Abstract
Angiogenesis, the expansion of the vascular bed, is an important component in remodeling of tissues and organs. Such remodeling is essential for coping with substantial and sustained increase in the demands for supply of oxygen and nutrients and the timely removal of waste products. The vasculature, and its effectiveness in systemic delivery to all parts of the body, regulates the distribution of immune cells and the delivery of therapeutics as well as the dissemination of disease. Therefore, the vascular bed is possibly one of the key organs involved in homeostasis, in health and disease. The critical role of the vasculature in health, and the accessibility to noninvasive probing by MRI, renders MRI as a modality of choice for monitoring the vasculature and its adaption to challenges.
Keywords: Angiogenesis, MRI, Permeability, Vascular remodeling, Molecular imaging
1. Vascular remodeling and angiogenesis
The vascular system is required to cope with acute and sustained alterations in the requirements of organs to oxygen and nutrients. Such adaptation and responsiveness lies at the heart of maintaining cellular homeostasis in face of changing environment. Acute changes in demand can be accommodated by local changes in blood flow and perfusion, through vascular constriction or dilation, and changes in the permeability of the vessel wall. Beyond the impact on cellular metabolism, such changes can enhance immune response or block blood flow locally by activation of the coagulation cascade. The underlying unifying theme is that these responses locally alter blood flow due to localized challenge such as exercise [2]. This can be contrasted with systemic effects associated with changes in cardiac output or hormonal stimuli as observed for example for the effects of the hormone relaxin during pregnancy [3].
Prolonged local changes in tissue demand results in local structural change in the capillary bed, through angiogenesis, namely branching and sprouting of new vessels thus locally expanding the blood volume and vascular surface area. Typically, beyond the rise in capillary volume and surface area, local induction of angiogenesis often significantly alters the spatial organization of the capillary bed [4]. Structural changes can include increased density of bifurcations and vessel branching, and often increase the tortuosity of vascular segments. While a mature well ordered vasculature is often fractal in nature, presenting similar structure across multiple scales, this is frequently not the case for sites of angiogenesis [5].
Structural changes in the vascular bed are reflected by corresponding changes in function. In particular, the functional parameters affected by angiogenesis include blood flow and perfusion, vasoreactivity in response to vasoactive challenges, and permeability of the vessel wall [6,7]. Such enhanced vascular leak can breach patency of vessels and break blood-tissue barriers (e.g. the bloodbrain barrier or the fetal-maternal barrier).
Structurefunction changes of the vessels are often accompanied by local changes in blood oxygenation and hemoglobin saturation. Less appreciated perhaps, is the impact of a change in vessel diameter on the local density of red blood cells, blood viscosity and hematocrit [8]. As red blood cells tend to flow in the fast moving center of the vessel while plasma flow is hindered by shear stress and wall interactions at the endothelial interface, changes in vessel diameter result in a change in the effective local hematocrit and viscosity of blood [9]. In the capillary environment, and particularly for sluggish blood flow in permeable dilated angiogenic vessels, blood cannot be regarded to be a classical viscous fluid. The local changes can result in induction of the coagulation cascade, in deposition of intravascular plaques and in activation of blood born and tissue resident immune cells.
Multiple types of cells take part in execution of the angiogenic program [10,11]. The best studied participants are vascular endothelial cells which line the vessels in nearly all organs and provide the smooth continuous inner lumen through which blood can flow. During angiogenesis, endothelial cells from the vessel wall must divide, migrate and adhere to form new vessels, without disrupting the flow of blood during this process. This is a remarkable feat for cells that typically remain dormant and survive with no cell division or cell death for many years. In special cases cells that are not endothelial in origin, adopt an endothelial phenotype and line blood vessels [12]. A physiological example for vascular mimicry is that of fetal trophoblast cells, which invade the maternal uterine spiral arteries and replace maternal endothelial cells, so as to control the flow of maternal blood into the maternal circulation of the placenta [13].
Plasticity of the vascular bed is enabled by the dissociation of perivascular contractile pericytes and vascular smooth muscle cells. These are the cells that constrict or dilate vessels in response to vasoactive challenges [14]. Fibroblasts and Immune cells of the stroma support angiogenesis by altering the production of growth factors, enzymes and structure of the extracellular matrix, so as to shift its property from quiescent, mature, anti angiogenic into a reactive proangiogenic provisional matrix. A significant role in activation of the extracellular matrix can be attributed to the elevated vascular permeability resulting in extravasation of plasma proteins and activation of coagulation to deposit a fibrin clot [15]. Multiple MRI tools were developed for detection of angiogenesis induced vascular permeability, vascular expansion and vascular maturation [16–18].
2. Vascular endothelial growth factor (VEGF)
It is remarkable that a single growth factor is required and sufficient for orchestrating vascular remodeling in multiple developmental, physiological and pathological processes, including the entire cascade from induction of vascular permeability, to endothelial cell proliferation, migration and tube formation. These activities are delivered by the master regulator of angiogenesis, vascular endothelial growth factor A (VEGFA; originally isolated as vascular permeability factor VPF) [19–21]. With delivery of oxygen being the most critical role of the vasculature, it makes sense the angiogenesis will be regulated by oxygen. Indeed, the role of oxygen as a mediator of vascular stability, whereby local hypoxia serves as a local signal for vascular expansion, was raised as hypothesis before the molecular mechanism of angiogenesis was identified [22].
With the discovery of VEGF as the primary mediator of angiogenesis, it’s regulation by hypoxia was discovered in both pathogenic as well as developmental and physiological angiogenesis [23,24]. The molecular machinery includes intracellular mechanism for oxygen sensing and its transmission for modulating the expression of VEGF. Hypoxia inducible factor 1 (HIF1) was implicated in mediating the cellular response to oxygen deficiency. This link between hypoxia, HIF, VEGF and angiogenesis was demonstrated in numerous systems and revealed by MRI analysis of vascular remodeling [25–28].
3. Receptors and cell surface markers of angiogenesis
VEGF activates angiogenesis by binding tyrosine kinase VEGF receptors found on the surface of endothelial cells [29]. The primary receptor active in induction of angiogenesis is VEGFR2. Among the other family members, VEGFR1 has higher affinity to VEGF and can thus act as an anti angiogenic VEGF trap, particularly when expressed as a soluble secreted protein (soluble Flt). Another family of vascular cell surface receptors is the Tie receptors, which respond to angiopoietins, and affect the interaction of endothelial cells with perivascular pericytes [30]. Ang1 promotes vascular survival and proliferation while Ang 2 acts as an antagonist, inducing vascular pruning [31]. Transgenic mice showed consistency of the roles of VEGFR2/Flk1 and Tie2/Tek during early angiogenesis and during subsequent vascular marturation respectively [31–33].
Cell surface receptors were evaluated as targets for therapy and for molecular imaging. Suppression of VEGF signaling either by ligation of the ligand or by inhibition of the receptor is the primary approach for anti angiogenic therapy. Such treatments showed significant efficacy in reducing blindness due to macular degeneration [34], however their efficacy in blocking cancer progression was considerably lower than originally hoped for [35]. As targets for molecular imaging by MRI, the primary cell surface marker that was studied is αVβ3, an endothelial integrin, which is induced during angiogenesis but is also ubiquitously expressed by other cells [36]. Utilizing nanoparticle based contrast media, including iron oxide particles or Gd decorated liposomes, regions of angiogenesis could be highlighted by the enhanced retention of vasculature targeted imaging contrast media [36–41].
4. ECM building blocks and enzymes
The extracellular matrix (ECM) maintains the quiescent vasculature by providing potent anti angiogenic cues. Induction of angiogenesis is accompanied and regulated by extensive changes of the ECM to form a reactive stroma, which is part of the granulation tissue of the wound repair machinery. Tumors hijack this wound healing machinery, as elegantly described in the hypothesis raised by Herald Dvorak that tumors can be viewed as wounds that do not heal [42]. The molecular constituents of the reactive angiogenic stroma include the replacement of collagen by fibrin; breakdown of collagen by MMPs and breakdown of Heparan and hyaluronan by heparanase and hyaluronidase. These enzymatic reactions release adherent growth factors from the ECM thereby further enhancing the angiogenic response [43]. Smart sensors for detection of ECM remodeling in angiogenesis provide an attractive tool for detection of the role of the activated matrix [17].
5. Mammalian reproduction
The ovary undergoes cycles of follicular development, expansion, ovulation and formation of a corpus luteum. This ovarian cycle includes extensive remodeling of the vascular bed. The cumulus cells surrounding the oocyte in the core of the developing follicle are exposed to hypoxia with follicular expansion, consistent with induction of VEGF expression [16]. The corpus lutetium development includes further substantial angiogenesis. Vascular leak in the ovary is high, and can be detected by DCE MRI particularly with the use of macromolecular contrast media [16,44–47].
One of the key processes for survival of mammalian species is embryo implantation and pregnancy. Survival of the nonsyngeneic embryo in the uterus of immune competent mother is one of the major mysteries of immune tolerance. Clearly the maternal vascular adaptation to pregnancy must include the requirement for effective mechanism for delivery of nutrients and oxygen to the growing fetus, while maintaining strict isolation between the maternal circulation and that of the fetus.
Although not observed yet for human, enhanced vascular leak in implantation sites is readily observable by MRI in pregnant mice as early as day 4.5, namely a few hours after adhesion [48]. Furthermore, MRI can be used for detection of the impact of specific challenge including genetic alteration or ablation of immune cells on implantation [49,50]. Further in pregnancy, the placenta effectively separates between the fetal and maternal circulation. Contrast enhanced MRI shows the barrier functions towards macromolecular contrast media, which low MW contrast media can be transported across to the fetal circulation [51–54]. Remarkably, the maternal blood flows through the placenta in spaces lined by embryo derived trophoblast cells, which mimic and replace the maternal endothelial cells.
6. Cancer
The tumor angiogenic switch hypothesis was raised by Folkman et al. 45 years ago [55,56]. Briefly this hypothesis set the foundation for the field of cancer angiogenesis, by suggesting that cancer cells can progress in the absence of new blood vessels only to form microscopic, millimeter size dormant nodules. Induction of angiogenesis requires a new additional genetic change so as to induce the production of postulated TAF, tumor angiogenic factor, which will induce sprouting of capillaries, thus enabling the tumors to progress [56]. The major support for this theory is the prevalence of avascular microscopic tumor nodules which can be found in people who died from non-cancer causes [57], suggesting that malignant transformation is necessary but is not sufficient for clinical progression of cancer and that angiogenesis may be the dominant prognostic factor.
The prevalence of dormant tumors, which lie within the detection level provided by liquid biopsies (namely omics measurements of blood samples) or detectable by imaging, raise the problem of over diagnosis which can accompany early detection, and highlights the need for improved prognostic differential markers that can be used for surveillance as alternative to intervention. The induction of angiogenesis was suggested to provide such a marker which can be easily tracked by MRI.
When the tumors progress, angiogenesis is required to sustain the growing needs of the tumor. However, the elevated permeability and the irregular vascular patterning, with high tortuosity and low vasoreactivity result in sluggish blood flow and elevated interstitial fluid pressure [58–60]. Thus, tumors often exhibit both hypoxia and high angiogenesis and vessel permeability, both of which are associated with poor patient outcome [55,61]. Inhibition of angiogenesis results in vascular trimming and suppression of vascular leak, resulting in “normalization” of the vessels and enhanced response to radiation and chemotherapy [62]. This normalization of the vasculature could be detected in patients by MRI [63–65]
7. MRI of angiogenesis
7.1. Endogenous contrast mechanisms
MRI is highly sensitive to motion, a sensitivity that can be harnessed for monitoring the function of the blood vessels without administration of contrast media. Methods such as FLAIR and MR angiography rely on the impact of flow on the apparent R1 relaxation rate.
Those are effective for detection of motions across time scales of up to a few seconds and translation of blood water across centimeters. Complementary methods such as Pulsed Gradient spin echo measurements of diffusion, ADC and IVIM, rely on loss of coherence of magnetization on the XY plane due to incoherent translation associated with diffusion, convection or flow in randomly oriented capillaries, at a scale of microns to millimeters and time scale of seconds [66]. Both approaches detect the water signal and thus reflect plasma motion and provide information complementary to Doppler Ultrasound imaging.
The vascular bed generates extended and continuous structures within all tissues and thus affects its mechanical properties including stiffness and elasticity. The microcirculation was demonstrated to affect the propagation of shear waves and thus can be detected by MR Elastography [67].
Fibrosis and scar formation is often part of the resolution phase of angiogenesis. Fibrosis can be detected as the desmoplastic reaction in cancer, as well as the progressive damage in hypovascular regions of the heart. Collagen deposition in fibrotic regions can be detected by chemical exchange saturation transfer and magnetization transfer [68,69].
The susceptibility changes associated with release of oxygen from hemoglobin provides the widely popular BOLD contrast, which can be used as surrogate for detection of cognitive function via local changes in blood oxygenation [70,71]. The same contrast mechanism can be used for detection of changes in vascular function and maturation (ie increased vasoreactivity) during angiogenesis [72,73] or for detection of maternal to fetal oxygen transport across the placenta [74].
The effects of deoxyhemoglobin on susceptibility can be detected by SWI with particular sensitivity to veins and capillaries [75–77]. While deoxyhemoglobin generates T2* contrast, reporting on intravascular hypoxia, elevated tissue oxygenation can be detected through the effects of dissolved oxygen on T1 contrast (TOLD contrast; [78]). Using a combination of both contrast mechanisms upon exposure to hypoxia to hyperoxia it is possible to derive apparent hemoglobin dissociation values, showing the difference between fetohemoglobin and adult hemoglobin, and map oxygen transport across the placenta [74].
7.2. Contrast enhanced imaging of angiogenesis
Dynamic contrast enhanced MRI using low MW Gd chelates, is widely used for detection of tumors. Contrast enhancement is due to enhanced vascular leak and accumulation of the contrast material in the interstitial space. Pharmacokinetic analysis of contrast enhancement can aid in tumor detection, prognosis, differentiation of benign and malignant lesions, and monitoring response to therapy [79–81].
Permeability of blood vessels to plasma proteins is one of the hallmarks of angiogenesis. Classically, this was measured by extravasion of the albumin binding dye Evans Blue. An MRI biomarker revealing such permeability is provided in the preclinical analysis of the dynamics of accumulation of albumin covalently decorated with multiple GdDTPA groups. Albumin-GdDTPA is a blood pool agent with a long lifetime in circulation and very slow clearance. In angiogenic regions it extravasates to the interstitial extracellular space to create prolonged signal enhancement thus providing a sensitive measure of angiogenesis [82–85]. Its application for clinical imaging of cancer angiogenesis was extensively reviewed [91].
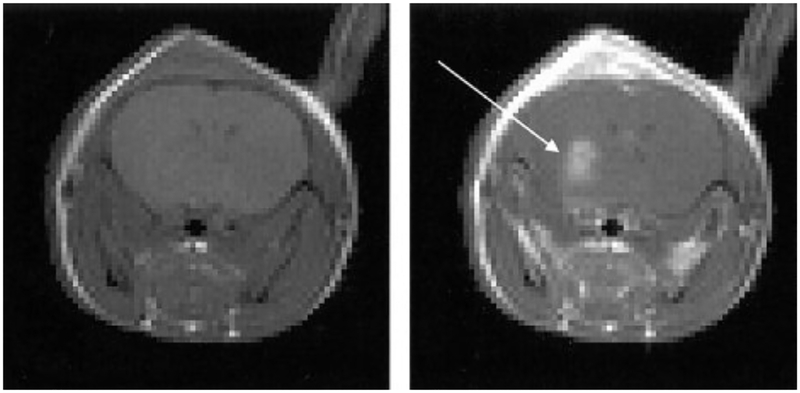
Marrying the ease of delivery of low MW contrast media, with the pharmaco-kinetic advantage of high MW blood pool agents, can be achieved with the use of albumin binding probes such as (MP-2269 and MS-325; Fig. 1) [1]. Using these probes extends the clearance time and enhances the selectivity for detection of a breach in the patency of the vascular wall.
Fig. 1.
Vascular permeability is one of the hallmarks of angiogenesis. Enhanced permeability of the vessels, detected using albumin binding contrast media, enabled early detection of mouse glioma tumors (Reproduced from Magn. Reson. Med. [1]).
Similar to protein based contrast media, nanoparticles can also be used for detection of the enhanced permeability of angiogenic blood vessels. Such nanoparticles can include dendrimers [92,93], iron-oxide magnetite based nanoparticles [94] and liposomes decorated or loaded with MR detectable payload [95]. By linking targeting moieties to the contrast media, it is possible to highlight endothelial cell surface markers that are enriched during angiogenesis. This approach was applied predominantly for targeting the αVβ3 integrin [36–40]. Ferumoxytol has been applied for vessel size mapping of angiogenesis in brain tumors [96].
8. Cellular tracking of angiogenesis
Angiogenesis occurs by local proliferation of resident endothelial cells, as well as by recruitment of bone marrow derived endothelial progenitor cells. Thus angiogenesis can be enhanced, for example in hypoxic poorly perfused tissues, by delivery of endothelial progenitor stem cells. Tracking exogenously delivered endothelial progenitor cells can be achieved by MRI by labeling of the cells with detectable contrast media, most often iron oxide nanoparticles, prior to their delivery [97–105]. Such labeling is diluted with cell death or with cell proliferation, and thus there is an advantage to develop reporter genes that can be genetically encoded to be expressed by the cells. Indeed, endothelial expression of ferritin as a reporter gene for MRI was demonstrated in transgenic mice [106]. Another approach included expression of biotinylated tagged protein on the surface of endothelial cells driven by the Tie2 promoter, and detection using avidin labeled contrast probes, showing vascular development in embryos and in tumors [107] [108].
9. Imaging angiogenesis by other modalities
Angiogenesis is an attractive target for imaging by most modalities. Clinical imaging of angiogenesis was demonstrated for contrast enhanced CT, nuclear imaging and ultrasound. Contrast enhanced CT reveals the architecture of blood vessels and their reorganization on tumors [109]. CT is also valuable for analysis of three dimensional vascular casts [110,111]. Such data sets are valuable for the development of algorithms for morphometric analysis of vascular beds. Nuclear imaging (PET and SPECT) provides molecular measures of angiogenesis relying on molecular biomarkers, in particular targeting VEGF receptors and endothelial cell surface integrins [112–115].
Ultrasound has long been a modality of choice for point of care analysis of the vasculature, particularly for cardiovascular and prenatal screening. The recent advancements in Super resolution US offer significant potential for detection of vascular remodeling and its impact on vascular structure and function [116]. An interesting companion technology in opto-acoustic imaging in which light is applied for generation of ultrasound signal. Multispectral opto acoustic imaging (MSOT) is endogenously sensitive to blood oxygenation and can be applied for mapping deoxyand oxyhemoglobin content in blood vessels as well as the distribution of contrast media [117,118].
In addition to the tools listed above, significant progress in preclinical analysis of angiogenesis was made possible by Intravital microscopy, showing cell migration, endothelial sprouting, association with stroma and immune cells and remodeling of the extracellular matrix [58,119–124]. Another interesting modality for imaging superficial angiogenesis is optical coherence tomography, which offers high resolution view of tissue anatomy and the vasculature using endogenous contrast for depth of up to 3 mm [125].
10. Outlook
Angiogenesis biomarkers are proven to be highly valuable in early detection of pathological processes, and can thus be used for aiding biopsy and surgical interventions. With better understanding of the underlying biology, angiogenesis based imaging readouts could aid in diagnosis and monitoring of disease progression as well as response to therapy
Funding
• This work was supported by the National Institutes of Health [1R01HD086323 and R01CA75334].
• This work was supported by Seventh Framework European Research Council Advanced Grant 232640-IMAGO.
• This work was supported by the Israel Science Foundation 326/14
• This work was supported by US-Israel Binational Science Foundation.
Footnotes
☆ “Advances and Applications of in vivo Magnetic Resonance”–Celebrating Two Decades of Joe Ackerman Editing JMR.
References
- [1].Adzamli K, Yablonskiy DA, Chicoine MR, Won EK, Galen KP, Zahner MC, Woolsey TA, Ackerman JJ, Albumin-binding MR blood pool agents as MRI contrast agents in an intracranial mouse glioma model, Magn. Reson. Med 49 (2003) 586–590. [DOI] [PubMed] [Google Scholar]
- [2].Hoier B, Hellsten Y, Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF, Microcirculation 21 (2014) 301–314. [DOI] [PubMed] [Google Scholar]
- [3].Devarakonda T, Salloum FN, Heart disease and relaxin: new actions for an old hormone, Trends Endocrinol. Metab (2018), 10.1016/j.tem.2018.02.008 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ribatti D, Nico B, Crivellato E, The development of the vascular system: a historical overview, Methods Mol. Biol 1214 (2015) 1–14. [DOI] [PubMed] [Google Scholar]
- [5].Grizzi F, Colombo P, Taverna G, Chiriva-Internati M, Cobos E, Graziotti P, Muzzio PC, Dioguardi N, Geometry of human vascular system: is it an obstacle for quantifying antiangiogenic therapies?, Appl Immunohistochem. Mol. Morphol 15 (2007) 134–139. [DOI] [PubMed] [Google Scholar]
- [6].Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF, Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine, EXS 79 (1997) 233–269. [DOI] [PubMed] [Google Scholar]
- [7].Laughlin MH, Bowles DK, Duncker DJ, The coronary circulation in exercise training, Am. J. Physiol. Heart Circ. Physiol 302 (2012) H10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fåhræus R, Lindqvist T, The viscosity of the blood in narrow capillary tubes, Am. J. Physiol 96 (1961) 562–568. [Google Scholar]
- [9].Secomb TW, Pries AR, Blood viscosity in microvessels: experiment and theory, CR Phys 14 (2013) 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Korn C, Augustin HG, Mechanisms of vessel pruning and regression, Dev. Cell 34 (2015) 5–17. [DOI] [PubMed] [Google Scholar]
- [11].Augustin HG, Kozian DH, Johnson RC, Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes, BioEssays 16 (1994) 901–906. [DOI] [PubMed] [Google Scholar]
- [12].Folberg R, Hendrix MJ, Maniotis AJ, Vasculogenic mimicry and tumor angiogenesis, Am. J. Pathol 156 (2000) 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fisher SJ, Why is placentation abnormal in preeclampsia?, Am J. Obstet. Gynecol 213 (2015) S115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Holm A, Heumann T, Augustin HG, Microvascular mural cell organotypic heterogeneity and functional plasticity, Trends Cell Biol (2018). [DOI] [PubMed] [Google Scholar]
- [15].Clark RA, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB, Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization, J. Invest. Dermatol 79 (1982) 264–269. [DOI] [PubMed] [Google Scholar]
- [16].Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C, Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle, Int. J. Exp. Pathol 78 (1997) 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Neeman M, Gilad AA, Dafni H, Cohen B, Molecular imaging of angiogenesis, J. Magn. Reson. Imag 25 (2007) 1–12. [DOI] [PubMed] [Google Scholar]
- [18].Narunsky L, Oren R, Bochner F, Neeman M, Imaging aspects of the tumor stroma with therapeutic implications, Pharmacol. Ther 141 (2014) 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR, Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors, Mol. Biol. Cell 3 (1992) 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrara N, The role of vascular endothelial growth factor in pathological angiogenesis, Breast Cancer Res. Treat 36 (1995) 127–137. [DOI] [PubMed] [Google Scholar]
- [21].Ferrara N, Bunting S, Vascular endothelial growth factor, a specific regulator of angiogenesis, Curr. Opin. Nephrol. Hypertens 5 (1996) 35–44. [DOI] [PubMed] [Google Scholar]
- [22].Adair TH, Gay WJ, Montani JP, Growth regulation of the vascular system: evidence for a metabolic hypothesis, Am. J. Physiol 259 (1990) R393–404. [DOI] [PubMed] [Google Scholar]
- [23].Shweiki D, Neeman M, Itin A, Keshet E, Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis, Proc. Natl. Acad. Sci. USA 92 (1995) 768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shweiki D, Itin A, Soffer D, Keshet E, Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis, Nature 359 (1992) 843–845. [DOI] [PubMed] [Google Scholar]
- [25].Ding JB, Chen JR, Xu HZ, Qin ZY, Effect of hyperbaric oxygen on the growth of intracranial glioma in rats, Chin. Med. J. (Engl.) 128 (2015) 3197–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He T, Smith N, Saunders D, Pittman BP, Lerner M, Lightfoot S, SilasiMansat R, Lupu F, Towner RA, Molecular MRI differentiation of VEGF receptor 2 levels in C6 and RG2 glioma models, Am. J. Nucl. Med. Mol. Imag 3 (2013) 300–311. [PMC free article] [PubMed] [Google Scholar]
- [27].Griffiths JR, McSheehy PM, Robinson SP, Troy H, Chung YL, Leek RD, Williams KJ, Stratford IJ, Harris AL, Stubbs M, Metabolic changes detected by in vivo magnetic resonance studies of HEPA-1 wild-type tumors and tumors deficient in hypoxia-inducible factor-1beta (HIF-1beta): evidence of an anabolic role for the HIF-1 pathway, Cancer Res 62 (2002) 688–695. [PubMed] [Google Scholar]
- [28].Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E, Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis, Nature 394 (1998) 485–490. [DOI] [PubMed] [Google Scholar]
- [29].Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z, Vascular endothelial growth factor (VEGF) and its receptors, FASEB J 13 (1999) 9–22. [PubMed] [Google Scholar]
- [30].Eklund L, Kangas J, Saharinen P, Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems, Clin. Sci. (Lond.) 131 (2017) 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thomas M, Augustin HG, The role of the Angiopoietins in vascular morphogenesis, Angiogenesis 12 (2009) 125–137. [DOI] [PubMed] [Google Scholar]
- [32].Augustin HG, Koh GY, Thurston G, Alitalo K, Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system, Nat. Rev. Mol. Cell Biol 10 (2009) 165–177. [DOI] [PubMed] [Google Scholar]
- [33].Fiedler U, Augustin HG, Angiopoietins: a link between angiogenesis and inflammation, Trends Immunol 27 (2006) 552–558. [DOI] [PubMed] [Google Scholar]
- [34].Shao J, Choudhary MM, Schachat AP, Neovascular age-related macular degeneration, Dev. Ophthalmol 55 (2016) 125–136. [DOI] [PubMed] [Google Scholar]
- [35].Tamura R, Tanaka T, Miyake K, Yoshida K, Sasaki H, Bevacizumab for malignant gliomas: current indications, mechanisms of action and resistance, and markers of response, Brain Tumor Pathol 34 (2017) 62–77. [DOI] [PubMed] [Google Scholar]
- [36].Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC, Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging, Nat. Med 4 (1998) 623–626. [DOI] [PubMed] [Google Scholar]
- [37].Kluza E, Jacobs I, Hectors SJ, Mayo KH, Griffioen AW, Strijkers GJ, Nicolay K, Dual-targeting of alphavbeta3 and galectin-1 improves the specificity of paramagnetic/fluorescent liposomes to tumor endothelium in vivo, J. Control. Release 158 (2012) 207–214. [DOI] [PubMed] [Google Scholar]
- [38].Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, Fayad ZA, Lowik CW, Kaijzel EL, Que I, Storm G, Strijkers GJ, Griffioen AW, Nicolay K, Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots, Angiogenesis 12 (2009) 17–24. [DOI] [PubMed] [Google Scholar]
- [39].van Tilborg GA, Mulder WJ, van der Schaft DW, Reutelingsperger CP, Griffioen AW, Strijkers GJ, Nicolay K, Improved magnetic resonance molecular imaging of tumor angiogenesis by avidin-induced clearance of nonbound bimodal liposomes, Neoplasia 10 (2008) 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van der Schaft DW, Storm G, Koning GA, Griffioen AW, Nicolay K, MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle, FASEB J 19 (2005) 2008–2010. [DOI] [PubMed] [Google Scholar]
- [41].Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F, Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner, Cancer Res 67 (2007) 1555–1562. [DOI] [PubMed] [Google Scholar]
- [42].Dvorak HF, Tumors: wounds that do not heal-redux, Cancer Immunol. Res 3 (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, Metzger S, Sarid R, Naggi A, Casu B, Ilan N, Vlodavsky I, Abramovitch R, Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models, FASEB J 19 (2005) 211–221. [DOI] [PubMed] [Google Scholar]
- [44].Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M, Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrast-enhanced MRI, Magn. Reson. Med 52 (2004) 741–750. [DOI] [PubMed] [Google Scholar]
- [45].Cohen Y, Dafni H, Avni R, Raz T, Biton I, Hemmings B, Neeman M, In search of signaling pathways critical for ovarian graft reception: Akt1 is essential for long-term survival of ovarian grafts, Fertil. Steril 101 (2014) 536–544. [DOI] [PubMed] [Google Scholar]
- [46].Tempel C, Neeman M, Spatial and temporal modulation of perfusion in the rat ovary measured by arterial spin labeling MRI, J. Magn. Reson. Imag 9 (1999) 794–803. [DOI] [PubMed] [Google Scholar]
- [47].Tempel C, Neeman M, Perfusion of the rat ovary: application of pulsed arterial spin labeling MRI, Magn. Reson. Med 41 (1999) 113–123. [DOI] [PubMed] [Google Scholar]
- [48].Plaks V, Kalchenko V, Dekel N, Neeman M, MRI analysis of angiogenesis during mouse embryo implantation, Magn. Reson. Med 55 (2006) 1013–1022. [DOI] [PubMed] [Google Scholar]
- [49].Plaks V, Gershon E, Zeisel A, Jacob-Hirsch J, Neeman M, Winterhager E, Rechavi G, Domany E, Dekel N, Blastocyst implantation failure relates to impaired translational machinery gene expression, Reproduction 148 (2014) 87–98. [DOI] [PubMed] [Google Scholar]
- [50].Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S, Uterine DCs are crucial for decidua formation during embryo implantation in mice, J. Clin. Invest 118 (2008) 3954–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Siauve N, Chalouhi GE, Deloison B, Alison M, Clement O, Ville Y, Salomon LJ, Functional imaging of the human placenta with magnetic resonance, Am. J. Obstet. Gynecol 213 (2015) S103–114. [DOI] [PubMed] [Google Scholar]
- [52].Deloison B, Millischer AE, Salomon LJ, Placental MRI: physiology and pathology, Gynecol. Obstet. Fertil 41 (2013) 394–403. [DOI] [PubMed] [Google Scholar]
- [53].Plaks V, Sapoznik S, Berkovitz E, Haffner-Krausz R, Dekel N, Harmelin A, Neeman M, Functional phenotyping of the maternal albumin turnover in the mouse placenta by dynamic contrast-enhanced MRI, Mol. Imag. Biol 13 (2011) 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tomlinson TM, Garbow JR, Anderson JR, Engelbach JA, Nelson DM, Sadovsky Y, Magnetic resonance imaging of hypoxic injury to the murine placenta, Am. J. Physiol. Regul. Integr. Comp. Physiol 298 (2010) R312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G, Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma, J. Natl. Cancer Inst 84 (1992) 1875–1887. [DOI] [PubMed] [Google Scholar]
- [56].Folkman J, Merler E, Abernathy C, Williams G, Isolation of a tumor factor responsible for angiogenesis, J. Exp. Med 133 (1971) 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Black WC, Welch HG, Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy, New Engl. J. Med 328 (1993) 1237–1243. [DOI] [PubMed] [Google Scholar]
- [58].Fukumura D, Duda DG, Munn LL, Jain RK, Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models, Microcirculation 17 (2010) 206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fukumura D, Jain RK, Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization, Microvasc. Res 74 (2007) 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fukumura D, Jain RK, Imaging angiogenesis and the microenvironment, APMIS 116 (2008) 695–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vaupel P, Mayer A, The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities, Antioxid. Redox Signal 22 (2015) 878–880. [DOI] [PubMed] [Google Scholar]
- [62].Carmeliet P, Jain RK, Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases, Nat. Rev. Drug Discov 10 (2011) 417–427. [DOI] [PubMed] [Google Scholar]
- [63].Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, Plotkin SR, Chi AS, Eichler AF, Dietrich J, Hochberg FH, Lu-Emerson C, Iafrate AJ, Ivy SP, Rosen BR, Loeffler JS, Wen PY, Sorensen AG, Jain RK, Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation, Proc. Natl. Acad. Sci. USA 110 (2013) 19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, Jain RK, Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion, Cancer Res 72 (2012) 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK, A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients, Cancer Res 69 (2009) 5296–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].LeBihan D, IVIM method measures diffusion and perfusion, Diagn. Imag. (San Franc.) 12 (1990) 133–136. [PubMed] [Google Scholar]
- [67].Juge L, Petiet A, Lambert SA, Nicole P, Chatelin S, Vilgrain V, Van Beers BE, Bilston LE, Sinkus R, Microvasculature alters the dispersion properties of shear waves–a multi-frequency MR elastography study, NMR Biomed 28 (2015) 1763–1771. [DOI] [PubMed] [Google Scholar]
- [68].Vandsburger M, Vandoorne K, Oren R, Leftin A, Mpofu S, Delli Castelli D, Aime S, Neeman M, Cardio-chemical exchange saturation transfer magnetic resonance imaging reveals molecular signatures of endogenous fibrosis and exogenous contrast media, Circ. Cardiovasc. Imag 8 (2015). [DOI] [PubMed] [Google Scholar]
- [69].Pumphrey A, Yang Z, Ye S, Powell DK, Thalman S, Watt DS, Abdel-Latif A, Unrine J, Thompson K, Fornwalt B, Ferrauto G, Vandsburger M, Advanced cardiac chemical exchange saturation transfer (cardioCEST) MRI for in vivo cell tracking and metabolic imaging, NMR Biomed 29 (2016) 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K, Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model, Biophys. J 64 (1993) 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ogawa S, Lee TM, Kay AR, Tank DW, Brain magnetic resonance imaging with contrast dependent on blood oxygenation, Proc. Natl. Acad. Sci. USA 87 (1990) 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Abramovitch R, Dafni H, Smouha E, Benjamin LE, Neeman M, In vivo prediction of vascular susceptibility to vascular susceptibility endothelial growth factor withdrawal: magnetic resonance imaging of C6 rat glioma in nude mice, Cancer Res 59 (1999) 5012–5016. [PubMed] [Google Scholar]
- [73].Gilead A, Neeman M, Dynamic remodeling of the vascular bed precedes tumor growth: MLS ovarian carcinoma spheroids implanted in nude mice, Neoplasia 1 (1999) 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Avni R, Golani O, Akselrod-Ballin A, Cohen Y, Biton I, Garbow JR, Neeman M, MR Imaging-derived oxygen-hemoglobin dissociation curves and fetal-placental oxygen-hemoglobin affinities, Radiology 280 (2016) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yadav BK, Krishnamurthy U, Buch S, Jella P, Hernandez-Andrade E, Yeo L, Korzeniewski SJ, Trifan A, Hassan SS, Haacke EM, Romero R, Neelavalli J, Imaging putative foetal cerebral blood oxygenation using susceptibility weighted imaging (SWI), Eur. Radiol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hu J, Yu Y, Juhasz C, Kou Z, Xuan Y, Latif Z, Kudo K, Chugani HT, Haacke EM, MR susceptibility weighted imaging (SWI) complements conventional contrast enhanced T1 weighted MRI in characterizing brain abnormalities of Sturge-Weber Syndrome, J. Magn. Reson. Imag 28 (2008) 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Haacke EM, Xu Y, Cheng YC, Reichenbach JR, Susceptibility weighted imaging (SWI), Magn. Reson. Med 52 (2004) 612–618. [DOI] [PubMed] [Google Scholar]
- [78].Huang CH, Shih YY, Siow TY, Hsu YH, Chen CC, Lin TN, Jaw FS, Chang C, Temporal assessment of vascular reactivity and functionality using MRI during postischemic proangiogenenic vascular remodeling, Magn. Reson. Imag 33 (2015) 903–910. [DOI] [PubMed] [Google Scholar]
- [79].Furman-Haran E, Feinberg MS, Badikhi D, Eyal E, Zehavi T, Degani H, Standardization of radiological evaluation of dynamic contrast enhanced MRI: application in breast cancer diagnosis, Technol. Cancer Res. Treat 13 (2014) 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Eyal E, Badikhi D, Furman-Haran E, Kelcz F, Kirshenbaum KJ, Degani H, Principal component analysis of breast DCE-MRI adjusted with a model based method, J. Magn. Reson. Imag 30 (2009) 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Eyal E, Degani H, Model-based and model-free parametric analysis of breast dynamic-contrast-enhanced MRI, NMR Biomed 22 (2009) 40–53. [DOI] [PubMed] [Google Scholar]
- [82].Raatschen HJ, Simon GH, Fu Y, Sennino B, Shames DM, Wendland MF, McDonald DM, Brasch RC, Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats, Radiology 247 (2008) 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Daldrup-Link HE, Brasch RC, Macromolecular contrast agents for MR mammography: current status, Eur. Radiol 13 (2003) 354–365. [DOI] [PubMed] [Google Scholar]
- [84].Brasch RC, Li KC, Husband JE, Keogan MT, Neeman M, Padhani AR, Shames D, Turetschek K, In vivo monitoring of tumor angiogenesis with MR imaging, Acad. Radiol 7 (2000) 812–823. [DOI] [PubMed] [Google Scholar]
- [85].Pham CD, Roberts TP, van Bruggen N, Melnyk O, Mann J, Ferrara N, Cohen RL, Brasch RC, Magnetic resonance imaging detects suppression of tumor vascular permeability after administration of antibody to vascular endothelial growth factor, Cancer Invest 16 (1998) 225–230. [DOI] [PubMed] [Google Scholar]
- [86].Pathak AP, McNutt S, Shah T, Wildes F, Raman V, Bhujwalla ZM, In vivo “MRI phenotyping” reveals changes in extracellular matrix transport and vascularization that mediate VEGF-driven increase in breast cancer metastasis, PLoS One 8 (2013) e63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bhujwalla ZM, Artemov D, Natarajan K, Solaiyappan M, Kollars P, Kristjansen PE, Reduction of vascular and permeable regions in solid tumors detected by macromolecular contrast magnetic resonance imaging after treatment with antiangiogenic agent TNP-470, Clin. Cancer Res 9 (2003) 355–362. [PubMed] [Google Scholar]
- [88].Vandoorne K, Addadi Y, Neeman M, Visualizing vascular permeability and lymphatic drainage using labeled serum albumin, Angiogenesis 13 (2010) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dafni H, Cohen B, Ziv K, Israely T, Goldshmidt O, Nevo N, Harmelin A, Vlodavsky I, Neeman M, The role of heparanase in lymph node metastatic dissemination: dynamic contrast-enhanced MRI of Eb lymphoma in mice, Neoplasia 7 (2005) 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dafni H, Landsman L, Schechter B, Kohen F, Neeman M, MRI and fluorescence microscopy of the acute vascular response to VEGF165: vasodilation, hyper-permeability and lymphatic uptake, followed by rapid inactivation of the growth factor, NMR Biomed 15 (2002) 120–131. [DOI] [PubMed] [Google Scholar]
- [91].Leach MO, Application of magnetic resonance imaging to angiogenesis in breast cancer, Breast Cancer Res 3 (2001) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kobayashi H, Reijnders K, English S, Yordanov AT, Milenic DE, Sowers AL, Citrin D, Krishna MC, Waldmann TA, Mitchell JB, Brechbiel MW, Application of a macromolecular contrast agent for detection of alterations of tumor vessel permeability induced by radiation, Clin. Cancer Res 10 (2004) 7712–7720. [DOI] [PubMed] [Google Scholar]
- [93].Kobayashi H, Brechbiel MW, Dendrimer-based macromolecular MRI contrast agents: characteristics and application, Mol. Imag 2 (2003) 1–10. [DOI] [PubMed] [Google Scholar]
- [94].Enochs WS, Harsh G, Hochberg F, Weissleder R, Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent, J. Magn. Reson. Imag 9 (1999) 228–232. [DOI] [PubMed] [Google Scholar]
- [95].Kuijten MM, Hannah Degeling M, Chen JW, Wojtkiewicz G, Waterman P, Weissleder R, Azzi J, Nicolay K, Tannous BA, Multimodal targeted high relaxivity thermosensitive liposome for in vivo imaging, Sci. Rep 5 (2015) 17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fredrickson J, Serkova NJ, Wyatt SK, Carano RA, Pirzkall A, Rhee I, Rosen LS, Bessudo A, Weekes C, de Crespigny A, Clinical translation of ferumoxytol-based vessel size imaging (VSI): feasibility in a phase I oncology clinical trial population, Magn. Reson. Med 77 (2017) 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wei MQ, Wen DD, Wang XY, Huan Y, Yang Y, Xu J, Cheng K, Zheng MW, Experimental study of endothelial progenitor cells labeled with superparamagnetic iron oxide in vitro, Mol. Med. Rep 11 (2015) 3814–3819. [DOI] [PubMed] [Google Scholar]
- [98].Chen C, Yu H, Xia R, Wang L, Ai H, Liu S, Xu Z, Xiao X, Gao F, Magnetic resonance tracking of endothelial progenitor cells labeled with alkyl polyethylenimine 2 kDa/superparamagnetic iron oxide in a mouse lung carcinoma xenograft model, Mol. Imag 13 (2014). [DOI] [PubMed] [Google Scholar]
- [99].Wang Q, Li K, Quan Q, Zhang G, R2* and R2 mapping for quantifying recruitment of superparamagnetic iron oxide-tagged endothelial progenitor cells to injured liver: tracking in vitro and in vivo, Int. J. Nanomed 9 (2014) 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shahnaz G, Kremser C, Reinisch A, Vetter A, Laffleur F, Rahmat D, Iqbal J, Dunnhaupt S, Salvenmoser W, Tessadri R, Griesser U, Bernkop-Schnurch A, Efficient MRI labeling of endothelial progenitor cells: design of thiolated surface stabilized superparamagnetic iron oxide nanoparticles, Eur. J. Pharm. Biopharm 85 (2013) 346–355. [DOI] [PubMed] [Google Scholar]
- [101].Wang XY, Ju S, Li C, Peng XG, Chen AF, Mao H, Teng GJ, Non-invasive imaging of endothelial progenitor cells in tumor neovascularization using a novel dual-modality paramagnetic/near-infrared fluorescence probe, PLoS One 7 (2012) e50575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chen J, Jia ZY, Ma ZL, Wang YY, Teng GJ, In vivo serial MR imaging of magnetically labeled endothelial progenitor cells homing to the endothelium injured artery in mice, PLoS One 6 (2011) e20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ju S, Qiu Y, Li C, Teng GJ, Ni Y, Multimodality imaging of endothelial progenitor cells with a novel multifunctional probe featuring positive magnetic resonance contrast and near-infrared fluorescence, Mol Imag 10 (2011) 359–369. [DOI] [PubMed] [Google Scholar]
- [104].Lee ES, Shuter B, Chan J, Chong MS, Ding J, Teoh SH, Beuf O, Briguet A, Tam KC, Choolani M, Wang SC, The use of microgel iron oxide nanoparticles in studies of magnetic resonance relaxation and endothelial progenitor cell labelling, Biomaterials 31 (2010) 3296–3306. [DOI] [PubMed] [Google Scholar]
- [105].Li Calzi S, Kent DL, Chang KH, Padgett KR, Afzal A, Chandra SB, Caballero S, English D, Garlington W, Hiscott PS, Sheridan CM, Grant MB, Forder JR, Labeling of stem cells with monocrystalline iron oxide for tracking and localization by magnetic resonance imaging, Microvasc Res 78 (2009) 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cohen B, Ziv K, Plaks V, Israely T, Kalchenko V, Harmelin A, Benjamin LE, Neeman M, MRI detection of transcriptional regulation of gene expression in transgenic mice, Nat. Med 13 (2007) 498–503. [DOI] [PubMed] [Google Scholar]
- [107].Suero-Abreu GA, Aristizabal O, Bartelle BB, Volkova E, Rodriguez JJ, Turnbull DH, Multimodal Genetic Approach for Molecular Imaging of Vasculature in a Mouse Model of Melanoma, Mol. Imag. Biol 19 (2017) 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bartelle BB, Berrios-Otero CA, Rodriguez JJ, Friedland AE, Aristizabal O, Turnbull DH, Novel genetic approach for in vivo vascular imaging in mice, Circ. Res 110 (2012) 938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kiessling F, Greschus S, Lichy MP, Bock M, Fink C, Vosseler S, Moll J, Mueller MM, Fusenig NE, Traupe H, Semmler W, Volumetric computed tomography (VCT): a new technology for noninvasive, high-resolution monitoring of tumor angiogenesis, Nat. Med 10 (2004) 1133–1138. [DOI] [PubMed] [Google Scholar]
- [110].Ehling J, Theek B, Gremse F, Baetke S, Mockel D, Maynard J, Ricketts SA, Grull H, Neeman M, Knuechel R, Lederle W, Kiessling F, Lammers T, Micro-CT imaging of tumor angiogenesis: quantitative measures describing micromorphology and vascularization, Am. J. Pathol 184 (2014) 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bauerle T, Bartling S, Berger M, Schmitt-Graff A, Hilbig H, Kauczor HU, Delorme S, Kiessling F, Imaging anti-angiogenic treatment response with DCE VCT, DCE-MRI and DWI in an animal model of breast cancer bone metastasis, Eur. J. Radiol 73 (2010) 280–287. [DOI] [PubMed] [Google Scholar]
- [112].Withofs N, Martinive P, Vanderick J, Bletard N, Scagnol I, Mievis F, Giacomelli F, Coucke P, Delvenne P, Cataldo D, Gambhir SS, Hustinx R, [(18) F]FPRGD2 PET/CT imaging of integrin alphavbeta3 levels in patients with locally advanced rectal carcinoma, Eur. J. Nucl. Med. Mol. Imag 43 (2016) 654–662. [DOI] [PubMed] [Google Scholar]
- [113].Withofs N, Signolle N, Somja J, Lovinfosse P, Nzaramba EM, Mievis F, Giacomelli F, Waltregny D, Cataldo D, Gambhir SS, Hustinx R, 18F-FPRGD2 PET/CT imaging of integrin alphavbeta3 in renal carcinomas: correlation with histopathology, J. Nucl. Med 56 (2015) 361–364. [DOI] [PubMed] [Google Scholar]
- [114].Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X, PET of vascular endothelial growth factor receptor expression, J. Nucl. Med 47 (2006) 2048–2056. [PubMed] [Google Scholar]
- [115].Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X, microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide, J. Nucl. Med 46 (2005) 1707–1718. [PubMed] [Google Scholar]
- [116].Dizeux A, Payen T, Le Guillou-Buffello D, Comperat E, Gennisson JL, Tanter M, Oelze M, Bridal SL, In vivo multiparametric ultrasound imaging of structural and functional tumor modifications during therapy, Ultras. Med. Biol 43 (2017) 2000–2012. [DOI] [PubMed] [Google Scholar]
- [117].Herzog E, Taruttis A, Beziere N, Lutich AA, Razansky D, Ntziachristos V, Optical imaging of cancer heterogeneity with multispectral optoacoustic tomography, Radiology 263 (2012) 461–468. [DOI] [PubMed] [Google Scholar]
- [118].Bao C, Beziere N, del Pino P, Pelaz B, Estrada G, Tian F, Ntziachristos V, de la Fuente JM, Cui D, Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers, Small 9 (2013) 68–74. [DOI] [PubMed] [Google Scholar]
- [119].Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE, Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging, Nat. Med 15 (2009) 1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Jain RK, Angiogenesis and lymphangiogenesis in tumors: insights from intravital microscopy, in: Cold Spring Harb. Symp. Quant. Biol, vol. 67, 2002, pp. 239–248. [DOI] [PubMed] [Google Scholar]
- [121].Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK, In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy, Nat. Med 7 (2001) 864–868. [DOI] [PubMed] [Google Scholar]
- [122].Boucher Y, Leunig M, Jain RK, Tumor angiogenesis and interstitial hypertension, Cancer Res 56 (1996) 4264–4266. [PubMed] [Google Scholar]
- [123].Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK, Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice, Cancer Res 52 (1992) 6553–6560. [PubMed] [Google Scholar]
- [124].Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P, Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model, Histochem. Cell Biol 130 (2008) 1147–1154. [DOI] [PubMed] [Google Scholar]
- [125].Larina IV, Larin KV, Justice MJ, Dickinson ME, Optical coherence tomography for live imaging of mammalian development, Curr. Opin. Genet. Dev 21 (2011) 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]