Abstract
Pyrethroids are synthetic insecticides that act acutely on voltage gated sodium channels to prolong channel opening and depolarization. Epidemiological studies find that exposure to pyrethroids are associated with neurological and developmental abnormalities in children. The long-term effects of type II pyrethroids, such as deltamethrin (DLM), on development have received little attention. We exposed Sprague-Dawley rats to DLM by gavage at doses of 0, 0.25, 0.5, and 1.0 mg/kg/day from postnatal day (P) 3–20 in a split-litter design. Following behavioral testing as adults, monoamine levels, release, and mRNA were assessed via high performance liquid chromatography, microdialysis, and qPCR, respectively. Long-term potentiation (LTP) was assessed at P25–35. Developmental DLM exposure resulted in deficits in allocentric and egocentric learning and memory, increased startle reactivity, reduced conditioned contextual freezing, and attenuated MK-801 induced hyperactivity compared with controls. Startle and egocentric learning were preferentially affected in males. Deltamethrin-treated rats exhibited increased CA1 hippocampal LTP, decreased extracellular dopamine release by microdialysis, reduced dopamine D1 receptor mRNA expression in neostriatum, and decreased norepinephrine levels in the hippocampus. The data indicate that neonatal DLM exposure has adverse long-term effects on learning, memory, startle, glutamatergic function, LTP, and norepinephrine.
Keywords: deltamethrin, development, pyrethroids, rats, cognition
Pyrethroids are synthetic analogs of pyrethrins derived from chrysanthemums. Pyrethroids bind to voltage gated sodium channels (VGSCs), slowing activation and inactivation that results in prolonged depolarization (Soderlund, 2012). There are two classes of pyrethroids: type I and type II (Soderlund, 2012). Both types prolong VGSC opening leading to repetitive action potentials, but type II pyrethroids prolong this effect compared with type I pyrethroids (Costa, 2013). This results in persistent depolarization, repetitive firing (Bradberry et al., 2005), and action potential blockade.
Pyrethroids are used in agriculture, households, lawns, schools, and parks, as well as directly on children for head lice, on pets for ticks and fleas, and on furniture for bedbugs. The United States Environmental Protection Agency restrictions on residential organophosphate pesticide use resulted in increased use of pyrethroids (Power and Sudakin, 2007; Williams et al., 2008). This increase is a concern for children as they are more susceptible to the effects of these compounds than adults (Landrigan et al., 1993); however, studies examining the effect of exposure to pyrethroids on brain development and behavior are limited. Neurodevelopmental and behavioral outcomes in children are altered after pyrethroid exposure as assessed by urinary pyrethroid metabolite levels, such as 3-phenoxybenzoic acid (3-PBA). A study in 1-year-old Chinese infants showed an inverse association between cognition, social adaptation, and motor function with 3-PBA levels collected from prenatal urine from the pregnant mothers (Xue et al., 2013). A positive association was reported between residential proximity to where pyrethroids were used before and during gestation and diagnosis of autism spectrum disorder or delayed cognitive and adaptive development in children (Shelton et al., 2014). Urinary 3-PBA levels in children identified from the National Health and Nutrition Examination Survey data were associated with higher prevalence of attention deficit hyperactivity disorder (Richardson et al., 2015) and increased hyperactive-impulsive symptoms in boys (Wagner-Schuman et al., 2015). Associations between pyrethroid metabolites in children and psychopathological disorders were also seen in a Canadian Health Measures Survey (Oulhote and Bouchard, 2013 ).
Rodent studies indicate that developmental exposure from embryonic day 0–21 to the type II pyrethroid deltamethrin (DLM) increases dopamine (DA) transporter and DA D1 receptor (DRD1) levels in the nucleus accumbens, while decreasing extracellular DA release in the striatum of adult mice (Richardson et al., 2015). In addition to alterations in DA biomarkers, Richardson et al. (2015) observed deficits in learning, memory, attention, and impulsivity, and increased open-field (OF) activity. By contrast, in adult rats, DLM alters serotonin as well as DA (Hossain et al., 2006, 2013). Gestational DLM exposure in rats causes deficits in learning and memory (L&M) when tested at 6–12 weeks of age, alters cholinergic circuitry, increases striatal 3, 4-dihydroxyphenylacetic acid (DOPAC) levels, alters OF activity and rearing, and reduces cytochrome P450s (Aziz et al., 2001; Johri et al., 2006; Lazarini et al., 2001). Deltamethrin exposure from postnatal day 10–16 alters muscarinic and nicotinic densities in hippocampus and cerebral cortex and increases spontaneous motor activity in mice (Eriksson and Fredriksson, 1991; Eriksson and Nordberg, 1990).
However, there are no data on developmental DLM exposure on striatal or hippocampal mediated L&M and other behaviors. Accordingly, the present study investigated the long-term effects of developmental exposure to DLM by gavage on postnatal days 3–20 for effects on L&M, anxiety, OF activity, startle, conditioned freezing, and activity after drug challenge.
MATERIALS AND METHODS
Animals
The protocol was approved by the Institutional Animal Care and Use Committee of Cincinnati Children’s Research Foundation and adhered to guidelines on the care and use of animals in research by the U.S. National Institutes of Health. Male and nulliparous female Sprague-Dawley rats (175–200 g upon arrival, CD IGS; strain No. 001, Charles River, Raleigh, North Carolina) were maintained on a freely available NIH-07 diet (LabDiet, Richmond, Indiana) and reverse osmosis filtered/UV sterilized water. Rats were acclimated to the AAALAC International accredited vivarium for 1–3 weeks before breeding. The vivarium was maintained on a 14–10 h light-dark cycle (lights on at 600 h) with controlled temperature (19°C ± 1°C) and humidity (50% ± 10%). Females were paired with males in wire bottom cages. The day a sperm plug was found was designated embryonic day 0, and females were placed in individual cages with standard bedding and stainless steel enclosure for enrichment (Vorhees et al., 2008). Day of birth was designated postnatal day 0 (P0). On P3 litters were culled to 4 males and 4 females using a random number table. On P7 rats were numbered using ear punches. Dams were weighed when offspring were P3 and weekly from P7 to the end of the experiment. Offspring were housed 2/cage of the same sex starting on P28 when separated from dams.
Treatment groups
Pups were randomly assigned to 4 treatment groups and given: 0 (corn oil [CO]), 0.25, 0.5, or 1.0 mg/kg DLM (Bayer Crop Science, Frankfurt, Germany > 99.9% pure). Deltamethrin was dissolved in corn oil (Acros Organics, Geel, Belgium) in a dosing volume of 5 ml/kg and administered once per day by gavage from P3–20. This period is roughly equivalent to third trimester to early postnatal development in humans and P7–10 approximates birth (Semple et al., 2013). Thirty-two litters were used for behavior and another 20 litters for long-term potentiation (LTP; P25–35). Ten of the former litters were used for high performance liquid chromatograph (HPLC) neurotransmitter determinations or DRD1 mRNA expression by qPCR and 12 litters for DA release by microdialysis (see Table 1).
Table 1.
Sample Size: Rats/Treatment/Sex
| Treatment (mg/kg DLM) | Males | Females |
|---|---|---|
| CO | 25 | 30 |
| 0.25 | 26 | 25 |
| 0.5 | 24 | 28 |
| 1.0 | 20 | 25 |
Behavioral testing
Testing began on P60. All behaviorally tested rats received all tests in the following order: (1) open-field (OF), (2) elevated zero maze (EZM), (3) straight channel swimming, (4) Morris water maze (MWM), (5) Cincinnati water maze (CWM), (6) acoustic and tactile startle (ASR/TSR), (7) pre-pulse inhibition of acoustic startle (PPI), (8) conditioned freezing, (9) OF with amphetamine (AMPH) challenge, and (10) OF with MK-801 challenge.
Open-field
On P60 rats were tested for locomotor activity in polycarbonate arenas (41 × 41 × 38 cm high, PAS system, San Diego Instruments, San Diego, California). Each arena had infrared photocells positioned in the X and Y coordinates spaced 2.5 cm apart. Rats were tested for 1 h, and data analyzed in 5-min intervals. For all tests except water mazes, the apparatus was cleaned between rats with EPA-approved, nontoxic, denaturing, antimicrobial agent Process NPD (Steris Corp, St Louis, Missouri).
Elevated zero maze
Elevated zero maze was conducted on P61. The apparatus was a circular runway constructed of gray textured aluminum with opposing closed and open quadrants. The runway is 10 cm wide, 100 cm i.d., 30 cm tall walls (infrared transparent walls on the inside) for the closed quadrants, 1.3 cm clear acrylic high curb for the open quadrants, and elevated 50 cm above the floor (Stoelting Co, Wood Dale, Illinois). Rats were placed in an enclosed quadrant and allowed to explore for 5 min. Movement was tracked through a camera mounted above the maze using Any-Maze software (Stoelting Co). Time spent in open and closed quadrants, latency to first open entry, and number of quadrants entered was analyzed.
Straight channel
Straight channel testing was conducted on P62. The apparatus was a 244 cm long × 15 cm wide × 50 cm deep channel filled halfway with water with a submerged platform at one end. Time to swim from one end to the other was recorded on 4 consecutive trials. Rats learn from this how to escape; latency to reach the goal is used to evaluate swimming ability, motivation, and swim speed.
Morris water maze
Rats began MWM testing on P63 (Vorhees and Williams, 2006, 2014, 2016). The apparatus is a circular pool (244 cm diameter × 51 cm deep) made of laminated black polyethylene with a conical bottom filled halfway with water (25 cm depth). On the walls surrounding the maze were distinctive distal cues (posters and geometric shapes). Rats were tested in 4 phases: acquisition, reversal, shift, and cued-random. The first 3 phases consisted of 4 trials/day for 6 days to find a hidden platform with a probe trial on day 7. The inter-trial interval (ITI) was 10–15 s. Rats not finding the platform within 2 min were placed on it for the ITI. The platform was positioned equidistant between the wall and the center and submerged approximately 2 cm below the surface. The platform position for acquisition was in the SW quadrant, for reversal in the NE quadrant, and for shift in the NW quadrant. Platform sizes were 10, 7, and 5 cm in diameter for acquisition, reversal, and shift, respectively. Probe trials lasted 45 s. Performance was tracked using Any-Maze. Latency, distance traveled, path efficiency, swim speed, and average heading error were analyzed on platform trials. On probe trials, average distance to reach the location where the platform used to be, swim speed, and average heading error were analyzed. After shift, rats were given cued-random testing. For this, black curtains were closed around the maze to block distal cues. Testing consisted of 4 trials/day for 2 days. The platform (10 cm) was marked with a yellow ball mounted on a stainless steel rod that extended 10 cm above the water. Platform and start positions were randomized on every trial during this phase and latency recorded.
Cincinnati water maze
Testing began the day following MWM on P86. The apparatus is a 10-unit multiple T water maze with dead-end T-shaped cul-de-sacs branching from a central path extending from the start to the goal where a submerged platform provided escape (Braun et al., 2015, 2016; Vorhees, 1987, 2008; Vorhees and Williams, 2016). Testing was conducted under infrared light using an infrared-sensitive camera mounted on the ceiling. The camera was connected to a monitor in an adjacent room where the experimenter counted errors. Rats were acclimated to the dark for no less than 5 min and were tested for 18 days, 2 trials/day (limit 5 min/trial). Rats not finding the platform within 5 min on trial-1 were rested for 5–10 min before trial-2 in a cage with absorbent towels. Rats reaching the goal on trial-1 received trial-2 immediately. Latency and errors (head and shoulder entries into the stem or arm of a T) were analyzed (Vorhees and Williams, 2016). Water was maintained at 21°C ± 1°C.
Acoustic and tactile startle and pre-pulse inhibition
Acoustic and tactile startle and pre-pulse inhibition were assessed for 2 days (P104–5). Testing was in SR-LAB apparatus (San Diego Instruments). Rats were placed in acrylic cylindrical holders (size large) mounted on a flat base plate and positioned inside a sound-attenuating cabinet with fan and light. Base plates have piezoelectric accelerometers attached to the underside to detect deflections. On P104, the ASR/TSR session consisted of 50 acoustic trials followed by 50 tactile trials. The tactile stimulus was a 20 ms, 60 psi air puff directed to the dorsum of the rat. The acoustic stimulus was a 20 ms, 120 dB SPL mixed frequency white noise burst (rise time 1.5 ms). On P105, rats were tested for PPI. Rats were given 100 trials in a 5 × 5 Latin square sequence of 25 trials repeated 4 times with pre-pulses of 0, 73, 77, or 82 dB. Pre-pulses preceded pulses by 70 ms from onset to onset and each stimulus lasted 20 ms. Maximum startle amplitude in mV (Vmax) was analyzed. The recording window was 100 ms and the ITI was 20 s. Testing began with a 5 min acclimation period prior to the start of trials.
Conditioned freezing
Testing was from P106–108. The test consisted of conditioning on day 1, assessing contextual memory on day 2, and cued memory on day 3. On day 1, rats were placed in an acrylic chamber 25 × 25 cm (San Diego Instruments) with a metal grid floor, LED light on the lid, and photocells to record movement. Test chambers were situated in sound-attenuating cabinets. Day 1 lasted 12 min and consisted of 6 min of acclimation followed by 6 min with an 82 dB 30 s tone paired with a 0.9 mA, 1 s foot-shock that occurred during the last 1 s of tone. Tone-shock pairing was repeated 3 times spaced 180 s apart. On day 2, rats were placed in the same apparatus for 6 min with no tone or foot-shock. On day 3, the rat was placed in a different, smaller, triangular black box for 6 min. For the first 3 min there was no stimulus; but for the last 3 min the tone was presented without foot-shock.
Amphetamine challenge
On P109 rats were tested for AMPH-induced locomotor activity in the OF apparatus. Rats were first given 30 min of habituation, followed by injection with physiological saline (3 ml/kg, sc) and tested for another 30 min. Rats were then administered (+)-AMPH sulfate (1.0 mg/kg in 3 ml/kg, sc, free base > 99% pure; Sigma-Aldrich, St Louis, Missouri) and placed back in the apparatus for 120 min. Dependent measures were total activity counts (successive beam breaks) and center time analyzed in 5 min intervals.
MK-801 challenge
One week following AMPH, rats were tested for MK-801 induced activity using the same procedure. Rats were first given 30 min to re-habituate, followed by 30 min after saline injection, and then 120 min following MK-801 injections (0.2 mg/kg in 3 ml/kg, sc, Sigma-Aldrich).
Neurotransmitters
One to two weeks following MK-801, rats were decapitated, brains removed and neostriatum and hippocampus dissected over ice, and stored at −80°C (Williams et al., 2007). For monoamines, tissues were weighed, sonicated in a 0.1 N perchloric acid (PCA), and centrifuged at 2100 × g for 13 min at 4°C; the collected supernatant (20 µl/sample) was loaded onto a Dionex UltiMate 3000 analytical autosampler (ThermoScientific) for injection into a HPLC with an electrochemical detector (ECD). The HPLC-ECD system consisted of an ESA 5840 pump, an ESA 5020 Guard Cell, a Supelco Supelcosil LC-18 column (15 cm × 4.6 mm, 3 μm; Sigma-Aldrich Co), and a Coulochem III ECD (ThermoScientific). The pump flow rate was 0.5 ml/min at 28°C. The guard cell potential was + 350 mV and the potential of the Coulochem III was −150 mV for E1 and + 250 mV for E2. Commercially available MD-TM mobile phase (ThermoFisher Scientific) was used that consisted of 89% water, 10% acetonitrile, and 1% sodium phosphate monobasic (monohydrate). Monoamine standards for norepinephrine (NE), DA, serotonin (5-HT) DOPAC, 5-hydroxyindoleacetic acid (5-HIAA), and homovanillic acid (HVA) were prepared in a solution of 0.1 N PCA. Monoamine standards were run individually as well as on a single chromatogram for peak verification. For a standard curve, chromatograms of all neurotransmitter standards were run at different concentrations.
qPCR
Tissue was collected as above. RNA was isolated from neostriatum and hippocampus of 10 control and 10 1.0 mg/kg DLM male rats. To extract hippocampal RNA, tissue was homogenized in 1 ml of TRIzol per 50–100 mg of tissue; 0.2 ml of chloroform per 1 ml of TRIzol reagent was added to the homogenate, vortexed, and incubated for 2–3 min then centrifuged at 12 000 × g for 15 min at 2°C–8°C. The RNA precipitate was isolated and 0.5 ml of isopropyl alcohol added, then incubated for 10 min at room temperature, and centrifuged for 10 min at 12 000 × g at 2°C–4°C. The supernatant was removed and the RNA pellet washed twice with 1 ml of 75% ethanol and centrifuged at 7500 × g for 5 min. The RNA pellet was dried and dissolved in autoclaved water. For neostriatum, RNA isolation was completed using the RNAqueous-Micro Kit filter for smaller tissue samples. RNA was quantified by Nanodrop (ThermoScientific, St Louis, Missouri). Reverse transcription (RT) reactions were performed using iScript RT supermix (Bio-Rad Laboratories, Inc.) combined with diluted RNA template (neostriatum concentration = 0.5 ng; hippocampal concentration = 2.0 ng) for a total volume of 20 μl. Reactions were carried out in a thermal cycler as follows: 5 min at 25°C, 20 min at 46°C, and 1 min at 95°C. qPCR contained 4 µl of cDNA, 2 µl of each primer (forward and reverse), and 10 µl SYBR Green Master Mix (Qiagen) in a 20 µl volume. The mixture was placed in 96-well plates and qPCR performed on an ABI Prism 7900HT analyzer (Applied Biosystems) using the following protocol: 50°C for 2 min, 95°C for 10 min, 50 cycles at 95°C for 15 s, and 60°C for 1 min. Primers were from Integrated DNA Technologies and selected based on primer efficiency determined to be 95%–100%. Rat primer sequences are shown in Table S1. Negative controls included qPCR in the absence of template. Ct values were determined using Applied Biosystems 7500 System Sequence detection software with a threshold set at 0.5. The denaturation curve showed a single peak, representative of a single PCR product. The average Ct values from quadruplicate repeats were calculated. These were averaged with values obtained from 2 independent qPCR experiments. Changes in mRNA were quantified using the ΔΔCt method with actin as reference (Livak and Schmittgen, 2001).
Table 2.
Primer Sequence for Each Gene
| Primer Name | Sequence | NM | Product Size |
|---|---|---|---|
| 5HT1A (Htr1a) F | GGTACTGGGCTATCACCGAC | NM_012585.1 | 221 |
| 5HT1A (Htr1a) R | CGGGATATAGAAAGCGCCGA | NM_012585.1 | 221 |
| 5HT2A (Htr2a) F | GCTGGGTTTCCTTGTCATGC | NM_017254.1 | 265 |
| 5HT2A (Htr2a) R | GATTGGCATGGATATACCTACAGA | NM_017254.1 | 265 |
| 5HT2C (Htr2c) F | GACGCTAGCGGGTTGTCA | NM_012765.3 | 280 |
| 5HT2C (Htr2c) R | GAAACAAGCGTCCACCATCG | NM_012765.3 | 280 |
| 5HT4 (Htr4) F | TGATGCTAATGTGAGTTCCAACGAG | NM_012853.1 | 231 |
| 5HT4 (Htr4) R | AACTCAATGGCACCGAAGGCA | NM_012853.1 | 231 |
| 5HT5A (Htr5a) F | CCAGGAAGACCAACAGCGTC | NM_013148.1 | 196 |
| 5HT5A (Htr5a) R | CAGCAGAGGACAAACACCCC | NM_013148.1 | 196 |
| 5HT6 (Htr6) F | GGTGCCATCTGCTTCACCTA | NM_024365.2 | 277 |
| 5HT6 (Htr6) R | ACACGGCCTGAGCTATGTTG | NM_024365.2 | 277 |
| 5HT7 (Htr7) F | CAACTGCCTGGTGGTGATCT | NM_022938.2 | 256 |
| 5HT7 (Htr7) R | CCCAAGGTACCTGTCGATGC | NM_022938.2 | 256 |
| Actin F | AGATCAAGATCATTGCTCCTCCT | NM_031144.3 | 415 |
| Actin R | ACGCAGCTCAGTAACAGTCC | NM_031144.3 | 415 |
| B2m F | CGAGACCGATGTATATGCTTGC | NM_012512.2 | 445 |
| B2m R | GTCCAGATGATTCAGAGCTCCA | NM_012512.2 | 445 |
| Bdnf F | TGATGCTCAGCAGTCAA | NM_001270630.1 | 160 |
| Bdnf R | CACTCGCTAATACTGTCAC | NM_001270630.1 | 160 |
| DAT F | GCTATGCTGGAAGCTGGTCA | NM_012694.2 | 222 |
| DAT R | ATGGCATAGGCCAGTTTCTCC | NM_012694.2 | 222 |
| Drd1 F | GTCCACTCTCCTGGGCAATAC | NM_012546.2 | 196 |
| Drd1 R | TACCCAGATGTTACAAAAGGGAC | NM_012546.2 | 196 |
| Drd2 F | GAAGACACCACTCAAGGGCA | NM_012547.1 | 220 |
| Drd2 R | ATCAGGGAGAGTGAGCTGGT | NM_012547.1 | 220 |
| Drd5 F | CCACATGATACCGAATGCAG | NM_012768.1 | 146 |
| Drd5 R | CACAGTCAAGCTCCCAGACA | NM_012768.1 | 146 |
| Nav 1.1 (Scn1a) F | ATCTTTACCAACTGACATTGCGTGC | NM_030875.1 | 237 |
| Nav 1.1 (Scn1a) R | TTGCTGCCGCCGCCT | NM_030875.1 | 237 |
| Nav 1.2 (Scn2a) F | AGTGGAGAGATGGACGCTCT | NM_012647.1 | 237 |
| Nav 1.2 (Scn2a) R | TTCTTTGATGGGCGTTCCCT | NM_012647.1 | 237 |
| Nav 1.3 (Scn3a) F | ACAAGAAGCTGTGCTCTCCC | NM_013119.1 | 236 |
| Nav 1.3 (Scn3a) R | GGTGGTACCGTTACTGTTGC | NM_013119.1 | 236 |
| Nav 1.6 (Scn8a) F | GGCCGTAGGAAATCTGGTGT | NM_019266.2 | 240 |
| Nav 1.6 (Scn8a) R | ATCAGCATGTTCAGGGTGGG | NM_019266.2 | 240 |
| Nav β1 (Scn1b) F | TGTCACGTCTACCGTCTCCT | NM_001271045.1 | 245 |
| Nav β1 (Scn1b) R | GCCAGGTATTCCGAGGCATT | NM_001271045.1 | 245 |
| Nav β2 (Scn2b) F | CACAGCCCACCCGCCTAA | NM_012877.1 | 280 |
| Nav β2 (Scn2b) R | TGGAGGAACATCTCCTCTGAGC | NM_012877.1 | 280 |
| Nav β3 (Scn3b) F | CCTCCGTGGTCTCGGAAATC | NM_139097.3 | 221 |
| Nav β3 (Scn3b) R | CTCAGCACTCAGATCACCTCAA | NM_139097.3 | 221 |
| Nav β4 (Scn4b) F | CTTGCTTCGTGAGGAACCCC | NM_001008880.1 | 219 |
| Nav β4 (Scn4b) R | CGAGACACTCCTTCTTCTTCTCTC | NM_001008880.1 | 219 |
| NR1 (Grin1) F | TCTGACAAGAGTATCCACCTGAG | NM_001270602.1 | 245 |
| NR1 (Grin1) R | GGTCCGCGCTTGTTGTCATA | NM_001270602.1 | 245 |
| SERT F | AGCAGTCTGAAGAACAGACCA | NM_013031.1 | 269 |
| SERT R | ACCCCTTGTCGGCTTTAGTG | NM_013031.1 | 269 |
| TPH F | CTAGAGGATGTGCCGTGGTT | NM_173839.2 | 182 |
| TPH R | GGAATGGGCTGGCCATATTT | NM_173839.2 | 182 |
NM, identification number and product size for each gene.
Long-term potentiation
Between P25–35, male and female rats previously treated with 1 mg/kg DLM or CO and not behaviorally tested were decapitated and brains dissected and placed in ice-cold artificial cerebrospinal fluid (aCSF: 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4/H2O, 1 mM MgCl2/6H2O, 10 mM glucose, 2 mM CaCl2, 26 mM NaHCO3) saturated with 95% O2/5% CO2 as described (Amos-Kroohs et al., 2017). Sections were chilled for 1–2 min, trimmed, and mounted on a vibratome (Vibratome 1500, Warner Instruments, Hamden, Connecticut) using super glue. Ice-cold, oxygenated aCSF was added to the stage containing tissue. Parasagittal hippocampal sections (350 µm) were cut and placed in a bath of oxygenated aCSF at 32°C. Slices rested for at least 1 h. Long-term potentiation was measured in CA1 using a MED64 multielectrode system (Alpha Med Sciences, Kadoma, Japan) with an 8 × 8 array of contacts. Electrode arrays were 50 × 50 µm and spaced 150 µm apart (Shimono et al., 2002). Pulses were delivered dorsally, and excitatory postsynaptic potentials (EPSPs) downstream were obtained until a stable baseline was obtained that lasted at least 10 min. Once a stable baseline was achieved, a theta burst [tetanus = 100 Hz in 10 bursts (4 pulses/burst) delivered at a frequency of 5 Hz for 2 s] was applied and field EPSPs and EPSP slopes were recorded for 90 min. The mean baseline value was calculated and percent change from baseline after the theta burst were the data analyzed.
Microdialysis
A subset of male rats (> P60) were used to examine AMPH stimulated DA release in the N. accumbens via microdialysis. Rats were implanted with a stainless steel guide cannula under isoflurane (2%–4%; IsoThesia; Butler Animal Health Supply, Dublin, Ohio) anesthesia 72 h prior to the insertion of a dialysis probe. On the morning of the dialysis experiment, a concentric style dialysis probe was inserted through the guide cannula into the N. accumbens such that the tip of the probe was located at the following coordinates: A/P, 1.2 mm relative to bregma; L, 0.8 mm; V, −8.4 mm (Paxinos et al., 1985). The probes are connected to an infusion pump set to deliver Dulbecco’s phosphate buffered saline (2 µl/min) and an acclimation period of 3 h followed. Four baseline samples were then obtained prior to the administration of AMPH (2 mg/kg, ip, dissolved in saline in a dosing volume of 1 ml/kg). Dopamine in dialysis samples was quantified by HPLC similar to those described (Nair and Gudelsky, 2004 ). Placement of dialysis probes were verified in post mortem coronal sections.
Data analyses
Some developmental effects of DLM were reported previously (Hossain et al., 2013, 2015; Richardson et al., 2015), therefore, we hypothesized deficits for these outcomes. These data were analyzed using a priori methods. Pre-planned comparisons used Dunnett’s test to compare each DLM group with CO. For these Dunnett p-values are given, not F-ratios. However, to test for interactions, mixed linear ANOVAs were used (SAS Proc Mixed, SAS Institute 9.3, Cary, North Carolina). To control for litter, litter was a random factor in these models. For models with a repeated measure factor, the autoregressive-1 covariance structure was used. First-order Kenward-Roger degrees of freedom were calculated. Mortality was analyzed by Chi-square. Parametric data are presented as least square (LS) mean ± SEM. Statistical significance was set at p < .05.
RESULTS
Mortality and Body Weight
Deltamethrin did not increase mortality. Deltamethrin decreased growth as reflected in body weight in the 1.0 mg/kg (p < .0001) and 0.5 mg/kg (p < .0001) DLM groups during dosing, regardless of sex (Figure 1A). These weight differences in the 1.0 and 0.5 mg/kg DLM groups persisted into adulthood compared with the CO group (Figure 1B). There was a treatment × age interaction on body weight during treatment: P3–20 [F(51, 3538) = 16.85, p < .001] and afterward: P21–119 [F(42, 2568) = 2.85, p < .001]. Differences in body weight between treated and control rats emerged around P7 (p < .01) and increased from P8–20 (p < .0001) (Figure 1A). Differences continued for the remainder of the experiment in the 1.0 and 0.5 mg/kg DLM groups until the end of the study at P119 (p < .0001) (Figure 1B). The 0.25 mg/kg DLM group did not differ from CO controls. In addition to treatment-related effects on body weight, sex main effects [P3–20: F(1, 190) = 7.16, p < .001; P21–119: F(1, 186) = 1121.49, p < .0001] were observed during and following dosing, with females having lower body weights than males. Sex × treatment [F(3, 186) = 6.98, p < .001], sex × age [F(14, 2533) = 126.27, p < .0001], and sex × treatment × age [F(42, 2568) = 1.76, p < .001] interactions were also observed from P21–119. Further analyses of the sex × treatment × age interaction showed decreased body weights for the male and female 0.5 and 1.0 mg/kg groups at all ages with the exception that on P21 there were no differences in males or in the 0.5 mg/kg female group compared with the female CO group.
Figure 1.
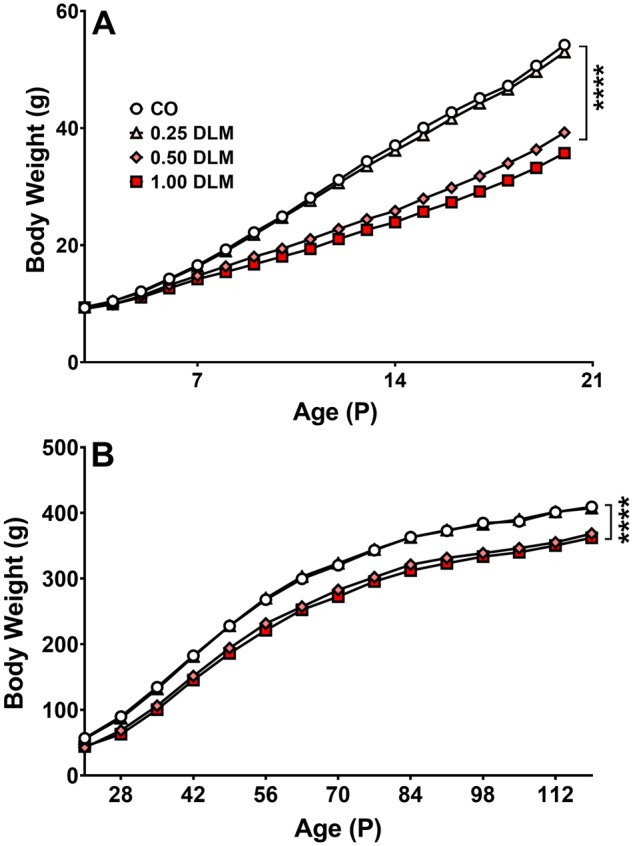
Body weight. A, Average daily body weight during dosing from P3–20 (corn oil, CO: n = 60 total, 29 males, 31 females; 0.25 mg/kg DLM: n = 53 total, 27 males, 26 females; 0.5 mg/kg DLM: n = 54 total, 25 males, 29 females; 1.0 mg/kg DLM: n = 51 total, 24 males, 27 females). B, Average weekly body weight following dosing from P21–119 (corn oil, CO: n = 57 total, 28 males, 29 females; 0.25 mg/kg DLM: n = 53 total, 27 males, 26 females; 0.5 mg/kg DLM: n = 50 total, 23 males, 27 females; 1.0 mg/kg DLM: n = 45 total, 20 males, 25 females). ****p < .0001 compared with its respective control group. DLM, deltamethrin.
Open-Field and EZM
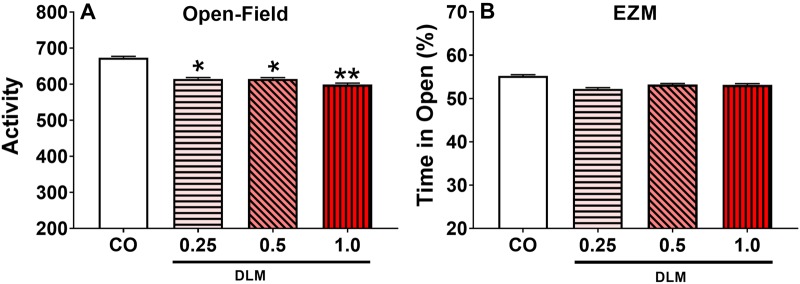
Deltamethrin-exposed rats, regardless of dose, displayed reduced OF activity [treatment: F(3, 298) = 3.15, p < .05] (Figure 2A); there were also main effects of interval [F(11, 2051) = 165.78, p < .0001] and sex [F(1, 294) = 5.75, p < .05] and an interval × sex interaction [F(11, 2051) = 2.97, p < .001], with females more active than males. In the EZM, there was no effect of treatment on latency to first quadrant entry, number of arm entries, or percent time in open quadrants (Figure 2B).
Figure 2.
Open-field (OF) locomotor activity and elevated zero maze (EZM). A, Average activity for 60 min OF test (corn oil, CO: n = 55 total, 25 males, 30 females; 0.25 mg/kg DLM: n = 51 total, 26 males, 25 females; 0.5 mg/kg DLM: n = 52 total, 24 males, 28 females; 1.0 mg/kg DLM: n = 45 total, 20 males, 25 females). B, Percent of time spent in the open arm of EZM (corn oil, CO: n = 55 total, 25 males, 30 females; 0.25 mg/kg DLM: n = 51 total, 26 males, 25 females; 0.5 mg/kg DLM: n = 52 total, 24 males, 28 females; 1.0 mg/kg DLM: n = 45 total, 20 males, 25 females). *p < .05; **p < .01 compared with CO. DLM, deltamethrin.
Straight channel swimming
No significant differences in swim latency were found [Control: 16.3 ± 0.9 s (n = 55); 0.25 mg/kg: 16.4 ± 1.0 s (n = 51); 0.5 mg/kg: 17.2 ± 0.9 s (n = 52); 1.0 mg/kg: 17.9 ± 1.0 s (n = 45)] suggesting no motor or motivation differences among the groups.
Morris Water Maze
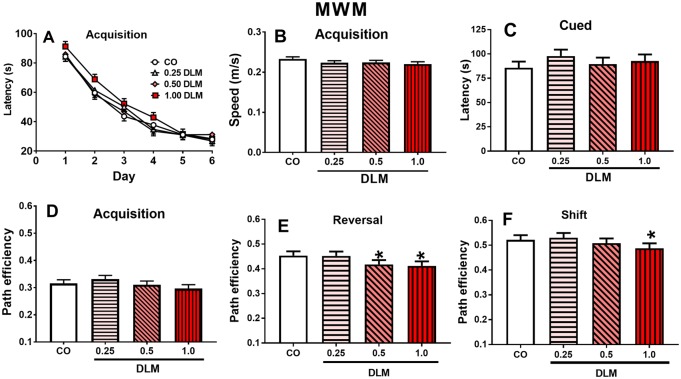
There was no main effect of DLM on MWM acquisition for latency, swim speed, or path efficiency (Figs. 3A, 3B, and 3D, respectively) or average heading error (not shown). In reversal, both the 1.0 mg/kg (p < .05) and 0.5 mg/kg (p < .05) DLM groups had reduced path efficiency compared with CO controls (Figure 3E); also, the 1.0 mg/kg (p < .05) and 0.5 mg/kg (p < .05) dose groups had increased average heading error compared with CO controls (not shown). In addition, there was a sex main effect [path efficiency: F(1, 280) = 55.71, p < .0001; average heading error: F(1, 274) = 63.56, p < .0001] and a sex × day interaction [path efficiency: F(5, 939) = 3.63, p < .01; average heading error: F(5, 938) = 5.19, p < .0001]. Path efficiency and average heading error were also affected during shift. For path efficiency, the 1.0 mg/kg DLM group had reduced path efficiency (p < .05) compared with CO controls (Figure 3F); there was also a sex main effect [F(1, 270) = 117.05, p < .0001], treatment × sex interaction [F(3, 270) = 2.72, p < .05], and sex × day interaction [F(5, 929) = 3.76, p < .01]. Slice-effect ANOVAs showed, the treatment × sex interaction was in 1.0 mg/kg DLM-treated males (p < .01) compared with CO males. For heading error, there was a sex main effect [F(1, 268) = 142.46, p < .0001], treatment × sex interaction [F(3, 268) = 3.05, p < .05)] and a sex × day interaction [F(5, 935) = 4.65, p < .001]. The treatment × sex interaction was attributable to the 1.0-mg/kg (p < .05) and 0.25-mg/kg-treated males (p < .05) having greater average heading errors compared with CO males. No effects were observed on probe trials (not shown) or cued-random trials (Figure 3C).
Figure 3.
Morris water maze (MWM). A, Acquisition latency across 6 days of testing (4 trials/day). B, Acquisition swim speed average for each treatment tested. C, Cued testing average latency. D–F, Acquisition, reversal, and shift average path efficiency for each treatment. (Corn oil, CO: n = 55 total, 25 males, 30 females; 0.25 mg/kg DLM: n = 51 total, 26 males, 25 females; 0.5 mg/kg DLM: n = 52 total, 24 males, 28 females; 1.0 mg/kg DLM: n = 45 total, 20 males, 25 females.) *p < .05; compared with CO. DLM, deltamethrin.
Cincinnati Water Maze
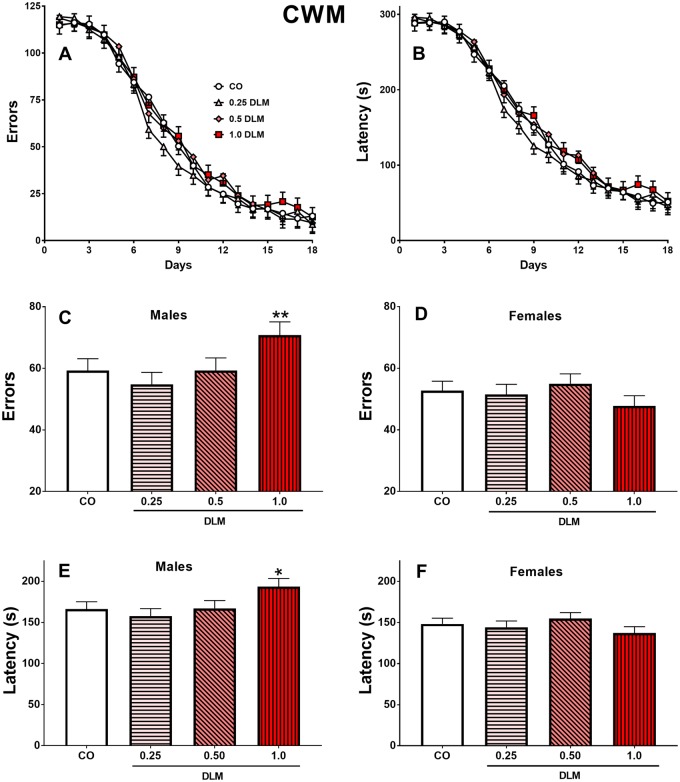
The learning curves for errors and latency are shown (Figs. 4A and 4B, respectively). No main effects were found, however, both latency and errors had significant main effects of sex [latency: F(1, 427) = 22.61, p < .0001; errors: F(1, 441) = 16.99, p < .0001] with males performing worse than females. There were, however, treatment × sex interactions [latency: F(3, 427) = 3.65, p < .01; errors: F(3, 441) = 3.73, p < .01]. Male 1 mg/kg DLM rats had increased errors (p < .05; Figure 4C) and latency (p < .01; Figure 4D) compared with CO males. Females showed no significant differences (Figs. 4D and 4F).
Figure 4.
Cincinnati water maze (CWM). A, Average errors made by each treatment across 18 days of testing (2 trials/day), males and females combined. B, Latency to find platform by each treatment across 18 days of testing (2 trials/day), males and females combined. C, Average errors made by treatment for males, D, for females. E, Average latency to find the platform for each treatment for males, F, for females. (Corn oil, CO: n = 55 total, 25 males, 30 females; 0.25 mg/kg DLM: n = 49 total, 24 males, 25 females; 0.5 mg/kg DLM: n = 49 total, 22 males, 27 females; 1.0 mg/kg DLM: n = 44 total, 20 males, 24 females.) *p < .05; **p < .01 compared with CO. DLM, deltamethrin.
Startle
For ASR/TSR, 1.0 mg/kg (p < .01) and 0.5 mg/kg (p < .05) DLM-treated rats displayed increased startle compared with CO controls. However, there was a treatment × sex [F(3, 138) = 3.23, p < .05] interaction. Males had increased startle responses [F(3, 138.5) = 5.92, p < .001] that were significant in the 0.5 mg/kg (p < .05) and 1.0 mg/kg (p < .001) DLM groups compared with CO males (Figure 5); there were no differences for females. There was also a main effect of stimulus type [F(1, 161) = 643.24, p < .0001] and a trend for stimulus type × treatment interaction [F(3, 161) = 2.64, p < .06]; no trend was seen on ASR trails. There were no main effects or interactions for PPI (not shown).
Figure 5.
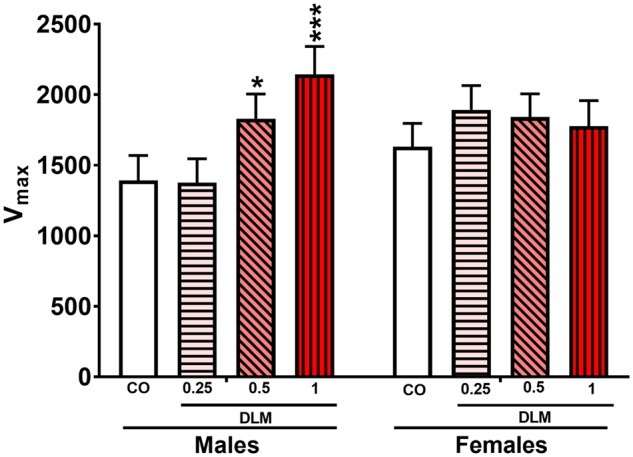
Acoustic and tactile startle response (ASR/TSR). A, Average startle response (both acoustic and tactile) made by each treatment for both males and females (corn oil, CO: n = 55 total, 25 males, 30 females; 0.25 mg/kg DLM: n = 51 total, 26 males, 25 females; 0.5 mg/kg DLM: n = 52 total, 24 males, 28 females; 1.0 mg/kg DLM: n = 45 total, 20 males, 25 females). *p < .05; ***p < .01; compared with CO. DLM, deltamethrin.
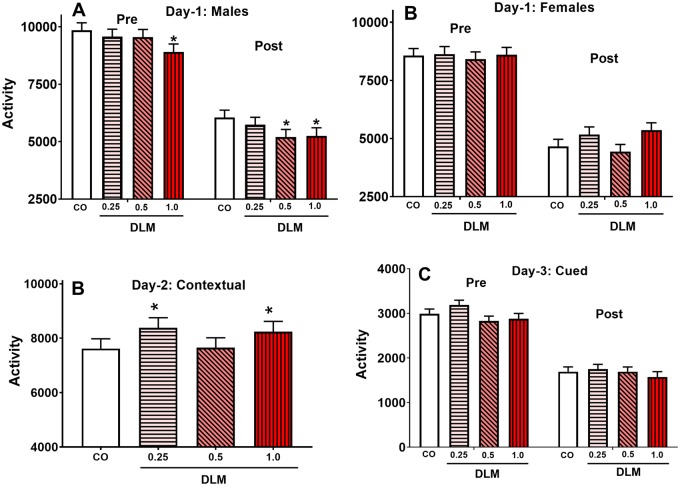
Conditioned Freezing
On day 1, there was no main effect of DLM, but there was a main effect for sex [F(1, 148) = 29.29, p < .0001] and interval [F(1, 174) = 895.01, p < .0001] and a treatment × sex interaction [F(3, 148) = 3.10, p < .05]. For the latter, 1.0 mg/kg DLM-treated males exhibited more freezing (p < .05) compared with CO controls (Figure 6A); there were no differences for females (Figure 6B). On day 2, the 1.0 and 0.25 mg/kg DLM groups showed reduced contextual memory compared with CO controls, regardless of sex (p < .04; Figure 6C). There were no effects on cued memory (Figure 6D).
Figure 6.
Conditioned freezing. A, Day 1, male average freezing behavior for both pre- and post-conditioned stimulus freezing. B, Day 1, female average freezing behavior for both pre- and post-conditioned stimulus freezing. C, Day 2, male and female rats were returned to the same test chamber to assess contextual freezing. D, Day 3, male and female rats were placed in a new chamber for 3 min with no stimulus (Pre) and remained in the chamber for 3 more minutes in presence of the tone (Post) to assess cued freezing. Note: scale of day 3 is different because the test chamber was half the size as that used on days 1 and 2 (corn oil, CO: n = 51 total, 23 males, 28 females; 0.25 mg/kg DLM: n = 47 total, 24 males, 23 females; 0.5 mg/kg DLM: n = 48 total, 22 males, 26 females; 1.0 mg/kg DLM: n = 43 total, 20 males, 23 females). *p < .05 compared with vehicle. DLM, deltamethrin.
Drug Challenge
Amphetamine
There were no treatment-related effects on OF activity during habituation or after saline. After AMPH, all groups increased activity, with an interval main effect [F(23, 3825) = 11.75, p < .0001] (Figure 7A). Although the high and low dose DLM groups trended higher than CO controls the effect was not significant (Figure 7A).
Figure 7.
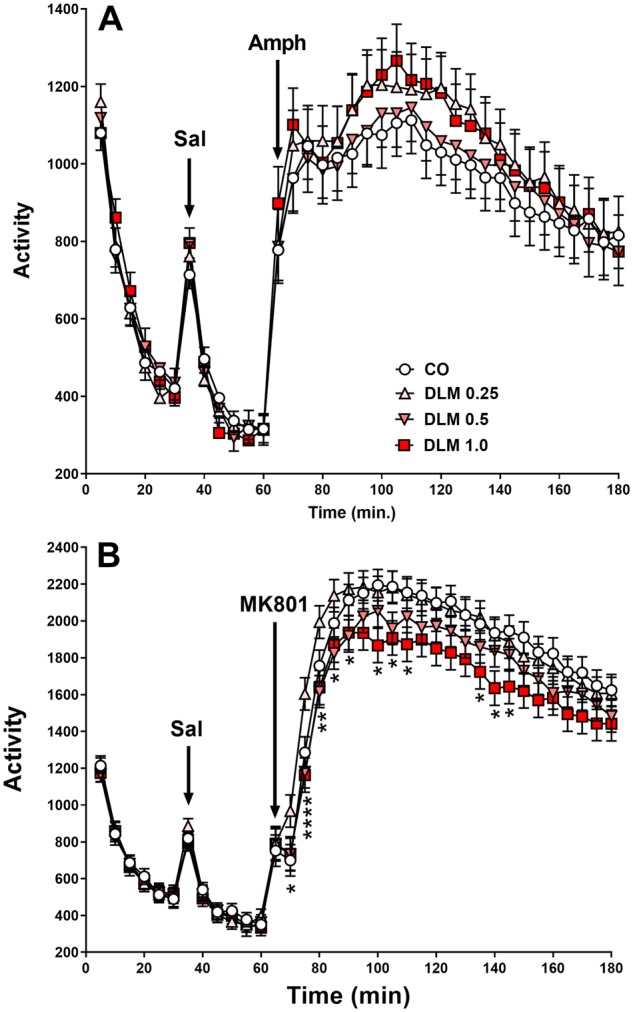
Drug challenges. A, Amphetamine (AMPH) challenge, rats were placed into locomotor chamber for a total of 3 h and examined for activity. Saline and AMPH (1.0 mg/kg in 3 ml/kg, sc) administration are noted by arrows for time of injection (corn oil, CO: n = 50 total, 22 males, 28 females; 0.25 mg/kg DLM: n = 46 total, 24 males, 22 females; 0.5 mg/kg DLM: n = 50 total, 23 males, 27 females; 1.0 mg/kg DLM: n = 42 total, 18 males, 24 females). B, MK-801 challenge, using a similar protocol to the AMPH challenge, rats were placed into locomotor chambers for a total of 3 h and examined for activity. Saline and MK-801 (0.2 mg/kg in 3 ml/kg, sc) administration are noted by arrows for time of injection (corn oil, CO: n = 55 total, 25 males, 30 females; 0.25 mg/kg DLM: n = 51 total, 26 males, 25 females; 0.5 mg/kg DLM: n = 51 total, 24 males, 27 females; 1.0 mg/kg DLM: n = 45 total, 20 males, 25 females. Activity is measured in beam breaks. *p < .05 compared with CO. DLM, deltamethrin.
MK-801
After a 1-week washout period, rats were tested after MK-801 challenge. There were no group differences prior to drug administration during habituation or saline (Figure 7B). MK-801 induced increased activity and in all groups (interval main effect [F(23, 4490) = 78.49, p < .0001]). Rats in the 1.0 mg/kg DLM group showed less drug-induced hyperactivity compared with CO control (treatment main effect: [F(3, 277) = 4.38, p < .01]); there were no differences in other groups. There was also a treatment × interval interaction [F(69, 4554) = 1.58, p < .01; Figure 7B]; this was attributable to the higher activity at 30 min in the 0.25 mg/kg DLM group than the other groups that had similar activity as CO controls. In addition, the 1.0 mg/kg DLM-treated rats exhibited lower levels of activity compared with CO controls by 30 min after MK-801 administration with 0.5 mg/kg DLM-treated rats in between.
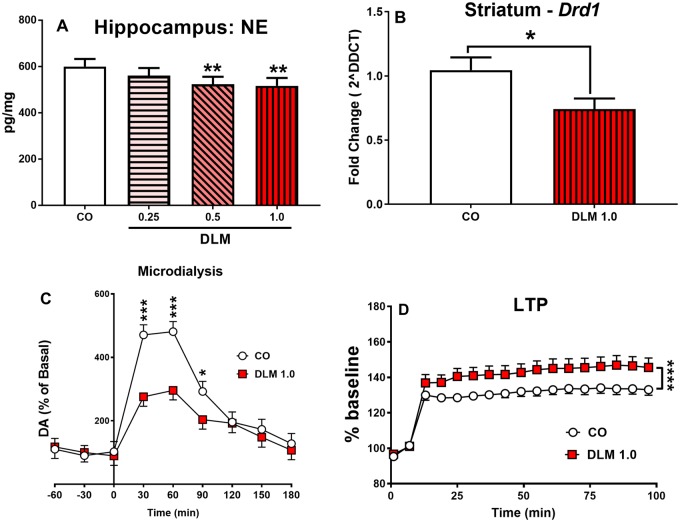
mRNA and monoamine analysis
For neostriatum and hippocampus there were no effects on brain levels of DA, DOPAC, HVA, 5-HT, or 5-HIAA. In the hippocampus, there was a decrease in NE in the 0.5 mg/kg (p < .05) and 1.0 mg/kg (p < .05) DLM groups compared with CO controls (Figure 8A). In addition DLM 1.0-mg/kg-treated males had reduced Drd1 mRNA expression in the neostriatum compared with controls [t(17) = 2.33, p < .05; Figure 8B]. No other mRNA changes detailed in Table S1 were observed.
Figure 8.
mRNA expression, monoamines, LTP and microdialysis: Norepinephrine concentrations in the hippocampus (A) measured by HPLC-ECD. (Corn oil, CO: n = 17 total, 8 males, 9 females; 0.25 mg/kg DLM: n = 17 total, 8 males, 9 females; 0.5 mg/kg DLM: n = 17 total, 8 males, 9 females; 1.0 mg/kg DLM: n = 14 total, 7 males, 7 females.) Only males treated with the highest dose of DLM or corn oil were assessed for mRNA expression (B), microdialysis (C), and males and females for LTP (D). Drd1 mRNA expression in the neostriatum of male rats treated with 1 mg/kg DLM or corn oil (B). (Corn oil, CO: n = 10 males; 1.0 mg/kg DLM: n = 9 males.) Amphetamine (AMPH; 2 mg/kg, ip) stimulated DA release in the N. accumbens in control and 1 mg/kg DLM-treated male rats (C). (Corn oil, CO: n = 7 males; 1.0 mg/kg DLM: n = 8 males.) LTP results for 1 mg/kg DLM-treated rats and corn oil treated rats over time (D). EPSP recordings presented as a percentage of the baseline. The tetanizing stimulus (arrow; tetanus = 100 Hz in 10 bursts [4 pulses/burst] delivered at a frequency of 5 Hz for 2 s) was delivered after 10 min of stable baseline, and then recorded for 90 min following stimulation. (Corn oil, CO: n = 16 total, 8 males, 8 females; 1.0 mg/kg DLM: n = 16 total, 8 males, 8 females.) *p < .05; **p < .01; ***p < .01; ****p < .0001 compared with vehicle. Abbreviations: DA, dopamine; DLM, deltamethrin; ECD, electrochemical detector; EPSP, excitatory postsynaptic potential; HPLC, high performance liquid chromatograph; LTP, long-term potentiation; N. accumbens, nucleus accumbens.
Microdialysis
No treatment-related differences were found for basal DA release. After an acute dose of AMPH (2 mg/kg), there were main effects of treatment [F(1, 27.3) = 6.94, p < .05] and time [F(8, 95.1) = 27.73, p < .0001], and a treatment × time interaction [F(8, 95.1) = 3.15, p < .01]. Dopamine release was significantly decreased in 1.0 mg/kg DLM-treated males compared with CO males at 30 (p < .001), 60 (p < .001), and 90 min (p < .05) following AMPH (Figure 8C).
Long-Term Potentiation
1.0 mg/kg DLM-treated rats showed increased LTP after a tetanizing stimulation compared with CO controls (Figure 8D) [treatment: F(1, 38.7) = 47.35, p < .0001]. No differences were found prior to the tetanizing stimulus.
DISCUSSION
Pyrethroids, such as DLM, affect VGSC acutely, however the long-term consequences of developmental exposure are poorly characterized. This is the case despite the fact that exposed children are found to have neuropsychological disorders in epidemiological studies, and mice developmentally exposed have long-term physiological and behavioral changes (Aziz et al., 2001; Oulhote and Bouchard, 2013; Richardson et al., 2015; Shelton et al., 2014; Wagner-Schuman et al., 2015; Xue et al., 2013). The present study builds on recent mouse developmental studies to include assessment of cognitive effects in rats.
The data show that developmental DLM results in cognitive and other behavioral deficits, several of which were sexually dimorphic. Most of the effects were seen at the highest dose (1 mg/kg), fewer at the mid dose (0.5 mg/kg), and the least at the lowest dose (0.25 mg/kg). The exposure period (P3–20) was designed to span the human equivalent of third trimester in utero brain development in humans extending into the early postnatal period. This is when the cortex reaches maximum growth (Bockhorst et al., 2008; Dobbing and Sands, 1979; Gottlieb et al., 1977; Herschkowitz et al., 1997; Khazipov et al., 2001; Kretschmann et al., 1986), when synaptogenesis (Levitt, 2003; Zagon and McLaughlin, 1977) and gliogenesis (Catalani et al., 2002; Kriegstein and Alvarez-Buylla, 2009) plateau, axonal and dendritic arborization increase (Baloch et al., 2009; Bockhorst et al., 2008; Cowan, 1979), and neurotransmitter and receptor systems become established (Hedner et al., 1986; Romijn et al., 1991). We administered DLM orally for consistency with other studies (Aziz et al., 2001; Hossain et al., 2015; Johri et al., 2006; Richardson et al., 2015) and because ingestion is the major route by which pregnant women and children are exposed.
Although DLM exposure reduced growth, it did not affect swim latency in the straight water channel test or swim speed in the MWM, hence, it was not a factor in the L&M deficits seen on egocentric learning in the CWM or spatial L&M in the MWM. Unlike these tests where performance and learning can be separated, we cannot rule out possible body weight effects on other outcomes, however, the weight differences at the time of testing for the 1 mg/kg group relative to the CO controls were small (Males: 16.8% and 12.9%, Females 12.7% and 8.6% at the beginning and end of testing, respectively). Moreover, for conditioned freezing there were differential effects of DLM on contextual (affected) versus cued memory (not affected) even though body weight differences were the same throughout this test; hence, body weight differences cannot account this outcome. Moreover, body weight differences cannot explain the effects of DLM on OF because all DLM groups were equally affected (reduced) on this test whereas body weight differences were dose-dependent. Similarly, during drug challenge, all DLM groups started out equally active despite body weight differences. After AMPH or MK-801 was given, activity changes were bidirectional (exaggerated after AMPH and attenuated after MK-801) whereas body weight changes were unidirectional (reduced). Although body weight reductions are common in developmental neurotoxicity studies, they rarely correlate with behavioral effects and the present study reinforces that dissociation.
We found that 1.0 and 0.5 mg/kg DLM-exposed rats have impaired allocentric reversal learning in the MWM. Impairments in the MWM are indicative of hippocampal dysfunction (Morris et al., 1982). These effects were selective: spatial acquisition was spared but deficits emerged during reversal and shift. Reversal reflects cognitive flexibility and the DLM-exposed rats were impaired at adjusting to the new platform location and on shift trials as retrograde interference increased. These deficits may reflect perseveration on previous habits in which they had difficulty extinguishing previously learned habits (Vorhees and Williams, 2006). Impaired cognitive flexibility may also reflect inhibitory control deficits, because part of switching to a new goal is inhibiting the impulse to go to the previous goal location. Hippocampal endoplasmic reticulum stress and learning deficits in the MWM were reported after DLM exposure (Hossain et al., 2015), however, ours is the first study to report deficits in cognitive flexibility. Also, in the Hossain et al. (2015) study, the dose of DLM was higher (3 mg/kg) than here and was in adult mice in which testing was done immediately following treatment (Hossain et al., 2015), whereas here there was > 30 days between exposure and testing. For conditioned freezing, we found contextual deficits in the 1.0 and 0.5 mg/kg DLM-treated groups, which is also a hippocampal mediated type of learning (Curzon et al., 2009).
Deltamethrin-treated rats also had altered LTP (1 mg/kg DLM). Long-term potentiation is a cellular substrate of spatial L&M (Bannerman et al., 1995; Bliss and Collingridge, 1993; Herring and Nicoll, 2016; Morris et al., 1986; Moser et al., 1998; Nicoll, 2017). In brain slices, after a tetanizing stimulus, DLM-treated rats showed increased LTP compared with CO controls. At first glance this may appear paradoxical. Most studies find diminished LTP in conjunction with memory impairment, whereas our data show increased LTP with impaired memory in the MWM. However, our study is not the first to show such effects. Studies examining mice deficient in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) Glu receptor 2 (GluR2) (Jia et al., 1996), reduced post synaptic density protein (PSD-95) (Migaud et al., 1998), reduced phosphodiesterase 4 D (Pde4d−/−) (Rutten et al., 2008), and disrupted Fmr2 (Gu et al., 2002) show increased LTP in conjunction with impaired memory in MWM, conditioned fear, and/or novel object recognition. As with many biological phenomena, too little or too much deviation from homeostasis leads to dysfunction. To better understand the effects of DLM on spatial L&M and LTP, in future studies we will assess CA1 LTP in rats at the same age we found spatial deficits to ensure that these effects coincide. Also, we plan to assess proteins involved in the LTP, including N-methyl-D-aspartate and AMPA receptors. We will also determine glutamate release in the hippocampus by microdialysis.
The 1.0-mg/kg-treated rats under-responded to the hyperactivity-inducing effects of MK-801 compared with CO controls. MK-801 is often used to examine hippocampal LTP-related behavior (Abraham and Mason, 1988; Coan et al., 1987; Gilbert and Mack, 1990). In an OF, MK-801 causes hyperactivity. The reduced activating effects of MK-801 in the 1.0 mg/kg DLM group suggest glutamatergic signaling dysregulation.
Deltamethrin administration from E0-P21 in mice increased DAT and DRD1 protein expression in the offspring (Richardson et al., 2015). Based on this we predicted that DLM would lead to CWM learning deficits because performance on this task depends on striatal DA (Braun et al., 2015, 2016; Vorhees and Williams, 2016). As predicted, increased latencies and errors were found and they were sexually dimorphic, with males affected. The reason for this male-specific effect is not known, but is consistent with male effects on dopaminergic markers and memory following developmental DLM exposure in mice (Richardson et al., 2015). We also found male-specific effects of DLM on ASR and TSR.
Deltamethrin-treated rats had decreased extracellular AMPH-stimulated DA release by microdialysis in the N. accumbens, a result similar to that found in mice developmentally treated with DLM (Richardson et al., 2015). In addition, DLM-treated rats had decreased Drd1 mRNA compared with CO controls, whereas increased DRD1 protein levels in DLM-treated male mice were seen previously (Richardson et al., 2015). Although not significant we observed a trend toward increased locomotor activity following AMPH administration in the 1.0 and 0.25 mg/kg DLM groups, a pattern consistent with changes in DRD1. This should be tested further using selective dopaminergic agonists, such as SKF-82958. Additional studies are also needed to understand the impact of DLM on other DA biomarkers. As for differences between our data and those of Richardson et al. (2015), we note there are differences in timing, dose, species, and measures (mRNA vs protein) that may explain or partially explain the different outcomes. To resolve this, we plan to assess additional DA protein biomarkers in our model. It may also be worth testing stimulated DA release in striatum and prefrontal cortex to see if DA effects that were found in the N. accumbens occur in other DA-rich regions.
Deltamethrin-exposed rats were less active in a novel environment (first OF test). This is similar to what Johri et al. (2006) reported after DLM, however, their exposure was prenatal. In mice given DLM throughout gestation and lactation, increased OF activity was seen but only in males (Richardson et al., 2015). The reason for differences between studies is not clear, but we note that Johri et al. (2006) and Richardson et al. (2015) exposed prenatally and/or both prenatally and postnatally whereas we exposed only postnatally.
Voltage gated sodium channels mediate the initial effects of DLM (Soderlund, 2012). Male mice exposed to 3 mg/kg DLM throughout gestation and lactation exhibit decreased VGSC subunit mRNA expression measured when they were adults (Magby and Richardson, 2017). We did not see similar alterations in VGSC gene expression by qPCR, suggesting that species may account for this difference in outcome.
The study also has limitations: (1) DLM doses were higher than the human reference dose (0.01 mg/kg) (Goodis, 2017). However, the doses are similar to those in the literature (Aziz et al., 2001; Hossain et al., 2015; Johri et al., 2006; Richardson et al., 2015), and below the LD50 for P11 rats of 5.1 mg/kg (Sheets et al., 1994). Pharmacokinetic modeling suggests that humans would have higher peak DLM brain concentrations compared to rats at comparable doses (Kim et al., 2010) including in young rats (Kim et al., 2010; Williams et al., 2019) which is relevant given that children clear pyrethroids slower than adults (Crow et al., 2007), however, more research is needed on dose comparability. (2) Our exposure was during the first 3 weeks after birth but there are no pharmacokinetic or pharmacodynamic data with this dosing regimen to help understand the internal level of exposure. (3) Biochemical assays were performed following behavior; the effect of this experience on neurochemical markers has yet to be determined. It is known that exercise and handling can affect monoamine levels in the hippocampus and striatum (Rabelo et al., 2015; Wang et al., 2013), but whether or how the tests used here affected biomarkers will require additional experiments. Nevertheless, all rats had the same tests and therefore were matched and our focus was on relative differences rather than absolute differences. Overall, the results indicate that developmental DLM causes long-term changes in behavior, cognition, LTP, some dopaminergic markers, and glutamatergic function. Further research to identify site(s) of CNS injury from this exposure is still needed. Disruption of dopaminergic pathways is one emerging aspect of the developmental effects of DLM, but there are likely to be others uncovered in future studies.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This research was supported by National Institute of Environmental Health Sciences T32 ES007051 (E.M.P.) and funds from the Division of Neurology, Cincinnati Children’s Research Foundation.
ACKNOWLEDGMENTS
Behavioral testing was conducted through the Animal Behavior Core of Cincinnati Children’s Research Foundation.
REFERENCES
- Abraham W. C., Mason S. E. (1988). Effects of the NMDA receptor/channel antagonists CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res. 462, 40–46. [DOI] [PubMed] [Google Scholar]
- Amos-Kroohs R. M., Davenport L. L., Atanasova N., Abdulla Z. I., Skelton M. R., Vorhees C. V., Williams M. T. (2017). Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory. Neurotoxicol. Teratol. 59, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M. H., Agrawal A. K., Adhami V. M., Shukla Y., Seth P. K. (2001). Neurodevelopmental consequences of gestational exposure (GD14-GD20) to low dose deltamethrin in rats. Neurosci. Lett. 300, 161–165. [DOI] [PubMed] [Google Scholar]
- Baloch S., Verma R., Huang H., Khurd P., Clark S., Yarowsky P., Abel T., Mori S., Davatzikos C. (2009). Quantification of brain maturation and growth patterns in C57BL/6J mice via computational neuroanatomy of diffusion tensor images. Cereb. Cortex 19, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D. M., Good M. A., Butcher S. P., Ramsay M., Morris R. G. (1995). Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature 378, 182–186. [DOI] [PubMed] [Google Scholar]
- Bliss T. V. P., Collingridge G. L. (1993). A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Bockhorst K. H., Narayana P. A., Liu R., Ahobila-Vijjula P., Ramu J., Kamel M., Wosik J., Bockhorst T., Hahn K., Hasan K. M., et al. (2008). Early postnatal development of rat brain: In vivo diffusion tensor imaging. J. Neurosci. Res. 86, 1520–1528. [DOI] [PubMed] [Google Scholar]
- Bradberry S. M., Thanacoody H. K., Watt B. E., Thomas S. H., Vale J. A. (2005). Management of the cardiovascular complications of tricyclic antidepressant poisoning: Role of sodium bicarbonate. Toxicol. Rev. 24, 195–204. [DOI] [PubMed] [Google Scholar]
- Braun A. A., Amos-Kroohs R. M., Gutierrez A., Lundgren K. H., Seroogy K. B., Skelton M. R., Vorhees C. V., Williams M. T. (2015). Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiol. Learn. Mem. 118, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. A., Amos-Kroohs R. M., Gutierrez A., Lundgren K. H., Seroogy K. B., Vorhees C. V., Williams M. T. (2016). 6-Hydroxydopamine-induced dopamine reductions in the nucleus accumbens, but not the medial prefrontal cortex, impair Cincinnati water maze egocentric and Morris water maze allocentric navigation in male Sprague Dawley rats. Neurotox. Res. 30, 199–212. [DOI] [PubMed] [Google Scholar]
- Catalani A., Sabbatini M., Consoli C., Cinque C., Tomassoni D., Azmitia E., Angelucci L., Amenta F. (2002). Glial fibrillary acidic protein immunoreactive astrocytes in developing rat hippocampus. Mech. Ageing Dev. 123, 481–490. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Saywood W., Collingridge G. L. (1987). MK-801 blocks NMDA receptor-mediated synaptic transmission and long term potentiation in rat hippocampal slices. Neurosci. Lett. 80, 111–114. [DOI] [PubMed] [Google Scholar]
- Costa L. G. (2013). Toxic effects of pesticides In Cassarett & Doull's Toxicology: The Basic Science of Poisons, 8th ed (Amdur M. O., Doull J., Klaassen C.D., Eds.), pp. 933–980. McGraw-Hill, New York, NY. [Google Scholar]
- Cowan W. M. (1979). The development of the brain. Sci. Am. 241, 113–133. [PubMed] [Google Scholar]
- Crow J. A., Borazjani A., Potter P. M., Ross M. K. (2007). Hydrolysis of pyrethroids by human and rat tissues: Examination of intestinal, liver and serum carboxylesterases. Toxicol. Appl. Pharmacol. 221, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P., Rustay N. R., Browman K. E. (2009). Cued and contextual fear conditioning for rodents In Methods of Behavior Analysis in Neuroscience, 2nd ed (Buccafusco J. J., Ed.). CRC Press/Taylor & Francis, Boca Raton, FL. [PubMed] [Google Scholar]
- Dobbing J., Sands J. (1979). Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83. [DOI] [PubMed] [Google Scholar]
- Eriksson P., Fredriksson A. (1991). Neurotoxic effects of two different pyrethroids, bioallethrin and deltamethrin, on immature and adult mice: Changes in behavioral and muscarinic receptor variables. Toxicol. Appl. Pharmacol. 108, 78–85. [DOI] [PubMed] [Google Scholar]
- Eriksson P., Nordberg A. (1990). Effects of two pyrethroids, bioallethrin and deltamethrin, on subpopulations of muscarinic and nicotinic receptors in the neonatal mouse brain. Toxicol. Appl. Pharmacol. 102, 456–463. [DOI] [PubMed] [Google Scholar]
- Gilbert M. E., Mack C. M. (1990). The NMDA antagonist, MK-801, suppresses long-term potentiation, kindling, and kindling-induced potentiation in the perforant path of the unanesthetized rat. Brain Res. 519, 89–96. [DOI] [PubMed] [Google Scholar]
- Goodis M. L. (2017). Deltamethrin; pesticide tolerances. Vol. 82, pp. 18574–18580. Document #: 2017-07816, Agency: Environmental Protection Agency (EPA). [Google Scholar]
- Gottlieb A., Keydar I., Epstein H. T. (1977). Rodent brain growth stages: An analytical review. Biol. Neonate 32, 166–176. [DOI] [PubMed] [Google Scholar]
- Gu Y., McIlwain K. L., Weeber E. J., Yamagata T., Xu B., Antalffy B. A., Reyes C., Yuva-Paylor L., Armstrong D., Zoghbi H., et al. (2002). Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J. Neurosci. 22, 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner J., Lundell K. H., Breese G. R., Mueller R. A., Hedner T. (1986). Developmental variations in CSF monoamine metabolites during childhood. Biol. Neonate 49, 190–197. [DOI] [PubMed] [Google Scholar]
- Herring B. E., Nicoll R. A. (2016). Long-term potentiation: From CaMKII to AMPA receptor trafficking. Annu. Rev. Physiol. 78, 351–365. [DOI] [PubMed] [Google Scholar]
- Herschkowitz N., Kagan J., Zilles K. (1997). Neurobiological bases of behavioral development in the first year. Neuropediatrics 28, 296–306. [DOI] [PubMed] [Google Scholar]
- Hossain M. M., DiCicco-Bloom E., Richardson J. R. (2015). Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol. Sci. 143, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. M., Suzuki T., Sato N., Sato I., Takewaki T., Suzuki K., Tachikawa E., Kobayashi H. (2006). Differential effects of pyrethroid insecticides on extracellular dopamine in the striatum of freely moving rats. Toxicol. Appl. Pharmacol. 217, 25–34. [DOI] [PubMed] [Google Scholar]
- Hossain M. M., Suzuki T., Richardson J. R., Kobayashi H. (2013). Acute effects of pyrethroids on serotonin release in the striatum of awake rats: An in vivo microdialysis study. J. Biochem. Mol. Toxicol. 27, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Agopyan N., Miu P., Xiong Z., Henderson J., Gerlai R., Taverna F. A., Velumian A., MacDonald J., Carlen P., et al. (1996). Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17, 945–956. [DOI] [PubMed] [Google Scholar]
- Johri A., Yadav S., Singh R. L., Dhawan A., Ali M., Parmar D. (2006). Long lasting effects of prenatal exposure to deltamethrin on cerebral and hepatic cytochrome P450s and behavioral activity in rat offspring. Eur. J. Pharmacol. 544, 58–68. [DOI] [PubMed] [Google Scholar]
- Khazipov R., Esclapez M., Caillard O., Bernard C., Khalilov I., Tyzio R., Hirsch J., Dzhala V., Berger B., Ben-Ari Y. (2001). Early development of neuronal activity in the primate hippocampus in utero. J. Neurosci. 21, 9770–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. B., Anand S. S., Kim H. J., White C. A., Fisher J. W., Tornero-Velez R., Bruckner J. V. (2010). Age, dose, and time-dependency of plasma and tissue distribution of deltamethrin in immature rats. Toxicol. Sci. 115, 354–368. [DOI] [PubMed] [Google Scholar]
- Kretschmann H. J., Kammradt G., Krauthausen I., Sauer B., Wingert F. (1986). Growth of the hippocampal formation in man. Bibl. Anat. 28, 27–52. [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Ann. Rev. Neurosci. 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P. J. M. D. R., Babich H. J., Boardman B., Bruckner J. V., Gallo M. A., Hutchings D. E., Jackson R. J., Karol M. H., Krewski D., Purvis G. A., et al. (1993). Pesticides in the Diets of Infants and Children. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Lazarini C. A., Florio J. C., Lemonica I. P., Bernardi M. M. (2001). Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. Neurotoxicol. Teratol. 23, 665–673. [DOI] [PubMed] [Google Scholar]
- Levitt P. (2003). Structural and functional maturation of the developing primate brain. J. Pediatr. 143, S35–S45. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Magby J. P., Richardson J. R. (2017). Developmental pyrethroid exposure causes long-term decreases of neuronal sodium channel expression. Neurotoxicology 60, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M., Charlesworth P., Dempster M., Webster L. C., Watabe A. M., Makhinson M., He Y., Ramsay M. F., Morris R. G., Morrison J. H., et al. (1998). Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O’Keefe J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Moser E. I., Krobert K. A., Moser M. B., Morris R. G. (1998). Impaired spatial learning after saturation of long-term potentiation. Science 281, 2038–2042. [DOI] [PubMed] [Google Scholar]
- Nair S. G., Gudelsky G. A. (2004). Protein kinase C inhibition differentially affects 3, 4-methylenedioxymethamphetamine-induced dopamine release in the striatum and prefrontal cortex of the rat. Brain Res. 1013, 168–173. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. (2017). A brief history of long-term potentiation. Neuron 93, 281–290. [DOI] [PubMed] [Google Scholar]
- Oulhote Y., Bouchard M. F. (2013). Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ. Health Perspect. 121, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C., Pennisi M., Topple A. (1985). Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J. Neurosci. Methods 13, 139–143. [DOI] [PubMed] [Google Scholar]
- Power L. E., Sudakin D. L. (2007). Pyrethrin and pyrethroid exposures in the United States: A longitudinal analysis of incidents reported to poison centers. J. Med. Toxicol. 3, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo P. C., Almeida T. F., Guimaraes J. B., Barcellos L. A., Cordeiro L. M., Moraes M. M., Coimbra C. C., Szawka R. E., Soares D. D. (2015). Intrinsic exercise capacity is related to differential monoaminergic activity in the rat forebrain. Brain Res. Bull. 112, 7–13. [DOI] [PubMed] [Google Scholar]
- Richardson J. R., Taylor M. M., Shalat S. L., Guillot T. S. 3rd, Caudle W. M., Hossain M. M., Mathews T. A., Jones S. R., Cory-Slechta D. A., Miller G. W. (2015). Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J. 29, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn H. J., Hofman M. A., Gramsbergen A. (1991). At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum. Dev. 26, 61–67. [DOI] [PubMed] [Google Scholar]
- Rutten K., Misner D. L., Works M., Blokland A., Novak T. J., Santarelli L., Wallace T. L. (2008). Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur. J. Neurosci. 28, 625–632. [DOI] [PubMed] [Google Scholar]
- Semple B. D., Blomgren K., Gimlin K., Ferriero D. M., Noble-Haeusslein L. J. (2013). Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106–107, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L. P., Doherty J. D., Law M. W., Reiter L. W., Crofton K. M. (1994). Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol. Appl. Pharmacol. 126, 186–190. [DOI] [PubMed] [Google Scholar]
- Shelton J. F., Geraghty E. M., Tancredi D. J., Delwiche L. D., Schmidt R. J., Ritz B., Hansen R. L., Hertz-Picciotto I. (2014). Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environ. Health Perspect. 122, 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K., Baudry M., Ho L., Taketani M., Lynch G. (2002). Long-term recording of LTP in cultured hippocampal slices. Neural Plast. 9, 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund D. M. (2012). Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 86, 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C. V. (1987). Reliability, sensitivity, and validity of behavioral indices of neurotoxicity. Neurotoxicol. Teratol. 9, 445–464. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Herring N. R., Schaefer T. L., Grace C. E., Skelton M. R., Johnson H. L., Williams M. T. (2008). Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: Effects of dose and rearing conditions. Int. J. Dev. Neurosci. 26, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2006). Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protoc. 1, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2014). Assessing spatial learning and memory in rodents. ILAR J. 55, 310–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2016). Cincinnati water maze: A review of the development, methods, and evidence as a test of egocentric learning and memory. Neurotoxicol. Teratol. 57, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Schuman M., Richardson J. R., Auinger P., Braun J. M., Lanphear B. P., Epstein J. N., Yolton K., Froehlich T. E. (2015). Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ. Health 14, 44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen X., Zhang N., Ma Q. (2013). Effects of exercise on stress-induced changes of norepinephrine and serotonin in rat hippocampus. Chin. J. Physiol. 56, 245–252. [DOI] [PubMed] [Google Scholar]
- Williams M. K., Rundle A., Holmes D., Reyes M., Hoepner L. A., Barr D. B., Camann D. E., Perera F. P., Whyatt R. M. (2008). Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000-2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect. 116, 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. T., Gutierrez A., Vorhees C. V. (2019). Effects of acute deltamethrin exposure in adult and developing Sprague Dawley rats on acoustic startle response in relation to deltamethrin brain and plasma concentrations. Toxicol. Sci. 168, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. T., Herring N. R., Schaefer T. L., Skelton M. R., Campbell N. G., Lipton J. W., McCrea A. E., Vorhees C. V. (2007). Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘foxy’) to adult rats: A new drug of abuse. Neuropsychopharmacology 32, 1404–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Li X., Su Q., Xu L., Zhang P., Kong Z., Xu J., Teng J. (2013). Effect of synthetic pyrethroid pesticide exposure during pregnancy on the growth and development of infants. Asia Pac. J. Public Health 25, 72s–79s. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. (1977). Effect of chronic maternal methadone exposure on perinatal development. Biol. Neonate 31, 271–282. [DOI] [PubMed] [Google Scholar]