disorder mimicking BPV (Bhattacharyya et al., 2017). In rare cases of intractable BPV, more extreme treatments such as surgical occlusion of the canal may be warranted, although this carries a risk of permanent hearing loss and imbalance (Ahmed et al., 2012). A retrospective review of 53 patients who underwent occlusion of the posterior canal reported hearing loss in 17% and vestibular loss in 10% of patients, post-operatively (Ahmed et al., 2012). Patients should be made aware that BPV can recur, but they should not restrict their daily activities. One study reported a recurrence rate of 16% at 6 months (Steenerson et al., 2005) and another reported 37% at 5 years (Sakaida et al., 2003).
6. BPV mimics
Positional vertigo and nystagmus are not always attributable to BPV. Here we discuss disorders that present with either a similar history to BPV or positional nystagmus that could be mistakenly attributed to BPV.
6.1. Orthostatic dizziness
Orthostatic hypotension can present as episodic vertigo or dizziness in some patients however symptoms only occur upon arising from a lying or sitting position and are not due to changes in head position relative to gravity (Bisdorff et al., 2009). Short duration nystagmus (<1 min), most often downbeating with a latency of several seconds, may occur during orthostatic challenging tests such as supine-standing and squatting-standing, but is not expected during typical provocative tests for BPV (Choi et al., 2015, Choi et al., 2015). Unlike BPV, patients with orthostatic hypotension are often asymptomatic and may report feeling better in bed. Diagnosis can be made based on blood pressure measurements while the patient is supine and standing.
6.2. Acute vestibular loss with enhanced spontaneous nystagmus in the supine position
Spontaneous vertigo can be aggravated by head movements however this is distinct from positional vertigo which is triggered by changes in head position. Spontaneous nystagmus due to other inner ear disorders, including vestibular neuritis, can enhance during positional testing and may be mistaken for positional nystagmus. For patients with spontaneous vertigo presenting to the emergency department, the brightly lit environment can suppress nystagmus usually observed in the upright position. Nystagmus observed on positional testing may prompt the examiner to misdiagnose BPV and offer fruitless repositioning manoeuvres, which will lead to unnecessary nausea and distress.
Both unilateral vestibular loss (UVL) and LC-BPV can present with horizontal spontaneous (or “pseudospontaneous”) nystagmus however the characteristics observed during positional testing are dissimilar. Firstly, nystagmus observed in UVL is unidirectional; moving the head to the left or right lateral positions does not change nystagmus direction. In contrast, LC-BPV yields direction-changing nystagmus. Secondly, nystagmus observed in UVL is persistent whereas the nystagmus observed in canalithiasis is paroxysmal.
6.3. Meniere’s disease
Meniere’s disease is characterised by spontaneous vertigo lasting minutes to hours. The accompanying horizontal nystagmus may spontaneously reverse direction during the course of an acute attack but is typically independent of the patient’s position (Fig. 6) (Bance et al., 1991). Positional nystagmus is less common in Meniere’s disease however both geotropic and apogeotropic horizontal positional nystagmus have been reported (Dobie et al., 1982, Lechner et al., 2014). Unlike BPV, fluctuating aural symptoms including tinnitus, low-frequency hearing loss and fullness are present in Meniere’s disease (Lopez-Escamez et al., 2015). BPV is a common co-morbidity (Karlberg et al., 2000).
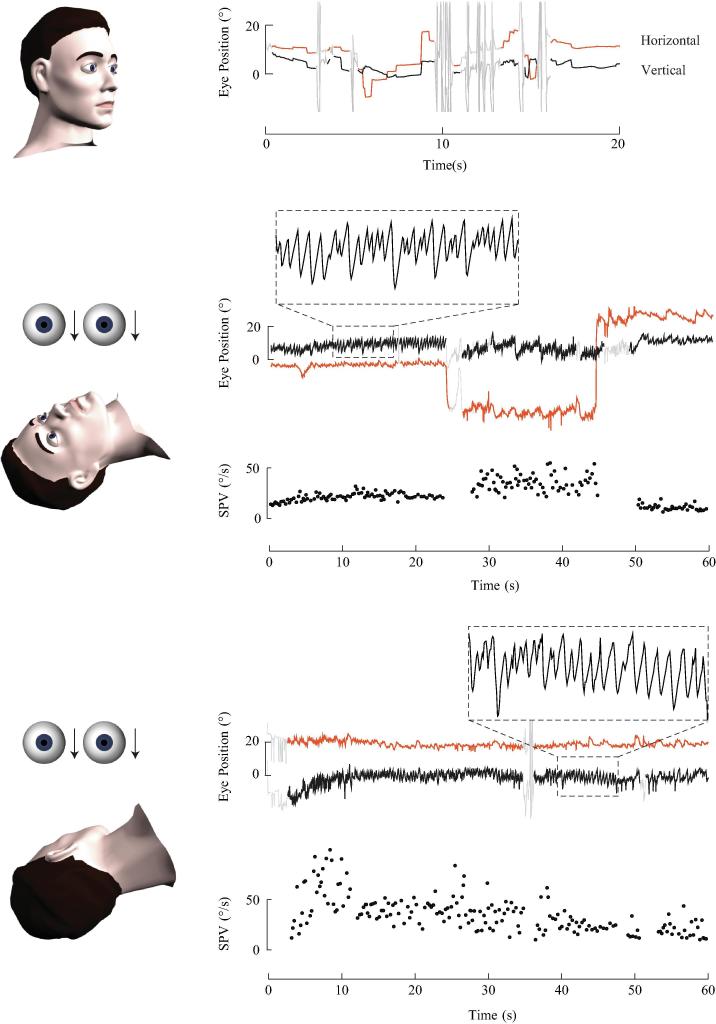
Fig. 6.
Horizontal eye position and slow phase velocity (SPV) during right and left roll tests in a subject with an acute attack of left-sided Meniere’s disease. This subject had right-beating spontaneous nystagmus. There was no change in nystagmus direction during the right and left roll tests. The slow phase velocity (SPV) profiles are flat and illustrate persistent right-beating nystagmus in either roll position.
6.4. Vestibular migraine
Although vertigo in vestibular migraine (VM) is typically spontaneous, positional vertigo can be a presenting symptom. The accompanying nystagmus can be vertical, horizontal or torsional in direction. It tends to be without latency, low velocity (2–7°/s) and persistent (Polensek and Tusa, 2010). The direction is not always specific to a single canal. Positional symptoms may be poorly lateralised. Polensek and Tusa (2010) found positional nystagmus in all patients with VM examined ictally. Horizontal positional nystagmus was the most common. Horizontal geotropic nystagmus of VM can be separated from lateral canalithiasis by its low velocity, persistent and symmetrical nature (Fig. 7) (Lechner et al., 2014). Horizontal apogeotropic nystagmus can closely resemble that of lateral cupulolithiasis (Lechner et al., 2014). Patients with VM may experience migraine features such as headache, photophobia, phonophobia or visual aura during episodes of vertigo (Lempert et al., 2012). If untreated, the symptomatic period for BPV may be weeks to months, while untreated VM lasts hours to days (von Brevern et al., 2004).
Fig. 7.
Horizontal eye position and slow phase velocity (SPV) during right and left roll tests in a subject with vestibular migraine. In the upright position, this subject had left-beating nystagmus. Right and left roll tests showed direction-changing horizontal nystagmus. With the right ear down, the nystagmus was right-beating. With the left ear down, the nystagmus was left-beating. The nystagmus was low velocity, persistent and did not have a crescendo-decrescendo slow phase velocity (SPV) profile, unlike lateral canalithiasis.
6.5. Vestibular paroxysmia
Vestibular paroxysmia is characterised by recurrent, brief attacks of vertigo lasting seconds to minutes. While these attacks can be triggered by changes in head position, they are more typically spontaneous (Strupp et al., 2016). The positional triggers are generally distinct from BPV (Strupp et al., 2016). Unlike BPV due to canalithiasis, a crescendo-decrescendo pattern of nystagmus is not seen (Brandt et al., 2016). Hyperacusis or unilateral tinnitus may be present during the attack and audio-vestibular tests may show unilateral hypofunction in between attacks (Hüfner et al., 2008). The condition is attributed to vascular compression of the vestibular nerve and is responsive to anticonvulsants such as carbamazepine (Brandt and Dieterich, 1994).
6.6. Vestibular schwannoma
Positional horizontal apogeotropic nystagmus has been described in cases of vestibular schwannoma and attributed to head movement causing further pressure on the vestibular nerve (Hong et al., 2008, Taylor et al., 2013). In a patient with atypical positional nystagmus, unilateral deficits on audio-vestibular function tests should trigger a search for a cerebellopontine angle lesion.
6.7. Central positional nystagmus
Rarely, positional vertigo and nystagmus may be due to lesions affecting the brainstem and cerebellum. Specifically, lesions of the cerebellar vermis, cerebellopontine angle, nodulus, superior cerebellar peduncle, medulla and fourth ventricle have been reported in patients with central positional nystagmus (CPN) (Macdonald et al., 2017). These lesions are thought to act by impairing transduction or central processing of graviceptive signals (Choi et al., 2018, Kim et al., 2012). The underlying pathologies can include ischemia, haemorrhage, space-occupying lesions and demyelinating disease (Cho et al., 2017). Generally other oculomotor abnormalities, neurological signs and symptoms will alert the clinician to the need for imaging, however occasionally positional vertigo and nystagmus are the only presenting symptom.
Typically, CPN has little or no latency. It can be persistent, making it difficult to separate from cupulolithiasis, or paroxysmal similar to canalithiasis (Macdonald et al., 2017). Persistent downbeat nystagmus is typically of central origin (Fig. 8). Paroxysmal downbeat nystagmus with and without a torsional component has been reported during straight head-hanging and Dix-Hallpike manoeuvres in central lesions including tumours, infarction and haemorrhage of the inferior cerebellar vermis, multiple system atrophy, CANVAS and antiepileptic drug intoxication (Choi et al., 2015, Choi et al., 2015). The nystagmus can closely resemble AC-BPV and even demonstrate reversal upon returning to the upright position. Short latency, pure torsional paroxysmal positional nystagmus has been reported in patients with intracranial tumours affecting the internal auditory canal or cerebellopontine angle (De Stefano et al., 2012). Choi et al., 2015, Choi et al., 2015 reported that in contrast to the crescendo-decrescendo slow phase velocity profile of BPV, central paroxysmal positional nystagmus tends to peak at the onset and decrease exponentially over time.
Fig. 8.
Eye position and vertical slow phase velocity (SPV) during Dix-Hallpike tests in a subject with a cerebellar arteriovenous malformation. In the upright position, no nystagmus was observed. Dix-Hallpike tests revealed a rapid onset of downbeat nystagmus with either ear down. The nystagmus was persistent, lasting more than one minute, unlike the characteristic nystagmus of canalithiasis.
Persistent horizontal apogeotropic nystagmus during supine roll testing has been reported in patients with cerebellar tumours (particularly in the region of the fourth ventricle and nodule), anterior inferior cerebellar artery (AICA) and posterior inferior cerebellar artery (PICA) territory infarct, nodular infarct and medullary infarct (Cho et al., 2017, Kim et al., 2012, Lee et al., 2014). These patients presented similar to lateral cupulolithiasis but were not responsive to repositioning. In a study comparing horizontal apogeotropic CPN to horizontal apogeotropic nystagmus in BPV, it was found that the modulation of nystagmus between sitting and supine positions may be a useful point of differentiation. Nystagmus intensity in sitting and supine positions was similar for patients with CPN but greater when supine compared to sitting for patients with LC-BPV (Choi et al., 2018). Horizontal geotropic nystagmus upon supine roll testing has also been reported in patients with a cerebellar peduncle tumour and lateral medullary infarct (Lee et al., 2014).
Several discriminators of BPV from CPN have been proposed. Pure torsional or pure vertical positional nystagmus are considered red flags for a central cause (Büttner et al., 1999). Although AC-BPV may appear as downbeat nystagmus without a visible torsional component, its rarity should lower the threshold for seeking a central cause. If the nystagmus is not in the direction expected for the stimulated canal plane, CPN is more likely (Büttner et al., 1999). Lack of fatigability with repeat positioning and non-reversal of nystagmus when changing from supine to sitting for BPV of the vertical canals, or from lying on either side for LC-BPV, should raise the possibility of an alternative diagnosis. Newly developed headaches and mismatch between the severity of vertigo and intensity of nystagmus should also raise the possibility of a central cause (Lee et al., 2014). Failure to respond to multiple repositioning manoeuvres should prompt investigation for a central origin. Horizontal apogeotropic and downbeat positional nystagmus are observed in less common BPV variants but often observed in central mimics, therefore merit particular attention (Nuti et al., 2016).
7. Conclusion
BPV is a frequently encountered cause of episodic vertigo in the neurology clinic and in primary care settings. In most cases, a diagnosis of BPV can be reached confidently with a typical history and careful examination of nystagmus during positional testing. Alternative diagnoses or comorbid conditions should always be considered when nystagmus patterns deviate from those of the most common BPV variants or persist after appropriate repositioning.
Acknowledgments
Acknowledgements
Miriam Welgampola is supported by grants from the National Health and Medical Research Council (APP1126976) and Garnett Passe and Rodney Williams Memorial Foundation.
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2019.03.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmed R., Pohl D., MacDougall H., Makeham T., Halmagyi G. Posterior semicircular canal occlusion for intractable benign positional vertigo: outcome in 55 ears in 53 patients operated upon over 20 years. J. Laryngol. Otol. 2012;126(7):677–682. doi: 10.1017/S0022215112000758. [DOI] [PubMed] [Google Scholar]
- Anagnostou E., Kouzi I., Spengos K. Diagnosis and treatment of anterior-canal benign paroxysmal positional vertigo: a systematic review. J. Clin. Neurol. 2015;11(3):262–267. doi: 10.3988/jcn.2015.11.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appiani G.C., Catania G., Gagliardi M., Cuiuli G. Repositioning maneuver for the treatment of the apogeotropic variant of horizontal canal benign paroxysmal positional vertigo. Otol. Neurotol. 2005;26(2):257–260. doi: 10.1097/00129492-200503000-00022. [DOI] [PubMed] [Google Scholar]
- Asprella-Libonati G. Diagnostic and treatment strategy of lateral semicircular canal canalolithiasis. Acta Otorhinolaryngol. Ital. 2005;25(5):277–283. [PMC free article] [PubMed] [Google Scholar]
- Asprella-Libonati G. Pseudo-spontaneous nystagmus: a new sign to diagnose the affected side in lateral semicircular canal benign paroxysmal positional vertigo. Acta Otorhinolaryngol. Ital. 2008;28(2):73–78. [PMC free article] [PubMed] [Google Scholar]
- Aw S., Todd M., Aw G., McGarvie L., Halmagyi G. Benign positional nystagmus: a study of its three-dimensional spatio-temporal characteristics. Neurology. 2005;64(11):1897–1905. doi: 10.1212/01.WNL.0000163545.57134.3D. [DOI] [PubMed] [Google Scholar]
- Balatsouras D.G., Korres S.G. Subjective benign paroxysmal positional vertigo. Otolaryngol. Head Neck Surg. 2012;146(1):98–103. doi: 10.1177/0194599811425158. [DOI] [PubMed] [Google Scholar]
- Balatsouras D.G., Koukoutsis G., Ganelis P., Korres G.S., Kaberos A. Diagnosis of single- or multiple-canal benign paroxysmal positional vertigo according to the type of nystagmus. Int. J. Otolaryngol. 2011;2011:1–13. doi: 10.1155/2011/483965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh R.W., Honrubia V., Jacobson K. Benign positional vertigo: clinical and oculographic features in 240 cases. Neurology. 1987;37(3):371–372. doi: 10.1212/wnl.37.3.371. [DOI] [PubMed] [Google Scholar]
- Baloh R.W. Reply to the letter from Lempert: Horizontal benign positional vertigo. Neurology. 1994;44(11):2214–2215. doi: 10.1212/wnl.44.11.2213-a. [DOI] [PubMed] [Google Scholar]
- Bance M., Mai M., Tomlinson D., Rutka J. The changing direction of nystagmus in acute Meniere's disease: pathophysiological implications. Laryngoscope. 1991;101(2):197–201. doi: 10.1288/00005537-199102000-00017. [DOI] [PubMed] [Google Scholar]
- Bertholon P., Bronstein A.M., Davies R.A., Rudge P., Thilo K.V. Positional down beating nystagmus in 50 patients: cerebellar disorders and possible anterior semicircular canalithiasis. J. Neurol. Neurosurg. Psychiatry. 2002;72(3):366–372. doi: 10.1136/jnnp.72.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N., Gubbels S.P., Schwartz S.R., Edlow J.A., El-Kashlan H., Fife T. Clinical practice guideline: benign paroxysmal positional vertigo (update) Otolaryngol. Head Neck Surg. 2017;156(3S):S1–S47. doi: 10.1177/0194599816689667. [DOI] [PubMed] [Google Scholar]
- Bisdorff A., Debatisse D. Localizing signs in positional vertigo due to lateral canal cupulolithiasis. Neurology. 2001;57(6):1085–1088. doi: 10.1212/wnl.57.6.1085. [DOI] [PubMed] [Google Scholar]
- Bisdorff A., von Brevern M., Lempert T., Newman-Toker D.E. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J. Vestib. Res. 2009;19:1–13. doi: 10.3233/VES-2009-0343. [DOI] [PubMed] [Google Scholar]
- Brandt T., Dieterich M. Vestibular paroxysmia: vascular compression of the eighth nerve? Lancet. 1994;343:798–799. doi: 10.1016/s0140-6736(94)91879-1. [DOI] [PubMed] [Google Scholar]
- Brandt T., Strupp M., Dieterich M. Vestibular paroxysmia: a treatable neurovascular cross-compression syndrome. J. Neurol. 2016;263(1):90–96. doi: 10.1007/s00415-015-7973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner U., Helmchen C., Brandt T. Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: a review. Acta Otolaryngol. 1999;119(1):1–5. doi: 10.1080/00016489950181855. [DOI] [PubMed] [Google Scholar]
- Cho B.-H., Kim S.-H., Kim S.-S., Choi Y.-J., Lee S.-H. Central positional nystagmus associated with cerebellar tumors: clinical and topographical analysis. J. Neurol. Sci. 2017;373:147–151. doi: 10.1016/j.jns.2016.12.050. [DOI] [PubMed] [Google Scholar]
- Choi J.-Y., Glasauer S., Kim J.H., Zee D.S., Kim J.-S. Characteristics and mechanism of apogeotropic central positional nystagmus. Brain. 2018;141(3):762–775. doi: 10.1093/brain/awx381. [DOI] [PubMed] [Google Scholar]
- Choi J.-Y., Kim J.H., Kim H.J., Glasauer S., Kim J.-S. Central paroxysmal positional nystagmus: characteristics and possible mechanisms. Neurology. 2015;84(22):2238–2246. doi: 10.1212/WNL.0000000000001640. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Seo J.D., Kim M.J., Choi B.Y., Choi Y., Cho B. Vertigo and nystagmus in orthostatic hypotension. Eur. J. Neurol. 2015;22(4):648–655. doi: 10.1111/ene.12622. [DOI] [PubMed] [Google Scholar]
- Choung Y.H., Shin Y.R., Kahng H., Park K., Choi S.J. ‘Bow and lean test' to determine the affected ear of horizontal canal benign paroxysmal positional vertigo. Laryngoscope. 2006;116(10):1776–1781. doi: 10.1097/01.mlg.0000231291.44818.be. [DOI] [PubMed] [Google Scholar]
- Cohen H.S. Side-lying as an alternative to the Dix-Hallpike test of the posterior canal. Otol. Neurotol. 2004;25(2):130–134. doi: 10.1097/00129492-200403000-00008. [DOI] [PubMed] [Google Scholar]
- De Stefano A., Kulamarva G., Dispenza F. Malignant paroxysmal positional vertigo. Auris Nasus Larynx. 2012;39(4):378–382. doi: 10.1016/j.anl.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Dobie R.A., Snyder J.M., Donaldson J.A. Electronystagmographic and audiologic findings in patients with Meniere's disease. Acta Otolaryngol. 1982;94(1–6):19–27. doi: 10.3109/00016488209128885. [DOI] [PubMed] [Google Scholar]
- Epley J.M. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol. Head Neck Surg. 1992;107(3):399–404. doi: 10.1177/019459989210700310. [DOI] [PubMed] [Google Scholar]
- Epley J.M. Human experience with canalith repositioning maneuvers. Ann. N. Y. Acad. Sci. 2001;942(1):179–191. doi: 10.1111/j.1749-6632.2001.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Faldon M., Bronstein A. Head accelerations during particle repositioning manoeuvres. Audiol. Neurootol. 2008;13(6):345–356. doi: 10.1159/000136153. [DOI] [PubMed] [Google Scholar]
- Foster C.A., Zaccaro K., Strong D. Canal conversion and reentry: a risk of Dix-Hallpike during canalith repositioning procedures. Otol. Neurotol. 2012;33(2):199–203. doi: 10.1097/MAO.0b013e31823e274a. [DOI] [PubMed] [Google Scholar]
- Gufoni M., Mastrosimone L., Di F.N. Repositioning maneuver in benign paroxysmal vertigo of horizontal semicircular canal. Acta Otorhinolaryngol. Ital. 1998;18(6):363–367. [PubMed] [Google Scholar]
- Gyo K. Benign paroxysmal positional vertigo as a complication of postoperative bedrest. Laryngoscope. 1988;98(3):332–333. doi: 10.1288/00005537-198803000-00019. [DOI] [PubMed] [Google Scholar]
- Hall S., Ruby R., McClure J. The mechanics of benign paroxysmal vertigo. J. Otolaryngol. 1979;8(2):151–158. [PubMed] [Google Scholar]
- Haynes D.S., Resser J.R., Labadie R.F., Girasole C.R., Kovach B.T., Scheker L.E., Walker D.C. Treatment of benign positional vertigo using the semont maneuver: efficacy in patients presenting without nystagmus. Laryngoscope. 2002;112(5):796–801. doi: 10.1097/00005537-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Hilton M.P., Pinder D.K. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst. Rev. 2014;2014(12):1–38. doi: 10.1002/14651858.CD003162.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.K., Choi H.G., Kim J.S., Koo J.W. A case of vestibular schwannoma presenting vertigo mimicking benign paroxysmalpositional vertigo. Korean J. Otorhinolaryngol-Head Neck Surg. 2008;51(7):664–667. [Google Scholar]
- Honrubia V., Baloh R.W., Harris M.R., Jacobson K.M. Paroxysmal positional vertigo syndrome. Am. J. Otol. 1999;20(4):465–470. [PubMed] [Google Scholar]
- House M.G., Honrubia V. Theoretical models for the mechanisms of benign paroxysmal positional vertigo. Audiol. Neurootol. 2003;8(2):91–99. doi: 10.1159/000068998. [DOI] [PubMed] [Google Scholar]
- Hüfner K., Barresi D., Glaser M., Linn J., Adrion C., Mansmann U. Vestibular paroxysmia Diagnostic features and medical treatment. Neurology. 2008;71(13):1006–1014. doi: 10.1212/01.wnl.0000326594.91291.f8. [DOI] [PubMed] [Google Scholar]
- Humphriss R.L., Baguley D.M., Sparkes V., Peerman S.E., Moffat D.A. Contraindications to the Dix-Hallpike manoeuvre: A multidisciplinary review. Int. J. Audiol. 2003;42(3):166–173. doi: 10.3109/14992020309090426. [DOI] [PubMed] [Google Scholar]
- Hunt W., Zimmermann E., Hilton M.P. Modifications of the Epley (canalith repositioning) manoeuvre for posterior canal benign paroxysmal positional vertigo (BPPV) Cochrane Database Syst. Rev. 2012;2012(4):1–41. doi: 10.1002/14651858.CD008675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Ito M., Takeda N., Uno A., Matsunaga T., Sekine K., Kubo T. Natural course of the remission of vertigo in patients with benign paroxysmal positional vertigo. Neurology. 2005;64(5):920–921. doi: 10.1212/01.WNL.0000152890.00170.DA. [DOI] [PubMed] [Google Scholar]
- Imai T., Takeda N., Ito M., Sekine K., Sato G., Midoh Y. 3D analysis of benign positional nystagmus due to cupulolithiasis in posterior semicircular canal. Acta Otolaryngol. 2009;129(10):1044–1049. doi: 10.1080/00016480802566303. [DOI] [PubMed] [Google Scholar]
- Ishiyama A., Jacobson K.M., Baloh R.W. Migraine and benign positional vertigo. Ann. Otol. Rhinol. Laryngol. 2000;109(4):377–380. doi: 10.1177/000348940010900407. [DOI] [PubMed] [Google Scholar]
- Karlberg M., Hall K., Quickert N., Hinson J., Halmagyi G.M. What inner ear diseases cause benign paroxysmal positional vertigo? Acta Otolaryngol. 2000;120(3):380–385. doi: 10.1080/000164800750000603. [DOI] [PubMed] [Google Scholar]
- Katsarkas A. Benign paroxysmal positional vertigo (BPPV): idiopathic versus post-traumatic. Acta Otolaryngol. 1999;119(7):745–749. doi: 10.1080/00016489950180360. [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Shin J.E., Chung J.W. The effect of canalith repositioning for anterior semicircular canal canalithiasis. ORL J. Otorhinolaryngol. Relat. Spec. 2005;67(1):56–60. doi: 10.1159/000084336. [DOI] [PubMed] [Google Scholar]
- Kim H.-A., Yi H.-A., Lee H. Apogeotropic central positional nystagmus as a sole sign of nodular infarction. Neurol. Sci. 2012;33(5):1189–1191. doi: 10.1007/s10072-011-0884-x. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Oh S.-Y., Lee S.-H., Kang J.H., Kim D.U., Jeong S.-H. Randomized clinical trial for apogeotropic horizontal canal benign paroxysmal positional vertigo. Neurology. 2012;78(3):159–166. doi: 10.1212/WNL.0b013e31823fcd26. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Oh S.-Y., Lee S.-H., Kang J.H., Kim D.U., Jeong S.-H. Randomized clinical trial for geotropic horizontal canal benign paroxysmal positional vertigo. Neurology. 2012;79(7):700–707. doi: 10.1212/WNL.0b013e3182648b8b. [DOI] [PubMed] [Google Scholar]
- Korres S., Balatsouras D.G., Kaberos A., Economou C., Kandiloros D., Ferekidis E. Occurrence of semicircular canal involvement in benign paroxysmal positional vertigo. Otol. Neurotol. 2002;23(6):926–932. doi: 10.1097/00129492-200211000-00019. [DOI] [PubMed] [Google Scholar]
- Lechner C., Taylor R.L., Todd C., Macdougall H., Yavor R., Halmagyi G.M., Welgampola M.S. Causes and characteristics of horizontal positional nystagmus. J. Neurol. 2014;261(5):1009–1017. doi: 10.1007/s00415-013-7223-5. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Kim E.S., Kim M., Chu H., Ma H.I., Lee J.S. Isolated horizontal positional nystagmus from a posterior fossa lesion. Ann. Neurol. 2014;76(6):905–910. doi: 10.1002/ana.24292. [DOI] [PubMed] [Google Scholar]
- Lempert T., Olesen J., Furman J., Waterston J., Seemungal B., Carey J. Vestibular migraine: diagnostic criteria. J. Vestib. Res. 2012;22(4):167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- Lempert T., Tiel-Wilck K. A positional maneuver for treatment of horizontal-canal benign positional vertigo. Laryngoscope. 1996;106(4):476–478. doi: 10.1097/00005537-199604000-00015. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez J.A., Carey J., Chung W.H., Goebel J.A., Magnusson M., Mandala M. Diagnostic criteria for Meniere's disease. J. Vestib. Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez J.A., Molina M.I., Gamiz M.J. Anterior semicircular canal benign paroxysmal positional vertigo and positional downbeating nystagmus. Am. J. Otolaryngol. 2006;27(3):173–178. doi: 10.1016/j.amjoto.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Macdonald N.K., Kaski D., Saman Y., Sulaiman A.A.-S., Anwer A., Bamiou D.-E. Central positional nystagmus: A systematic literature review. Front. Neurol. 2017;8(141):1–11. doi: 10.3389/fneur.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias J.D., Ellensohn A., Massingale S., Gerkin R. Vibration with the canalith repositioning maneuver: a prospective randomized study to determine efficacy. Laryngoscope. 2004;114(6):1011–1014. doi: 10.1097/00005537-200406000-00010. [DOI] [PubMed] [Google Scholar]
- Mandalà M., Santoro G.P., Libonati G.A., Casani A.P., Faralli M., Giannoni B. Double-blind randomized trial on short-term efficacy of the Semont maneuver for the treatment of posterior canal benign paroxysmal positional vertigo. J. Neurol. 2012;259(5):882–885. doi: 10.1007/s00415-011-6272-x. [DOI] [PubMed] [Google Scholar]
- Mostafa B.E., Youssef T.A., Hamad A.S. The necessity of post-maneuver postural restriction in treating benign paroxysmal positional vertigo: a meta-analytic study. Eur. Arch. Otorhinolaryngol. 2013;270(3):849–852. doi: 10.1007/s00405-012-2046-z. [DOI] [PubMed] [Google Scholar]
- Motamed M., Osinubi O., Cook J. Effect of mastoid oscillation on the outcome of the canalith repositioning procedure. Laryngoscope. 2004;114(7):1296–1298. doi: 10.1097/00005537-200407000-00029. [DOI] [PubMed] [Google Scholar]
- Nuti D., Masini M., Mandalà M. Benign paroxysmal positional vertigo and its variants. Handb. Clin. Neurol. 2016;137:241–256. doi: 10.1016/B978-0-444-63437-5.00018-2. [DOI] [PubMed] [Google Scholar]
- Nuti D., Nati C.A., Passali D. Treatment of benign paroxysmal positional vertigo: no need for postmaneuver restrictions. Otolaryngol. Head Neck Surg. 2000;122(3):440–444. doi: 10.1016/S0194-5998(00)70070-2. [DOI] [PubMed] [Google Scholar]
- Nuti D., Vannucchi P., Pagnini P. Benign paroxysmal positional vertigo of the horizontal canal: a form of canalolithiasis with variable clinical features. J. Vestib. Res. 1996;6(3):173–184. [PubMed] [Google Scholar]
- Oghalai J.S., Manolidis S., Barth J.L., Stewart M.G., Jenkins H.A. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol. Head Neck Surg. 2000;122(5):630–634. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- Oh H.J., Kim J.S., Han B.I., Lim J.G. Predicting a successful treatment in posterior canal benign paroxysmal positional vertigo. Neurology. 2007;68(15):1219–1222. doi: 10.1212/01.wnl.0000259037.76469.e4. [DOI] [PubMed] [Google Scholar]
- Parnes L.S., Agrawal S.K., Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV) CMAJ. 2003;169(7):681–693. [PMC free article] [PubMed] [Google Scholar]
- Parnes L.S., Price-Jones R.G. Particle repositioning maneuver for benign paroxysmal positional vertigo. Ann. Otol. Rhinol. Laryngol. 1993;102(5):325–331. doi: 10.1177/000348949310200501. [DOI] [PubMed] [Google Scholar]
- Pérez P., Franco V., Cuesta P., Aldama P., Alvarez M.J., Méndez J.C. Recurrence of benign paroxysmal positional vertigo. Otol. Neurotol. 2012;33(3):437–443. doi: 10.1097/MAO.0b013e3182487f78. [DOI] [PubMed] [Google Scholar]
- Polensek S.H., Tusa R.J. Nystagmus during attacks of vestibular migraine: an aid in diagnosis. Audiol. Neurootol. 2010;15(4):241–246. doi: 10.1159/000255440. [DOI] [PubMed] [Google Scholar]
- Prokopakis E., Vlastos I., Tsagournisakis M., Christodoulou P., Kawauchi H., Velegrakis G. Canalith repositioning procedures among 965 patients with benign paroxysmal positional vertigo. Audiol. Neurootol. 2013;18(2):83–88. doi: 10.1159/000343579. [DOI] [PubMed] [Google Scholar]
- Sakaida M., Takeuchi K., Ishinaga H., Adachi M., Majima Y. Long-term outcome of benign paroxysmal positional vertigo. Neurology. 2003;60(9):1532–1534. doi: 10.1212/01.wnl.0000061477.03862.4d. [DOI] [PubMed] [Google Scholar]
- Schuknecht H.F. Cupulolithiasis. Arch. Otolaryngol. 1969;90(6):765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- Semont A., Freyss G., Vitte E. Curing the BPPV with a liberatory maneuver. Adv. Oto-Rhino-Laryng. 1988;42:290–293. doi: 10.1159/000416126. [DOI] [PubMed] [Google Scholar]
- Song C.I., Kang B.C., Yoo M.H., Chung J.W., Yoon T.H., Park H.J. Management of 210 patients with benign paroxysmal positional vertigo: AMC protocol and outcomes. Acta Otolaryngol. 2015;135(5):422–428. doi: 10.3109/00016489.2014.993089. [DOI] [PubMed] [Google Scholar]
- Steddin S., Brandt T. Unilateral mimicking bilateral benign paroxysmal positioning vertigo. Arch. Otolaryngol. Head Neck Surg. 1994;120(12):1339–1341. doi: 10.1001/archotol.1994.01880360037007. [DOI] [PubMed] [Google Scholar]
- Steenerson R.L., Cronin G.W., Marbach P.M. Effectiveness of treatment techniques in 923 cases of benign paroxysmal positional vertigo. Laryngoscope. 2005;115(2):226–231. doi: 10.1097/01.mlg.0000154723.55044.b5. [DOI] [PubMed] [Google Scholar]
- Strupp M., Lopez-Escamez J.A., Kim J.-S., Straumann D., Jen J.C., Carey J. Vestibular paroxysmia: diagnostic criteria. J. Vestib. Res. 2016;26:409–415. doi: 10.3233/VES-160589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.L., Chen L., Lechner C., Aw S.T., Welgampola M.S. Vestibular schwannoma mimicking horizontal cupulolithiasis. J. Clin. Neurosci. 2013;20(8):1170–1173. doi: 10.1016/j.jocn.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Tirelli G., D'Orlando E., Giacomarra V., Russolo M. Benign positional vertigo without detectable nystagmus. Laryngoscope. 2001;111(6):1053–1056. doi: 10.1097/00005537-200106000-00022. [DOI] [PubMed] [Google Scholar]
- Vannucchi P., Giannoni B., Pagnini P. Treatment of horizontal semicircular canal benign paroxysmal positional vertigo. J. Vestib. Res. 1997;7(1):1–6. [PubMed] [Google Scholar]
- Vannucchi P., Pecci R., Giannoni B. Posterior semicircular canal benign paroxysmal positional vertigo presenting with torsional downbeating nystagmus: an apogeotropic variant. Int. J. Otolaryngol. 2012;2012:1–9. doi: 10.1155/2012/413603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi P., Pecci R., Giannoni B., Di Giustino F., Santimone R., Mengucci A. Apogeotropic posterior semicircular canal benign paroxysmal positional vertigo: some clinical and therapeutic considerations. Audiol. Res. 2015;5(130):38–43. doi: 10.4081/audiores.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brevern M., Bertholon P., Brandt T., Fife T., Imai T., Nuti D., Newman-Toker D. Benign paroxysmal positional vertigo: diagnostic criteria. J. Vestib. Res. 2015;25:105–117. doi: 10.3233/VES-150553. [DOI] [PubMed] [Google Scholar]
- von Brevern M., Radtke A., Clarke A.H., Lempert T. Migrainous vertigo presenting as episodic positional vertigo. Neurology. 2004;62(3):469–472. doi: 10.1212/01.wnl.0000106949.55346.cd. [DOI] [PubMed] [Google Scholar]
- von Brevern M., Radtke A., Lezius F., Feldmann M., Ziese T., Lempert T., Neuhauser H. Epidemiology of benign paroxysmal positional vertigo: a population based study. J. Neurol. Neurosurg. Psychiatry. 2007;78(7):710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacovino D.A., Hain T.C., Gualtieri F. New therapeutic maneuver for anterior canal benign paroxysmal positional vertigo. J. Neurol. 2009;256(11):1851–1855. doi: 10.1007/s00415-009-5208-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.