Abstract
BACKGROUND:
Minor recurrent aphthous stomatitis (MiRAS) is one of the most common, recurrent, and painful mucosal pathological condition. It is characterised by round or shallow oval ulcers, less than 10 mm in diameter, surrounded by a thin erythematous halo. It involves non-keratinized mucosa such as the labial and buccal mucosa, the ventral surface or borders of the tongue and the floor of the mouth, but it is uncommon to occur on the keratinised mucosa. It heals spontaneously within 10-14 days without scarring. There is no curative remedy to prevent its recurrence; also, available modalities only reduce the symptoms and severity of the lesion.
AIM:
Since these lesions may be extremely painful, we decided to estimate the pain-relieving and healing properties of low energy level laser therapy using diode laser 660 nm on MiRAS.
MATERIAL AND METHODS:
Twenty healthy patients suffering from minor aphthous ulcers were randomly selected from the Out-Patient Clinic of Oral Medicine Department, Faculty of Dentistry, Alexandria University. They were equally divided into two groups, study group who received 660 nm diode laser irradiation while the control group received placebo (sodium bicarbonate rinse). The visual analogue scale, size reduction, effectiveness indices and functional disorders were compared between the groups.
RESULTS:
Both groups presented a statistically significant difference from baseline to follow up periods. But, diode laser 660 nm treatment showed more remarkable improvements in reduction of healing time, pain and lesion size.
CONCLUSION:
We concluded that diode laser 660 nm should be further considered as an effective alternative therapeutic regimen to patients who suffer from recurrent aphthous stomatitis.
Keywords: Minor Aphthous Ulcers, Recurrent Aphthous Stomatitis Treatment, Diode Laser 660 nm
Introduction
The minor recurrent aphthous stomatitis (MiRAS) is one of the most common forms of the recurrent aphthous stomatitis (RAS) [1], [2]. It has a higher incidence in children and adult females [3], [4] with a notable association with the socioeconomic status [5]. It is known as a pathological condition which is presented by painful recurrent rounded or ovoid shallow mucosal ulceration, less than 10 mm in diameter, with yellow or grey floor surrounded by thin erythematous halo margins [2], [3]. It usually includes the non-keratinized mucosa such as the labial and buccal mucosa, the ventral surface and lateral borders of the tongue and the floor of the mouth. The exposure of nerve endings leads to pain, burning or soreness sensation [6] which affects the patient’s ability to eating and speaking. It heals spontaneously within 10-14 days; it recovers without leaving scars [2].
Although many studies have been devoted to clarifying the aetiology and pathogenesis of RAS revealing the relation to cell-mediated mechanisms [4], the specific immunopathogenesis is unknown [7]. However, local and systemic factors, genetic impacts, immunologic influences, and microbial causes may play a role in the pathogenesis of RAS. Since the aetiology is unidentified, the diagnosis is completely based on the history and clinical findings [8].
There is no curative remedy to avoid the recurrence of these ulcers; its management depends on the supportive protocol targeting the relief of RAS symptoms [8]. Though, varies medications have been developed including local and systemic anti-inflammatory, antibacterial and immunosuppressive agents, patients complained of considerable discomfort [8].
Recently, the application of the low-level laser therapy (LLLT) has been expanding for healing and pain control of various pathologic conditions [9], [10]. LLLT emits the light of a single wavelength through non-thermal or photochemical cell reactions which are called biostimulation reaction. Once the target cells have absorbed these photons, a cascade of biochemical events happens inducing rapid wound healing [11].
The diode laser is one of the LLLT where semi-conductor diode acts as the active medium to emit light [12]. It provides more benefits than other lasers because it has a small size, a wide range of spectrum and transmission through fibre optics [13]. Their application produces biomodulatory and analgesic effects on the tissue without thermal damage or ablation [14]. As these lesions may be tremendously painful, we aimed to estimate the pain-relieving ability and healing efficiency of LLLT using diode laser 660 nm on MiRAS as easy, less time-consuming and non-chemical therapy.
The purpose of this study was to evaluate the clinical effect of diode laser on the treatment of minor aphthous ulcer (MRAS).
Material and Methods
The study proposal was evaluated and accepted by the Ethical Committee of Faculty of Dentistry, Alexandria University, Alexandria, Egypt. Informed consent was accomplished after delivering all information to the study cases.
Recruitment of subjects
Twenty patients with clinically diagnosed MiRAS of both sexes were selected from the outpatient clinics of the Oral Medicine Department, Faculty of Dentistry, Alexandria University in randomised parallel study design. They were chosen according to the following criteria.
Inclusion criteria
- Patients’ age ranges from 20 to 40 years.
- Patients had minor aphthous ulcers with onset less than two days.
- No treatment with any modality for at least 1 month before the start of the clinical trial.
- Willingness to participate in the present clinical study.
Exclusion criteria
- Patients who had physiological or mental diseases.
- Patient under anticoagulants, anti-inflammatory or immunosuppressant medications.
- Pregnant or postmenopausal females.
- Smoker patients.
Pre-study assessment
Detailed pre-operative data were documented from all allocated patients including their names, ages, occupations, medical histories and dental histories. Also, the onset, duration and severity of the pain of the ulcer were reported. Extra-oral examinations were done including the dermal and ocular examinations to exclude Behçet syndrome. However, the intraoral examination and diagnosis of MiRAS were based on clinical features of solitary shallow round mucosal ulcers surrounded by inflammatory halos, with the size of less than 10 mm in diameter [4] and positive history of recurrence.
Study procedure
A randomised selection was made to the included patients, and they were separated into two equal groups; Group A (study group) contained ten patients who received diode laser treatment. They were guided to wear protective eyeglasses matched to our laser wavelength to avoid radiation hazards. Then, diode laser (wavelength 660 nm, intensity 100-130 mW/cm, energy 4 J/cm2) was performed in the first visit only as shown in Figure 1. The laser beam was delivered using an optical fibre of 2 mm diameter which was applied perpendicularly to the mucosa. The ulcer and 2 mm of its surrounding borders were illuminated with several spots (40 sec/spot). The technique was painless and did not necessitate any anaesthesia. While Group B (control group) included ten patients who received a placebo. They were instructed to rinse a diluted sodium bicarbonate oral 4 times/day (one teaspoon in a medium glass of water).
Figure 1.
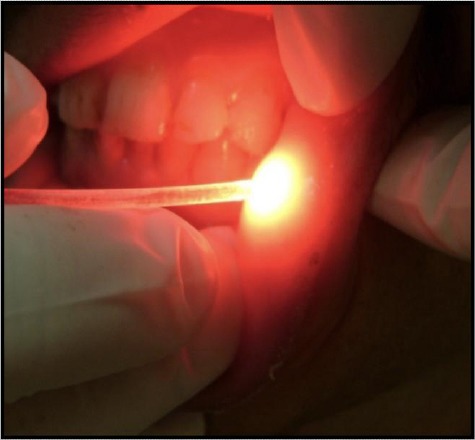
Minor aphthous lesion during phototherapy using diode laser 660 nm
Clinical Evaluation
The responses of group A and group B were assessed according to symptoms (pain) scores and sign scores (lesion size) using:
A) Subjective pain assessment of the ulcer
A visual analogue scale (VAS) was used consisting of a 10-cm horizontal line starting with a pole of no pain to reach the end pole presenting the unbearable pain [15]. Individuals were requested to mark the point on the horizontal line that best signifies their pain level.
B) Objective assessment of the ulcer sizes
The index ulcer’s size was estimated on the treatment of day one, four and six. The maximum and minimum diameters of an oval-shaped ulcer were measured by a calibrated periodontal probe (University of Michigan 0 probe) as shown in Figure 2. Then, the two dimensions were calculated to exemplify the cross-sectional areas of the lesion.
Figure 2.
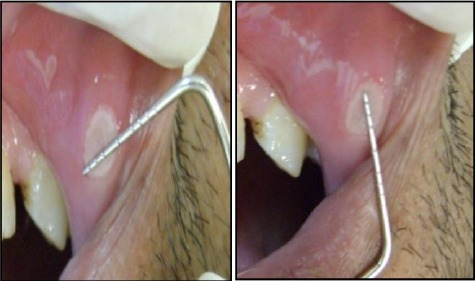
The measurements of the largest and smallest diameters of MiRAS on the labial mucosa of the upper lip, using a calibrated periodontal probe
C) The effectiveness indices (EI) assessment of healing
The effectiveness indices (EI) of the pain improvement and ulcer size were analyzed with a formula EI = [(V1 – V4 or V6) ÷ V1] × 100 % (16) where V4 and V6 referred to the values which were measured at day 4 visit and day 6 visit, while V1 referred to the baseline value which was measured before the study entry.
The effectiveness indices were evaluated on a 4-Rank scale [17]:
Rank (1): Heal: EI ≥ 95%.
Rank (2): Marked improvement: El < 95%, but ≥ 70%.
Rank (3): Moderate improvement: EI < 70%, but ≥ 30%.
Rank (4): No improvement: EI < 30%.
D) Post-operative complications’ assessment
Participants were asked to grade the VAS of the severity of their postoperative complications (such as the presence of pain, oedema, and functional disorders) in numbers from 1 to 10 in the first, second and third visits, according to the following:
1-2 no postoperative complications;
3-5 mild postoperative complications;
5-7 moderate postoperative complications;
8-10 severe postoperative complications.
The patients were reviewed for follow up periods from day 1 to day 6. Clinical photographs were taken before treatment and during follow up periods.
Statistical analysis
Statistical analyses were implemented by the SPSS software (SPSS Inc, Chicago, IL). The Kolmogorov–Smirnov test was performed to analyse the data distribution. Results were expressed as median or mean ± SD and t-test to appraise the significance of any variances between the two study groups. All correlations were estimated, and the statistical significance was set at p < 0.05.
Results
In this clinical trial, 20 patients (13 males and 7 females) with clinically diagnosed MiRAS were allocated equally into 2 groups. Table 1 shows the patients characterisation regarding the sex and age distribution; no statistically significant differences were observed between the study and control groups in sex or age distribution (p = 0.66 and 0.49 respectively).
Table 1.
Sex and age distribution of the study sample
| Study group | Control group | Test | p-value | ||
|---|---|---|---|---|---|
| Sex | Male | 6 (60) | 7 (70) | - | 0.66 |
| Female | 4 (40) | 3 (30) | |||
| Age | Min-max | 21 - 40 | 21 - 40 | 0.71 | 0.49 |
| Mean ± SD | 30.1 ± 6.9 | 28.1 ± 5.8 | |||
Table 2 displays the comparison between the two sample groups regarding the pain scores at varies follow up visits. The differences in VAS pain score between the study and control groups at the beginning of days 1, 2 and 3 were 8.00 ± 0.51, 9.00 ± 0.58 and 8.38 ± 0.72 respectively. However, the mean indices at day 4 in the study and control groups were 91.82 ± 18.34 and 8.00 ± 13.98 while at day 6, all cases in the study group had effectiveness index = “100” and the mean in the control group was 48.00 ± 31.55. The differences between the study and control groups in the effectiveness indices for pain after the two periods were statistically significant (p < 0.0001 for both).
Table 2.
Comparison of VAS pain score between the two study groups at different follow-up periods
| Effectiveness index Mean ± SD | Z of MWU test | P-value | ||
|---|---|---|---|---|
| Study group | Control group | |||
| At day 1 | 2.00 ± 1.61 | 10.00 ± 0 | 4.14 | < 0.0001* |
| At day 2 | 1.00 ± 1.84 | 10.00 ± 0 | 4.23 | < 0.0001* |
| At day 3 | 0.82 ± 1.83 | 9.20 ± 1.40 | 4.12 | < 0.0001* |
| At day 4 | 91.82 ± 18.34 | 8.00 ± 13.98 | 4.12 | < 0.0001* |
| At day 6 | 100.00 ± 0 | 48.00 ± 31.55 | 3.55 | < 0.0001* |
Statistically significant at p ≤ 0.05; Mann Whitney U test used instead of t-test.
Table 3 demonstrates the ulcers’ sizes between the comparative groups. At the beginning of the treatment, there was no statistically significant difference between both groups on day 1 (p = 0.76). Whereas, the differences in ulcers’ sizes between the study and control groups at days 4 and 6 were statistically significant (p = 0.008 and 0.001 respectively). The differences in the study group in ulcer size between day1 and each of day 4 and day 6 were statistically significant (p = 0.002 and 0.007 respectively). However, the differences in the control group in ulcer size between day1 and day 4 were not statistically significant (p = 0.34) whereas the difference between day 1 and day 6 was statistically significant (p = 0.01).
Table 3.
Comparison of ulcer size in mm2 between the two study groups at different follow-up periods
| Ulcer size Mean ± SD | t-test | p-value | |||
|---|---|---|---|---|---|
| Study group | Control group | Difference | |||
| Day 1 | 6.56 ± 3.17 | 6.10 ± 3.18 | 0.46 ± 1.46 | 0.31 | 0.76 |
| Day 4 | 2.44 ± 1.59 | 5.80 ± 3.01 | 3.36 ± 1.12 | 2.98 | 0.008* |
| Day 6 | 0.17 ± 0.50 | 2.90 ± 1.73 | 2.73 ± 0.57 | 3.38 | 0.001* |
| Paired t test (1-4 days) | 4.47 | 1.00 | |||
| p value | 0.002* | 0.34 | |||
| WSR test (1- 6 days) | 2.68 | 2.53 | |||
| p value | 0.007* | 0.01* | |||
Statistically significant at p ≤ 0.05; Mann Whitney U test used instead of t-test; WSR test: Wilcoxon signed ranks test.
Table 4 exhibits the levels of effectiveness index of pain reduction in the two study groups after 4 and 6 days in which at day 4, all cases in the study group showed either “heal” or “moderate improvement “compared to the control group whose cases showed “moderate” or “no improvement” (p < 0.0001*). While at the sixth day, all cases in the study group were completely “healed”, which was in contrast with the control group where 2 cases were healed and the remaining cases showed either “moderate” or “no improvement” (p = 0.001*).
Table 4.
Comparison of levels of effectiveness index for pain reduction between the two study groups at different follow-up periods
| Levels of effectiveness index N (%) | χ2p-value | |||
|---|---|---|---|---|
| Study group | Control group | |||
| After 4 days | Score 1 (heal) | 9 (81.8) | 0 | 18.33 < 0.0001* |
| Score 2 (marked improvement) | 0 | 0 | ||
| Score 3 (moderate improvement) | 1 (18.2) | 1 (10) | ||
| Score 4 (no improvement) | 0 | 9 (90) | ||
| After 6 days | Score 1 (heal) | 10 (100) | 2 (20) | 14.22 0.001* |
| Score 2 (marked improvement) | 0 | 0 | ||
| Score 3 (moderate improvement) | 0 | 5 (50) | ||
| Score 4 (no improvement) | 0 | 3 (30) | ||
| WSR test | 1.41 | 2.46 | ||
| p value | 0.16 | 0.01* | ||
Statistically significant at P ≤ 0.05; WSR test: Wilcoxon signed ranks test.
Table 5 represents the comparison of levels of effectiveness index of the size reduction between the sample groups in the fourth and sixth days. At day 4, there was a statistically significant difference in the levels of effectiveness index between the two groups. The study group showed one case healed and the remaining cases showed either marked or moderate improvement (Figure 3, and 4) compared to the control cases which showed one case had moderate improvement, and the remaining cases showed no improvement (p = 0.001*). While at the day 6, there was a statistically significant difference in the levels of effectiveness index between the two groups, the study group showed one case had remarkable improvement, and the remaining cases were healed, but the control cases showed one case had healed and most of the remaining cases showed either moderate or no improvement (p = 0.004*).
Table 5.
Comparison of levels of effectiveness index for size reduction between the two study groups at different follow-up periods
| Levels of effectiveness index N (%) | χ2p-value | |||
|---|---|---|---|---|
| Study group | Control group | |||
| After 4 days | Score 1 (heal) | 1 (11.1) | 0 | 15.56 < 0.001* |
| Score 2 (marked improvement) | 3 (22.2) | 0 | ||
| Score 3 (moderate improvement) | 6 (66.7) | 1 (10) | ||
| Score 4 (no improvement) | 0 | 9 (90) | ||
| After 6 days | Score 1 (heal) | 8 (88.9) | 1 (10) | 13.43 0.004* |
| Score 2 (marked improvement) | 2 (11.1) | 1 (10) | ||
| Score 3 (moderate improvement) | 0 | 6 (60) | ||
| Score 4 (no improvement) | 0 | 2 (20) | ||
| WSR test | 2.60 | 2.46 | ||
| p value | 0.009* | 0.01* | ||
Statistically significant at p ≤ 0.05; WSR test: Wilcoxon signed ranks test.
Figure 3.
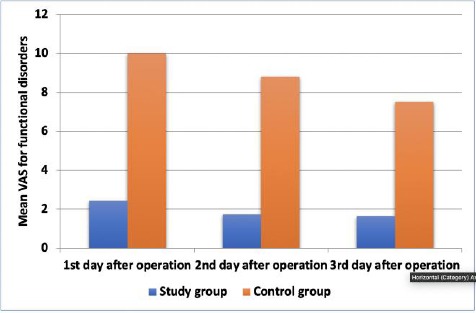
Comparison of functional disorders between the two study groups at different follow-up periods
Table 6 and Figure 3 illustrate the comparison of functional disorders between the two study groups at various follow-up periods. The mean functional disorders scores in the study group were 2.45 ± 1.29 on the first day, 1.73 ± 1.43 in the second day and 1.64 ± 1.43 on the third day. In the control group the mean scores were 10.00 ± 0 on the first day, 8.80 ± 1.14 in the second day and 7.50 ± 2.42 on the third day. The differences in functional disorder scores between the study and control groups at days 1, 2 and 3 after operation were 7.55, 7.07 and 5.86 respectively.
Table 6.
Comparison of functional disorders between the two study groups at different follow-up periods
| VAS for functional disorders Mean ± SD | t-test | p-value | |||
|---|---|---|---|---|---|
| Study group | Control group | Difference | |||
| 1st day after operation | 2.45 ± 1.29 | 10.00 ± 0 | 7.55 (6.68, 8.41) | 19.35 | < 0.0001* |
| 2nd day after operation | 1.73 ± 1.43 | 8.80 ± 1.14 | 7.07 (5.89, 8.26) | 12.52 | < 0.0001* |
| 3rd day after operation | 1.64 ± 1.43 | 7.50 ± 2.42 | 5.86 (3.99, 7.74) | 6.68 | < 0.0001* |
| Paired t test (1-2 days) | 2.67 | 3.34 | |||
| p value | 0.02* | 0.01* | |||
| Paired t test (1-3 days) | 3.11 | 3.27 | |||
| p value | 0.01* | 0.01* | |||
Statistically significant at p ≤ 0.05.
In the study group, the decrease in the functional disorder scores between day 1 and day 2 as well as between day 1 and day 3 was statistically significant (p = 0.02 and 0.01 respectively).
In the control group, the differences between day 1 and day 2 as well as between day 1 and day 3 were statistically significant (p = 0.01* for both). The differences between both groups at days 1, 2 and 3 were statistically significant (p < 0.0001 for all).
Discussion
In our research, individuals on regular systemic steroid therapy or other immunosuppressant drugs were excluded to eliminate any effect of the drugs on ulcers healing or pain reduction. Also, teenagers, pregnant and postmenopausal women were prohibited from the study to avoid the effect of hormonal changes on the healing process. Also, smokers were not included because smoking increases the expression of TNF- α cytokine which has a role in aphthous ulceration [17].
The ages of enrolled individuals in our study ranged between 21-40 years for study and control groups with a mean age of 30.1 ± 6.9 and 28.1 ± 5.8 respectively; it is the most common range in many studies [18], [19]. Nevertheless, sex and age distributions had no statistically significant differences between the study and control groups.
In the present study, each patient in the control group (group A) received sodium bicarbonate mouth rinse as it provides a buffering activity to the acidic nature of the damaged mucosal cells and can decrease the pain [20]. While in the study group (group B), each patient received diode laser irradiation which was applied in the first visit only. Then, we compared VAS scores, ulcer sizes, effective indices and functional disorders between the two groups.
On comparing the VAS pain score between the two study groups at different follow-up periods, statistically, significant differences were observed between both groups as all patients of the study group confirmed the complete or partial reduction of pain after a single session of the diode laser application. This may be due to the pain-relieving action of the laser therapy because the laser irradiation can reach the exposed nerve endings easily, causing a blockage of nociceptive signals in primary afferent neurons and also can intensify the natural analgesics such as opioid peptides, reduction by releasing histamine [21].
Also, the level of effectiveness index of pain reduction at day 4 in the study group showed complete healing in 9 cases and one case of moderate improvement while in control group, there was a moderate improvement in one case and no improvements in the other nine cases. However, at day 6, all cases in the study group had effectiveness index = “100” as the ten patients were completely healed as shown in Figure 4 (a, b, c, and d). While the mean in the control group was 48.00 ± 31.55 including 2 complete healing cases, 5 moderate improved cases and 3 cases without any improvement as shown in Figure 5 (a, b, and c). The explanation of this decrease in effectiveness index in the control group is related to the liquid consistency that lacks the sticking potential on the mucosa for a long time causing a mild and momentary effect.
Figure 4.
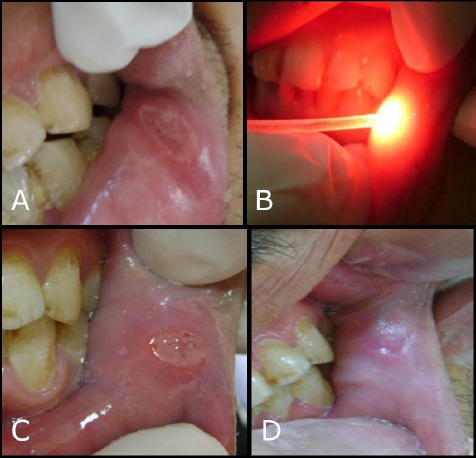
Preoperative and postoperative treatment of MiRAS by diode laser in the study group; a) Minor aphthous lesion on lower labial mucosa before phototherapy; b) Minor aphthous lesion during phototherapy using diode laser 660 nm; c) the Fourth day of diode laser application; d) Sixth day of diode laser application
Figure 5.
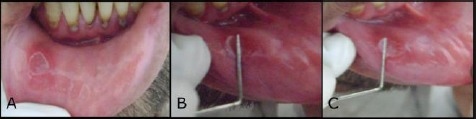
MiRAS in lower labial mucosa of the control group; a) MiRAS on the lower labial mucosa (preoperative); b) fourth day of follow up visits; c) Sixth day of follow up visits
These results were in agreement with a randomised controlled clinical trial which was conducted by Colvard and Kuo [22] who used carbon dioxide laser in an ablative manner to treat RAS, but local anaesthesia was mandatory in their procedure. Also, our outcomes were in corresponding to Zand et al., [23], [24] and Prasad et al., [25] who evaluated the efficacy of a single-session, non-thermal, sub-ablative carbon dioxide laser irradiation in relieving the pain of MiRAS. These studies concluded that high power laser should be included as an alternative modality for the treatment of MiRAS, due to its ability of pain elimination. Nevertheless, our study was different as we used diode laser as LLLT in a non-ablative, non-thermal manner and without anaesthesia.
On comparison of the effectiveness index for ulcer size reduction between the two groups, the mean index at day 4 in the study and control groups were 64.20 ± 20.62 and 3.33 ± 10.54. In the study group, there were one completely healed lesion, three lesions with marked improvements and six lesions with moderate improvements in the ulcer sizes. While at day 6, they were 97.22 ± 8.33 and 53.06 ± 31.44 respectively. The differences were statistically significant (p < 0.0001 and 0.001) respectively where a complete healing was reported in 8 patients, and 2 patients showed marked improvements in the study group, on the contrary, the control group showed only one case with complete healing, one with marked improvement, six with moderate improvements and two patients didn’t show any improvement.
These findings revealed that phototherapy had a higher effective index than sodium bicarbonate mouth rinse which in turn gave the superiority to diode laser in the reduction of ulcer size and pain. Regression of ulcer size and healing enhancement may be caused by the capillary vasodilation and re-epithelization stimulation which increase the mitotic activity, the proliferation of fibroblasts, the collagen synthesis and the epithelial proliferation. Also, the host modulation effect of mast cells and the reduction of prostaglandin E2 promote healing activity [21], [26]. These outcomes were corresponding with Rodriguez et al., (27) who measured the effectiveness index of ulcer’s size on day 0 (when the intervention began) and on treatment day 2 and 5 to evaluate the short-term benefits of the intervention as RAS has a self-limited natural history. Also, the findings were in accordance with De Souza et al., [28] who reported that application of LLLT daily can reduce pain and regress the ulcers after four days as well as Khademi H et al., [29] who revealed that there was a reduction in pain intensity and healing time when using LLLT than other treatments.
Moreover, our findings were confirmed by 2 clinical studies of diode laser; one was done by Aggarwal et al., [30] and Hazeem et al., [31] who used 810 nm and 940 nm diode lasers in a controlled split-mouth study and concluded that laser was effective in the healing process and pain relief. And the other study was done by Lalabonova et al., in 2014 [32] who conducted that 658nm diode laser with exposure time 1.14 minutes can manage the pain and inflammation symptoms of the aphthous ulcer, but the documented long time was a disadvantage in their study because it increased the risk of health peril and time consuming on dental chair.
The comparison of functional disorders between the two study groups at different follow up periods showed statistically significant (p < 0.0001*) between first, second, the third day after study procedures. These were in corresponding with Tezel [34] who concluded that patients treated with the Nd: YAG laser had less post-treatment pain, fewer functional complications (eating and speech) and faster healing after 5 days. However, Nd: YAG laser required prolonged irradiation times (2-3 min) that may be a health hazard to the patient and the clinician [33], [34]. The outcomes of our present study showed that one application of diode laser 660 nm gave a more remarkable reduction in healing time, pain and lesion size than the sodium bicarbonate rinse, consequently, it is very effective in the treatment of RAS.
In conclusion, despite the numerous therapeutic choices available for the management of RAS, there is no precise treatment due to the variegated causative factors of RAS. The effectiveness of its management depends on the acceleration time of relieving the sign and symptoms of the ulcers such as pain and inflammation. Our conclusions represent the diode laser 660 nm as an applicable effective alternative modality in the reduction of pain, ulcer size and healing time. However, the study sample reflected the findings in these enrolled groups of participants only, additional trails with larger samples are required to verify these outcomes.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Shulman JD. An exploration of point, annual, and lifetime prevalence in characterising recurrent aphthous stomatitis in USA children and youths. J Oral Pathol Med. 2004;33:558–66. doi: 10.1111/j.1600-0714.2004.00241.x. https://doi.org/10.1111/j.1600-0714.2004.00241.x PMid:15357677. [DOI] [PubMed] [Google Scholar]
- 2.Altenburg A, Abdel Naser MB, Seeber H, Abdallah M, Zouboulis CC. Practical aspects of management of recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2007;21:1019–26. doi: 10.1111/j.1468-3083.2007.02393.x. https://doi.org/10.1111/j.1468-3083.2007.02393.x PMid:17714120. [DOI] [PubMed] [Google Scholar]
- 3.Miller MF, Ship II. A retrospective study of the prevalence and incidence of recurrent aphthous ulcers in a professional population, 1958-1971. Oral Surg Oral Med Oral Pathol. 1977;43:532–7. doi: 10.1016/0030-4220(77)90105-0. https://doi.org/10.1016/0030-4220(77)90105-0. [DOI] [PubMed] [Google Scholar]
- 4.Jurge S, Kuffer R, Scully C, Porter SR. Recurrent aphthous stomatitis. Oral Dis. 2006;12:1–21. doi: 10.1111/j.1601-0825.2005.01143.x. https://doi.org/10.1111/j.1601-0825.2005.01143.x PMid:16390463. [DOI] [PubMed] [Google Scholar]
- 5.Crivelli MR, Aguas S, Adler I, Quarracino C, Bazergue P. Influence of socio-economic status on oral mucosa lesion prevalence in schoolchildren. Community Dent Oral Epidemiol. 1988;16:58–60. doi: 10.1111/j.1600-0528.1988.tb00556.x. https://doi.org/10.1111/j.1600-0528.1988.tb00556.x PMid:3422621. [DOI] [PubMed] [Google Scholar]
- 6.Scully C, Felix DH. Oral medicine-update for the dental practitioner. Aphthous and other common ulcers. British Dent Journal. 2005;199(5):259–264. doi: 10.1038/sj.bdj.4812649. https://doi.org/10.1038/sj.bdj.4812649 PMid:16155535. [DOI] [PubMed] [Google Scholar]
- 7.Ship JA. Recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141–7. doi: 10.1016/s1079-2104(96)80403-3. https://doi.org/10.1016/S1079-2104(96)80403-3. [DOI] [PubMed] [Google Scholar]
- 8.Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis:a consensus approach. J Am Dent Assoc. 2003;134:200–7. doi: 10.14219/jada.archive.2003.0134. https://doi.org/10.14219/jada.archive.2003.0134 PMid:12636124. [DOI] [PubMed] [Google Scholar]
- 9.Mester AF, Mester A. Wound-healing. Laser Therapy. 1989;1:7–15. https://doi.org/10.5978/islsm.89-RE-01. [Google Scholar]
- 10.Walker J. Relief from chronic pain by low power laser irradiation. Neurosci Lett. 1983;43:339–44. doi: 10.1016/0304-3940(83)90211-2. https://doi.org/10.1016/0304-3940(83)90211-2. [DOI] [PubMed] [Google Scholar]
- 11.Ohta A, Abergel RP, Uitto J. Laser modulation of human immune system:inhibition of lymphocyte proliferation by a gallium-arsenide laser at low energy. Lasers Surg Med. 1987;7:199–201. doi: 10.1002/lsm.1900070211. https://doi.org/10.1002/lsm.1900070211 PMid:2956471. [DOI] [PubMed] [Google Scholar]
- 12.Ricci L, Weidemüller M, Esslinger T, Hemmerich A, Zimmermann C, Vuletic V, et al. A compact grating- stabilized diode laser system for atomic physics. Opt Commun. 1995;117:541–9. https://doi.org/10.1016/0030-4018(95)00146-Y. [Google Scholar]
- 13.Moritz A, Gutknecht N, Doertbudak O, Goharkhay K, Schoop U, Schauer P, et al. Bacterial reduction in periodontal pockets through irradiation with diode laser, A pilot study. J Clin Laser Med Surg. 1997;15:33–7. doi: 10.1089/clm.1997.15.33. https://doi.org/10.1089/clm.1997.15.33 PMid:9467340. [DOI] [PubMed] [Google Scholar]
- 14.Scully C, and Porter SR. Oral mucosal disease:recurrent aphthous stomatitis. Bri Dent J. 2008;46(3):198–206. doi: 10.1016/j.bjoms.2007.07.201. https://doi.org/10.1016/j.bjoms.2007.07.201 PMid:17850936. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg M, Glick M. Orofacial pain:Burket's Oral Medicine Diagnosis and Treatment, tenth edition. Vol. 310. Hamilton: BC Decker Inc; 2003. [Google Scholar]
- 16.Liu J, Zeng X, Chen Q, Cai Y. An evaluation on the efficacy and safety of Amlexanox oral adhesive tablets in the treatment of recurrent minor aphthous ulceration in a Chinese cohort:A randomized, double blind controlled, unparallel clinical trial. Oral Surg Oral Med Oral Path Oral Radiol Endod. 2006;102:475–81. doi: 10.1016/j.tripleo.2005.12.014. https://doi.org/10.1016/j.tripleo.2005.12.014 PMid:16997114. [DOI] [PubMed] [Google Scholar]
- 17.Tuzun B, Wolf R, Tuzun Y, Serdaroglu S. Recurrent aphthous stomatitis and smoking. Int J Dermatol. 2000;39:358–60. doi: 10.1046/j.1365-4362.2000.00963.x. https://doi.org/10.1046/j.1365-4362.2000.00963.x PMid:10849126. [DOI] [PubMed] [Google Scholar]
- 18.Zain RB. Oral recurrent aphthous ulcers/stomatitis:prevalence in Malaysia and an epidemiological update. J Oral Sci. 2000;42:15–9. doi: 10.2334/josnusd.42.15. https://doi.org/10.2334/josnusd.42.15 PMid:10808270. [DOI] [PubMed] [Google Scholar]
- 19.Rivera-Hidalgo F, Shulman JD, Beach MM. The association of tobacco and other factors with recurrent aphthous stomatitis in an US adult population. Oral Dis. 2004;10:335–45. doi: 10.1111/j.1601-0825.2004.01049.x. https://doi.org/10.1111/j.1601-0825.2004.01049.x PMid:15533208. [DOI] [PubMed] [Google Scholar]
- 20.Sircus M. Sodium bicarbonate - rich man's poor man's cancer treatment. 2nd ed. Ch 1. IMVA publications; 2009. pp. 10–11. [Google Scholar]
- 21.Laakso EL, Richardson CR, Cramond T. Factors affecting low level laser therapy. Aust J Physio. 1993;39:95–99. doi: 10.1016/S0004-9514(14)60473-6. https://doi.org/10.1016/S0004-9514(14)60473-6. [DOI] [PubMed] [Google Scholar]
- 22.Colvard M, Kuo P. Managing aphthous ulcers:laser treatment applied. J Am Dent Assoc. 1991;122:51–3. doi: 10.1016/s0002-8177(91)26017-1. https://doi.org/10.1016/S0002-8177(91)26017-1. [DOI] [PubMed] [Google Scholar]
- 23.Zand N, Ataie-Fashtami L, Djavid GE, Fateh M, Alinaghizadeh M-R, Fatemi S-M, et al. Relieving pain in minor aphthous stomatitis by a single session of non- thermal carbon dioxide laser irradiation. Lasers Med Sci. 2009;24:515–20. doi: 10.1007/s10103-008-0555-1. https://doi.org/10.1007/s10103-008-0555-1 PMid:18408986. [DOI] [PubMed] [Google Scholar]
- 24.Zand N, Fateh M, Ataie-Fashtami L, Djavid GE, Fatemi S-M, Shirkavand A. Promoting wound healing in minor recurrent aphthous stomatitis by non-thermal, non-ablative CO2laser therapy:a pilot study. Photomed Laser Surg. 2012;30:719. doi: 10.1089/pho.2012.3301. https://doi.org/10.1089/pho.2012.3301 PMid:23113511. [DOI] [PubMed] [Google Scholar]
- 25.Prasad S, Pai A. Assessment of immediate pain relief with laser treatment in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:189–93. doi: 10.1016/j.oooo.2013.02.011. https://doi.org/10.1016/j.oooo.2013.02.011 PMid:23622766. [DOI] [PubMed] [Google Scholar]
- 26.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation:a review. J Clin Periodontol. 1996;23:492–6. doi: 10.1111/j.1600-051x.1996.tb00580.x. https://doi.org/10.1111/j.1600-051X.1996.tb00580.x PMid:8783057. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez M, Rubio JA, Sanchez R. Effectiveness of two oral pastes for the treatment of recurrent aphthous stomatitis. Oral Dis. 2007;13:490–4. doi: 10.1111/j.1601-0825.2006.01327.x. https://doi.org/10.1111/j.1601-0825.2006.01327.x PMid:17714352. [DOI] [PubMed] [Google Scholar]
- 28.De Souza TO, Martins MA, Bussadori SK, Fernandes KP, Tanji EY, Mesquita-Ferrari RA, et al. Clinical evaluation of low-level laser treatment for recurring aphthous stomatitis. Photomed Laser Surg. 2010;28:S85–8. doi: 10.1089/pho.2009.2661. https://doi.org/10.1089/pho.2009.2661 PMid:20950190. [DOI] [PubMed] [Google Scholar]
- 29.Khademi H, Shirani AM, Nikegbal F. Effect of low power laser in the treatment of recurrent oral aphthous. Shiraz Univ Dent J. 2009;10:160–2. [Google Scholar]
- 30.Aggarwal H, Singh MP, Nahar P, Mathur H, Gv S. Efficacy of low-level laser therapy in treatment of recurrent aphthous ulcers - a sham controlled, split mouth follow up study. J Clin Diagn Res. 2014;8(2):218–221. doi: 10.7860/JCDR/2014/7639.4064. https://doi.org/10.7860/JCDR/2014/7639.4064 PMid:24701539 PMCid:PMC3972568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazeem MI, Rajab M sh, Badeia RA. Treatment of Recurrent Aphthous Stomatitis with 940nm Diode Laser. Tikrit J Dent Sci. 2013;1:77–82. [Google Scholar]
- 32.Lalabonova H, Daskalov H. Clinical assessment of the therapeutic effect of low-level laser therapy on chronic recurrent aphthous stomatitis. Biotechnol Equip. 2014;28:929–33. doi: 10.1080/13102818.2014.966526. https://doi.org/10.1080/13102818.2014.966526 PMid:26019580 PMCid:PMC4433909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezel A, Kara C, Balkaya V, Orbak R. An evaluation of different treatments for recurrent aphthous stomatitis and patient perceptions:Nd:YAG laser versus medication. Photomed Laser Surg. 2009;27:101–6. doi: 10.1089/pho.2008.2274. https://doi.org/10.1089/pho.2008.2274 PMid:18687056. [DOI] [PubMed] [Google Scholar]
- 34.Arabaci T, Kara C, Ciçek Y. Relationship between periodontal parameters and Behçet's disease and evaluation of different treatments for oral recurrent aphthous stomatitis. J Periodontal Res. 2009;44:718–25. doi: 10.1111/j.1600-0765.2008.01183.x. https://doi.org/10.1111/j.1600-0765.2008.01183.x PMid:19076988. [DOI] [PubMed] [Google Scholar]


