Abstract
Background
Tafenoquine, an 8-aminoquinoline, is now indicated for causal prophylaxis against all human malarias and as radical curative (anti-relapse) treatment against Plasmodium vivax and Plasmodium ovale. As with other 8-aminoquinolines, tafenoquine causes hemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency (hemizygous males and homozygous females) and is contraindicated in this population. Those with intermediate G6PD activity (heterozygous females) are also at risk for hemolysis. Awareness of how to prescribe tafenoquine in relation to G6PD status is needed so it can be used safely.
Methods
A standard literature search was performed on varying combinations of the terms tafenoquine, Arakoda, Kodatef, Krintafel, Kozenis, primaquine, G6PD deficiency, malaria prophylaxis and radical cure. The data were gathered and interpreted to review how tafenoquine should be prescribed in consideration of the G6PD status of an individual and traveller.
Results
Tafenoquine should only be given to those with G6PD activity >70% of the local population median. Qualitative G6PD tests are sufficient for diagnosing G6PD deficiency in males. However, in females quantitative G6PD testing is necessary to differentiate deficient, intermediate and normal G6PD statuses. Testing for G6PD deficiency is mandatory before tafenoquine prescription. Measures can be taken to avoid tafenoquine administration to ineligible individuals (i.e. due to G6PD status, age, pregnancy and lactation). Primaquine is still necessary for some of these cases. This review provides actions that can be taken to diagnose and manage hemolysis when tafenoquine is given inadvertently to ineligible individuals.
Conclusion
Attention to G6PD status is required for safe prescription of tafenoquine. A high index of suspicion is needed if hemolysis occurs. Clinicians should seek evidence-based information for the management and treatment of iatrogenicy hemolysis caused by 8-aminoquinolines.
Keywords: 8-aminoquinolines, malaria prophylaxis, causal prophylaxis, radical cure, presumptive anti-relapse treatment (PART), tafenoquine, glucose-6-phosphate dehydrogenase deficiency
History of the 8-aminoquinoline antimalarials
Plasmodium falciparum and Plasmodium vivax are the most common causes of human malaria. Both are endemic in tropical areas, but the majority of P. vivax cases occur outside Sub-Saharan Africa.1 The standard treatment for P. falciparum is schizonticidal artemisinin combination therapy against blood-stage infection. When used in combination with a gametocytocidal single dose of primaquine, transmission is blocked.2 However, blood-stage treatment alone does not cure P. vivax (or P. ovale). This is due to the hypnozoite stage (Figure 1), which lies dormant in the liver until triggered to develop into a relapsed blood-stage infection.4,5 Without anti-relapse (radical cure) treatment against hypnozoites, relapses increase the rate of infection.6
Figure 1.
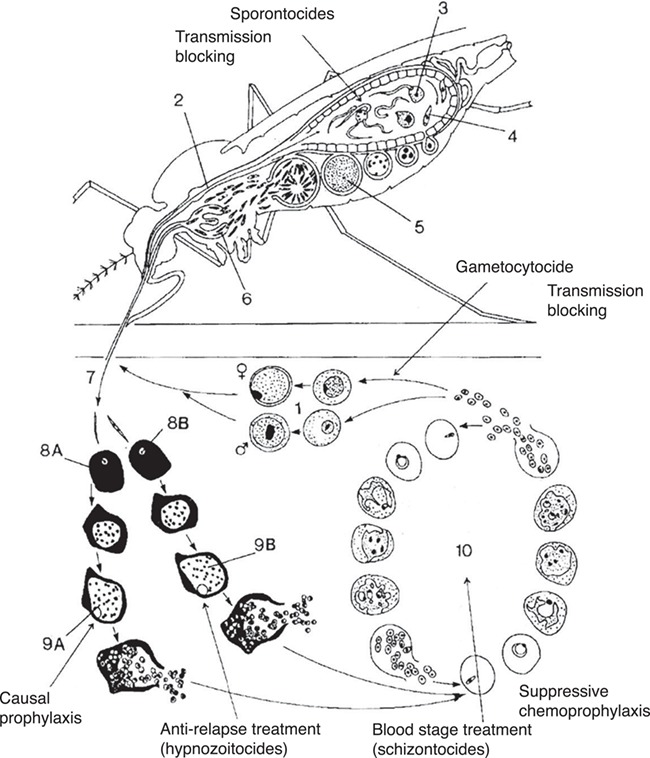
The sexual gametocytes transmit infection and complete the life cycle from humans to mosquitoes. 2–6: The sporogonic stages are where gametes form oocysts in the mosquito mid-gut then proceed to become sporozoites in the salivary glands. 7: The infective sporozoites are inoculated into humans during a mosquito bite.8A and 9A: Sporozoites travel to the liver and pre-erythrocytic schizogony occurs here. The resulting tissue schizonts rupture and releases merozoites into the bloodstream; causal prophylactic drugs act here. 8B and 9B: In P. vivax and P. ovale, some sporozoites will separately differentiate into dormant hypnozoites. When triggered to ‘wake up’, the latent hypnozoites will undergo pre-erythrocytic schizogony; ‘radical cure’ targets this life-cycle stage. 10: Asexual intraerythrocytic schizogony is the blood stage of infection when symptoms occur and where blood schizontocidal drugs act.This figure was reproduced and modified from Figure 1 in Peters, 1999,3 ‘based on an original figure by Andrea Darlow’ under SAGE publications licence number 4519190237646.
The transmission-blocking gametocytocidal effect and radical curative efficacy of the 8-aminoquinoline drugs have been recognized for nearly a century.7,8 Early analogues of 8-aminoquinolines had significant side effects and prompted a search for alternative radical curative agents by the World War II antimalarial program. This resulted in the introduction of primaquine in 1950.9 Since then, primaquine has been the only gametocytocidal and radical curative treatment available for common use. Very recently, tafenoquine, a primaquine analogue with a long terminal half-life developed 40 years ago,10 was approved by the US Food and Drug Administration. Tafenoquine, like primaquine, causes hemolysis in glucose-6-phosphate dehydrogenase (G6PD)-deficient individuals, the most common genetic disorder in humans. In this article we review practical points for the safe use of tafenoquine focusing on the G6PD status of the traveller.
An unusual development history has led tafenoquine succinate to be approved in two separate formulations (of the same active pharmaceutical ingredient) from two separate manufacturers. The 60° Pharmaceuticals manufactures Arakoda® and Kodatef® for causal prophylaxis in the USA and Australia, respectively. Partnering with Medicines for Malaria Venture, GlaxoSmithKline (GSK) manufactures Krintafel® and Kozenis® for radical cure of P. vivax, also in the USA and Australia, respectively.
Indications for tafenoquine prescription
Tafenoquine works in three ways to prevent malaria infections (Figure 1 and Table 1):
Causal prophylaxis for all human malarias except for prevention of relapses caused by P. vivax or P. ovale—Arakoda® (US trade name) and Kodatef® (Australian trade name) manufactured by 60° Pharmaceuticals (60P). Blood-stage infection is prevented by action against tissue schizonts in the liver. This contrasts with suppressive chemoprophylaxis where schizonticidal drugs (such as mefloquine and doxycycline) are used against blood-stage parasites.
Radical cure against P. vivax malaria relapses arisingfrom hypnozoites, after an acute primary symptomatic blood-stage infection has already occurred—Krintafel® (US trade name) and Kozenis® (Australian trade name) manufactured by GSK. The acute bloodstage infection should already be treated with a schizonticide, and radical cure can be given during the blood-stage treatment or after it is complete.
Presumptive anti-relapse therapy (PART, previously termed post-exposure prophylaxis or terminal prophylaxis) against P. vivax malaria relapses in asymptomatic individuals with recent exposure—the final post-exposure dose of tafenoquine given during causal prophylaxis (Arakoda® and Kodatef®) can act as PART in place of primaquine. However, the actual anti-relapse tafenoquine regimen is a 300-mg single dose. Neither Krintafel®/Kozenis® nor Arakoda®/Kodatef® currently are licenced for PART. A traveller taking a schizonticidal drug for suppressive chemoprophylaxis during travel could reasonably use a 300-mg dose of any preparation of tafenoquine in this way instead of primaquine.12
Table 1.
Indications and contraindications for tafenoquine as compared to primaquine
| Primaquine; radical curea and PART | Tafenoquine; radical cure (Krintafel®)b | Causal prophylaxis (Kozenis®)c | Causal prophylaxis (Arakoda®)b | Causal prophylaxis (Kodatef®)b | |
|---|---|---|---|---|---|
| Recommended dosing | Daily regimen: 0.25 or 0.5 mg/kg per day × 14 days or weekly regimen: 0.75 mg/kg per week × 8 weeks | 300-mg single dose (two 150-mg tablets) | 300-mg single dose (two 150-mg tablets) | Loading dose: 200-mg daily for days (two 100-mg tablets); maintenance dose: 200 mg weekly while in the malarious area (start 7 days after the last loading dose). Final dose is 7 days after the last maintenance dose. Maximum duration: 6 months | Loading dose: 200 mg daily for 3 days [two 125.5 mg (100-mg base) tablets]; maintenace dose: 200 mg weekly while in the malarious area (start 7 days after the last loading dose). Final dose is 7 days after the last maintenance dose. Maximum duration: 6 months |
| Age limitations | ≥6 months | ≥16 years | Not available | ≥18 years | 18 years |
| Use in pregnancy | Contraindicated | Not recommended but not contraindicated, avoid pregnancy or use effective contraception for 3 months after the dose | Contraindicated | Not recommended but not contraindicated, avoid pregnancy or use contraception for 3 months after the last dose | Contraindicated, use effective contraception during malaria prevention administration and for five half-lives (3 months) after the end of treatment |
| Use in lactation | Contraindicated if G6PD status of the breastfeeding infant is deficient or unknown | Contraindicated if infant G6PD status is abnormal or unknownd | Contraindicated if infant G6PD status is abnormal or unknown | Contraindicated if infant G6PD status is abnormal or unknownd | Contraindicated |
| Administration after food | Recommended | Recommended | Not available | Recommended | Can be taken with or without food. Taken with food may be associated with better gastrointestinal tolerance |
| G6PD testing requirements | When G6PD status is not known and G6PD testing is not available, a decision to prescribe primaquine must be based on an assessment of the risks and benefits of adding primaquine | G6PD test is required | G6PD testing is required, withhold if G6PD enzyme levels <70% of normal | G6PD test is required | G6PD test is required |
| G6PD deficiency | Daily dosing is contraindicated | Contraindicated in G6PD deficiency or unknown | Contraindicated in G6PD deficiency | Contraindicated in G6PD deficiency or unknown | Contraindicated in G6PD deficiency or unknown |
| Dosing in G6PD deficiency | Weekly regimen (0.75 mg/kg per day for 8 weeks) with close medical supervision | None | None | None | None |
aInformation taken from the World Health Organization malaria guidelines 2015.11
bInformation taken from the package inserts of the respective manufacturers (Supplement Figures 1–3).
cPackage inserts are not available. Information taken from the press release, from the section titled ‘Important safety information’ (Supplement Figure 4)
dThe warning label in the package insert advises women not to breastfeed a G6PD deficiency infant or infant with unknown G6PD status during treatment and for 3 months after the last dose.
Although tafenoquine has gametocytocidal activity,13,14 it is not indicated currently as a transmission-blocking drug against P. falciparum for which primaquine is prescribed as a single low dose in both G6PD-normal and G6PD-deficient individuals. Both 8-aminoquinolines are effective against blood schizont stage of the P. vivax life cycle, but the parasite clearance is slower than with chloroquine and neither should be used as mono-therapy against blood-stage infections.15,16
The main advantage of tafenoquine over primaquine is its slow elimination (the terminal half-life is 12–17 days).17–19 A single 300-mg dose is efficacious for radical cure, a loading dose of 200 mg for 3 days and maintenance dose of 200 mg weekly are necessary for causal prophylaxis, and a single 200-mg dose 7 days after the last maintenance dose suffices for PART if tafenoquine was used during travel. The convenient practical tafenoquine regimens potentially will improve drug effectiveness due to increased adherence, in contrast to primaquine where adherence is challenged by long periods of daily dosing.
Non-hemolytic adverse effects of tafenoquine
The side effect profile of tafenoquine is similar to primaquine. Tafenoquine is associated with gastrointestinal symptoms,15,18 which can be alleviated if food is taken before the dose.20 Moderate methemoglobinemia (absolute levels, >10%) can occur when higher doses of tafenoquine (>200 mg) are given daily or weekly.15,18,20–22 When given as a single dose for radical cure moderate methemoglobin elevations are rare and have been reported in <0.01% (3/740) study subjects in total.22–27 In these studies, symptoms were not associated with methemoglobin elevations. Corneal deposits (vortex keratopathy due to the lipid properties of the drug) not affecting vision were seen in 25% of subjects given tafenoquine for 6 months and after cessation resolved spontaneously within 12 weeks in 95% of affected subjects and within 48 weeks in 100% of subjects.28 The manufacturer indicates that a prophylaxis study of 1-year duration is underway.
Hemolytic effects of tafenoquine according G6PD status
Tafenoquine, like primaquine, causes hemolysis in individuals with G6PD deficiency, the most common genetic disorder in humans. G6PD-normal heterozygous females with intermediate G6PD activity are also at risk for tafenoquine-induced hemolysis27 (see below). Here, the advantage of the rapidly eliminated primaquine is clear; it can be stopped as soon as hemolysis is suspected.
Biology of G6PD deficiency
The rate limiting G6PD enzyme catalyzes the first step in the pentose phosphate pathway during which NADPH is generated.29 NADPH is essential for reducing oxidized glutathione, which is formed inside the red blood cell (RBC) during oxidative stress, and maintaining the normal reduced state of the RBC.30 In the presence of primaquine or tafenoquine, G6PD-deficient RBCs have limited reductive capacity (from decreased NADPH byproduct) and are susceptible to hemolysis caused by the oxidative 8-aminoquinolines.
The G6PD gene lies on the X chromosome so that males with a mutation (hemizygote) and females with mutated genes on both X chromosomes (homozygote) will have full phenotypic expression of the deficiency. For females with one normal and one mutated G6PD gene (heterozygote) the situation is different. During early embryogenesis in a female, one X chromosome in each somatic cell is randomly inactivated so either the maternal or paternal genes are expressed. This is called X-chromosome inactivation (lyonization) and results in G6PD heterozygous females having a mixture of G6PD-normal and G6PD-deficient red cells at varying ratios with total G6PD activity levels ranging from deficient to normal31 (Figure 2).
Figure 2.
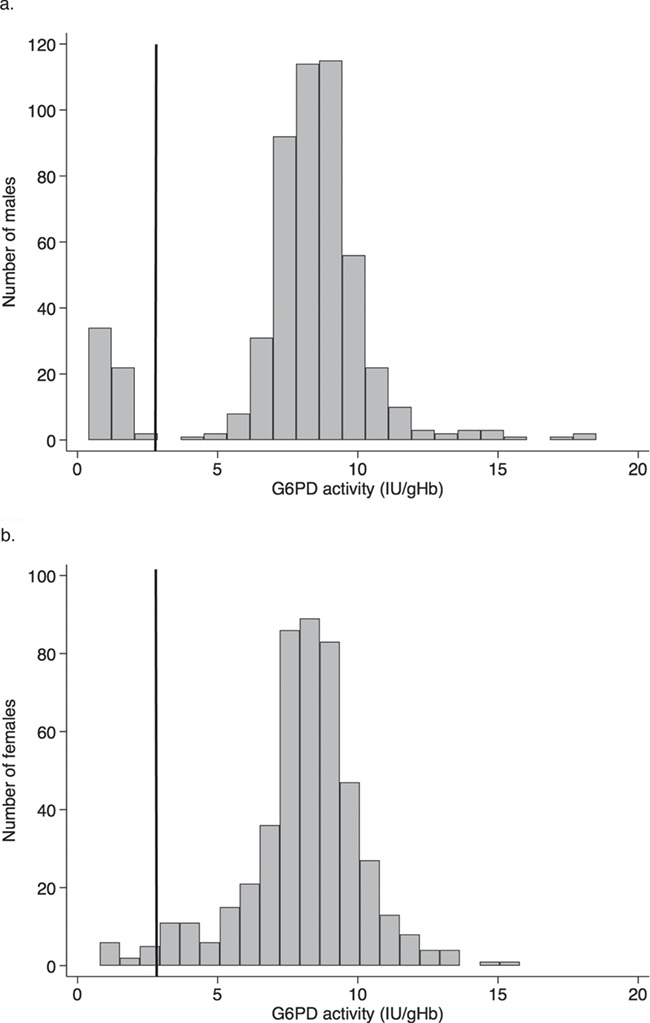
The solid black line indicates the threshold for diagnosing G6PD deficiency (≲30% of the population median). Figure 2a shows 2 distinct populations in males (G6PD normal and deficient). Figure 2b shows that some females will have intermediate G6PD activity as seen by the continuous distribution. This figure was adapted from Oo et al.32
Diagnosis of G6PD deficiency
An individual’s G6PD status is assessed by genotyping, protein characterization or enzymatic activity. The best assessment for a drug’s hemolytic risk is the enzymatic activity detected in blood (expressed as IU/gHb or RBC). The gold standard laboratory analysis for G6PD quantitative enzymatic activity is the spectrophotometric assay,33 which requires an equipped laboratory, trained technicians and expensive reagents.
The G6PD fluorescent spot test is a rapid diagnostic test (RDT) where phenotype is determined by hemolysing a small quantity of blood and supplying the substrate for the enzymatic reaction that produces NADPH. This is then spotted on filter paper and examined under long-wavelength UV light to visualize the naturally fluorescent NAPDH. When NADPH levels are reduced, there is no fluorescence. Other phenotypic G6PD RDTs commercially available are the CareStart™ G6PD and BinaxNOW® G6PD, which are lateral flow devices. These three tests are used for the qualitative measurement of G6PD activity, i.e. results are binary with deficiency diagnosed on a single activity threshold of ≲30%. Thus, anyone with ≳30% activity, including G6PD heterozygous females, will be diagnosed as G6PD normal. While the qualitative test is sufficient for identifying individuals with G6PD activity ≲30%, to know the G6PD activity level of most heterozygous females (with intermediate activity) requires a quantitative test. Point-of-care technologies now available are the ‘Standard G6PD’ by SD Biosensor34,35 and the ‘CareStart™ G6PD Biosensor’ by AccessBio.36,37 These tests provide a quantitative reading of G6PD activity similar to the gold standard spectrophotometric assay in a fraction of the time and cost. The ‘Standard G6PD’ can measure hemoglobin, but the ‘CareStart™ G6PD Biosensor’ does not so it would need to be ordered separately. Females with intermediate G6PD activity (≳30% to ≲80% of the adjusted population median38) at risk for hemolysis from 8-aminoquinolines27,39–41 can be identified with these quantitative tests. When quantitative G6PD tests are ordered from reference laboratories, a value for G6PD activity and a reference range are given in the results. Practitioners should not administer tafenoquine to male or females with G6PD activity levels below the laboratory’s reference range, which corresponds to an intermediate or deficient G6PD status.
Because the G6PD activity measurement is normalized by the hemoglobin from the same blood sample, severe anaemia results in an artificially elevated G6PD activity level42 and possibly an incorrect diagnosis of G6PD normality. In situations where the G6PD status by phenotypic test is uncertain and the diagnosis is required, genotype can help identify the individuals who should not take an 8-aminoquinoline (hemizygous males and homozygous females) and those who could be treated alternatively with primaquine (heterozygous females). Otherwise, the G6PD phenotypic test can be repeated once the hemoglobin is at steady state (~3 months after a blood transfusion or 4–6 weeks after a hemolytic episode).
The assessment of whether a drug will have a hemolytic effect relies on the G6PD phenotypic test. An exception is in a small minority of G6PD variants that are associated chronic non-spherocytic hemolytic anaemia. Such individuals should not receive an 8-aminoquinoline regardless of G6PD phenotype because of ongoing hemolysis and risk for severe hemolytic exacerbations.43
Outcomes when tafenoquine has been inadvertently administered to G6PD deficient individuals
To date, published studies using tafenoquine have included only G6PD-normal individuals. Some studies included specifically only those with a G6PD activity >70% of the population median, which is the upper limit of the intermediate threshold. For males, a G6PD qualitative test is highly sensitive and specific for the diagnosis of G6PD deficiency. No G6PD-deficient males have been reported to have received tafenoquine in any published clinical trials whereas two G6PD deficient females have. Both were in the same study,44 mistakenly diagnosed as G6PD normal on a qualitative test, randomized to a 400-mg regimen (loading then weekly dose) and experienced hemoglobin reductions of >3 g/dL. One was heterozygous and the other homozygous for the (A-) G6PD variant. The G6PD heterozygous female required blood transfusions while the G6PD homozygous female was asymptomatic. Hematologic recoveries occurred without further intervention. These observations confirm that G6PD deficiency is an absolute contraindication for tafenoquine. Testing for G6PD deficiency is mandatory before prescribing tafenoquine.
Outcomes when tafenoquine has been administered to G6PD-normal individuals (including heterozygotes with intermediate activity)
Of the 6337 individuals included in 27 published human trials on tafenoquine, there were 783 females in 18 studies (Table 2). Excepting the two G6PD-deficient cases described previously, all were G6PD-normal (≳30% activity) females as diagnosed by a qualitative G6PD test. Nineteen were G6PD heterozygous females with intermediate activity (40–80%) as diagnosed by a quantitative G6PD test. Two of them were receiving daily tafenoquine (450 mg for 2 days). They developed a hemoglobin reduction >2.5 g/dL at Day 10 and were subsequently found to be heterozygous for the Santa Maria and (A-) G6PD variants.18
Table 2.
Summary table of trials using tafenoquine that included female subjects
| Publication (first author, year of publication) | Number of females in tafenoquine groups | Malaria statusa | Tafenoquine frequency | Tafenoquine dose in base mg | G6PDb status of females | Study location | Hematologic findings |
|---|---|---|---|---|---|---|---|
| Walsh, 1999 | 17 | Pv monoinfection | Daily and/or weekly, or single | 300 or 500* | G6PD normal | Thailand and Myanmar | No clinically important abnormalities |
| Lell, 2000 | 176 | Any malaria or healthy | Daily | 25, 50, 100 or 200* | G6PD normal | Gabon | Hb at Day 28 was slightlyc (0.4 g/dL) but significantly lower in the TQ 200 mg base groupd |
| Shanks, 2001 | 65 | Healthy | Daily and/or weekly | 200 or 400 | G6PD normal, two G6PD deficients subsequently identified (quantitative) |
Kenya | Two G6PD deficient females had >3 g/dL hb reduction; one transfused and one asymptomatic. Hb changes in the other females not reported |
| Nasveld, 2002 | 51 | Healthy | Daily or twice daily | 200 or 400 | G6PD normal | Australia (Papua New Guineae) | Not reported |
| Hale, 2003 | 131 | Any malaria or healthy | Daily and/or weekly | 25, 50, 100 or 200* | G6PD normal | Ghana | Trend of more frequent Hb reductiond to <8 g/dL as TQ dose increases |
| Walsh, 2004 | 21 | Pv monoinfection | Daily or single dose | 300 or 600* | G6PD normal | Thailand and Myanmar | No clinically important abnormalities |
| Charles, 2007 | 14 | Healthy | Daily and/or weekly | 200 | G6PD normal | Australia (Timor-Lestee) | Not reported |
| Kitchener, 2007 | 1 | Healthy | Daily and weekly | 200 | G6PD normal | Australia (Papua New Guineae) | Not reported |
| Elmes, 2008 | 28 | Healthy | Daily or twice daily | 200 or 400* | G6PD normal | Australia (Bougainville, Timor-Lestee) | Not reported |
| Leary, 2009 | 33 | Healthy | Daily and weekly | 200* | G6PD normal | UK and USA | Not reported |
| Nasveld, 2010 | 14 | Healthy | Daily and weekly | 200* | G6PD normal | Australia (Timor-Lestee) | Hct reduction of 15%c from baseline was more frequent with TQ (20%) and 2 subjects had clinically significant reductionsd (<85% LLN) |
| Miller, 2013 | 18 | Healthy | Daily | 450*,f | G6PD normal, two G6PD intermediates subsequently identified (quantitative) | USA | Two G6PD heterozygotes with intermediate G6PD activity had Hb reduction >2.5 g/dL. Trend of greater frequency of TQ treated subjects with mild Hb reductions (>.5–≤2.5 mg/dL) |
| Llanos-Cuentas, 2014 | 56 | Pv monoinfection | Single dose | 50, 100, 300 or 600* | >70% G6PD activity (quantitative) | Brazil, India, Peru and Thailand | >2.5 g/dL reduction or >25% fractional decline from baseline more frequent with TQd |
| Rueangweerayut, 2017 | 17 | Healthy | Single dose | 100 or 300* | 40–80% G6PD activity (quantitative) | Thailand | Dose-dependent Hb reduction (>3 g/dL) with TQ |
| Fukuda, 2017 | 9 | Pv monoinfection | Daily | 400* | G6PD normal | Thailand | Not reported |
| Green, 2016 | 16 | Healthy | Single dose | 300* | >70% G6PD activity (quantitative) | USA | No Hb decline >2.0 g/dL in any group |
| Llanos-Cuentas, 2019 | 52 | Pv monoinfection | Single dose | 300* | >70% G6PD activity (quantitative) | Brazil, Colombia, Peru, Thailand and Vietnam | Frequency of Hb reduction >3 g/dL or >30% fractional from baseline similar in TQ (2.4%) and PQ (1.2%) groupsd |
| Lacerda, 2019 | 64 | Pv monoinfection | Single dose | 300* | >70% G6PD activity (quantitative) | Brazil, Cambodia, Ethiopia, Peru, the Philippines and Thailand | Frequency of Hb reduction >3 g/dL greater in TQ (5.4%) compared with other groups (~1.5%)d |
*Non-tafenoquine groups were included in the study.
aStudy subjects with malaria received a schizonticidal drug in addition to tafenoquine.
bUnless specified, all females were G6PD normal by a qualitative G6PD test.
cThe value presented was not specified (i.e. mean, median, absolute or fractional) in the publication.
dHemoglobin or hematocrit reductions were not stratified by sex.
eThis is the location of the malaria exposure.
fThe publication does not specify whether this dose is in base mg.
Hb, hemoglobin; Hct, hematocrit; TQ, tafenoquine; PQ, primaquine; LLN, lower limit of normal.
The other 17 were enrolled in a single study where they were randomized to receive 100-, 200- or 300-mg tafenoquine as a single dose.27 A dose-dependent hemolysis was observed; three of three G6PD heterozygous females receiving the 300-mg single dose experienced a hemoglobin reduction >3 g/dL, which was similar to the primaquine group (15 mg daily for 14 days).
Of the remaining 764 G6PD-normal females receiving tafenoquine, 188 of them were enrolled in studies (single dose tafenqouine only) that included only persons with a G6PD activity >70% of the population median. In the studies where only a G6PD qualitative test was performed (daily or weekly tafenoquine only), 7 of 13 studies included information on whether hemolysis occurred. Hemoglobin reductions (either greater in value or more frequent) were observed during tafenoquine administration in four of seven studies, but this was not stratified by sex so whether hemolysis was more frequent or severe in females (possibly due to undetected intermediate activity) is unknown. Nonetheless, dose-dependent hemolysis was observed in single dose tafenoquine trials, which means that the hemolytic risk in G6PD-intermediate females potentially is greater in the daily loading doses used for causal prophylaxis. Thus, quantitative G6PD testing must be performed before prescribing tafenoquine. Tafenoquine should only be given if the G6PD activity is >70% of the local population median. The current product labelling of Arakoda®/Kodatef® and Krintafel®/Kozenis® does not specify explicitly at which threshold of G6PD activity tafenoquine should be prescribed so clinicians should not rely entirely on product information for guidance when navigating around G6PD status and tafenoquine.
Overall considerations before prescribing tafenoquine
When considering tafenoquine for a traveller, certain aspects of the medical history are important. Have they experienced hemolysis before? Is there a history of an hemoglobinopathy or underlying chronic disease? This information might be useful if hemolysis occurs unexpectedly during tafenoquine use in an individual. Blood dyscrasias or chronic diseases may slow hematologic recovery or exacerbate hemolysis.45 In addition, tafenoquine has not been studied in persons with kidney or liver disease so it would be sensible to follow these individuals closely. What medications are currently taken, and do they cause hemolysis? Two excellent resources that have collected available evidence on hemolytic medications are Youngster et al.46 and Luzzatto and Seneca.47 Some drugs on the ‘potentially hemolytic’ or ‘have been considered unsafe’ lists may not cause significant hemolysis alone in therapeutic doses but may when used in supratherapeutic doses,48,49 in combination with other hemolytic drugs50 or in the presence of other hemolytic stresses such as infection.42
Since the G6PD gene lies on the X chromosome, it is important to know the traveller’s genetic sex so the appropriate G6PD test can be used. However, this may not be necessary if a quantitative test is always utilized. The clinician should also confirm whether a traveller is breastfeeding and determine the G6PD status of their infant. In G6PD unknown or deficient infants, the unanimous recommendation is for temporary cessation of breastfeeding during tafenoquine dosing and for 3 months after the final dose. In reality, not breastfeeding can be harmful (risk of waterborne disease or contaminated infant formula). A very reasonable alternative is to use a primaquine regimen with the requirement that the infant is >28 days old.51
Baseline hematologic testing usually is not necessary unless there are abnormal clinical findings but could include a complete blood count (CBC) for underlying anaemia or signs of a blood dyscrasia. Subsequent confirmation with diagnostic tests, such as hemoglobin typing, may be needed. In males, a G6PD qualitative test is sufficient, whereas for females, a G6PD quantitative test is necessary. G6PD testing is best done when the traveller’s hemoglobin is at their steady state (i.e. 3 months after a blood transfusion, 4–6 weeks after a severe illness); consultation with a hematologist may be helpful. For the cautious clinician, it is prudent to perform the urine pregnancy test after 4 weeks of effective contraception before prescribing tafenoquine to a female traveller. This avoids drug exposure in an early undetected pregnancy. If reliable contraception has been used for <4 weeks, a combination of urine pregnancy testing, serum beta-HCG sampling or transvaginal ultrasound can be performed; consider consultation with an obstetrician.
A plasma tafenoquine concentration of ≥80 ng/mL has been designated as the therapeutic threshold and provides a 2-fold margin of protection during causal prophylaxis.52,53 After the first 200-mg loading dose of tafenoquine for causal prophylaxis, a therapeutic drug level is reached within 2 hours.17–19 Thus, it is reasonable to start tafenoquine the week before travel.12 As stated in the product information, PART should be given 7 days after the last weekly maintenance dose taken for causal prophylaxis.
Tafenoquine eligibility and precautions
Tafenoquine eligibility can be stratified by sex. Males are diagnosed qualitatively as either G6PD normal or deficient. Tafenoquine can be prescribed to the former but not to the latter. G6PD-deficient males should receive an alternative anti-malarial for causal prophylaxis and the weekly primaquine regimen for radical cure or PART (Table 3). Females should be diagnosed with a quantitative G6PD test. For tafenoquine, dosing is divided into three categories: >70% (normal), ≥30 to ≤ 70% (intermediate) and <30% (deficient). G6PD-normal females can be treated with tafenoquine. G6PD-deficient females should receive an alternative anti-malarial for causal prophylaxis and the weekly primaquine regimen for radical cure or PART. G6PD-intermediate females will require an alternative anti-malarial for causal prophylaxis (like G6PD deficient females) and can receive daily primaquine regimens (up to 0.5 mg/kg per day) for radical cure or PART (unlike G6PD deficient females) (Table 3).
Table 3.
Eligibility for tafenoquine by sex and G6PD status

|
aThe threshold for normality is ≥30% for G6PD qualitative tests.
bMales are either G6PD deficient or normal; therefore, they will not have intermediate states and will not need quantitative testing.
cFemales with a quantitative G6PD activity ≥30% to <70% are considered to be intermediate for G6PD deficiency and are at risk for hemolysis. Do not use tafenoquine in this group.
dIn G6PD-deficient individuals, use primaquine 0.75 mg base/kg per dose weekly for 8 weeks. In G6PD intermediate individuals, use daily primaquine (no more than 0.5 mg/kg per day; the specific regimen depends on national guidelines). If G6PD intermediate status cannot be determined (i.e. reference laboratory results do not specify), then use the weekly regimen as for G6PD deficient individuals
All individuals who are prescribed tafenoquine should be counselled on the signs of hemolysis (i.e. dark-coloured urine and jaundice) and signs and symptoms of anaemia (i.e. pallor, fatigue, tachycardia or dyspnea with mild exertion). Safety is further enhanced by requesting the traveller to begin tafenoquine for causal prophylaxis ≥2 weeks prior to travel so that any adverse events are managed early and where medical care is familiar.
Assessing the rate of tafenoquine ineligibility in travellers
Without further data, it is not possible to give a valid number or proportion of travellers who would be ineligible to receive tafenoquine.54 To quantify this requires several pieces of evidence. Within the region-specific travelling population (tourists, students and military), one needs, for the numerator (only among travellers to malaria endemic countries), the rates of (i) G6PD deficiency and variants, (ii) G6PD intermediate status (<70% G6PD activity), (iii) pregnancy, (iv) rate and duration of breastfeeding and (v) rate of persons age ≤ 16 or ≤ 18 years old that travel. For the denominator, one needs (vi) the number of travellers to malaria endemic countries and (vii) the rate of becoming ineligible while overseas. In males, one could remove (ii), (iii), (iv) and (vii) when assessing tafenoquine ineligibility. Even so, reliable evidence for (v) and (vi) are not available. It has been estimated that in a population with a G6PD deficiency prevalence of >10% and fertility rates of 2.4–3.4 per woman, assuming the average reproductive female has three children who survive at least 2 years and breastfeeding duration is 2 years, and excluding infants <6 months old, up to 20% of individuals would be ineligible to receive tafenoquine or primaquine.11 While this may not be applicable to every traveller, it can give an idea of the rate of tafenoquine ineligibility and how that number is obtained.
When tafenoquine inadvertently is prescribed to an ineligible individual
G6PD deficient, intermediate or unknown status
If tafenoquine is given inadvertently to an ineligible individual due to G6PD status, travel should be delayed for 2 weeks after the first dose. Significant hemolysis might occur and the nadir hemoglobin may occur between Days 3 and 14.18,27,44
G6PD-intermediate individuals who receive tafenoquine may or may not develop clinically relevant hemolysis. Active monitoring will avoid unexpected events. The nadir hemoglobin reduction in females with intermediate G6PD activity taking daily and single dose tafenoquine is between Days 6 and 14.18,27 It would thus be reasonable to have close follow-up during the nadir period with daily vital signs, pulse oximeter monitoring, symptom reporting and hemoglobin and serum bilirubin monitoring. If G6PD-deficient individuals receive tafenoquine, it should be assumed that that they will experience hemolysis. When it occurs, it may be noted early around Day 3, as shown by the two G6PD-deficient females receiving 400-mg tafenoquine daily.44 As soon as hemolysis is suspected in a G6PD-deficient individual, they should be admitted to the hospital so interventions can be performed immediately if needed. In all cases of tafenoquine-associated hemolysis, the investigations may include CBC, serum haptoglobin and a peripheral smear to confirm intravascular hemolysis, hemoglobin typing to diagnose a concomitant hemoglobinopathy, urine dipstick for microscopic hematuria (a normal urinalysis in the presence of hematuria suggests intravascular hemolysis) or kidney and liver function tests to assess end organ dysfunction. Any hematologic measurements should be taken before a blood transfusion is given; otherwise, the results will be contaminated by the donor. If the G6PD status is unknown, the clinician should proceed as if the individual was G6PD deficient. A G6PD qualitative test may be normal and quantitative test transiently elevated during severe anaemia, so testing for G6PD genotype is indicated. Since there are over 180 G6PD-deficient genotypes55 the result may be wildtype (normal) if the individual has a variant that is not tested for; consider checking for other variants. If primaquine-associated hemolysis is suspected, stop the treatment. The hemoglobin tends to rise in the following few days but should be confirmed with daily measurements.
Blood transfusions should be given according to the clinical presentation and local guidelines. G6PD testing of the donor blood is highly recommended (qualitative for male and quantitative for female donors), so further hemolysis does not occur in the recipient. The risk of further tafenoquine-induced hemolysis with G6PD-deficient or G6PD-intermediate donor blood is acute and irreversible kidney injury in the recipient.
Pregnancy and lactation
While the 2015 World Health Organization malaria guidelines56 clearly state that primaquine is contraindicated in pregnancy, the guidelines for tafenoquine use in pregnancy are not as clearly defined (Table 1). Under the product label, Kodatef® is contraindicated in pregnancy, but for Arakoda® and Krintafel®, it is ‘not recommended’ (Supplementary Figures 1–3). The concern is a presumed risk of hemolysis in a G6PD-deficient fetus. To date, there is no evidence that tafenoquine is safe in pregnancy; therefore, it should not be prescribed in pregnancy.
There is now evidence that there is minimal primaquine excretion into breast milk.51 Thus, primaquine could be used in women breastfeeding children who are at least 28 days old, although guidelines have yet to change. Studies of tafenoquine in breast milk are lacking so it remains contraindicated in mothers with G6PD deficiency or unknown status who are breastfeeding infants (Table 1) until evidence is available otherwise.57
Children
The safety of tafenoquine in children is not yet established. The current age requirement is ≥16 years old for Krintafel®/Kozenis® and ≥18 years old for Arakoda®/Kodatef®.
If tafenoquine is given to a pregnant woman, stop the drug immediately. Since tafenoquine is slowly eliminated, the drug exposure to the fetus may be prolonged. If the G6PD genotype of both parents are known, the G6PD status of the infant may be deduced. There is no evidence to support termination of pregnancy if G6PD deficiency is suspected in the fetus. If a lactating mother receives tafenoquine and the breastfeeding infant’s G6PD status is unknown or deficient, the mother should stop tafenoquine. If breastfeeding cannot be stopped, the infant’s hemoglobin or hematocrit could be monitored until 3 months (~5 half-lives) after the last maternal dose as an additional safety measure. In some situations, the mother may be able to stop breastfeeding, but she should be counselled on safe use of water, fully air drying feeding equipment and risk of substandard infant formula during travel. Tafenoquine can be resumed in the lactating mother if the infant’s G6PD status is subsequently known.
Conclusion
The efficacy and, more importantly, the dosing regimen of tafenoquine makes it a good alternative for causal prophylaxis and an excellent alternative for PART and has the potential to transform the approach to radical cure. However, the interaction of tafenoquine with G6PD-deficient and G6PD-intermediate states cannot guarantee safety in each and every individual; safety can be maximized with appropriate G6PD testing and correct interpretation. As with primaquine, clinicians should be careful when prescribing tafenoquine, astute when assessing subsequent clinical complaints and knowledgeable if hemolysis is suspected.
Author contributions
C.S.C. performed the literature review and wrote the original draft. D.O.F. provided ancillary literature and provided sequential revisions of the drafts. All authors read and approved the final manuscript.
Funding
C.S.C. is supported by the Wellcome Trust.
Conflict of interest: D.O.F. receives salaried compensation from Shoreland Travax, an online clinical decision support tool.
Supplementary Material
Acknowledgements
Many thanks and appreciation to Germana Bancone and Gonzalo Domingo for providing their expert opinions, Professor Sir Nicholas J White for sharing his intelligent insight and Professor François Nosten for contributing his discerning observations.
References
- 1. Battle KE, Karhunen MS, Bhatt S et al. Geographical variation in plasmodium vivax relapse. Malar J 2014; 13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 2012; 56:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Peters W. The evolution of tafenoquine---antimalarial for a new millennium?. J R Soc Med 1999; 92:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White NJ. Determinants of relapse periodicity in plasmodium vivax malaria. Malar J 2011 Jan; 10:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shanks GD, White NJ. The activation of vivax malaria hypnozoites by infectious diseases. Lancet Infect Dis 2013; 13:900–6. [DOI] [PubMed] [Google Scholar]
- 6. Coatney GR, Cooper WC, Eyles DE et al. Observations on the use of pentaquine in the prevention and treatment of Chesson strain vivax malaria. J Natl Malar Soc 1950; 9:222–33. [PubMed] [Google Scholar]
- 7. Manson-Bahr P. The action of plasmochin on malaria. Proceedings of the Royal Society of Medicine 1927; 191–926. [PMC free article] [PubMed]
- 8. Krauss W. A resume of studies upon plasmochin. South Med J 1929; 22:359–62. [Google Scholar]
- 9. Edgcomb JH, Arnold J, Yount EH et al. Primaquine, SN 13272, a new curative agent in vivax malaria; a preliminary report. J Natl Malar Soc 1950; 9:285–92. [PubMed] [Google Scholar]
- 10. Gutteridge WE. Antimalarial drugs currently in development. J R Soc Med 1989; 82:63–8. [PMC free article] [PubMed] [Google Scholar]
- 11. Watson J, Taylor WRJ, Bancone G et al. Implications of current therapeutic restrictions for primaquine and tafenoquine in the radical cure of vivax malaria. PLoS Negl Trop Dis 2018; 12:e0006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baird JK. Tafenoquine for travelers’ malaria: evidence, rationale and recommendations. J Travel Med 2018; 25:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coleman RE, Clavin AM, Milhous WK. Gametocytocidal and sporontocidal activity of antimalarials against Plasmodium berghei ANKA in ICR mice and Anopheles stephensi mosquitoes. Am J Trop Med Hyg 1992; 46:169–82. [DOI] [PubMed] [Google Scholar]
- 14. Ponsa N, Sattabongkot J, Kittayapong P et al. Transmission-blocking activity of tafenoquine (WR-238605) and artelinic acid against naturally circulating strains of Plasmodium vivax in Thailand. Am J Trop Med Hyg 2003; 69:542–7. [PubMed] [Google Scholar]
- 15. Fukuda MM, Krudsood S, Mohamed K et al. A randomized, double-blind, active-control trial to evaluate the efficacy and safety of a three day course of tafenoquine monotherapy for the treatment of Plasmodium vivax malaria. PLoS One 2017; 12:e0187376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pukrittayakamee S, Vanijanonta S, Chantra A et al. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis 1994; 169:932–5. [DOI] [PubMed] [Google Scholar]
- 17. Edstein MD, Kocisko DA, Brewer TG et al. Population pharmacokinetics of the new antimalarial agent tafenoquine in Thai soldiers. Br J Clin Pharmacol 2001; 52:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller AK, Harrell E, Ye L et al. Pharmacokinetic interactions and safety evaluations of coadministered tafenoquine and chloroquine in healthy subjects. Br J Clin Pharmacol 2013; 76:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charles BG, Miller AK, Nasveld PE et al. Population pharmacokinetics of tafenoquine during malaria prophylaxis in healthy subjects. Antimicrob Agents Chemother 2007; 51:2709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh DS, Looareesuwan S, Wilairatana P et al. Randomized dose-ranging study of the safety and efficacy of WR 238605 (tafenoquine) in the prevention of relapse of Plasmodium vivax malaria in Thailand. J Infect Dis 1999; 180:1282–7. [DOI] [PubMed] [Google Scholar]
- 21. Walsh DS, Eamsila C, Sasiprapha T et al. Efficacy of monthly tafenoquine for prophylaxis of Plasmodium vivax and multidrug-resistant P. falciparum malaria. J Infect Dis 2004; 190:1456–63. [DOI] [PubMed] [Google Scholar]
- 22. Walsh DS, Wilairatana P, Tang DB et al. Randomized trial of 3-dose regimens of tafenoquine (WR238605) versus low-dose primaquine for preventing Plasmodium vivax malaria relapse. Clin Infect Dis 2004; 39:1095–103. [DOI] [PubMed] [Google Scholar]
- 23. Llanos-Cuentas A, Lacerda MVG, Rueangweerayut R et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 2014; 383:1049–58. [DOI] [PubMed] [Google Scholar]
- 24. Green JA, Mohamed K, Goyal N et al. Pharmacokinetic interactions between tafenoquine and dihydroartemisinin-piperaquine or artemether-lumefantrine in healthy adult subjects. Antimicrob Agents Chemother 2016; 60:7321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Llanos-Cuentas A, Lacerda MVG, Hien TT et al. Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019; 380:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacerda MVG, Llanos-Cuentas A, Krudsood S et al. Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med 2019; 380:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rueangweerayut R, Bancone G, Harrell EJ et al. Hemolytic potential of tafenoquine in female volunteers heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PD mahidol variant) versus G6PD-normal volunteers. Am J Trop Med Hyg 2017; 97:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leary KJ, Riel MA, Roy MJ et al. A randomized, double-blind, safety and tolerability study to assess the ophthalmic and renal effects of tafenoquine 200 mg weekly versus placebo for 6 months in healthy volunteers. Am J Trop Med Hyg 2009; 81:356–62. [PubMed] [Google Scholar]
- 29. Glader B. Hereditary hemolytic aneamis due to red blood cell enzyme disorders In: Greer JP, Foerster J, Rodgers GM, et al. (eds). Wintrobe’s Clinical Hematology, 12th edn Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2009. 933–55. [Google Scholar]
- 30. Pandolfi PP, Sonati F, Rivi R et al. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 1995; 14:5209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bancone G, Kalnoky M, Chu CS et al. The G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjects. Sci Rep 2017; 7:9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oo NN, Bancone G, Maw LZ et al. Validation of G6PD point-of-care tests among healthy volunteers in Yangon. Myanmar PLoS One 2016; 11:e0152304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beutler E, Blume KG, Kaplan JC et al. International committee for standardization in haematology: recommended methods for red-cell enzyme analysis*. Br J Haematol 1977; 35:331–40. [DOI] [PubMed] [Google Scholar]
- 34. Pal S, Bansil P, Bancone G et al. Evaluation of a novel quantitative test for glucose-6-phosphate dehydrogenase deficiency: bringing quantitative testing for glucose-6-phosphate dehydrogenase deficiency closer to the patient. Am J Trop Med Hyg 2018; 00:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alam MS, Kibria MG, Jahan N et al. Field evaluation of quantitative point of care diagnostics to measure glucose-6-phosphate dehydrogenase activity. PLoS One 2018; 13:e0206331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weppelmann TA, von ME, Wilfong TD et al. Field trial of the CareStart biosensor analyzer for the determination of glucose-6-phosphate dehydrogenase activity in Haiti. Am J Trop Med Hyg 2017; 97:1262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bancone G, Gornsawun G, Chu CS et al. Validation of the quantitative point-of-care CareStart biosensor for assessment of G6PD activity in venous blood. PLoS One 2018; 13:e0196716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ley B, Luter N, Espino FE et al. The challenges of introducing routine G6PD testing into radical cure: a workshop report. Malar J 2015; 14:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chu CS, Bancone G, Moore KA et al. Haemolysis in G6PD heterozygous females treated with primaquine for Plasmodium vivax malaria: a nested cohort in a trial of radical curative regimens. PLoS Med 2017; 14:e1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tarlov AR, Brewer GJ, Carson PE et al. Primaquine sensitivity. Glucose-6-phosphate dehydrogenase deficiency: an inborn error of metabolism of medical and biological significance. Arch Intern Med 1962; 109:209–34. [DOI] [PubMed] [Google Scholar]
- 41. Alving AS, Kellermeyer RW, Tarlov AR et al. Biochemical and genetic aspects of primaquine-sensitive hemolytic anemia. Ann Intern Med 1958; 49:240. [DOI] [PubMed] [Google Scholar]
- 42. Chu CS, Bancone G, Soe NL et al. The impact of using primaquine without prior G6PD testing: a case series describing the obstacles to the medical management of haemolysis. Wellcome Open Res 2019 Feb 6; 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luzzatto L. Glucose 6-phosphate dehydrogenase deficiency: from genotype to phenotype. Hematol J 2006; 91:1303–6. [PubMed] [Google Scholar]
- 44. Shanks GD, Oloo AJ, Aleman GM et al. A new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malaria. Clin Infect Dis 2001; 33:1968–74. [DOI] [PubMed] [Google Scholar]
- 45. Pootrakul P, Sirankapracha P, Hemsorach S et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood 2000; 96:2606–12. [PubMed] [Google Scholar]
- 46. Youngster I, Arcavi L, Schechmaster R et al. Medications and glucose-6-phosphate dehydrogenase deficiency; an evidence-based review. Drug Saf 2010; 33:713–26. [DOI] [PubMed] [Google Scholar]
- 47. Luzzatto L, Seneca E. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol 2014; 164:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rees DC, Kelsey H. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ 1993; 306:841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mehta JB, Singhal SB, Mehta BC. Ascorbic-acid-induced haemolysis in G-6-PD deficiency. Lancet 1990; 336:944. [DOI] [PubMed] [Google Scholar]
- 50. Kheng S, Muth S, Taylor WRJ et al. Tolerability and safety of weekly primaquine against relapse of Plasmodium vivax in Cambodians with glucose-6-phosphate dehydrogenase deficiency. BMC Med 2015; 13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gilder ME, Hanpithakphong W, Hoglund RM et al. Primaquine pharmacokinetics in lactating women and breastfed infant exposures. Clin Infect Dis 2018; 67:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Edstein MD, Kocisko DA, Walsh DS et al. Plasma concentrations of tafenoquine, a new long-acting antimalarial agent, in Thai soldiers receiving monthly prophylaxis. Clin Infect Dis 2003; 37:1654–8. [DOI] [PubMed] [Google Scholar]
- 53. Kitchener S, Nasveld P, Edstein MD. Short report: tafenoquine for the treatment of recurrent Plasmodium vivax malaria. Am J Trop Med Hyg 2007; 76:494–6. [PubMed] [Google Scholar]
- 54. Davlantes A, Tan KR, Arguin PM. Quantifying malaria risk in travellers: a quixotic pursuit. J Travel Med 2017; 24:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Minucci A, Moradkhani K, Hwang MJ et al. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the “old” and update of the new mutations. Blood Cells Mol Dis 2012 Mar 15; 48:154–65. [DOI] [PubMed] [Google Scholar]
- 56. World Health Organization Guidelines for the Treatment of Malaria, 3rd edn Geneva: World Health Organization, 2015. [Google Scholar]
- 57. Saito M, Gilder ME, McGready R et al. Antimalarial drugs for treating and preventing malaria in pregnant and lactating women. Expert Opin Drug Saf 2018; 17:1129–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


