Abstract
BACKGROUND:
Glial Fibrillary Acidic Protein (GFAP) is a protein produced by astrocytes in response to brain injury, which then penetrates the cerebrospinal fluid and the blood stream.
AIM:
We sought to determine whether GFAP serum level in acute ischemic stroke could predict clinical outcome.
METHODS:
As much as 64 patients with first-ever ischemic stroke had their GFAP serum level measured at 72 hours after onset. The National Institute of Health Stroke Scale (NIHSS) was assessed during the 72 hours of onset, the seventh day, and followed up 1 month after.
RESULTS:
There were 46 men and 18 women included in the study. Mean age was 58.3 years old, and nearly half of them (46.9%) were between 50-59 years old. More than half (58.7%) presented with moderate to a severe stroke and mean GFAP serum level was 0.113 ± 0.029 ng/mL. GFAP serum levels had a significant correlation with NIHSS after 1 month (p = 0.04, r = 0.259).
CONCLUSION:
There is a significant correlation between GFAP serum levels with stroke severity scale after 1 month of stroke onset.
Keywords: GFAP, Clinical outcome, Ischemic stroke
Introduction
As the third leading cause of morbidity and disability in the world, the global burden of stroke-affected all populations throughout countries worldwide [1], [2]. In Indonesia stroke is the leading cause of death, with a prevalence of 12 per mil. It is estimated that the tendency of increasing prevalence would also increase the burden of disease, and further decrease the nation’s productivity and economic capacity [3], [4], [5].
GFAP is a specific intermediate filament type III produce by astrocytes. In brain injury, astrocytes will release GFAP which then penetrates the cerebrospinal fluids (CSF) and blood stream. Coherently, the extent of infarct volume should correlate to GFAP level. GFAP concentration in serum is higher in haemorrhagic stroke compared to ischemic stroke. Several studies show that an increase in GFAP serum concentrations in acute stroke shows an unfavourable outcome [1], [6].
The difference between populations and stroke severity may produce a different result. Hence we sought to determine whether GFAP serum concentrations in 72 hours of onset would correlate with patient’s neurological deficit at that time, day 7 and 1 month later.
Methods
Patients with acute ischemic stroke, the onset of ≤ 72 hours were included in the study. Patient’s demographic data and stroke risk factors were documented. Neurologic deficits were assessed using The National Institute of Health Stroke Scale (NIHSS) at that time and followed up after 1 month of onset. All patients were treated with antiplatelet and statins along with early rehabilitation. Patients with a history of previous stroke, traumatic brain injury, brain tumour, infection, degenerative brain disease, liver failure, kidney failure, chronic heart disease and acute myocardial infarct were excluded. Patients receiving thrombolytic were also excluded.
All patients underwent brain MRI 1.5T to confirm ischemic stroke. A blood sample was taken ≤ 72 hours of onset. As much as 3 cc were drawn and inserted in a tube. The tube was kept at room temperature for 30-45 minutes, then centrifuged 3000 rpm for 15 minutes. Blood was then divided into 3 sample cup as much as 0.3 cc each. Sample cup was labelled then stored at -70°C. All sample cups were sent to the laboratory using a cool box with dry ice. GFAP biomarker was measured using Quantitative Enzyme Immunoassay (ELISA) method.
Data analysis
Data were collected using the provided research form. Data was then edited, coded and organised using SPSS version 20.0 software. Statistical analysis was done using the statistic test which appropriates with research variables measurement scale. Spearman correlation test was used to test the correlation between these 2 variables. The significant level of this analysis was set at 5%.
Ethical clearance
Ethical clearance was obtained from the Mochtar Riady Institute for Nanotechnology Ethics Committee Number 072/MRIN-EC/08/2014.
Results
As much as 64 patients were included in the study. Mean age was 58.8 years old with male predominance (62%). Most patients were between 50-59 years old. Almost all patients have hypertension (93.75%), and most came with Lacunar anterior circulation infarct (LACI). Over half of the patients had moderate NIHSS (Table 1).
Table 1.
Demographic data and patients’ characteristics
| Variable | N | % |
|---|---|---|
| Gender | ||
| Male | 46 | 71.88 |
| Female | 18 | 28.13 |
| Age group (years) | ||
| 30-39 | 1 | 1.56 |
| 40-49 | 10 | 15.63 |
| 50-59 | 30 | 46.88 |
| 60-69 | 10 | 15.63 |
| 70-79 | 11 | 17.19 |
| 80-89 | 2 | 3.13 |
| BMI | ||
| Underweight | 1 | 1.56 |
| Normal | 36 | 56.25 |
| Overweight | 20 | 31.25 |
| Obese | 7 | 10.94 |
| Diabetes | ||
| Yes | 17 | 26.56 |
| No | 47 | 73.44 |
| Hypertension | ||
| Yes | 60 | 93.75 |
| No | 4 | 6.25 |
| Dyslipidemia | ||
| Yes | 53 | 82.81 |
| No | 11 | 17.19 |
| Smoking | ||
| Yes | 17 | 26.56 |
| No | 47 | 73.44 |
| Atrial fibrillation | ||
| Yes | 5 | 7.81 |
| No | 59 | 92.19 |
| Type of Stroke (Bamford) | ||
| LACI | 49 | 76.56 |
| Non-LACI | 15 | 23.43 |
Stroke severity was assessed using the NIHSS, categorised as mild if NIHSS was 0-3, moderate if NIHHS was 4-15 and severe if NIHSS was 16-42. NIHSS was scored in 72 onsets, at day 7 of onset, and after 30 days of onset, as seen in Table 2. Mean NIHSS is shown in Figure 1.
Table 2.
Stroke severity scale of patients using NIHSS
| Stroke Severity Scale | n | Percentage (%) | |
|---|---|---|---|
| NIHSS 72 hours | |||
| Mild (0-3) | 24 | 37.5 | |
| Moderate (4-15) | 38 | 59.4 | |
| Severe (16-42) | 2 | 3.1 | |
| NIHSS day 7 | |||
| Mild (0-3) | 35 | 54.7 | |
| Moderate (4-15) | 28 | 43.8 | |
| Severe (16-42) | 1 | 1.6 | |
| NIHSS day 30 | |||
| Mild (0-3) | 55 | 85.9 | |
| Moderate (4-15) | 9 | 14.1 | |
| Severe (16-42) | 0 | 0 | |
Figure 1.
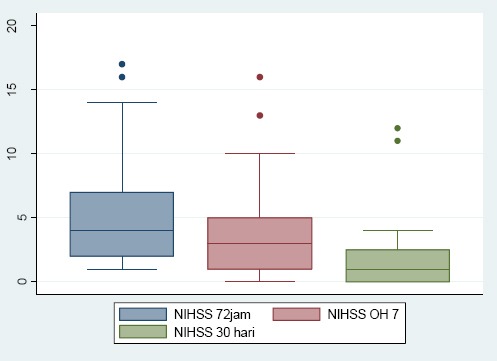
Mean NIHSS on 72 hours of onset, day 7 and day 30
In 72 hours of stroke onset, serum GFAP was sampled in 64 patients, and the mean value was 0.113 ± 0.029 (0.011-0.248) ng/L.
Spearman correlation test was utilised to see if there was a correlation between GFAP serum concentration in the acute phase and stroke severity scale at 72 hours, day 7, and day 30. GFAP serum had a positive correlation to stroke severity on 72 hours, day 7 and day 30. This correlation seems to be more intense towards day 30 (Table 3).
Table 3.
Correlation between GFAP serum concentration and stroke severity
| NIHSS 72 hours | NIHSS day 7 | NIHSS day 30 | |
|---|---|---|---|
| r | 0.223 | 0.255 | 0.266 |
| p | 0.076 | 0.042 | 0.034 |
To analyse the relevancy of GFAP level in the acute phase and stroke severity, we set a cut-off value using Roc analysis (area under the curve/ AUC), (Table 4).
Table 4.
Roc Analysis of GFAP serum level and NIHSS
| Stroke Severity | Cut Off | Sen (%) | Spe (%) | LR+ | LR- | AUC |
|---|---|---|---|---|---|---|
| NIHSS 72 hours | ≥ 0.112 | 55.26 | 72.7 | 2.02 | 0.61 | 0.65 |
| NIHSS day 7 | ≥ 0.112 | 68 | 69.7 | 2.24 | 0.46 | 0.71 |
| NIHSS day 30 | ≥ 0.114 | 66.67 | 74 | 2.56 | 0.45 | 0.66 |
By obtaining this cut-off, GFAP serum level was categorised into low and high. We perform another correlation test using Spearman to know the correlation between the level of GFAP and categorised stroke severity. After the stroke severity was categorised as mild and moderate/severe, the relation is most robust when compared to day 7 (Table 5).
Table 5.
Correlation between categorised level of GFAP and categorised stroke severity
| Stroke Severity | Correlation | ||||
|---|---|---|---|---|---|
| Mild | Moderate-Severe | r | p | ||
| 72 hours | < 0.112 | 19 | 18 | 0.071 | 0.018 |
| ≥ 0.112 | 6 | 21 | |||
| Day 7 | < 0.112 | 25 | 12 | 0.303 | 0.015 |
| ≥ 0.112 | 10 | 17 | |||
| Day 30 | < 0.114 | 40 | 5 | 0.131 | 0.303 |
| ≥ 0.114 | 15 | 4 | |||
The level of GFAP serum shows a positive correlation towards stroke severity in 72 hours and day 7. However, this correlation was not seen in NIHSS day 30.
Discussion
Astrocyte is a special glial cell which is abundant and reacted to brain insult known as “reactive astrogliosis”, which act as a sign of structural damage [7].
In the event of ischemic stroke, oxygen levels in brain tissue decreased. This disrupts the viability of surrounding tissues, damaging neurons, astrocytes and blood-brain barrier. The destruction of astrocytes releases GFAP protein which is an astrocyte’s cytoskeleton. GFAP enters the blood vessels through a damaged blood-brain barrier [8]. GFAP is a part of astrocyte cytoskeletons and has big molecular size (40-54 kDa). The more extended the damage, the higher the elevation of serum GFAP. The higher concentration is commonly seen in intracerebral haemorrhage rather than ischemic stroke [9]. In this study, we sought to understand the pathomechanism of brain injury in acute stroke by analysing GFAP – a product of astrocyte and looking into stroke severity after 1 month of onset.
This study consists of 64 acute stroke patients, and most frequent risk factors were hypertension, dyslipidemia, and smoking. This finding has a similar profile compared with the previous study by Kusuma in 2009 and Mihardja in 2016. Most strokes have the clinical syndrome of LACI (Lacunar Cerebral Infarct) as seen in Table 1 [3], [10]. Most of the patients came with moderate stroke severity (Table 2). During follow up, the percentage of moderate and severe stroke decreased, and by day 30, the majority of patients had a mild stroke (Figure 1). Traditionally, lacunar infarcts were considered to have a more favourable outcome. However, in 2003 Norrving in his research found that the case fatality rate increased at long term follow up. Patients with lacunar infarct also had an increased risk for recurrence and high risks for cognitive impairment and dementia. Silent lacunae also double the risk of dementia in the elderly. And for that, lacunar infarct should be considered an ominous marker of small-vessel disease of the brain. In this study, follow up was carried out until day 30 and it may be equitable that we acquire a decent outcome [11], [12].
In less than 72 hours, GFAP serum concentration was 0.113 ± 0.029 ng/L. This finding does not differ much from a study by Wunderlich in 2006, which found a mean GFAP of 0.15 ng/mL [11]. Hermann in 2000 discovered that the highest level of GFAP was on day 4, whereas Wunderlich found that on 48 hours of stroke onset GFAP was at its highest, and stayed high until day 5 [13], [14].
Several studies have tried to obtain GFAP cutoff in various brain injury. Ren in 2016 found that 0.34 ng/ml is a cutoff to differentiate between ischemic and hemorrhagic stroke, and also associated with stroke severity [1]. Nylen in 2007 uses 0.15 ng/mL as a cutoff to predict unfavourable outcome in aneurysmal subarachnoid haemorrhage [15]. Okonkwo in 2013 obtained optimal cutoff of 0.68 ng/mL to distinguish the presence and severity of CT Scan findings in traumatic brain injury patients [16].
In this study, we obtain a cut off value for GFAP using Roc analysis and then analyse stroke severity using those cut-offs (Table 4 and Table 5). GFAP cut-off for 72 hours and day 7 was 0.112 and at day 30 was 0.114. This cutoff is relatively lower than the previous study; however, seems reasonable considering the different research design and cases.
After we categorised the level of GFAP, it shows a positive correlation with stroke severity at 72 hours and on day 7. This shows that in acute stroke until day 7, serum GFAP of 0.112 ng/mL and more is considered high, and correlates with more severe stroke on 72 hours until day 7.
Spearman test shows a positive correlation between serum GFAP at less than 72 hours and stroke severity on day 7 and day 30. The correlation seems to be more substantial towards day 30 (Table 3). This finding was consistent with Wunderlich who acclaimed that serum GFAP 96 hours after stroke onset correlates with clinical severity 3 months later [13]. This finding shows that patients with higher serum GFAP does not necessarily have higher stroke severity on admission, but shows more severe stroke on follow up at day 7 and day 30. The more intense correlation towards day 30 made us believe that if we had extended the follow up to a much longer period, the correlation might have been more robust.
In conclusion, GFAP serum concentration in acute ischemic stroke has a positive correlation with clinical severity stroke scale in acute settings (< 72 hours), day 7, and day 30. GFAP could be utilised as a predictor of clinical outcome 1 month after stroke. We propose a GFAP serum level of 0.112 ng/mL as a cut off for high-level GFAP serum concentration, which might predict stroke severity until day 7.
There are some limitations to this study. The sample size was rather small, and GFAP concentration was measured only once at the acute phase. Stroke severity was also analysed using only one stroke scale, without integrating cognitive assessment. Follow up was done only 30 days after, which may reveal a different result if it had been longer.
We expect other studies in the future that included all the aspects of our limitations and hopefully broaden our understanding of GFAP in stroke cases.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Ren C, et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Scientific Reports. 2016;6:24588. doi: 10.1038/srep24588. https://doi.org/10.1038/srep24588 PMid:27074724 PMCid:PMC4830936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojaghihaghighi S, Vahdati SS, Mikaeilpour A, Ramouz A. Comparison of neurological clinical manifestation in patient with hemorrhagic and ischemic stroke. World J Emerg Med. 2017;8(1):34–8. doi: 10.5847/wjem.j.1920-8642.2017.01.006. https://doi.org/10.5847/wjem.j.1920-8642.2017.01.006 PMid:28123618 PMCid:PMC5263033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusuma Y, Venketasubramanian Kiemas LS, Misbach J. Burden of stroke in Indonesia. Int J Stroke. 2009;4(5):379–80. doi: 10.1111/j.1747-4949.2009.00326.x. https://doi.org/10.1111/j.1747-4949.2009.00326.x PMid:19765126. [DOI] [PubMed] [Google Scholar]
- 4.Riset Kesehatan Dasar. Badan Penelitian dan Pengembangan Kesehatan Kementrian Kesehatan. 2013;3:91. [Google Scholar]
- 5.Strong K, Matheus C, Bonita R. Preventing Stroke:sacing lives around the world. Lancet Neurology. 2007;6:182–7. doi: 10.1016/S1474-4422(07)70031-5. https://doi.org/10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 6.Foerch C, Kurnaz IA, Ozilgen M. Astrocyte-neuron lactate shuttle may boost more ATP supply to the neuron under hypoxic condition. BMC Syst Biol. 2009;5:162. doi: 10.1186/1752-0509-5-162. https://doi.org/10.1186/1752-0509-5-162 PMid:21995951 PMCid:PMC3202240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofroniew MV, Vinters HV. Astrocytes:biology and pathology. Acta Neuropathological. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. https://doi.org/10.1007/s00401-009-0619-8 PMid:20012068 PMCid:PMC2799634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MH, et al. Clinical usefulness of Serum Glial Fibrillary Acidic Protein in Patients with Acute Ischemic Stroke. Journal of Neurocritical Care. 2009;2(2):50–5. [Google Scholar]
- 9.Surjawan Y, As'ad S, Ranakusuma TAS, Wijaya A. The different pattern of blood S100B protein and GFAP concentrations in ischemic stroke. Med J Indones. 2013;22:215–20. https://doi.org/10.13181/mji.v22i4.602. [Google Scholar]
- 10.Mihardja L, et al. Death rate and risk factors of stroke as underlying cause of death in Padang Pariaman district, West Sumatera province. Buletin Penelitian Kesehatan. 2016;44(4):227–36. [Google Scholar]
- 11.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2(4):238–45. doi: 10.1016/s1474-4422(03)00352-1. https://doi.org/10.1016/S1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Prins ND, denHeijer T, Hofman A, Koudstaal PJ, Breteler M. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22. doi: 10.1056/NEJMoa022066. https://doi.org/10.1056/NEJMoa022066 PMid:12660385. [DOI] [PubMed] [Google Scholar]
- 13.Wunderlich MT, Wallesch CW, Goertler M. Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. European journal of Neurology. 2006;13:1118–23. doi: 10.1111/j.1468-1331.2006.01435.x. https://doi.org/10.1111/j.1468-1331.2006.01435.x PMid:16987165. [DOI] [PubMed] [Google Scholar]
- 14.Hermann M, Vos P, Wunderlich MT, de Brujin CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke:A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31(11):2670–7. doi: 10.1161/01.str.31.11.2670. https://doi.org/10.1161/01.STR.31.11.2670. [DOI] [PubMed] [Google Scholar]
- 15.Nylén K, et al. Serum glial fibrillary acidic protein is related to focal brain injury and outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:1489–94. doi: 10.1161/STROKEAHA.106.478362. https://doi.org/10.1161/STROKEAHA.106.478362 PMid:17395862. [DOI] [PubMed] [Google Scholar]
- 16.Okonkwo DO, et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury:results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma. 2013;30(17):1490–7. doi: 10.1089/neu.2013.2883. https://doi.org/10.1089/neu.2013.2883 PMid:23489259 PMCid:PMC3751263. [DOI] [PMC free article] [PubMed] [Google Scholar]


