Abstract
Tetrabromobisphenol A (TBBPA, CAS No. 79-94-7) is a brominated flame retardant used in 90% of epoxy coated circuit boards. Exposures to TBBPA can induce neurotoxicity and disrupt MAPK, estrogen, thyroid, and PPAR-associated signaling pathways. Because these pathways also regulate transporters of the central nervous system barriers, we sought to determine the effect of TBBPA on the expression and activity of 3 ATP binding cassette (ABC) transporters of the blood-brain barrier (BBB). Using a confocal based assay, we measured the ex vivo and in vivo effects of TBBPA on P-glycoprotein (P-gp), breast cancer resistant protein (BCRP), and multidrug resistance-associated protein 2 (MRP2) transport activity in rat brain capillaries. Our rationale for using a rat model was based on tissue availability, ease of handling, and availability of historical TBBPA toxicokinetic data. We found that TBBPA (1–1000 nM) exposure had no significant effect on multidrug resistance-associated protein 2 transport activity in either sex, suggesting TBBPA does not compromise the physical integrity of the BBB. However, low concentrations of TBBPA (1–100 nM) significantly decreased breast cancer resistant protein transport activity in both sexes. Additionally, TBBPA exposures (1–100 nM), elicited a sex-dependent response in P-gp transport: increasing transport activity in males and decreasing transport activity in females. All TBBPA dependent changes in transport activity were dose- and time-dependent. Inhibitors of either transcription or translation abolished the TBBPA dependent increases in male P-gp transport activity. Western blot and immunofluorescent assays confirmed the TBBPA dependent P-gp increases expression in males and decreases in females. Antagonizing PPAR-γ abolished the TBBPA dependent increases in males but not the decreases in females. However, the decreases in female P-gp transport were blocked by an ER-α antagonist. This work indicates that environmentally relevant concentrations of TBBPA (1–100 nM) alter ABC transporter function at the BBB. Moreover, permeability changes in the BBB can alter brain homeostasis, hinder central nervous system drug delivery, and increase the brain’s exposure to harmful xenobiotic toxicants.
Keywords: tetrabromobisphenol A, P-glycoprotein, ABC transporters, blood-brain barrier, brominated flame retardants, estrogen receptor alpha, PPAR gamma
Brominated flame retardants (BFR) are industrial organobromine compounds applied to a variety of consumer and building products to increase their ignition temperature. Tetrabromobisphenol A (2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol, TBBPA, CAS no. 79-94-7) is the most ubiquitous BFR due to its industrial use in the epoxy lamination of digital circuit boards. The global production volume of TBBPA exceeds 170 kilotons, making it the highest of all BFR produced (ECB, 2006). The TBBPA is found in water, soil, sediments, sewage sludge, and house dust (Wang et al., 2015). Documented human exposures indicate that TBBPA is found in human breast milk throughout the world (Cariou et al., 2008). In mammals, TBBPA is readily absorbed by the gastrointestinal epithelium, transported to the liver, and subjected to sulfate and glucuronide conjugation via phase II metabolism (Knudsen et al., 2014, 2018). Therefore, TBBPA bioavailability is largely limited by first pass metabolism. Even with a relatively short half-life (2 days) and low overt toxicity, in vivo and in vitro data associate TBBPA’s biological interactions that include neurologic, tumorigenic, estrogen, thyroid, and PPAR signaling pathways (Burk et al., 2010; Mahringer and Fricker, 2010; More et al., 2017; Wang et al., 2014).
The brain’s first line of defense against endogenous and xenobiotic toxicants is denial of entry. This task is assigned to the brain’s vascular system by the formation of a blood-brain barrier (BBB). The BBB is a component of the neurovascular unit formed by the tri-lamination of endothelial cells, pericytes, and astrocytic feet. The BBB selectively modulates central nervous system entry and exit of drugs, metabolites, xenobiotics, and neurotoxicants. To perform these tasks, the BBB relies on 2 essential components: (1) Tight junction proteins that “mortar” adjacent endothelial cells together to prevent paracellular movement of harmful solutes from the blood to the brain and (2) luminal (blood) facing ATP-driven xenobiotic efflux pumps that remove or limit brain entry of foreign or unwanted molecules from the blood. P-glycoprotein (P-gp, MDR1, ABCB1), breast cancer resistance protein (BCRP, ABCG2), and multidrug resistance-associated protein 2 (MRP2, ABCC2) are 3 important members of the ATP binding cassette (ABC) transporter superfamily that serve as xenobiotic efflux pumps protecting the brain. Of the 3, P-gp is the most studied due to its high level of expression and its wide and diverse range of substrates. Studies of P-gp indicate that it is dynamically regulated through both genomic and non-genomic (signaling) mechanisms (Cannon et al., 2012; Mesev et al., 2017; Miller and Cannon, 2014). In vivo and ex vivo studies show that xenobiotic and endogenous metabolites can activate specific signaling pathways to induce or repress transport of P-gp and other ABC transporters at the BBB (Chan et al., 2017; Miller and Cannon, 2014; Wang et al., 2011, 2014).
Given the propensity of TBBPA to be neurotoxic and dysregulate signaling pathways both in vivo and in vitro, we sought to determine how TBBPA exposure might alter the expression and activity of important xenobiotic efflux transporters at the BBB. Here, we show that in vivo and ex vivo TBBPA exposures produce a sex-specific response; where P-gp transport increases in males and decreases in females. Furthermore, we show this response is dependent on peroxisome proliferator-activated receptor gamma (PPAR-γ) activity in males and estrogen signaling through ER-α in females. We also found the TBBPA decreases BCRP transport for both sexes whereas eliciting no changes in MRP2 transport. These important findings indicate that exposure to relatively low and environmentally relevant concentrations of TBBPA rapidly influence the permeability of the BBB in a sex-specific manner by modulating xenobiotic ABC transporters.
MATERIALS AND METHODS
Materials
P-glycoprotein fluorescent substrate [N- ε-(4-Nitrobenzofurazan-7-yl)-D-Lys8] cyclosporine A (NBD-CSA) was custom-synthesized by R. Wenger (Sandoz, Basel, Switzerland). Breast cancer resistance protein fluorescent substrate, BODIPY® FL Prazosin was purchased from ThermoFisher. The TBBPA, DMSO, BCRP inhibitor KS-176, MRP2 fluorescent substrate Texas Red (Sulforhodamine 101), and ß-actin mouse monoclonal antibody A1978 were purchased from Sigma-Aldrich. P-glycoprotein inhibitor PSC-833, PPAR-γ inhibitor GW9662, and the ER-α antagonist, ICI 182780, were purchased from Tocris Bioscience. E2-estradiol was kindly provided by K. Korach (NIEHS—NIH, Research Triangle Park, North Carolina). P-glycoprotein rabbit monoclonal antibody ab170904, and BCRP rat monoclonal antibody ab24115 were purchased from Abcam. Secondary antibodies Alexafluor 647 goat anti-mouse IgG and Alexafluor 647 goat anti-rabbit IgG were purchased from ThermoFisher Scientific. IRDye® 800CW goat anti-rat IgG was purchased from Licor. Tissues for Western blot analysis were processed in CelLytic MT Mammalian Tissue Lysis/Extraction Reagent with complete Mini protease inhibitor (Roche Diagnostics). Ten-well Invitrogen NuPAGE 4%–12% Bis-Tris Gels NP0321 and PDVF Immobilon-FL membranes (Millipore) were used for the western blotting. Immunohistochemistry (IHC) antibodies were P-gp mouse monoclonal antibody C219 and Alexa Fluor 488 goat anti-mouse IgG antibody, both purchased from ThermoFisher.
Animals
The Animal Care and Use Committee at the National Institute of Environmental Health Sciences approved all animal experiments according to NIH guidelines. We reported all data in compliance with the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines. We purchased Male and female Hsd: Sprague Dawley (SD) rats (age 15–20 weeks) from Envigo (Raleigh, North Carolina). Animals were housed in an AAALAC-approved animal care facility (approximately 49% humidity, approximately 72°F room temperature, 12 h light/dark cycle) for 7 days prior to use. Animals were provided food (NIH No. 31) and water (Durham, North Carolina) ad libitum and euthanized by CO2 inhalation immediately before tissue collections.
Animal dosing for in vivo studies
Each animal received a single oral dose of TBBPA (purchased from Sigma-Aldrich Chemistry) by gavage, 250 mg/kg (4 ml/kg). Dosing vehicle was sesame oil (Sigma-Aldrich). Doses were chosen to match a previous published TBBPA study (Knudsen et al., 2007) and to provide a brain exposure at 3–6 h of 2.0 nM/g of brain tissue.
Transport assay for ex vivo studies
Brain tissue was harvested following euthanasia by CO2 and placed on ice in PBS containing glucose/pyruvate. Capillaries were isolated, pooled, and transport assayed as previously described (Chan and Cannon, 2017). Steady-state transport activity was measured as luminal accumulation of a fluorescent substrate specific for each transporter, 2 µM NBD-CSA, BODIPY® FL Prazosin, and Texas Red for P-gp, BCRP, and MRP2, respectively. Background fluorescence was subtracted from P-gp, BCRP, and MRP2 using specific inhibitors, 10 µM PSC-833 and 20 µM KS-176, and 20 µM MK-571, respectively. Where indicated, TBBPA dissolved in DMSO (Sigma-Aldrich) was added to the chamber slides, with or without inhibitors or antagonist (10 nM GW 9662, 10 nM ICI, pretreated 15 min) and incubated at appropriate time (1–6 h). All non-treated vehicle controls (NT) samples contained the vehicle, DMSO, in a volume equal to that used in the treated samples. Capillaries were imaged using a Zeiss Inverted confocal laser scanning microscope, with a 40 × 1.2 numeric aperture water‐immersion objective, with functional 488 nm, and 561 nm laser and detector lines (Carl Zeiss, LSM 710 confocal microscope). Within each experiment, all microscope parameters, including pinhole diameter, photomultiplier gain, laser power, and digital gain, were identical. A minimum of 15 capillaries per treatment were imaged in each experiment, which was performed 3–4 times. Fluorescence intensity in the capillary lumen was quantified using FIJI/ImageJ analysis software.
Western Blotting
Isolated capillaries were treated with TBBPA in 1 ml PBS for 6 h. After treatment, capillaries were centrifuged for 15 min at a relative centrifugal force of 1860 × g and pellets were frozen at −80°C until use. Two hundred microliters of lysis buffer with protease inhibitor was added to each pellet. These samples were kept on ice and vortexed for 10 s every 10 min for 90 min. Additionally, samples were sonicated for 20 s at 20, 40, and 60 min. Samples were centrifuged at 10 000 × g for 30 min to isolate the nuclear pellet. Twenty microliters of lysis buffer was added to the nuclear pellet. The denucleated supernatant was centrifuged at 100 000 × g for 90 min. Thirty microliters of lysis buffer was added to the membrane pellet. The membrane, nuclear, and cytosolic fractions were stored at −80°C. Quantification of protein concentration was determined as described by the ThermoScientific Coomassie Protein Assay Kit 23200, a modified version of the Bradford assay (Bradford, 1976). Bovine serum albumin protein standards were provided by the kit. Western blotting was performed according to the manufacturer’s instructions. Protein was transferred to membranes and hybridized overnight at 4°C in 1× PBS with 0.1% Tween with 1:200 P-gp and BCRP primary antibodies and 1:5000 ß-actin primary antibody. The membrane was incubated at 25°C for 3 h in 1× PBS with 1:10 000 secondary antibodies, and then imaged with FluorChem M (ProteinSimple, San Jose, California, USA).
Immunofluorescence
Isolated capillaries were plated and treated per transport assay. Following treatment, capillaries were fixed for 15 min with 1 ml 3% paraformaldehyde/0.2% Glutaraldehyde in PBS, rinsed, permeabilized for 30 min with 1 ml 0.1% Triton X, rinsed, blocked 30 min with 1 ml 1% bovine serum albumin, and rinsed. Capillaries were incubated with 1:200 P-gp primary antibody overnight with 0.1% Tween in PBS. No specific binding of primary antibody was removed by 3 PBS washes. Next secondary antibody was added at 1:10 000 and incubated for 3 h at 37°C. Secondary antibody was removed by 3 washes with PBS and fluorescence was imaged with confocal microscopy and quantified using Image J software. Nonspecific background fluorescence of no-primary controls was subtracted to determine specific fluorescence.
Statistical analysis
Data are expressed as mean ± standard error. Statistical analyses between groups were calculated by one-way analysis of variance with Tukey-Kramer post-test (multiple comparisons) or Student’s unpaired t test with GraphPad Prism 7 software (GraphPad Software, San Diego, California). P < .05 was considered a statistically significant difference between means.
RESULTS
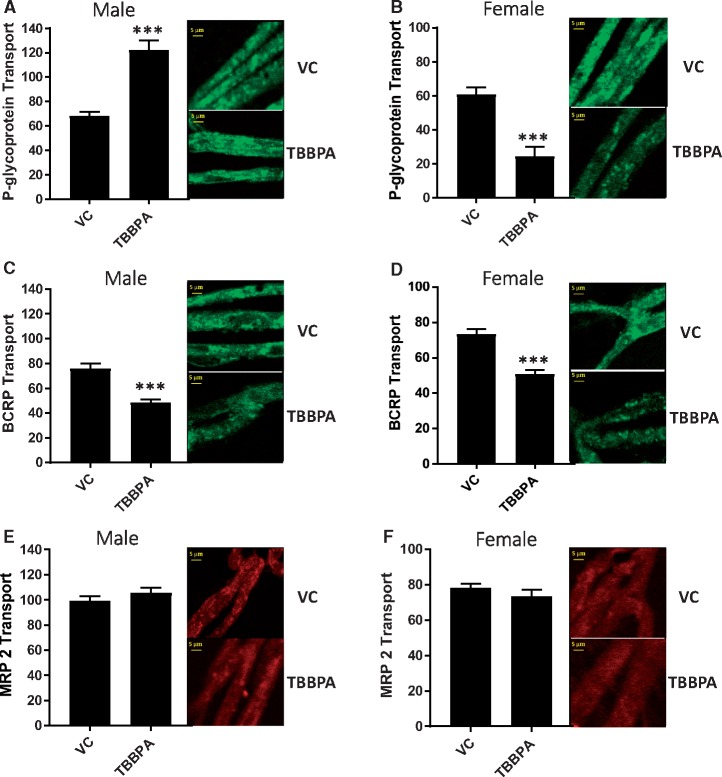
Using an established steady-state confocal microscopy-based transport assay (Chan and Cannon, 2017) we measured the effect of TBBPA on 3 important ABC efflux transporters at the BBB. To accomplish this, freshly isolated brain capillaries from male and female SD rats were exposed to 100 nM of TBBPA for 6 h. P-glycoprotein, BCRP, and MRP2 transport activity was measured by the steady state luminal accumulation of the fluorescent substrates, NBD-CSA, BODIPY FL prazosin, and Texas Red, respectively. We determined specific transport activity by subtracting non-specific luminal fluorescence following treatment with inhibitors selective for each transporter (P-gp-PSC833, BCRP-KO143, and MRP2-MK571.) Figures 1A–F show representative confocal images of male and female rat brain capillaries exposed to 100 nM TBBPA for 6 h and graphically depict the measured levels of luminal fluorescence indicative of P-gp, BCRP, and MRP2 transport activity. Indicated in Figures 1A and 1B, TBBPA significantly increased P-gp transport activity relative to vehicle treated controls in males and significantly decreased P-gp transport in females. Additionally, TBBPA significantly decreased BCRP transport activity in both male and female capillaries (Figs. 1C and 1D) but produced no significant changes in MRP2 transport activity in either sex (Figs. 1E and 1F). These data indicate that ex vivo exposures to low concentrations of TBBPA produces transporter activity specific changes without restricting energy (ATP) availability or compromising the physical integrity of the capillary. Most significantly, we observed sex-specific TBBPA dependent changes in P-gp transport activity.
Figure 1.
Changes in ABC transport activity in male and female rat brain capillaries after 6-h exposure to 100 nM TBBPA; VC—vehicle control, TBBPA—100 nM TBBPA. A, (left) Male P-gp transport activity with (right) confocal images. B, (left) Female P-gp transport activity with (right) confocal images. C, (left) Male BCRP transport activity with (right) confocal images. D, (left) Female BCRP transport activity with (right) confocal images. E, (left) Male MRP2 transport activity with (right) confocal images. F, (left) Female MRP2 transport activity with (right) confocal images. Each bar represents the mean value for 15–20 capillaries from a single preparation (pooled tissue from 3 to 7 rats, 15–20 weeks old); variability is shown as SE bars. Units are arbitrary fluorescence intensity. Statistical comparisons: ***significantly lower than control, p < .001. Scale bar in each panel equals 10 µm in length. Abbreviations: BCRP, breast cancer resistant protein; MRP2, multidrug resistance-associated protein 2; P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
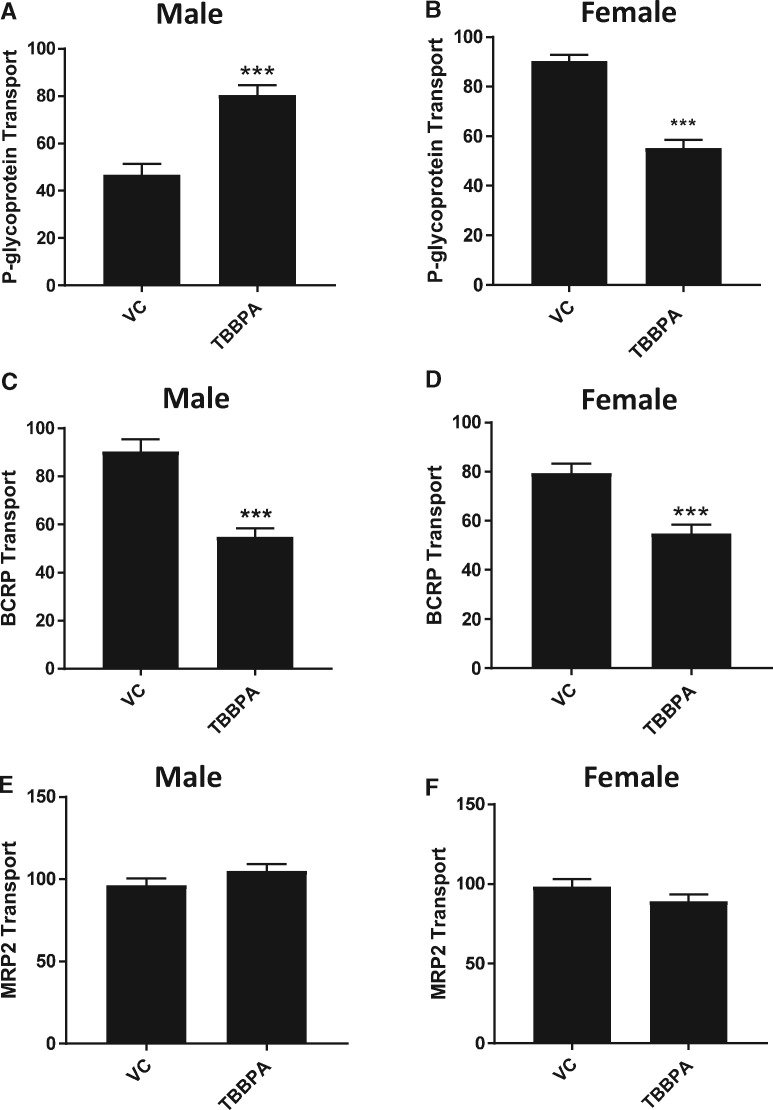
To confirm these findings in vivo, we delivered TBBPA (250 mg/kg) by oral gavage to male and female SD rats and measured P-gp, BCRP, and MRP2 transport activity 6 h later. This dose was chosen to deliver a comparable in vivo dose of TBBPA based on published pharmacokinetic studies (Knudsen et al., 2007). In males, P-gp transport activity was significantly increased relative to vehicle treated controls (Figure 2A). Breast cancer resistance protein transport activity was reduced and MRP2 transport activity was no different than vehicle treated controls (Figs. 2C and 2E). In females, both P-gp and BCRP levels were decreased (Figs. 2B and 2D). Again, female MRP2 transport activity did not differ from vehicle treated controls (Figure 2F). Together, the ex vivo and in vivo data indicate that exposures to TBBPA modulates 2 important ABC transporters at the BBB. Moreover, rats exposed to TBBPA in vivo conserve the unique sex-specific P-gp response seen ex vivo.
Figure 2.
In vivo effects of TBBPA on P-gp, BCRP, and MRP2 transport in male and female rat brain capillaries; VC—vehicle control, TBBPA—250 mg/kg by gavage. A, Male P-gp transport activity. B, Female P-gp transport activity. C, Male BCRP transport activity. D, Female BCRP transport activity. E, Male MRP2 transport activity. F, Female MRP2 transport activity. Each bar represents the mean value for 15–20 capillaries from a single preparation (pooled tissue from 5 to 7 rats, 15–20 weeks old); variability is shown as SE bars. Units are arbitrary fluorescence. Statistical comparisons: ***significantly different than control, p < .001. Abbreviations: BCRP, breast cancer resistant protein; MRP2, multidrug resistance-associated protein 2; P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
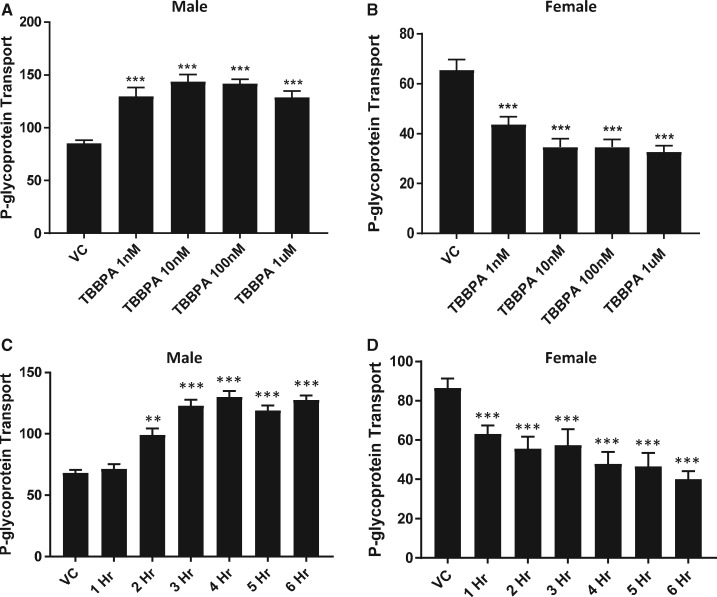
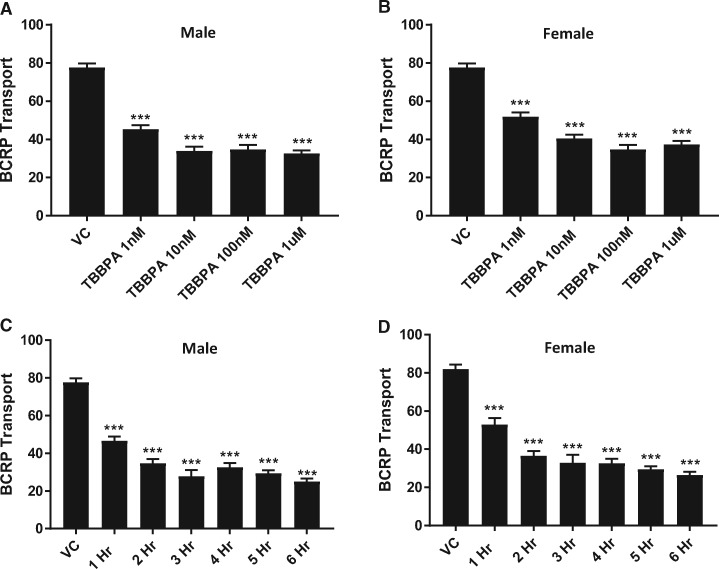
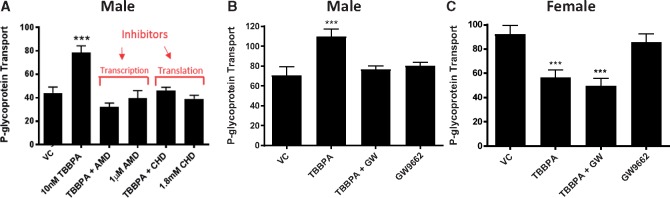
To investigate changes in transport at the BBB with increasing dose, we exposed male and female rat brain capillaries to increasing concentrations of TBBPA (1–1000 nM) for 6 h and measured P-gp and BCRP transport activity. As shown in Figure 3A, P-gp transport activity in male capillaries increased significantly in a dose-dependent manner. Consistent with our earlier findings, P-gp transport activity of TBBPA treated female capillaries significantly decreased in a dose-dependent manner (Figure 3B). Breast cancer resistance protein transport activity in both male and female capillaries significantly decreased with dose (Figs. 4A and 4B). To gain insight into the kinetics of the observed changes in transport activity, we exposed male and female rat brain capillaries to 100 nM TBBPA and measured the changes of P-gp and BCRP transport activity at hourly intervals for 6 h. Figure 3C shows that P-gp transport activity in male capillaries exposed to 100 nM TBBPA increased to a maximum in 3 h and remained high for the remaining 3 h. P-glycoprotein transport activity in female brain capillaries significantly decreased in 1 h and remained low for the remaining 5 h of treatment (Figure 3D). The kinetics of BCRP transport in TBBPA exposed capillaries decreased in both male and female in the first hour and remained low for the remainder of the treatment period (Figs. 4C and 4D). These data indicate that TBBPA exposures at relatively low doses (1–100 nM) rapidly and uniquely altered the activities of two important ABC transporters at the BBB.
Figure 3.
TBBPA dependent changes in P-gp transport activity in rat brain capillaries are sex-, dose-, and time dependent. P-gp transport activity in capillaries exposed to 100 nM TBBPA for 4 h; (A) male and (B) female capillaries. Time-dependent response of 100 nM TBBPA on (C) male and (D) female P-gp transport activity. Each bar represents the mean value for 15–20 capillaries from a single preparation (pooled tissue from 5 to 7 rats, 15–20 weeks old); variability is shown as SE bars. Units are arbitrary fluorescence. Statistical comparisons: ***significantly different than control, p < .001. Abbreviations: P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
Figure 4.
TBBPA dependent decreases in BCRP transport activity are dose- and time dependent. Dose dependent decreases at 4 h of TBBPA on (A) male and (B) female BCRP activity. (C) Male and (D) female BCRP transport activity exhibits time-dependent decreases from 100 nM TBBPA exposure. Shown are mean ± SEM for 15–20 capillaries from single preparation (pooled brains from 4 to 7 rats, 15–20 weeks old). ***p < .001, significantly different than control. Abbreviations: BCRP, breast cancer resistant protein; TBBPA, tetrabromobisphenol A.
Next, we examined the male TBBPA dependent increases in P-gp transport activity to determine if they were due to de novo protein synthesis. To accomplish this, we co-treated freshly isolated capillaries with TBBPA and inhibitors of transcription or translation and subsequently measured P-gp transport activity. Figure 5A shows increases in P-gp transport activity in response to 100 nM TBBPA exposure was abolished by inhibiting transcription (actinomycin D) or translation (cycloheximide), whereas neither inhibitor alone affected P-gp transport activity, supporting the notion that TBBPA-dependent increases in P-gp transport activity in male capillaries requires protein expression.
Figure 5.
TBBPA induced P-gp transport requires transcription, translation and PPAR-γ. A, In males, pretreatment with an inhibitor of transcription, 1 μM actinomycin D (AMD), or translation, 1.8 mM cycloheximide (CHD), blocks TBBPA increases in P-gp activity. B, In males, co-treatment with 10 nM GW9662 and 100 nM TBBPA blocks P-gp induction. C, In females, co-treatment with 10 nM GW9662 and 100 nM TBBPA does not reverse TBBPA dependent decreases in P-gp induction. Shown are mean ± SEM for 10–20 capillaries from single preparation (pooled brains from 3 to 5 rats, 15–20 weeks old). ***p < .001, significantly different than control. Abbreviations: P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
Because TBBPA exposure is associated with the activation of PPAR-γ (Hoffmann et al., 2017; Honkisz and Wojtowicz, 2015; Watt and Schlezinger, 2015), we co-treated male and female rat brain capillaries ex vivo with TBBPA and a PPAR-γ inhibitor, GW9662 (10 nM), and measured their effects on P-gp transport. Figure 5B shows that inhibition of PPAR-γ by the selective inhibitor, GW9662, abolishes the TBBPA dependent increases in P-gp transport activity in male rat brain capillaries. In contrast, inhibition of PPAR-γ with GW9662 had no effect on female P-gp transport activity (Figure 5C).
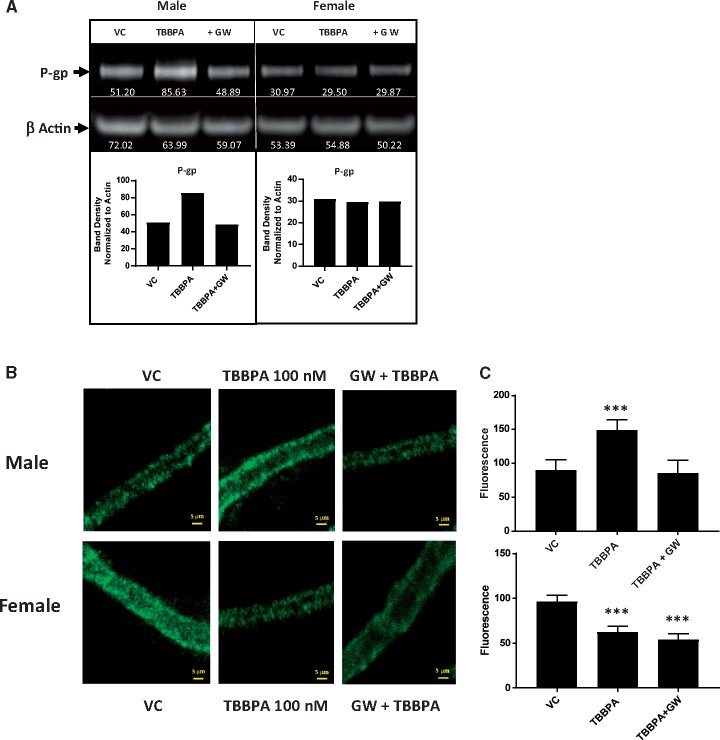
To further assess the role of PPAR-γ in the TBBPA induced changes in P-gp protein levels in rat brain capillaries, we performed western blots and immunofluorescent detection assays. We used Western blots to measure the P-gp protein levels in male and female capillaries exposed to 100 nM TBBPA with or without the PPAR-γ antagonist, GW9662 (10 nM). Shown in Figure 6A, male P-gp levels increased in response to TBBPA exposure relative to vehicle treated controls. Importantly, these increases were blocked by PPAR-γ inhibition with GW9662 (Figure 6A). Consistent with the Western blot results, immunofluorescence detection assays using the C-219 primary anti-P-gp monoclonal antibody show that TBBPA exposure in males increases P-gp on the luminal membranes of brain capillaries. However, co-treating the capillaries with the 100 nM TBBPA and the PPAR-γ antagonist, GW9662 (10 nM), abolishes this increase (Figs. 6B and 6C). We observed mixed results in females. Our Western blots detected no significant changes in brain capillary lysates. In our immunofluorescence detection assays, TBBPA decreased the P-gp luminal membrane fluorescence and GW9662/TBBPA co-treatment did not reverse the decrease. These data are consistent with the notion that TBBPA exposure elicits a P-gp sex-specific response; increasing in males and decreasing in females. They also show the increased response in males is PPAR-γ dependent.
Figure 6.
Inhibition of PPAR-γ blocks TBBPA mediated increases in P-gp expression in males. A, Western blot containing proteins from male (left) and female (right) rat brain capillaries. Non-treated vehicle controls (NT), TBBPA 100 nM exposed capillaries (TBBPA), and co-treated with TBBPA + 10 nM GW 9668 (+ GW). B, (top) Immunofluorescent images showing TBBPA increases are blocked by inhibition of PPAR-γ in males. Band density was determined by densitometry using ImageJ software. White numbers under P-gp bands in blot were normalized to the density of actin bands to control for minor loading variances. Graphs depict normalized density values of P-gp band in Western blot. B, (bottom) Immunofluorescent images showing TBBPA decreases in females are insensitive to PPAR-γ inhibition. Graphical analysis of P-gp immunofluorescence in males (C, top) and females (C, bottom). Shown are mean fluorescence ± SEM for 10–20 capillaries from single preparation (pooled brains from 3 to 5 rats, 15–20 weeks old). ***p < .001, significantly different than control. Scale bar equals 10 µm in length. Abbreviations: P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
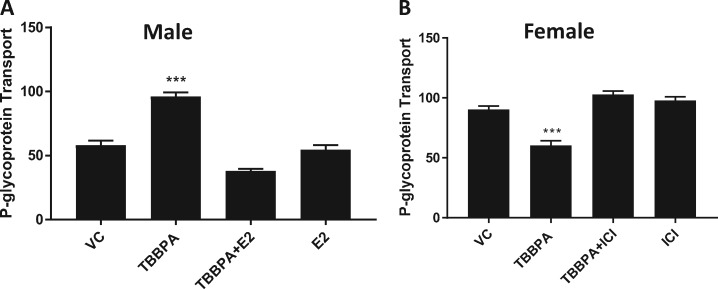
Estrogen, signaling through its ER-α receptor, can activate or repress PPAR-γ (Hoffmann et al., 2017, 2018; Riu et al., 2011; Wang and Kilgore, 2002). Figure 7A shows that treating male capillaries with 1 nM 17ß-estradiol (E2) blocks the TBBPA dependent increases in P-gp transport. Furthermore, co-treating female capillaries with TBBPA and the ER-α antagonist, ICI (10 nM), blocked the TBBPA induced decreases in P-gp transport (Figure 7B). These data taken together implicate a role for cross talk between signaling pathways involving PPAR-γ and ER-α in P-gp sex-specific responses to TBBPA.
Figure 7.
ER-α signaling suppresses PPAR-γ mediated TBBPA induction of P-gp transport. A, 1 nM E2 blocks TBBPA induction of P-gp transport in male rat brain capillaries. B, Antagonizing ER-α signaling with 10 nM ICI blocks TBBPA mediated decreases in female rat brain capillaries. Graphs are mean fluorescence ± SEM for 10–20 capillaries from single preparation (pooled brains from 3 to 5 rats, 15–20 weeks old). ***p < .001, significantly different than control. Abbreviations: P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
DISCUSSION
The ubiquitous environmental contaminant, TBBPA, is a BFR that disrupts biological signaling pathways important in tumorigenesis, development, and metabolism (Hoffmann et al., 2017; Pollock et al., 2017; Sanders et al., 2016; Strack et al., 2007; Yang et al., 2016). Tetrabromobisphenol A is known to modulate the signals in pathways involving estrogen and PPAR. These pathways are also important in regulating the basal activity of transporters at the BBB (Mahringer and Fricker, 2010; More et al., 2017). In this study we investigated the potential for TBBPA to dysregulate the xenobiotic protective efflux transporters of the BBB. We focused our study on P-gp, BCRP, and MRP2; three important ABC transporters in the BBB. These blood-facing, efflux transporters are localized on the luminal membrane of the brain microvessels (capillaries) and function to regulate the movement of endogenous and xenobiotic molecules to and from the brain. To accomplish this, we exposed male and female rat brain capillaries in vivo or ex vivo to environmentally-relevant concentrations of TBBPA (1–1000 nM) and measured its effects on expression and transport activity of P-gp, BCRP, and MRP2. We observed sex-dependent changes in P-gp transport, decreases in BCRP transport, and no changes in MRP2 transport. We concluded two things from these findings: (1) TBBPA exposure alters gene expression and signaling pathways that affects xenobiotic transporters of the BBB uniquely and in a context specific manner and (2) TBBPA does not compromise the physical integrity of the BBB. Had it done so, we would have observed differences in MRP2 transport between vehicle treated and TBBPA treated capillaries.
TBBPA exposures produced a unique sex-specific response in P-gp transport activity and expression: increasing in males and decreasing in females. Additionally, TBBPA exposure rapidly (1–6 h) decreased BCRP transport in both sexes at relatively low concentrations 1–100 nM. Furthermore, both male and female decreases in BCRP transport activity were associated with reduced luminal protein. Reductions in BCRP expression and activity could have significant biological consequences, considering its endogenous substrates include heme, porphyrins, riboflavin, and estrogenic metabolites. Moreover, the observed changes in both P-gp and BCRP transporter function could significantly compromise the BBB’s ability to protect the brain from xenobiotics, harmful endogenous metabolites, and neurotoxic chemotherapeutic drugs. They also function to reduce exposures to harmful metabolites and toxicants in the intestine, liver, and kidney and contribute to the protective barriers of the placenta (fetus), retina, and testes.
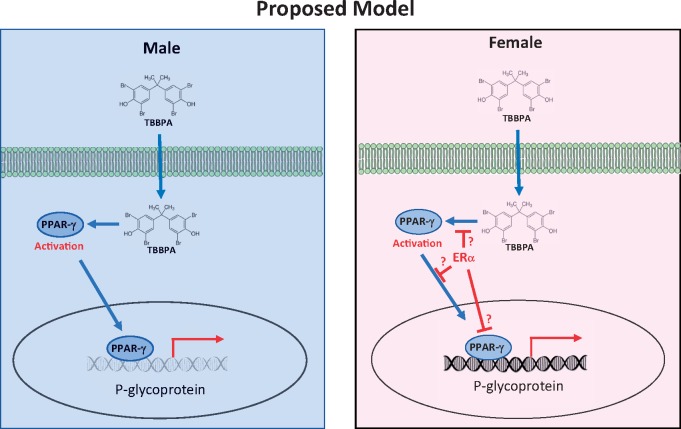
This work identified and characterized a novel sex-specific P-gp response to TBBPA. To understand the sex-dependent response of P-gp to low concentrations of TBBPA, we carried out a series of experiments. Initially we showed that, in males, the TBBPA dependent increases in transport activity were associated with increases in membrane bound P-gp. We conclude these increases were due to de novo protein synthesis because inhibition of either transcription or translation blocked this increased response. Additionally, our Western blot and immunofluorescence data confirmed males had increased P-gp protein levels in TBBPA treated capillaries relative to untreated controls. Interestingly, P-gp protein levels in female capillaries treated with TBBPA were unchanged in Western blots but significantly lower in immunofluorescence assays. The observed discordance in our assays has been previously ascribed to signaling based mechanisms that regulate intracellular vesicular trafficking of P-gp to and from the luminal membrane (Hawkins et al., 2010; Tome et al., 2016). We also found that inhibiting PPAR-γ with an antagonist, GW9668, blocked the TBBPA-mediated increases of P-gp protein in males but had no effect on the decreases in females. To further investigate the P-gp decreased response to TBBPA in females, we relied on previous findings that showed PPAR signaling effects could be either positively or negatively influenced by estrogen (Hoffmann et al., 2018; Houston et al., 2003; Wang and Kilgore, 2002). We reasoned if PPAR-γ is required to maintain basal expression levels of P-gp, increases in PPAR-γ function or activity in males could increase P-gp transport expression and activity. Furthermore, in females, where estrogen signaling through ER-α is more robust, repression of PPAR-γ activation or its downstream signaling effects could result in decreases of P-gp basal transport activity. This model (Figure 8) could explain the sex-specific response we observed. As predicted, in males, the increased response to TBBPA was blocked by antagonizing PPAR-γ. Additionally, the decreases in P-gp transport activity in females were muted by the addition of an ER-α antagonist (10 nM ICI). These results support the notion that PPAR-γ signaling is suppressed by estrogen signaling through ER-α. The precise mechanism of how this occurs is unknown. In Figure 8 (right) we proposed 3 possible alternatives to explain the sex-dependent bi-direction effect of TBBPA on P-gp activity. We propose that increases in ER-α activation or signaling in females could: (1) reduce PPAR-γ activation, (2) reduce PPAR-γ, nuclear translocation, or (3) reduce PPAR-γ promoter binding. Any of these are plausible events and would lead to a reduction in PPAR-γ dependent P-gp expression in a cellular context where ER-α signaling is increased (eg, females). To date, all reports of ER-α/PPAR-γ “crosstalk” tend to favor our third alternative. The most relevant work of Wang and Kilgore (2002) indicates that ER-α signaling in MCF-7 breast cancer cells inhibits basal and stimulated PPAR-γ signals mediated reporter gene activity. They also show that repression of PPAR-γ signaling by ER-α requires the presence of a DNA binding domain. However, in their report antagonism of ER-α and PPAR-γ did not block the repressive effect of ER-α. In contrast to their findings, we show that antagonist of ER-α or PPAR-γ blocks the TBBPA dependent effects on the transporters. Furthermore, our work indicates that E2, added exogenously to male capillaries, represses TBBPA mediated induction of P-gp (Figure 7A). Although additional work will be required to better understand the mechanism, our work remains important because it identifies a novel sex-specific P-gp response to an important environmentally relevant xenobiotic, TBBPA. This work also illustrates, for the first time, how low-level exposures to TBBPA can rapidly modulate the expression and/or activity of important ABC transporters at the BBB.
Figure 8.
Proposed model of sex-dependent effects of TBBPA on P-gp transport. Left-Males, TBBPA activation of PPAR-γ leads to increased expression and transport. Right-Females, ER-α signaling in females represses PPAR-γ causing a decrease in P-gp expression and transport. Abbreviations: P-gp, P-glycoprotein; TBBPA, tetrabromobisphenol A.
FUNDING
Intramural Research Program of National Institutes of Health/National Cancer Institute (Project ZIA BC 011476).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Burk O., Brenner S. S., Hofmann U., Tegude H., Igel S., Schwab M., Eichelbaum M., Alscher M. D. (2010). The impact of thyroid disease on the regulation, expression, and function of ABCB1 (MDR1/P glycoprotein) and consequences for the disposition of digoxin. Clin. Pharmacol. Ther. 88, 685–694. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., Peart J. C., Hawkins B. T., Campos C. R., Miller D. S. (2012). Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc. Natl. Acad. Sci. U.S.A. 109, 15930–15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R., Antignac J.-P., Zalko D., Berrebi A., Cravedi J.-P., Maume D., Marchand P., Monteau F., Riu A., Andre F., et al. (2008). Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 73, 1036–1041. [DOI] [PubMed] [Google Scholar]
- Chan G. N., Cannon R. E. (2017). Assessment of ex vivo transport function in isolated rodent brain capillaries. Curr. Protoc. Pharmacol. 76, 7.16.1–7.16.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. N. Y., Evans R. A., Banks D. B., Mesev E. V., Miller D. S., Cannon R. E. (2017). Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model. Neurosci. Lett. 639, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECB (2006) European Union Risk Assessment Report—2,2′,6,6′-Tetrabromo-4,4′-Isopropylidenediphenol (Tetrabromobisphenol-A or TBBP-A) (CAS: 79-94-7) Part II—Human Health. European Chemicals Bureau, United Kingdom. [Google Scholar]
- Hawkins B. T., Sykes D. B., Miller D. S. (2010). Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J. Neurosci. 30, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Fiedor E., Ptak A. (2017). Bisphenol A and its derivatives tetrabromobisphenol A and tetrachlorobisphenol A induce apelin expression and secretion in ovarian cancer cells through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Toxicol. Lett. 269, 15–22. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Rak A., Ptak A. (2018). Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicol. Lett. 291, 61–69. [DOI] [PubMed] [Google Scholar]
- Honkisz E., Wojtowicz A. K. (2015). The role of PPARgamma in TBBPA-mediated endocrine disrupting effects in human choriocarcinoma JEG-3 cells. Mol. Cell. Biochem. 409, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston K. D., Copland J. A., Broaddus R.R., Gottardis M. M., Fischer S. M., Walker C. L. (2003). Inhibition of proliferation and estrogen receptor signaling by peroxisome proliferator-activated receptor gamma ligands in uterine leiomyoma. Cancer Res. 63, 1221–1227. [PubMed] [Google Scholar]
- Knudsen G. A., Hall S. M., Richards A. C., Birnbaum L. S. (2018). TBBPA disposition and kinetics in pregnant and nursing Wistar Han IGS rats. Chemosphere 192, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen G. A., Jacobs L. M., Kuester R. K., Sipes I. G. (2007). Absorption, distribution, metabolism and excretion of intravenously and orally administered tetrabromobisphenol A [2, 3-dibromopropyl ether] in male Fischer-344 rats. Toxicology 237, 158–167. [DOI] [PubMed] [Google Scholar]
- Knudsen G. A., Sanders J. M., Sadik A. M., Birnbaum L. S. (2014). Disposition and kinetics of tetrabromobisphenol A in female Wistar Han rats. Toxicol. Rep. 1, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahringer A., Fricker G. (2010). BCRP at the blood-brain barrier: Genomic regulation by 17beta-estradiol. Mol. Pharm. 7, 1835–1847. [DOI] [PubMed] [Google Scholar]
- Mesev E. V., Miller D. S., Cannon R. E. (2017). Ceramide 1-phosphate increases P-glycoprotein transport activity at the blood-brain barrier via prostaglandin E2 signaling. Mol. Pharmacol. 91, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. S., Cannon R. E. (2014). Signaling pathways that regulate basal ABC transporter activity at the blood- brain barrier. Curr. Pharm. Des. 20, 1463–1471. [DOI] [PubMed] [Google Scholar]
- More V. R., Campos C. R., Evans R. A., Oliver K. D., Chan G. N. Y., Miller D. S., Cannon R. E. (2017). PPAR-alpha, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression. J. Cereb. Blood Flow Metab. 37, 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T., Mantella L., Reali V., deCatanzaro D. (2017). Influence of tetrabromobisphenol A, with or without concurrent triclosan, upon bisphenol A and estradiol concentrations in mice. Environ. Health Perspect. 125, 087014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A., le Maire A., Grimaldi M., Audebert M., Hillenweck A., Bourguet W., Balaguer P., Zalko D. (2011). Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: The case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci. 122, 372–382. [DOI] [PubMed] [Google Scholar]
- Sanders J. M., Coulter S. J., Knudsen G. A., Dunnick J. K., Kissling G. E., Birnbaum L. S. (2016). Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol. Appl. Pharmacol. 298, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S., Detzel T., Wahl M., Kuch B., Krug H. F. (2007). Cytotoxicity of TBBPA and effects on proliferation, cell cycle and MAPK pathways in mammalian cells. Chemosphere 67, S405–S411. [DOI] [PubMed] [Google Scholar]
- Tome M. E., Herndon J. M., Schaefer C. P., Jacobs L. M., Zhang Y., Jarvis C. K., Davis T. P. (2016). P-glycoprotein traffics from the nucleus to the plasma membrane in rat brain endothelium during inflammatory pain. J. Cereb. Blood Flow Metab. 36, 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Abualnaja K. O., Asimakopoulos A. G., Covaci A., Gevao B., Johnson-Restrepo B., Kumosani T. A., Malarvannan G., Minh T. B., Moon H.-B., et al. (2015). A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 83, 183–191. [DOI] [PubMed] [Google Scholar]
- Wang X., Campos C. R., Peart J. C., Smith L. K., Boni J. L., Cannon R. E., Miller D. S. (2014). Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J. Neurosci. 34, 8585–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hawkins B. T., Miller D. S. (2011). Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 25, 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kilgore M. W. (2002). Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol. Cell. Endocrinol. 194, 123–133. [DOI] [PubMed] [Google Scholar]
- Watt J., Schlezinger J. J. (2015). Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 331, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ni W. W., Yu L., Cai Z., Yu Y. J. (2016). Toxic effects of tetrabromobisphenol A on thyroid hormones in SD rats and the derived-reference dose. Biomed. Environ. Sci. 29, 295–299. [DOI] [PubMed] [Google Scholar]