Abstract
Diffuse-type tenosynovial giant cell tumor (D-TGCT), otherwise known as pigmented villonodular synovitis, is a locally aggressive tumor which can show multiple recurrences but is rarely associated with metastasis. A handful of studies have elucidated the imaging features and clinical course in metastatic D-TGCT with malignant transformation on histology. However, only 5 cases of metastatic D-TGCT with benign histological features have been reported in the literature, with the clinical course and prognosis reported in only 1 case. Therefore, relatively little is known about the implications of histologically benign metastasis on the role of imaging, management, and clinical outcomes. We report a case of a 51-year-old female with recurrent D-TGCT localized to the knee that metastasized to the lymph nodes and soft tissue 3 years after above-the-knee amputation and 16 years after initial diagnosis of localized D-TGCT, despite benign histologic features on lymph node excision. This case highlights the necessity of timely MRI imaging to prevent delayed diagnosis, the role of histological findings on treatment response, and clinical outcomes associated with metastasized D-TGCT.
Keywords: D-TGCT, PVNS, Metastasis, Histologically benign, Tenosynovial giant cell tumor
Introduction
Tenosynovial giant cell tumors (TGCT) are locally invasive tumors of synovial origin that can involve joints, tendon sheaths, and bursae [1], [2]. Per the 2013 World Health Organization guidelines [2], TGCT is further classified into localized-type TGCT (L-TGCT) and diffuse-type TGCT (D-TGCT) according to growth pattern and behavior. L-TGCT, otherwise known as giant cell tumor of the tendon sheath, is typically confined to the synovium or tendon sheath and most commonly involves fingers and toes. D-TGCT, otherwise known as pigmented villonodular synovitis, is characterized by infiltrative growth, propensity for local recurrence, and predilection for involving the knee joint.
On histology, L-TGCT and D-TGCT are nearly indistinguishable and are characterized by growth of histiocyte-like cells associated with giant cells, foam cells, and hemosiderin laden cells [3]. Although extremely rare, D-TGCT has been known to metastasize, in most cases after undergoing malignant transformation on histology. The definition of malignant D-TGCT has been widely debated and controversial, but it is generally accepted that transformation occurs in about 3% of cases [4]. Approximately 30 cases of malignant D-TGCT have been described in the literature [5], [6], half of which involved metastases [5], [7]. Metastases very rarely occurs with histologically benign disease and to our knowledge, only 5 of these cases have been reported [1], [8], [9], [10], [11].
Patients with D-TGCT typically present with swelling around the affected joint or tendon sheath, pain that can lead to joint dysfunction and multiple recurrences after local excision. Benign D-TGCT was diagnosed at an average age of 39.5 years, while malignant D-TGCT was diagnosed at an average age of 60.9 years, with a slight female predilection according to 1 review [7]. Another review found that patients with malignant D-TGCT survived a median of 21.5 months after diagnosis with malignant D-TGCT; all 6 of these patients also had lung metastases [6].
Although studies have attempted to elucidate the treatment options and prognosis of metastasized D-TGCT with malignant transformation, the treatment and clinical course of metastatic D-TGCT with histologically benign features are relatively unknown. To our knowledge, only 5 cases of metastatic spread of histologically benign disease have been published in the literature [1], [8], [9], [10], [11], with documentation of disease course and outcome only in 1 case [11]. We report a case of D-TGCT with metastases to the lymph node and soft tissue despite benign histologic features on lymph node excision. This case highlights the role of imaging in timely diagnosis and follow-up, and the implications of histological findings of metastasized D-TGCT on treatment options and clinical course.
Case report
A 51-year-old female with history of recurrent D-TGCT of the left lower extremity presented to oncology clinic in 2016 to establish care at our institution due to insurance changes. She was initially diagnosed with biopsy-confirmed D-TGCT in 2000 at an outside institution, after presenting with left knee pain and swelling. Despite radiation treatment and multiple tumor debulking surgeries in the early 2000s, the mass continued to recur. Due to progressive destruction of the knee joint, she received a knee replacement in 2008. After D-TGCT recurred a few years later, she underwent a left above-the-knee amputation in 2012 at an outside institution. She was asymptomatic until a few years later, when she noticed an enlarged palpable left inguinal lymph node, not in proximity to the amputation stump, which was biopsied by fine need aspiration in February 2016 at an outside institution. This was diagnosed as metastatic D-TGCT on histology.
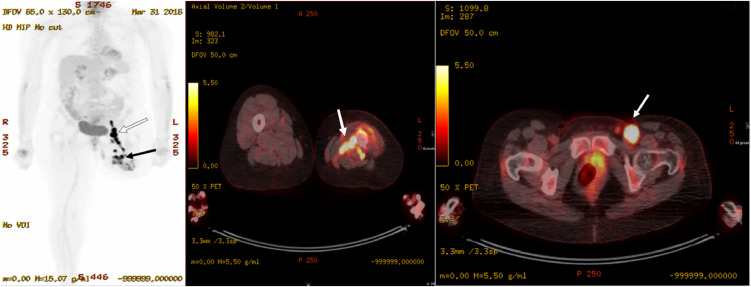
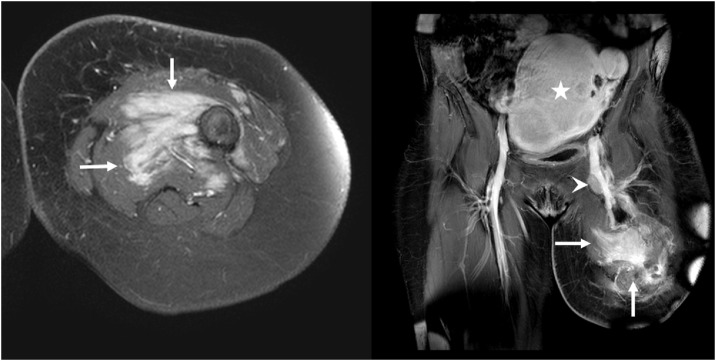
Patient was transferred to our institution for her care in March 2016. The physical exam was notable for left above-the-knee amputation with mild edema at the stump. At least 2 enlarged left inguinal lymph nodes were palpated, the largest being about 3 cm. Subsequent 18 fluorodeoxyglucose (18F-FDG) position emission tomography-computed tomography (PET/CT) demonstrated hypermetabolic soft tissue tracking within deep fascial planes of the anteromedial left thigh proximal to the amputation stump (Fig. 1), with maximal standardized uptake value (SUV) measuring 19.8. In addition, multiple enlarged left inguinal lymph nodes (up to 2.8 × 1.2 cm) are demonstrated with intense FDG uptake (maximal SUV 24.2) (Fig. 1). Contrast-enhanced MRI demonstrated an enhancing infiltrative mass along the medial left femur with circumferential extension, measuring 6.9 × 7.6 × 7.3 cm (Fig. 2) and multiple enlarged, enhancing left inguinal lymph nodes, the largest of which measured 1.2 × 2.0 cm. Pathology report from the biopsy (Fig. 3) of the enlarged lymph nodes revealed cells with eccentric, hyperchromatic nuclei, nucleoli, irregular nuclear contours, and abundant eosinophilic cytoplasm, with a background of scattered chronic inflammatory cells and fibrotic stroma. These cells stained positive for alpha-1 antitrypsin, CD68, and vimentin. No giant cells, foam cells, siderophages, necrosis, or mitotic figures were identified. She underwent left groin lymph node dissection on May 17, 2016 and partial excision of the mass. Pathology results were consistent with metastatic D-TGCT without anaplastic features to suggest sarcoma.
Fig. 1.
3D Maximal intensity projection (HD MIP) (left) and axial fused (middle, right) FDG PET/CT images obtained in March 2016 demonstrate increased metabolic activity in the mass along the left femur in the anteromedial thigh proximal to the amputation stump (SUV = 19.8) (black arrow, left and white arrow middle) and in the enlarged left inguinal lymph node (SUV = 24.2) (white arrow, left and white arrow, right).
Fig. 2.
Initial T1-weighted fat-sat contrast enhanced axial (left) and coronal (right) MRI demonstrate enhancing infiltrative mass along the anteromedial left thigh proximal to the amputation stump (arrows, left and right), corresponding to the increased metabolic activity on PET-CT images. The enlarged deep inguinal lymph node is along the left femoral vein (arrowhead, right). Incidental note is made of enlarged uterine with fibroids (star, right).
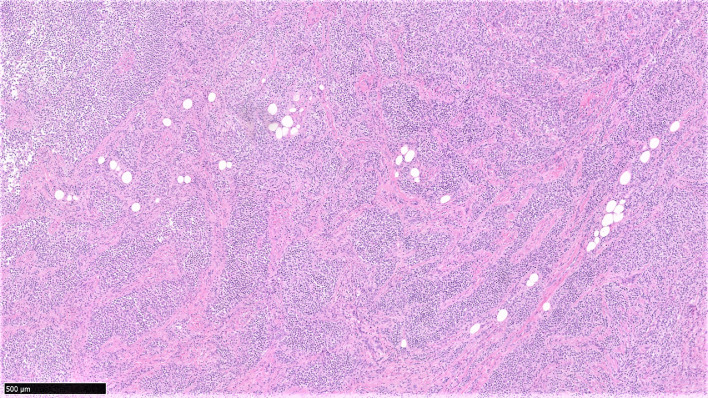
Fig. 3.
H&E stained tissue section at 5× magnification shows D-TGCT forming solid areas with multinodular architecture separated by fibrous septa and zones of stromal fibrosis.
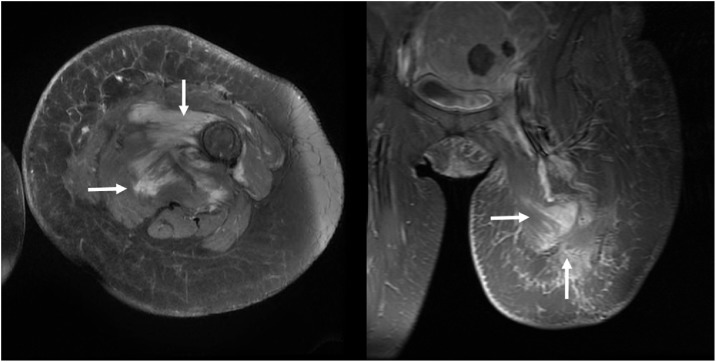
Postoperatively, she recovered uneventfully and followed up with an oncologist who initially did not start medications due to asymptomatic presentation. However, an MRI in June 2017 showed suspected metastatic relapse, evidenced by an irregularly marginated infiltrative mass along the distal femur and medial thigh, with the largest component being 6.9 × 7.7 cm. Her oncologist then started her on imatinib 400 mg PO. An MRI ordered 6 months later (January 2018) to evaluate her response showed slight improvement, with a reduction in the largest component from 6.9 × 7.7 cm to 6 × 7.4 cm (Fig. 4). A subsequent MRI another 6 months later in July 2018 showed that the mass was stable and the same size as before. She was last seen in clinic in December 2018 with no acute complaints or symptoms.
Fig. 4.
Six months after patient started imatinib treatment, T1-weighted fat-sat contrast enhanced axial (left) and coronal (right) MRI demonstrate slightly decreased mass compared with the MRI prior to treatment shown in Fig. 2 (arrows, left and right).
Discussion
The differential diagnosis for D-TGCT is broad due to the nonspecific finding of synovial hyperplasia and joint effusions, which can mimic common pathologies such as arthritis and other tumors (eg hemangioma, chondromatosis). Combined with the vague clinical presentation, this can often lead to significant delays in diagnosis (2–3 years on average) [12], which highlights the importance of appropriate imaging and biopsy for timely diagnosis and characterization of D-TGCT.
Results from radiographs, knee arthroscopy, and ultrasound are often nondiagnostic, as the findings that present in D-TGCT are nonspecific and common in infection and other pathologies such as tumors [13], [14]. In particular, common radiographic features of D-TGCT include joint effusion, soft-tissue swelling, and extrinsic erosion of bone. Arthrography demonstrates diffuse synovial thickening with nodular or villous extensions. Ultrasound reveals heterogeneous, echogenic masses associated with joint effusion and a thickened, hypoechoic synovium with increased blood flow on Doppler imaging [14].
CT demonstrates synovial thickening sometimes with slightly higher attenuation than muscle due to hemosiderin deposits. In addition, low attenuation joint effusion is seen. However, CT has insufficient soft tissue contrast resolution to evaluate the extent of the lesion [14]. MRI is the modality of choice for D-TGCT due to its ability to obtain detailed definition of the lesion and the extent of soft tissue and bone involvement [13].
D-TGCT on MRI is characterized by low signal intensity areas of nodular masses with synovial thickening and cystic bone lesions sometimes intermixed with sparser foci of high signal intensity on both T1- and T2-weighted images representing lipid-laden macrophages or lower concentrations of hemosiderin [5], [12], [15]. These areas are surrounded by joint effusion, which appears as low signal on T1 and high signal on T2-weighted images [13]. In addition, gradient-echo images classically accentuate the susceptibility related to the underlying hemosiderin deposition known as “blooming” of the hemosiderin-laden nodular lesions [5], [13], [14]. MRI may provide a critical role in characterizing the lesion for surgical treatment [12].
To our knowledge, this case report is the second case of metastasized TGCT with benign histological features that included MRI and PET/CT correlation in follow-up of the patient. In the other case [11], 1 year follow-up with PET/CT of the left lung found metastases to the left lung, back, right thigh, and inguinal/femoral lymph nodes, and final follow-up 1 year later resulted in slightly increased lesion sizes despite being asymptomatic.
As shown in previous reports [12], 18F-FDG PET/CT in our case demonstrated hypermetabolic soft tissue activity with focal intense FDG uptake at the tumor site. This suggests the sensitivity of PET-CT in the detection of D-TGCT, but its role in diagnosis is unclear as the focal FDG uptake of D-TGCT is often nonspecific and can be mistaken for malignancy. Our case highlights the necessity of considering benign lesions in the differential for hypermetabolic foci and the need of obtaining a biopsy for histological characterization. This is consistent with previous reports that 18F-FDG PET/CT has not been completely dependable in differentiating between benign and malignant lesions [12].
Our lymph node biopsy possessed the distinctive histologic features of D-TGCT, characterized by sheets of large uniform epithelioid cells (Fig. 5) with abundant eosinophilic cytoplasm and eccentric nuclei, and infiltration and entrapment of adipose tissue (Fig. 6). However, the histological findings did not meet the criteria for malignant D-TGCT set by Bertoni et al [16] or the Armed Forces Institute of Pathology [17]. Per Bertoni et al, our biopsy met 3 out of the 6 criteria: (1) nodular lesions, infiltrative growth pattern, (2) cells with large nuclei with eosinophilic cytoplasm and prominent nucleoli, and (3) fewer giant cells, xanthoma cells, and inflammatory cells. However, our tissue histology did not meet the other criteria: (1) large plump or round to oval cells, (2) lack of normal zonal patterns of maturation of D-TGCT, and (3) areas of necrosis.
Fig. 5.
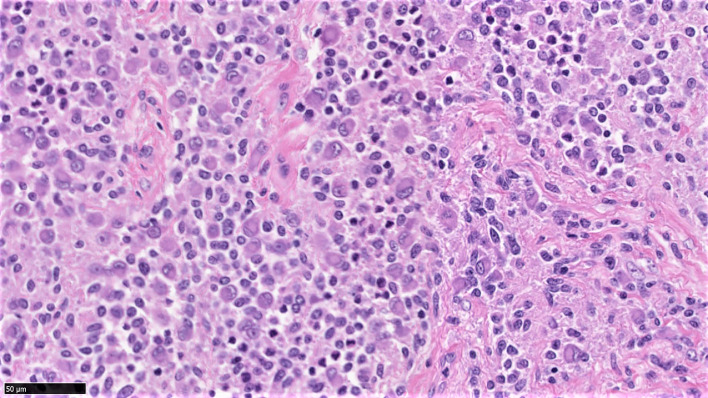
H&E-stained tissue section at 40× magnification shows distinctive histologic features of D-TGCT, namely sheets of large uniform epithelioid cells with abundant eosinophilic cytoplasm, and eccentric nuclei, and few or no giant cells.
Fig. 6.
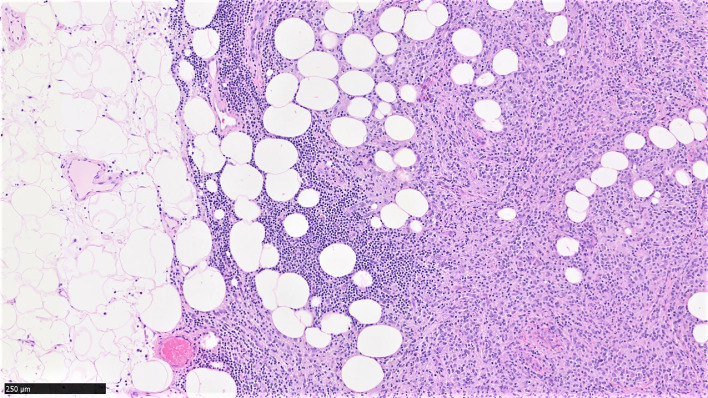
H&E stained tissue section at 10× magnification showing invasion of adjacent tissues, as illustrated by infiltration and entrapment of adipose tissue.
Furthermore, the Armed Forces Institute of Pathology [17] requires 5 out of 8 criteria to be considered malignant: (1) diffuse pleomorphism, (2) prominent nucleoli, (3) high nuclear cytoplasmic ratio, (4) mitoses of 10/10 hpfs, (5) necrosis, (6) discohesion of cells, (7) paucity of giant cells, and (8) diffuse growth pattern. Our histological findings only met 3 criteria (prominent nucleoli, paucity of giant cells, and diffuse growth pattern). Notably, we did not identify necrosis, mitotic figures, or anaplastic features.
Our case also sheds light on the progression from localized D-TGCT to metastasized D-TGCT in the absence of malignant transformation. In our patient, metastasis was discovered 16 years after the initial diagnosis of localized D-TGCT, similar to a recently reported case in 2015 [1], in which metastasis was discovered 18 years after initial synovectomy. In other cases of metastatic D-TGCT with benign features, the discovery of metastasis occurred in variable time spans including several years [9], 49 years [8], 5 years [10], and 8 years [11] after initial surgery, showing that benign D-TGCT may have an indolent, prolonged course before metastasis is discovered. Some hypothesized that surgical manipulation may cause passive metastatic seeding via the bloodstream [11]. Similar to another case [1], metastasized D-TGCT was discovered despite a total knee amputation 3 years prior, which suggests that total knee amputation for our patient was either too late or might not be effective in preventing metastasis. This highlights the need for further studies on the pathogenesis of metastatic seeding to determine the threshold of suspicion of metastasis in patients with a history of D-TGCT when present with a painful lymph node.
Given the extremely rare incidence of metastatic D-TGCT with histologically benign features, well-defined treatment guidelines have not been developed. However, treatment for localized, benign TGCT almost always necessitates excision with wide margins to avoid high risk for local recurrence, while malignant TGCT is often treated with cytotoxic chemotherapy or medical therapies that target the CSF1 gene, which is often overexpressed due to fusion with a promoter in a t(1;2) translocation [18], [19]. The effectiveness of these treatments is still being studied. In a case series of 6 patients by Nakayama et al [6], cytotoxic therapy based on anthracycline and gemcitable/docetaxel showed benefit in 3 out of 4 and 2 out of 3 patients respectively, while medical treatment with a tyrosine kinase inhibitor (TKI), eg sunitinib, showed stable disease in only 1 out of 5 patients. In a previous study by Cassier et al [18] in which 27 patients (25 with benign TGCT, 2 with malignant TGCT) were treated with imatinib, 74% had stable disease and 19% had partial response.
In the presented case, our patient was treated with imatinib following metastatic relapse of D-TGCT, with a slightly decreased tumor size on MRI 6 months later. This is consistent with the cytostatic effect of imatinib on histologically benign disease and the potentially higher prognostic value based on histological characterization compared to metastatic status. Metastatic D-TGCT with histologically benign features in other cases was also treated conservatively with either no medications [11] or imatinib [1]. In the only other published case in which follow-up was documented [11], the tumor size increased slightly without medications. In our case, the tumor size decreased slightly following imatinib treatment. These 2 cases suggest that imatinib might be an appropriate treatment with histologically benign lesions, in contrast to histologically malignant lesions, which are less responsive to TKI.
Despite treatment, the risk of local recurrence is estimated at up to 70% [4], with an average of 5 years before recurrence. Recurrence often occurs in the knee joint after previous operations, with a 46% recurrence after synovectomy [13]. Given its aggressive course in the 30–40 cases previously published in literature, malignant D-TGCT has a poor prognosis with a 33%–50% mortality rate and a median duration of 21.5 months to live after diagnosis [6]. In comparison, metastasizing benign D-TGCT seems has a much more favorable prognosis than malignant D-TGCT. Based on 1 report and our case, benign histological features indicate indolent disease progression and carry a much more favorable prognosis than malignant D-TGCT, with both patients asymptomatic 2 years after follow-up.
In summary, we present the fifth case of metastasized D-TGCT with histologically benign features and the second case documenting patient outcome and response to treatment. Our case highlights the role of imaging in obtaining detailed definition of the lesion and its extent, and the necessity of regular follow-up imaging to increase the sensitivity of detecting local recurrence and distant metastasis as surveillance for relapsed or distant metastases after surgery, as metastatic relapse was found 4 months after surgical excision in our case. Clinicians should have a high degree of clinical suspicion due to aggressive behavior despite benign cytologic features, and radiologists should consider including both malignant and benign D-TGCT in the differential diagnosis in patients who underwent previous surgeries for localized D-TGCT. Finally, this case suggests that benign histological findings on biopsy could indicate responsiveness to medical therapy with TKIs and a much better prognosis compared to findings of malignant transformation. Nevertheless, further studies are needed to elucidate the pathogenesis of metastatic D-TGCT, the possible role of surgeries in facilitating metastatic seeding, and treatment outcomes with medical therapies.
Footnotes
Acknowledgment: Special thanks to Dr. John Groth for histology contributions.
Disclosure: No disclosures.
Conflict of Interest Statement: The Research Open Access Publishing (ROAAP) Fund of the University of Illinois at Chicago provided financial support towards the open access publishing fee for this article.
References
- 1.Righi A., Gambarotti M., Sbaraglia M., Frisoni T., Donati D., Vanel D. Metastasizing tenosynovial giant cell tumour, diffuse type/pigmented villonodular synovitis. Clin Sarcoma Res. 2015;5(1) doi: 10.1186/s13569-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Righi A., Gambarotti M., Sbaraglia M., et al. Metastasizing tenosynovial giant cell tumour, diffuse type/pigmented villonodular synovitis. Clin Sarcoma Res. 2015;5(1). doi:10.1186/s13569-015-0030-2 [DOI] [PMC free article] [PubMed]
- 2.Somerhausen N.S., van den Rijn M. Tenosynovial giant cell tumour, diffuse type. In: Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F., editors. WHO classification of tumours of soft tissue and bone. 4th ed. IARC Press; Lyon: 2013. pp. 102–103. [Google Scholar]; Somerhausen N.S., van den Rijn M.Tenosynovial giant cell tumour, diffuse type. In: Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F., eds. WHO classification of tumours of soft tissue and bone. 4th ed.Lyon: IARC Press; 2013:102–103.
- 3.Ushijima M., Hashimoto H., Tsuneyoshi M., Enjoji M. Giant cell tumor of the tendon sheath (Nodular Tenosynovitis) a study of 207 cases to compare the large joint group with the common digit group. Cancer. 1986;57(4):875–884. doi: 10.1002/1097-0142(19860215)57:4<875::aid-cncr2820570432>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]; Ushijima M., Hashimoto H., Tsuneyoshi M., Enjoji M.Giant cell tumor of the tendon sheath (Nodular Tenosynovitis) a study of 207 cases to compare the large joint group with the common digit group. Cancer. 1986;57(4):875–884. doi:10.4324/9780203414866_chapter_4 [DOI] [PubMed]
- 4.Sistla R., J V S V, Afroz T. Malignant pigmented villonodular synovitis–a rare entity. J Orthop Case Rep. 2014;4(4):9–11. doi: 10.13107/jocr.2250-0685.214. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sistla R., J V S V, Afroz T.Malignant pigmented villonodular synovitis–a rare entity. J Orthop Case Rep. 2014;4(4):9–11. doi:10.13107/jocr.2250-0685.214 [DOI] [PMC free article] [PubMed]
- 5.Yoon H.J., Cho Y.A., Lee J.I.L., Hong S.P., Hong S.D. Malignant pigmented villonodular synovitis of the temporomandibular joint with lung metastasis: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(5):e30–e36. doi: 10.1016/j.tripleo.2010.11.031. [DOI] [PubMed] [Google Scholar]; Yoon H.J., Cho Y.A., Lee J.I.L., Hong S.P., Hong S.D.Malignant pigmented villonodular synovitis of the temporomandibular joint with lung metastasis: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(5):e30–e36. doi:10.1016/j.tripleo.2010.11.031 [DOI] [PubMed]
- 6.Nakayama R., Jagannathan J.P., Ramaiya N., Ferrone M.L., Raut C.P., Ready J.E. Clinical characteristics and treatment outcomes in six cases of malignant tenosynovial giant cell tumor: initial experience of molecularly targeted therapy. BMC Cancer. 2018;18(1):1296. doi: 10.1186/s12885-018-5188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nakayama R., Jagannathan J.P., Ramaiya N., et al. Clinical characteristics and treatment outcomes in six cases of malignant tenosynovial giant cell tumor: initial experience of molecularly targeted therapy. BMC Cancer. 2018;18(1):1296. doi:10.1186/s12885-018-5188-6 [DOI] [PMC free article] [PubMed]
- 7.Li C.F., Wang J.W., Huang W.W., Hou C.C., Chou S.C., Eng H.L. Malignant diffuse-type tenosynovial giant cell tumors: a series of 7 cases comparing with 24 benign lesions with review of the literature. Am J Surg Pathol. 2008;32(4):587–599. doi: 10.1097/PAS.0b013e318158428f. [DOI] [PubMed] [Google Scholar]; Li C.F., Wang J.W., Huang W.W., et al. Malignant diffuse-type tenosynovial giant cell tumors: a series of 7 cases comparing with 24 benign lesions with review of the literature. Am J Surg Pathol. 2008;32(4):587–599. doi:10.1097/PAS.0b013e318158428f [DOI] [PubMed]
- 8.Choong P.F.M. Pigmented villonodular synovitis. Monoclonality and metastasis–a case for neoplastic origin. Acta Orthop Scand. 1995;66(1):64–68. doi: 10.3109/17453679508994643. [DOI] [PubMed] [Google Scholar]; Choong P.F.M.Pigmented villonodular synovitis. Monoclonality and metastasis–a case for neoplastic origin? Acta Orthop Scand. 1995;66(1):64–68. [DOI] [PubMed]
- 9.Enzinger, N. F.Benign tumors and tumorlike lesions of synovial tissue. Soft Tissue Tumors. 1995:735–755.
- 10.Somerhausen N.S., Fletcher C.D. Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am J Surg Pathol. 2000;24(4):479–492. doi: 10.1097/00000478-200004000-00002. [DOI] [PubMed] [Google Scholar]; Somerhausen N.S., Fletcher C.D.Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am J Surg Pathol. 2000;24(4):479–492. [DOI] [PubMed]
- 11.Asano N., Yoshida A., Kobayashi E., Yamaguchi T., Kawai A. Multiple metastases from histologically benign intraarticular diffuse-type tenosynovial giant cell tumor: a case report. Hum Pathol. 2014;45(11):2355–2358. doi: 10.1016/j.humpath.2014.06.025. [DOI] [PubMed] [Google Scholar]; Asano N., Yoshida A., Kobayashi E., Yamaguchi T., Kawai A.Multiple metastases from histologically benign intraarticular diffuse-type tenosynovial giant cell tumor: a case report. Hum Pathol. 2014;45(11):2355–2358. doi:10.1016/j.humpath.2014.06.025 [DOI] [PubMed]
- 12.Elumogo C.O., Kochenderfer J.N., Civelek A.C., Bluemke D.A. Pigmented villonodular synovitis mimics metastases on fluorine 18 fluorodeoxyglucose position emission tomography-computed tomography. Quant Imaging Med Surg. 2016;6(2):218–223. doi: 10.21037/qims.2016.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elumogo C.O., Kochenderfer J.N., Civelek A.C., Bluemke D.A.Pigmented villonodular synovitis mimics metastases on fluorine 18 fluorodeoxyglucose position emission tomography-computed tomography. Quant Imaging Med Surg. 2016;6(2):218–223. doi:10.21037/qims.2016.01.04 [DOI] [PMC free article] [PubMed]
- 13.Ofluoglu O. Pigmented villonodular synovitis. Orthop Clin North Am. 2006;37(1):23–33. doi: 10.1016/j.ocl.2005.08.002. [DOI] [PubMed] [Google Scholar]; Ofluoglu O.Pigmented villonodular synovitis. Orthop Clin North Am. 2006;37(1):23–33. doi:10.1016/j.ocl.2005.08.002 [DOI] [PubMed]
- 14.Murphey M.D., Rhee J.H., Lewis R.B., Fanburg-Smith J.C., Flemming D.J., Walker E.A. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28(5):1493–1518. doi: 10.1148/rg.285085134. [DOI] [PubMed] [Google Scholar]; Murphey M.D., Rhee J.H., Lewis R.B., Fanburg-Smith J.C., Flemming D.J., Walker E.A.Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28(5):1493–1518. doi:10.1148/rg.285085134 [DOI] [PubMed]
- 15.Garner H.W., Ortiguera C.J., Nakhleh R.E. Pigmented villonodular synovitis. Radiographics. 2008;28(5):1519–1523. doi: 10.1148/rg.285075190. [DOI] [PubMed] [Google Scholar]; Garner H.W., Ortiguera C.J., Nakhleh R.E.Pigmented villonodular synovitis. Radiographics. 2008;28(5):1519–1523. doi:10.1148/rg.285075190 [DOI] [PubMed]
- 16.Bertoni F., Unni K.K., Beabout J.W., Sim F.H. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis) Am J Surg Pathol. 1997;21(2):153–163. doi: 10.1097/00000478-199702000-00004. [DOI] [PubMed] [Google Scholar]; Bertoni F., Unni K.K., Beabout J.W., Sim F.H.Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153–163. [DOI] [PubMed]
- 17.Fanburg-Smith J.C., Miettinen M. Malignant giant cell tumors of the tendon sheath: histologic classification with clinical correlation. Clin Exp Pathol. 1998;46:16A. [Google Scholar]; Fanburg-Smith J., Miettinen M.Malignant tenosynovial giant cell tumors (MGCTTS). In: Scientific expansions. Nice, France: International Academy of Pathology; 1998.
- 18.Cassier P.A., Gelderblom H., Stacchiotti S., Thomas D., Maki R.G., Kroep J.R. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118(6):1649–1655. doi: 10.1002/cncr.26409. [DOI] [PubMed] [Google Scholar]; Cassier P.A., Gelderblom H., Stacchiotti S., et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118(6):1649–1655. doi:10.1002/cncr.26409 [DOI] [PubMed]
- 19.Cupp J.S., Miller M.A., Montgomery K.D., Nielsen T.O., O’Connell J.X., Huntsman D. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol. 2007;31(6):970–976. doi: 10.1097/PAS.0b013e31802b86f8. [DOI] [PubMed] [Google Scholar]; Cupp J.S., Miller M.A., Montgomery K.D., et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides: Am J Surg Pathol. 2007;31(6):970–976. doi:10.1097/PAS.0b013e31802b86f8 [DOI] [PubMed]