Abstract
Objective
Prescription opioid abuse continues to be a public health concern. Oxycodone ARIR is an immediate-release (IR) oxycodone tablet composed of multiple overlapping barriers that deter manipulation of the tablet for non-oral abuse.
Design
This randomized, double-blind, double-dummy, active- and placebo-controlled, four-way crossover, intranasal human abuse potential study assessed the pharmacodynamics and pharmacokinetics of crushed intranasal oxycodone ARIR compared with crushed intranasal IR oxycodone and intact oral oxycodone ARIR.
Outcome Measures
Pharmacodynamic end points included mean maximum drug liking (Emax), as measured by subjects on a bipolar 100-mm visual analog scale (primary), and desire to take the drug again, overall drug liking, drug high, and good effects (secondary). Pharmacokinetic assessments included peak concentration and time to peak concentration.
Results
Twenty-nine subjects completed the treatment phase. Crushed intranasal oxycodone ARIR demonstrated a significant reduction of 46.9% and 23.4% in drug liking Emax compared with crushed intranasal IR oxycodone and intact oral oxycodone ARIR, respectively (P < 0.0001 for both). Significant reductions also were observed in desire to take the drug again, drug high, overall drug liking, and good effects when comparing crushed intranasal oxycodone ARIR with crushed intranasal IR oxycodone and intact oral oxycodone ARIR (P < 0.001 for all). Crushed intranasal oxycodone ARIR exhibited lower peak oxycodone plasma concentrations and slower time to peak concentration compared with crushed intranasal IR oxycodone and intact oral oxycodone ARIR. All treatments were well tolerated; adverse effects were typical of opioids or intranasal administration.
Conclusions
These data indicate that oxycodone ARIR has the potential to reduce abuse via the intranasal route.
Keywords: Abuse-Deterrent, Drug Liking, Intranasal, Opioid, Oxycodone
Introduction
Introduction of an abuse-deterrent formulation of extended-release (ER) oxycodone (OxyContin, Purdue Pharma, L.P., Stamford, CT, USA) significantly reduced reports of misuse and abuse of ER oxycodone; however, early epidemiologic evidence suggests that this decrease was accompanied by a concomitant increase in abuse of non-abuse-deterrent formulations, such as immediate-release (IR) oxycodone [1]. In the United States, 90% of all prescriptions for opioids are for IR formulations [2]. The population-adjusted rates of intentional abuse and diversion are 4.6 times and 6.1 times greater, respectively, for IR opioids than for ER opioids [2]. Results of a recent study of advanced opioid abusers indicated that when choosing an opioid for abuse, 66% preferred IR opioids compared with only 4% who preferred ER opioids [3]. Therefore, development of an abuse-deterrent IR formulation is an important next step in combatting the opioid overdose crisis.
Non-oral routes of administration, such as intranasal insufflation and intravenous (IV) injection, are particularly concerning because they are associated with significantly higher morbidity and mortality [4]. Data from the RADARS System Poison Center Program show that the relative risk of death or a major, life-threatening effect (e.g., overdose) relative to a single instance of oral abuse is 2.2 times greater for each instance of intranasal abuse and 2.6 times greater for each instance of IV abuse [4]. Snorting and injection are common routes of IR oxycodone abuse among individuals entering substance abuse treatment [5,6]. Between Q1 2015 and Q4 2016, 52% of IR oxycodone abusers reported abuse via intranasal administration, and 28% reported abuse via IV administration (Inflexxion, unpublished data). In addition to overdose and death, abuse via these nonoral routes introduces risks for serious health concerns, including HIV infection, hepatitis C virus, and nasal necrosis and perforation [7–13].
Abuse-resistant IR oxycodone (oxycodone ARIR, RoxyBond, Daiichi Sankyo, Inc., Basking Ridge, NJ, USA) is an abuse-deterrent tablet formulated with SentryBond technology, which consists of multiple overlapping barriers that deter manipulation of the tablet for non-oral abuse [14,15]. Oxycodone ARIR is the first abuse-deterrent IR opioid formulation approved by the US Food and Drug Administration, and it was developed via the 505(b)(2) pathway. In vitro laboratory data show that relative to IR oxycodone, oxycodone ARIR has increased resistance to cutting, crushing, grinding, or breaking using select household tools; resists extraction in select household and laboratory solvents; and forms a viscous material that resists passage through a needle [14,15]. In this human abuse potential study, we assess the pharmacodynamics (PD) and pharmacokinetics (PK) of intranasal administration of crushed oxycodone ARIR compared with intranasal administration of crushed IR oxycodone (Roxicodone, Mallinckrodt Brand Pharmaceuticals, Inc., Hazelwood, MO, USA) and oral administration of intact oxycodone ARIR.
Methods
Subjects
This study included healthy male and female subjects between the ages of 18 and 55 years. Subjects were eligible to participate if they were not physically dependent on opioids (based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, criteria and a naloxone challenge test) but had used opioids for nontherapeutic purposes (i.e., recreational drug use) at least 10 times during the preceding year and at least once in the 12 weeks before screening. In addition, subjects must have used a drug intranasally on at least three occasions during the year prior to screening. Subjects were excluded if they had participated in, were currently participating in, or were seeking treatment for substance-related disorders, or if they had a history or presence of drug or alcohol dependence (excluding nicotine and caffeine). With the exception of tetrahydrocannabinol, subjects were excluded if they had positive urine drug screen results at screening, during the qualification period, or at admission for the treatment period. Subjects who tested positive for opioids, amphetamines, cocaine, or benzodiazepines at screening were eligible for enrollment in the study provided the drug screen results at check-in for the qualification and treatment periods were negative. Pregnant or nursing subjects or those planning to become pregnant also were excluded. In addition, subjects were excluded if they had a history or presence of clinically significant cardiovascular, pulmonary, hepatic, renal, hematologic, gastrointestinal, endocrine, immunologic, dermatologic, neurologic, oncologic, or psychiatric disorder, or any other condition that would, in the opinion of the investigator, jeopardize the subject’s safety or compromise the validity of the study results. Subjects provided written informed consent to participate in the study.
Study Design and Treatment
This randomized, double-blind, double-dummy, active- and placebo-controlled, single-dose, four-way crossover study was conducted at a single site (PRA Health Sciences, Salt Lake City, UT, USA). The study consisted of a screening period, a qualification period, a study drug treatment period, and a follow-up period (Figure 1). The qualification period consisted of a four-night inpatient, double-blind qualifying session during which a naloxone challenge test was administered to evaluate subjects for signs of opiate withdrawal. Subjects were given an initial dose of naloxone hydrochloride (HCl) 0.2 mg by IV bolus. If no evidence of withdrawal occurred within 30 seconds, as assessed by a Clinical Opiate Withdrawal Scale score of less than 5, an additional 0.6 mg of naloxone HCl was administered. Subjects who did not exhibit symptoms of withdrawal during the five-minute observation period proceeded to the drug discrimination test, which assessed whether they could distinguish oxycodone from placebo and discern differing oxycodone doses. The drug discrimination test was a three-way crossover, 1:1:1 ratio, double-blind, computer-generated randomized design during which subjects received a single intranasal dose each of IR oxycodone (15-mg crushed tablet), IR oxycodone (30-mg crushed tablet), and placebo powder. Each dose was separated by at least 24 hours. The investigator made a blinded assessment as to whether subjects could distinguish oxycodone from placebo and oxycodone doses by using a bipolar drug-liking visual analog scale (VAS).
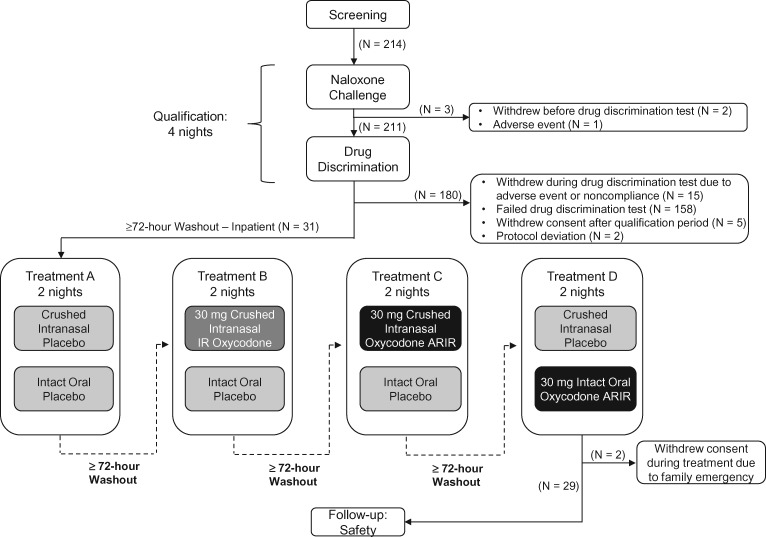
Figure 1.
Subject disposition through all treatment phases.
Subjects who passed the naloxone challenge and drug discrimination test underwent a minimum 72-hour washout period. They then entered the treatment period, which consisted of an 11-night inpatient stay with a minimum 72-hour washout period between each treatment. Subjects received each of the four treatments according to a computer-generated randomized 1:1:1:1 ratio based on a Williams design. A statistician or designee who was not involved in the conduct of the study generated all randomization codes before the start of the study. Treatments included placebo, crushed IR oxycodone (30 mg), crushed oxycodone ARIR (30 mg), and intact oxycodone ARIR (30 mg). All treatments were matched with placebo tablets and/or powder to ensure that the treatment regimens were visually similar. To ensure blinding, subjects were dosed individually in a private room with a closed door. A posttreatment follow-up was performed seven to 10 days after the last dose was administered. This study was conducted in accordance with the Good Clinical Practice guideline, as defined by the International Council on Harmonisation; the Declaration of Helsinki; and all applicable federal and local regulations. Prior to study initiation, the New England Institutional Review Board reviewed and approved the protocol.
Pharmacodynamic Assessments
Bipolar Drug Liking Visual Analog Scale
The primary end point was the mean maximum effect (Emax) for drug liking of crushed intranasal oxycodone ARIR compared with crushed intranasal IR oxycodone. Secondary comparisons for the primary end point included Emax for intact oral oxycodone ARIR compared with crushed intranasal IR oxycodone and crushed intranasal oxycodone ARIR. Drug liking was measured on a 0–100-mm bipolar VAS, where a score of 0 represents strong disliking, 50 represents a neutral response (neither like nor dislike), and 100 represents strong liking. Subjects completed the bipolar drug liking VAS within five minutes postdose, and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours postdose. The overall mean and median, as well as the percentage reduction in Emax for each subject, were calculated as follows:
where ci, ti, and pi are the Emax values for the control (crushed intranasal IR oxycodone), test (crushed intranasal oxycodone ARIR), and placebo, respectively, from the ith subject, and N is the sample size.
Overall Drug Liking Visual Analog Scale
The overall drug-liking VAS assessed the global perception of drug liking (i.e., the subjective effects over the entire course of the drug experience, including any carryover effects). Subjects responded to the statement “Overall, my liking for this drug is:” by marking a single vertical line on a 0–100-point bipolar VAS; a score of 0 indicates “strong disliking,” a score of 50 indicates “neither like nor dislike,” and a score of 100 indicates “strong liking.” Overall drug liking was assessed at 12 and 24 hours postdose.
Take Drug Again Assessment
The take drug again assessment VAS was used to assess each subject’s desire to use the drug again. Subjects responded to the statement “Would you want to take the drug you just received again, if given the opportunity?” by marking a single vertical line on the 0–100-point bipolar VAS anchored on the left with “definitely would not” (score of 0), in the middle with “do not care” (score of 50), and on the right with “definitely would” (score of 100). The desire to take the drug again was assessed at 12 and 24 hours postdose.
Drug Effects Questionnaire
Subjects completed the eight-item drug effects questionnaire (DEQ) by using a unipolar 0–100-mm VAS, anchored on the left by “none” (score of 0) and on the right by “extremely” (score of 100). Questions included the following: “Do you feel any drug effects?”; “Does the drug have good effects?”; “Does the drug have any bad effects?”; “How high are you now?”; “Does the drug make you feel sick?”; “Do you have any nausea?”; “Does the drug make you sleepy?”; and “Does the drug make you dizzy?” Subjects completed the DEQ during the treatment period at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours postdose. The questions regarding drug high, sickness, nausea, sleepiness, and dizziness were asked within one hour before dosing as well.
Ease of Snorting Visual Analog Scale
Subjects were asked about the ease of snorting the drug five minutes after insufflating each dose. They responded to the statement “Snorting the drug was:” by marking a single vertical line on a 0–100-point unipolar VAS anchored on the left by “very easy” (score of 0) and on the right by “very difficult” (score of 100).
Nasal Effects Assessment
Subjects were asked to use a four-point Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) to rate the following “at this moment” items: any intranasal irritation, nasal burning, runny nose/nasal discharge, facial pain/pressure, nasal congestion, or need to blow nose. The investigator assessed nasal effects predose and within five minutes and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours postdose.
Pharmacokinetic Assessments
Blood samples were obtained within one hour predose and at 0.25, 0.5, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 8, 12, and 24 hours postdose. In subjects who experienced emesis within one hour after dosing, PK samples were not collected for the duration of the treatment period. Plasma oxycodone concentrations were assessed by means of a validated liquid chromatography, tandem mass spectrometry method (calibration range = 0.398–99.620 ng/mL); the lower limit of quantitation was 0.398 ng/mL. PK parameters, including maximum observed plasma concentration (Cmax), time to Cmax (Tmax), and terminal half-life (t1/2), were calculated using a noncompartmental model (Phoenix WinNonlin 6.2, Certara, Princeton, NJ, USA). Linear trapezoidal estimation was used to calculate area under the plasma concentration time curve from 0 hours to the last measurable concentration (AUC0-t), extrapolated to infinity (AUC0-∞), and at various times during the study (e.g., AUC0–0.5 h, AUC0–1 h, AUC0–2 h). The abuse quotient (AQ; Cmax/Tmax), a measure associated with drug liking and abuse potential [16,17], was also calculated.
Safety Assessments
Adverse events (AEs) were recorded using the Medical Dictionary for Regulatory Activities (MedDRA), version 17.0. Complete physical examinations and vital sign measurements were performed at screening and at the follow-up visit; 12-lead electrocardiography was performed at screening, at check-in to the qualification period, and at follow-up; and other laboratory tests were performed at screening, at admission to the clinic the day before the treatment period began, and at the follow-up visit.
Statistical Analysis
Assuming a 22% dropout rate during treatment, 36 subjects would need to enter the treatment period to ensure that 28 subjects completed the study. With 28 subjects, this study was powered to detect a difference between treatments with at least 90% power.
Demographics and baseline data, PD parameters, and PK parameters were summarized using descriptive statistics. PD parameters, including the primary end point of maximum drug liking, were estimated by means of standard noncompartmental methods. Analysis of variance (ANOVA) models were used to assess the differences in PK parameters. The SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) linear mixed-effects model procedure (PROC MIXED) was used to construct the ANOVA models, with the PK parameter as the dependent variable; treatment, sequence, and period as fixed effects; and subject nested within sequences as a random effect. With the exception of Tmax, t1/2, and Ke, PK parameters were log-transformed. All significance testing was two-tailed using α = 0.05.
Results
Subjects
Two hundred fourteen subjects participated in and passed the naloxone challenge, and three subjects withdrew before the drug discrimination test. Of the 211 subjects who underwent the drug discrimination test, 31 passed and entered the treatment phase (safety population), 158 failed the drug discrimination test, and 22 withdrew during or after the test (Figure 1). During the treatment phase, two subjects withdrew consent but are included in the PK analysis; 29 subjects who completed the study are included in the PD analysis. Most subjects were white men, and the mean age of subjects was 24.4 years (Table 1). In accordance with the inclusion criteria for the study, all subjects had used opioids recreationally in the previous 12 weeks; among men, the mean number of times was 16.4, and among women, 17.4. All intranasal doses were completely (100%) insufflated, as confirmed by an intranasal check.
Table 1.
Baseline demographics and characteristics (safety population)
| Parameter | Subjects (N = 31) | |
|---|---|---|
| Age, y | ||
| Mean (SD) | 24.4 (5.12) | |
| Median (range) | 23 (19–40) | |
| Sex, No. (%) | ||
| Male | 26 (83.9) | |
| Female | 5 (16.1) | |
| Race, No. (%) | ||
| White | 29 (93.5) | |
| Black or African American | 1 (3.2) | |
| Other | 1 (3.2) | |
| Ethnicity, No. (%) | ||
| Not Hispanic or Latino | 26 (83.9) | |
| Hispanic or Latino | 5 (16.1) | |
| Body mass index, kg/m2 | ||
| Mean (SD) | 23.6 (3.29) | |
| Median (range) | 23 (19.1–32.3) | |
| Recreational drug use during the 12 wk before study | Subjects using, No. (%) | Mean (SD) times taken, No. |
| Cannabinoids | ||
| Men | 23 (88.5) | 150.0 (233.98) |
| Women | 4 (80) | 60.0 (34.64) |
| Opioids | ||
| Men | 25 (96.2) | 16.4 (16.14) |
| Women | 5 (100) | 17.4 (21.82) |
| Benzodiazepines | ||
| Men | 2 (7.7) | 7.0 (4.24) |
| Women | 2 (40) | 1.5 (0.71) |
| Stimulants | ||
| Men | 12 (46.2) | 5.3 (4.33) |
| Women | 2 (40) | 6.0 (5.66) |
Pharmacodynamic Assessments
Drug Liking
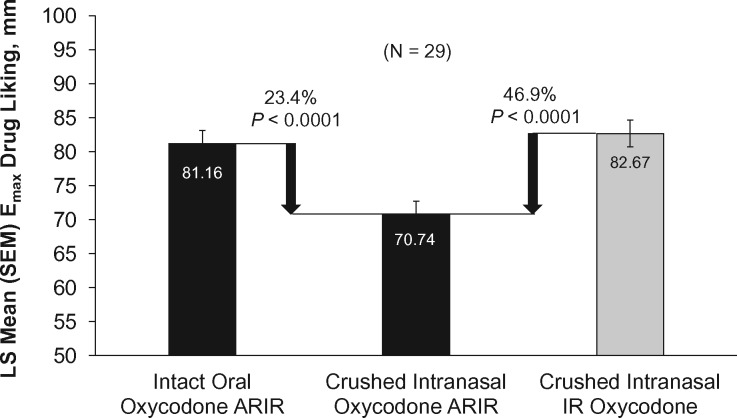
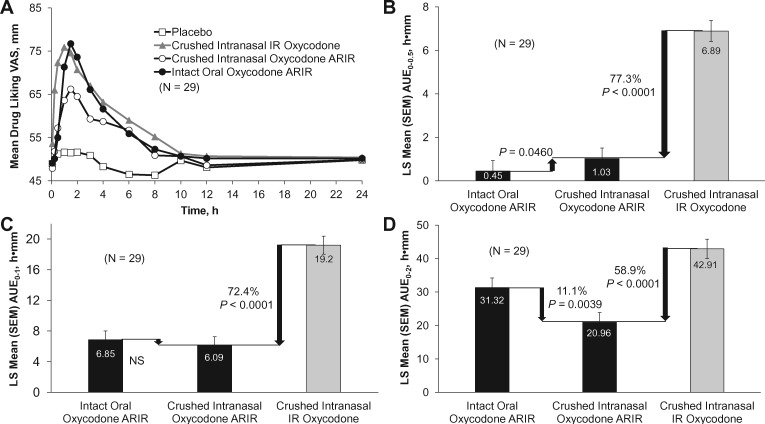
There was a significant reduction of 46.9% in drug liking Emax for crushed intranasal oxycodone ARIR compared with crushed intranasal IR oxycodone (70.74 mm vs 82.67 mm, P < 0.0001) (Figure 2). Drug liking was 23.4% lower for crushed intranasal oxycodone ARIR than for intact oral oxycodone ARIR (P < 0.0001). Drug liking over time for each treatment is shown in Figure 3A. Regarding early mean drug liking area under the drug curve effect (AUE), crushed intranasal oxycodone ARIR was significantly lower for AUE0–0.5 h (77.3% reduction, P < 0.0001), AUE0–1 h (72.4% reduction, P < 0.0001), and AUE0–2 h (58.9% reduction, P < 0.0001) compared with crushed intranasal IR oxycodone (Figure 3B–D). Drug liking also was significantly lower for crushed intranasal oxycodone ARIR than for intact oral oxycodone ARIR at two hours (P = 0.0039).
Figure 2.
Maximum (Emax) drug liking scores. Emax scores are least squares (LS) means ± standard error. The mean percentage reduction was calculated based on (ci – ti)/|ci–pi| × 100%; i = 1, 2, . . . , n. IR = immediate release; SEM = standard error of the mean.
Figure 3.
(A) Mean drug liking over time and early mean drug liking area under the drug curve effect (AUE) (B) over the first 30 minutes (AUE0–0.5), (C) over the first hour (AUE0–1), and (D) over the second hour (AUE0–2). The mean percentage reduction was calculated based on (ci– ti)/|ci – pi| × 100%; i = 1, 2, . . . , n. IR = immediate release; NS = not significant; SEM = standard error of the mean.
Other Drug Effects
Scores for “overall drug liking” and “take drug again” were significantly reduced for crushed intranasal oxycodone ARIR compared with crushed intranasal IR oxycodone or intact oral oxycodone ARIR (P < 0.0001 for all) (Table 2). The study results also showed significant reductions in subjects’ Emax with crushed intranasal oxycodone ARIR for any effects (P < 0.0001), good effects (P < 0.0001), and drug high (P < 0.0001) relative to results for crushed intranasal IR oxycodone and intact oral oxycodone ARIR (Table 2). There were no significant differences between any treatments in assessments of bad effects, sickness, nausea, or dizziness. Additionally, no significant differences were found in any of the secondary end points when comparing crushed intranasal IR oxycodone with intact oral oxycodone ARIR.
Table 2.
Secondary end points (N = 29)
| Crushed Intranasal IR Oxycodone vs Crushed Intranasal Oxycodone ARIR |
Intact Oral Oxycodone ARIR vs Crushed Intranasal Oxycodone ARIR |
Crushed Intranasal IR Oxycodone vs Intact Oral Oxycodone ARIR |
||||
|---|---|---|---|---|---|---|
| Secondary End Point | LS Means, mm | P value | LS Means, mm | P value | LS Means, mm | P Value |
| Overall drug liking | 80.78 vs 63.84 | <0.0001 | 78.16 vs 63.84 | 0.0004 | 80.78 vs 78.16 | NS |
| Take drug again | 82.07 vs 61.83 | <0.0001 | 76.72 vs 61.83 | 0.0014 | 82.07 vs 76.72 | NS |
| Drug effects questionnaire | ||||||
| Any effects | 64.55 vs 38.26 | <0.0001 | 62.79 vs 38.26 | <0.0001 | 64.55 vs 62.79 | NS |
| Good effects | 68.15 vs 39.97 | <0.0001 | 64.06 vs 39.97 | <0.0001 | 68.15 vs 64.06 | NS |
| Drug high | 66.10 vs 38.34 | <0.0001 | 65.78 vs 38.34 | <0.0001 | 66.10 vs 65.78 | NS |
| Bad effects | 12.73 vs 15.73 | NS | 13.22 vs 15.73 | NS | 12.73 vs 13.22 | NS |
| Sick | 7.73 vs 6.39 | NS | 9.49 vs 6.39 | NS | 7.73 vs 9.49 | NS |
| Nausea | 7.36 vs 6.38 | NS | 4.94 vs 6.38 | NS | 7.36 vs 4.94 | NS |
| Dizzy | 9.78 vs 6.86 | NS | 10.38 vs 6.86 | NS | 9.78 vs 10.38 | NS |
IR = immediate release; LS = least squares; NS = not significant.
Subjects found crushed oxycodone ARIR significantly more difficult to snort than crushed IR oxycodone (P < 0.0001). For nasal effects, crushed oxycodone ARIR caused significantly more irritation, burning, runny nose/discharge, facial pain/pressure, and nasal congestion than did crushed IR oxycodone (P < 0.0001 for all).
Pharmacokinetic Assessments
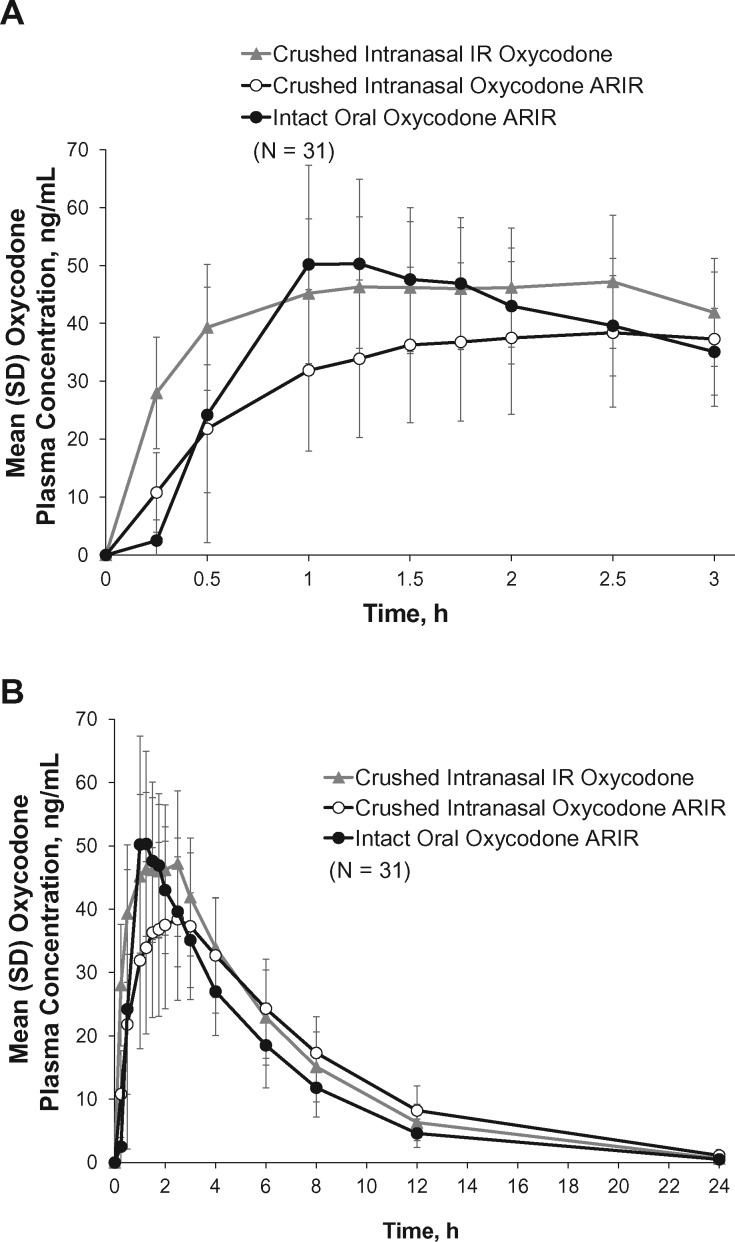
The least squares (LS) mean Cmax was lower for intranasally administered crushed oxycodone ARIR (40.04 ng/mL) than for intranasal administration of crushed IR oxycodone (55.56 ng/mL) and oral administration of intact oxycodone ARIR (56.97 ng/mL) (Table 3, Figure 4). Based on LS means, the oxycodone Cmax was 28% lower for crushed intranasal oxycodone ARIR than for crushed intranasal IR oxycodone. Crushed intranasal oxycodone ARIR exhibited a 35% longer median Tmax relative to crushed intranasal IR oxycodone. Oxycodone plasma concentrations were higher for crushed intranasal IR oxycodone than for crushed intranasal oxycodone ARIR for the first three hours after administration (Figure 4A). From four hours onward, the concentrations were similar and decreased in parallel (Figure 4B). Early oxycodone exposure (AUC0–0.5 h) was 57% lower for crushed intranasal oxycodone ARIR and 78% lower for intact oral oxycodone ARIR than for crushed intranasal IR oxycodone. The AQs (Cmax/Tmax) for crushed intranasal oxycodone ARIR were 43% and 53% lower than that for crushed intranasal IR oxycodone and intact oral oxycodone ARIR, respectively (21.93 vs 38.42 vs 46.81 ng/mL/h, respectively).
Table 3.
Pharmacokinetic parameters for oxycodone after administration of crushed intranasal oxycodone ARIR, crushed intranasal IR oxycodone, or intact oral oxycodone ARIR (N = 31)
| Least Squares Means*,† |
|||
|---|---|---|---|
| Parameter | Intact Oral Oxycodone ARIR | Crushed Intranasal Oxycodone ARIR | Crushed Intranasal IR Oxycodone |
| Cmax, ng/mL | 56.97 | 40.04 | 55.56 |
| Tmax, h | 1.3 | 2.3 | 1.7 |
| AUC0-t, ng • h/mL | 265.38 | 309.21 | 330.77 |
| AUC0-∞, ng • h/mL | 271.98 | 318.82 | 337.91 |
| ke, h−1 | 0.22 | 0.18 | 0.21 |
| t1/2, h | 3.29 | 3.97 | 3.46 |
| AUC0–0.5 h, ng • h/mL | 1.34 | 3.69 | 9.70 |
| AUC0–1, ng • h/mL | 15.57 | 15.04 | 29.84 |
| AUC0–2 h, ng • h/mL | 62.95 | 47.79 | 75.30 |
| AUC0–8 h, ng • h/mL | 211.21 | 212.43 | 254.11 |
| AUC0–12 h, ng • h/mL | 243.39 | 261.31 | 296.30 |
| AUC0–24 h, ng • h/mL | 268.13 | 310.85 | 332.94 |
AUC0–0.5, 0–1, 0–2, 0–8, 0–12, 0–24 = area under the plasma concentration-time curve from 0 h to 0.5 h, 1 h, 2 h, 8 h, 12 h, and 24 h; AUC0-t = area under the plasma concentration-time curve from 0 h to the last measurable concentration above the lower limit of quantification; AUC0-∞ = area under the plasma concentration-time curve from 0 h to infinity; Cmax = maximum observed plasma concentration; IR = immediate release; ke = elimination rate constant; Tmax = time associated with Cmax; t1/2 = half-life.
Values for ke and t1/2 are untransformed.
Values for Tmax are the untransformed median.
Figure 4.
Mean plasma concentration time profile of oxycodone for each treatment (A) for the first three hours postdose and (B) for 24 hours postdose. Error bars represent standard deviation. IR = immediate release.
Safety
At least one AE was experienced by 46.7% of subjects with crushed IR oxycodone, 41.9% of subjects with intact oxycodone ARIR, 26.7% of subjects with crushed oxycodone ARIR, and 3.4% of subjects with placebo. Most treatment-emergent AEs were typical of opioid use or related to intranasal administration of the drugs; the most common AEs were generalized pruritus, nausea, and vomiting (Table 4). All treatments were well tolerated, with most AEs being mild or moderate in severity. No subjects withdrew from the study owing to AEs during the treatment period, and there were no serious AEs or deaths.
Table 4.
Treatment-related adverse events occurring in the treatment period (safety population)
| Subjects, No. (%) |
|||
|---|---|---|---|
| Parameter | Crushed Intranasal IR Oxycodone (N = 30) | Crushed Intranasal Oxycodone ARIR (N = 30) | Intact Oral Oxycodone ARIR (N = 31) |
| Pruritus, generalized | 7 (23.3) | 2 (6.7) | 4 (12.9) |
| Nausea | 4 (13.3) | 4 (13.3) | 2 (6.5) |
| Vomiting | 2 (6.7) | 3 (10.0) | 2 (6.5) |
| Pruritus | 0 | 0 | 4 (12.9) |
| Headache | 1 (3.3) | 0 | 1 (3.2) |
| Hiccups | 2 (6.7) | 0 | 0 |
| Irritability | 2 (6.7) | 0 | 0 |
| Dizziness | 0 | 1 (3.3) | 0 |
| Eye pain | 0 | 1 (3.3) | 0 |
| Hot flush | 1 (3.3) | 0 | 1 (3.2) |
| Lacrimation increased | 0 | 1 (3.3) | 0 |
| Muscle spasms | 0 | 0 | 1 (3.2) |
| Nasal congestion | 0 | 1 (3.3) | 0 |
| Retching | 0 | 1 (3.3) | 0 |
| Somnolence | 1 (3.3) | 0 | 0 |
IR = immediate release.
Discussion
The introduction of an abuse-deterrent ER opioid formulation led to a significant reduction in reports of misuse and abuse of ER oxycodone [1]. However, early epidemiologic evidence suggests that this decrease was accompanied by a concomitant increase in abuse of non-abuse-deterrent formulations, such as IR oxycodone [1]. Abuse-deterrent ER opioid formulations are designed primarily to prevent dose-dumping and deter the abuser from converting an ER opioid to an IR opioid. Because IR opioids are much more commonly prescribed, diverted, and abused than ER opioids [2,3], and are often abused by means of nonoral routes of administration [5,6,8,11], abuse-deterrent formulations of IR opioids are needed. However, this is a difficult task, given that IR opioids are formulated for fast release of the active ingredient to provide immediate pain relief to patients. Ideally, an abuse-deterrent IR opioid formulation would need to hinder or slow extraction on tampering, as well as deter administration through alternative, and riskier, routes (e.g., intranasal, IV) by making physical manipulation more difficult and achieving lower and slower release of the opioid through nonoral routes of abuse.
Oxycodone ARIR is the first and only abuse-deterrent IR opioid formulation approved by the US Food and Drug Administration. In vitro studies have demonstrated that oxycodone ARIR tablets are difficult to physically manipulate, and the drug resists extraction by means of commonly used household and laboratory solvents, as well as passage through a needle because a viscous material is formed when placed in a liquid environment [14,15]. Results of this intranasal human abuse potential study indicate that crushed intranasal oxycodone ARIR is associated with a significant reduction in maximum drug liking, early drug liking, and take drug again scores relative to crushed intranasal IR oxycodone. Drug liking also was significantly lower for crushed intranasal oxycodone ARIR compared with intact oral oxycodone ARIR. Furthermore, crushed intranasal oxycodone ARIR exhibited lower peak oxycodone plasma concentrations and slower time to peak concentration compared with intact oral oxycodone ARIR and crushed intranasal IR oxycodone. Crushed intranasal oxycodone ARIR exhibited a lower AQ compared with both crushed intranasal IR oxycodone and intact oral oxycodone ARIR. Taken together, these data indicate that physical manipulation and intranasal administration of oxycodone ARIR tablets slow the IR characteristics of the drug, thereby significantly reducing maximum drug liking, early drug liking, and other PD factors including desire to take the drug again, any effects, good effects, and drug high.
Evaluating the results of human abuse potential studies can be challenging because they rely heavily on the self-reported subjective effects of the drugs and the associated variability inherent in the test measures themselves. Conclusively translating these data to a real-world population is also difficult. However, a meta-analysis reported that a 5-mm reduction in overall drug-liking Emax for an abuse-deterrent formulation of ER oxycodone would be expected to produce a 10.1% reduction in the nonmedical use rate [18]. Here we report that crushed intranasal oxycodone ARIR led to a 16-mm reduction in overall drug liking Emax compared with crushed intranasal IR oxycodone. Another group reported that a clinically important difference in drug high Emax constituted an 8.8- to 10.2-mm difference between treatments [19]. In this intranasal human abuse potential study, crushed intranasal oxycodone ARIR exhibited a 28-mm lower drug high Emax than did crushed intranasal IR oxycodone. These data strongly support the notion that oxycodone ARIR has the potential to reduce nonmedical use; however, long-term epidemiologic studies are needed to assess the real-world abuse potential of oxycodone ARIR.
The safety profile of all treatments in this study was consistent with the known AE profile associated with use of opioid-containing medications and intranasal administration the most common AEs were generalized pruritus, nausea, and vomiting. Crushed intranasal oxycodone ARIR tended to be associated with fewer AEs than did intact oral oxycodone ARIR and crushed intranasal IR oxycodone.
Because most opioid abusers begin by using IR products [5,20], abuse-deterrent IR opioid formulations such as oxycodone ARIR may deter abuse and hopefully prevent progression to more dangerous routes of abuse. However, as long as non-abuse-deterrent opioids are available, abusers might migrate to these or other illicit drugs (e.g., heroin) [21,22]. Abuse-deterrent opioid formulations are only one component of a comprehensive opioid risk management plan—including provisions for prescription drug monitoring programs, Centers for Disease Control and Prevention guidelines, prescribing guidelines, safe disposal guidelines, physician and patient education, and substance abuse treatment plans, among others—that requires multiple stakeholders to play a role in reducing opioid abuse. As always, maintaining judicious prescribing habits is important as misuse of abuse-deterrent IR formulations through intranasal, IV, or oral routes is still possible, including ingesting higher-than-prescribed numbers of intact tablets.
Conclusions
The results of this intranasal human abuse potential study indicate that crushed intranasal oxycodone ARIR is associated with lower and slower oxycodone plasma concentrations concurrent with significantly reduced drug liking compared with crushed intranasal IR oxycodone and intact oral oxycodone ARIR. Taken together, these data indicate that oxycodone ARIR has the potential to reduce misuse and abuse via the intranasal route of administration.
Acknowledgments
The authors acknowledge Manish Shah and Ray DiFalco of Cerovene for their role in this study. Medical writing assistance (funded by Daiichi Sankyo, Inc.) was provided by Kelly M. Cameron, PhD, ISMPP CMPP, of JB Ashtin, who developed the first draft based on an author-approved outline and assisted in incorporating author revisions.
Funding sources: This study was funded by Inspirion Delivery Sciences, LLC (Morristown, NJ, USA). Medical writing assistance was funded by Daiichi Sankyo, Inc. (Basking Ridge, NJ, USA)
Disclosure and conflicts of interest: Dr. Webster is an employee of PRA Health Sciences; he has received honoraria for consulting or service on advisory boards from Alcobra, Daiichi Sankyo, Egalet, Elysium, Inspirion, Insys, Kempharm, Pain Therapeutics, Pfizer, Shionogi, and Teva. Dr. Smith was an employee of PRA Health Sciences at the time of the study; he is currently an employee of KalVista Pharmaceuticals, Inc. Ms. Pantaleon, Mr. Iverson, Dr. Kinzler, and Dr. Aigner are full-time employees of, or consultants for, Inspirion Delivery Sciences, LLC.
References
- 1. Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF.. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med 2014;15(3):440–51. [DOI] [PubMed] [Google Scholar]
- 2. Iwanicki JL, Severtson SG, McDaniel H, et al. Abuse and diversion of immediate release opioid analgesics as compared to extended release formulations in the United States. PLoS One 2016;11(12):e0167499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cicero TJ, Ellis MS, Kasper ZA.. Relative preferences in the abuse of immediate-release versus extended-release opioids in a sample of treatment-seeking opioid abusers. Pharmacoepidemiol Drug Saf 2017;26(1):56–62. [DOI] [PubMed] [Google Scholar]
- 4. Green JL, Bucher Bartelson B, Le Lait MC, et al. Medical outcomes associated with prescription opioid abuse via oral and non-oral routes of administration. Drug Alcohol Depend 2017;175:140–5. [DOI] [PubMed] [Google Scholar]
- 5. Budman SH, Grimes Serrano JM, Butler SF.. Can abuse deterrent formulations make a difference? Expectation and speculation. Harm Reduct J 2009;6(1):8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH.. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J 2011;8(1):29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D.. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction 2012;107(7):1318–27. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. HIV surveillance report, 2015, volume 27. 2016. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed November 2017).
- 9. Conrad C, Bradley HM, Broz D, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015;64:443–4. [PMC free article] [PubMed] [Google Scholar]
- 10. Greene D. Total necrosis of the intranasal structures and soft palate as a result of nasal inhalation of crushed OxyContin. Ear Nose Throat J 2005;84:512, 514, 516. [PubMed] [Google Scholar]
- 11. Lankenau SE, Kecojevic A, Silva K.. Associations between prescription opioid injection and hepatitis C virus among young injection drug users. Drugs (Abingdon Engl) 2015;22(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surratt H, Kurtz SP, Cicero TJ.. Alternate routes of administration and risk for HIV among prescription opioid abusers. J Addict Dis 2011;30(4):334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yewell J, Haydon R, Archer S, Manaligod JM.. Complications of intranasal prescription narcotic abuse. Ann Otol Rhinol Laryngol 2002;111(2):174–7. [DOI] [PubMed] [Google Scholar]
- 14. Kinzler ER, DiFalco R, Pantaleon C, Iverson M, Aigner S.. In vitro evaluation of a novel immediate release formulation of oxycodone (RoxyBond™) for the potential for abuse via injection. Postgrad Med 2016;128(suppl 2):46–7.26635068 [Google Scholar]
- 15. Kinzler ER, Pantaleon C, Iverson M, Aigner S.. Oxycodone ARIR (RoxyBond™) is resistant to physical manipulation techniques commonly used by opioid abusers. Postgrad Med 2017;129(suppl 1):16. [Google Scholar]
- 16. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T 2012;37:412–8. [PMC free article] [PubMed] [Google Scholar]
- 17. Webster LR, Bath B, Medve RA.. Opioid formulations in development designed to curtail abuse: Who is the target? Expert Opin Investig Drugs 2009;18(3):255–63. [DOI] [PubMed] [Google Scholar]
- 18. White AG, LeCates J, Birnbaum HG, et al. Positive subjective measures in abuse liability studies and real-world nonmedical use: Potential impact of abuse-deterrent opioids on rates of nonmedical use and associated healthcare costs. J Opioid Manag 2015;11(3):199–210. [DOI] [PubMed] [Google Scholar]
- 19. Eaton TA, Comer SD, Revicki DA, et al. Determining the clinically important difference in visual analog scale scores in abuse liability studies evaluating novel opioid formulations. Qual Life Res 2012;21(6):975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy 2012;23(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coplan PM, Kale H, Sandstrom L, Landau C, Chilcoat HD.. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol Drug Saf 2013;22(12):1274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372(3):241–8. [DOI] [PubMed] [Google Scholar]