Abstract
AIM:
Verifying if physical therapy, neurostimulation techniques, aerobic fitness and video games can induce neural plasticity making it possible for cortical reorganisation, motor recovery in patients, improvement of cognitive functions and transfer of spatial knowledge in the everyday living environment.
METHODS:
There have been revised scientific articles respectively focused on the role of pain, the role of physical therapy, neurostimulation techniques and video games in cortical reorganisation. Articles related to the role of pain have taken in the study subjects with pain, to observe its role in cortical reorganisation. Studies related to physical therapy and neurostimulation techniques after cerebrovascular accident consisted of the involvement of these subjects which exposed to different neurostimulations. Also, related to cognition and video games subjects exposed to these interventions for cognitive benefits.
RESULTS:
From all articles reviewed there have been effective results of neurostimulation techniques, aerobic fitness and video games in cortical reorganisation inducing neural plasticity (p < 0.05) toward motor recovery, improvement of executive functions and transfer of spatial knowledge.
CONCLUSION:
Rehabilitation through locomotor training and neurostimulation techniques, improves mobility in subjects after a cerebrovascular accident due to cortical reorganisation. Also, through aerobic fitness and video games, there have been improvements in cognitive functions. This way, rehabilitation dedicated to the promotion of well-being and health urges beneficial neuroplastic changes in brain corresponding in functional improvement.
Keywords: Cortical reorganisation, Neural plasticity, Aerobic fitness, Video games, Rehabilitation
Introduction
The brain is a vital organ for our existence. For many years, scientists have known that neurons can form new connections during the first years of human life, but after this phase, it has been believed that the brain is completely formed and is relatively unchangeable. They have found that nerve fibres keep growing and innervate cerebral cortex areas in children from three years old till puberty; even observations have shown that brain generates new neurons in adults in an important region for memory and learning [1]. The whole this can be explained through neural plasticity/neuroplasticity [2] which is as intriguing as a complex concept.
The purpose of this review is to verify if physical therapy, neurostimulation techniques, aerobic fitness and video games can induce neural plasticity making it possible for cortical reorganisation, motor recovery in patients, improvement of cognitive functions and transfer of spatial knowledge in the everyday living environment.
The road through which the role of rehabilitation will be understood in neuroplastic changes will contribute in understanding that the expanding of cortical neuroplastic changes is one of the most important neurophysiologic characteristics that correspond with the level of functional improvement [3].
In this way, we can note that this topic is current. It deserves special attention to be treated by many professionals in the scientific field, paving the way in understanding the role of rehabilitation in the brain’s adaptability toward wellbeing and health promotion.
Material and Methods
To realise this review, the articles have been carefully selected in terms of explaining the basic purpose and giving details for neuroplasticity inducement through different rehabilitation techniques. The main subjects of neural plasticity term in our review have been subjects with pain, blinds, amputated subjects and CVA subjects. We revise how neural plasticity can be induced through physical training, neuromodulations and pain influencing in volume representation, cognitive function improvement, physical mobility and spatial knowledge transferring in everyday environments especially in blinds.
For this review, we have selected those articles which:
- Mainly focused on real results in human, since most of the studies remain using animal models.
- Articles that took subjects with pain specifically LBP, Phantom-limb pain, complex regional pain syndrome type 1 to verify if this kind of neurological pain can induce neural plasticity while excluding other types of pain.
- Articles that focus on post-stroke subjects with mild/severe impairment and not those articles which focused on subjects with a low impairment since we aim to focus on the brain’s ability in severe problems from a stroke.
- Articles that took in the study blinds to better understand the role of neuroplasticity in spatial knowledge transferring.
- Articles that took in the study old subjects with/without cognition disorders so we could understand a comparison between these subjects and see what happens in the plasticity of the brain with age.
And we excluded articles that focused on neuroplasticity but took in the study subjects with psychiatric disorders, subjects with cancer, heart problems, MRI phobia/other electrostimulation techniques since we consider these kinds of patients as a category to be reviewed at another time.
For this review articles have been taken from official sources like PubMed Central page, where mostly information was taken from various journals (Nature Medicine, PNAS USA, Brain, The Journal of Gerontology, The Journal of Neuroscience, Frontiers in Human Neuroscience, Clinical Neurophysiology, Neuroscience Letters, Electroencephalography and Clinical Neurophysiology, Journal of Neurology Neurosurgery & Psychiatry, Proceeding Biological Sciences, International Journal of Neuropsychopharmacology, Psychology and Aging, PLoS One), Wiley Online Library and IEEE Xplore- Digital Library.
Results
Do pain and physical dysfunction play a role in a cortical reorganisation, thus enabling neuroplastic changes?
In the study of Tsao et al., (2008) [4] it was seen that reorganisation of the motor cortex was related to postural control deficit in LBP. This study investigated the extension and motor input organisation of trunk muscles in subjects with/without pain. It was observed that there was no difference in the motor threshold between left and right TrA (transversus abdominis). Moreover, the GC localisation of TrA muscle for LBP was more posterior and laterally from the localisation of GC in control group which brought lower activation of TrA. Similar connections were found for the representation volume, raise of representation volume related to a slowly start of TrA EMG (r > 0.57, P < 0.001).
The study of Pleger et al., (2004) [5] shows that mean sustained pain levels are related to the differences of representation in somatosensory cortex, in complex regional pain syndrome type-1, probably reflecting two non-exclusive mechanisms: (a) persistent nociceptive inputs can interrupt with cortical reinforcements of sensory perception, progressively lowering representation zone of the affected limb from pain, and (b) a possible elimination of S1 activity can in itself support the experience of pain.
There exists a strong relationship between the quantity of cortical reorganisation that happens after the proximal extremity amputation and the intensity of phantom limb pain according to Flor et al., (1995) [6]. This study shows that phantom limb pain induces cortical reorganisation (r = 0.93), but non-painful phantom phenomenon after arm amputation does not induce it. These data indicate that phantom limb pain can be related and a consequence of neuroplastic changes in primary somatosensory cortex.
Lotze et al., (2001) [7] in their study show that phantom limb pain in amputated subjects affects in a high-level reorganisation of M1/S1 cortex. Patients with phantom limb pain showed higher activation in contralateral M1/S1 cortex (Z = 4.19) and a large mouth representation. The difference was not significant for patients with the non-painful phantom phenomenon. In imaginary hand movements, patients with phantom limb pain showed higher activation of hand zone in M1/S1 and the contralateral lip of the amputated part.
The study of Seminowicz et al., (2011) [8] consists in demonstration of the fact that effective treatment of chronic LBP, reducing pain and physic disability affects in cortical thickness increase specifically on prefrontal, dorsolateral medial cortex evidencing anatomical and functional change of brain. Data indicate that functional and structural brain abnormalities specifically on the prefrontal, dorsolateral medial cortex are reversible, suggesting that chronic pain treatment can return normal functions in a human’s brain.
Does physical therapy, stimulation techniques and motor skills training increase in adaptive plasticity and motor recovery after a cerebral vascular accident?
The study of Wilkins et al., (2017) [9] shows that there exists an improvement of neural plasticity in moderate to severe chronic stroke following a device-assisted task-specific arm/hand intervention. Subjects demonstrated a change of cortical activity related to hand opening from contralesional hemisphere to ipsilesional hemisphere [t(7) = 3.09, p = 0.02] through the intervention; also a rise of grey matter in the ipsilesional somatosensory primary cortex but a low density of grey matter in the contralesional somatosensory primary cortex. Subjects also demonstrated a higher density of grey matter in the thalamus in the lesional hemisphere.
Another study is the study of Cauraugh and Kim, (2003) [10] which demonstrates that active neuromuscular stimulation and blocked/random practice schedules affect in motor recovery after CVA. There were noticed faster motor reaction times which shows significant effects of the limb [F(2, 28) = 3.64, p = 0.039], and less variability in the sustained muscular contraction task.
Figure 1.
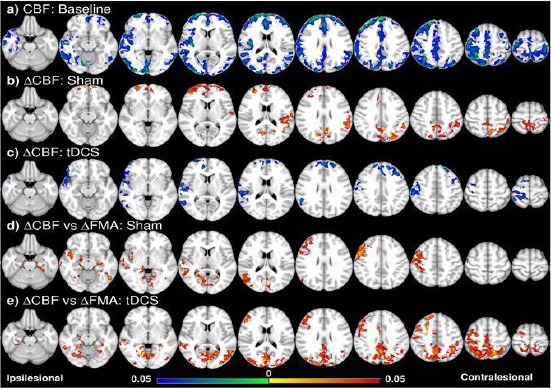
Voxel-wise CBF analysis and behavioural correlation [13]. (a) CBF of all the patients (tDCS and sham groups together) is lower especially in the ipsilesional hemisphere compared to the control at the baseline. (b) CBF increase in the frontal and contralesional side after training in the sham group. (c) CBF decrease in the frontal and ipsilesional side after training in the tDCS group. (d) ∆CBF positively correlates with ∆FMA in the sham group in the ipsilesional side, including the pre/postcentral cortices, angular gyrus, lateral occipital cortex, and middle temporal gyrus, and the ventral occipital lobes in both sides; (e) in the tDCS group, positive correlation between ∆CBF and ∆FMA is in similar regions as well as the posterior and superior part of the two hemispheres including the posterior parietal cortices. Colorbar represents p-value with hot colour as an increase (or positive correlation) and cold colour as decrease (or negative correlation) (p<0.05, FWE corrected)
Combined training MI-BCI (Pfurtscheller & Neuper, 2001) [11] with tDCS (Brunoni et al., 2011) [12] in chronic stroke subjects affects in neuroplasticity growth through long-term ipsilesional motor growth and white matter integrity between motor areas Hong et al., (2017) [13]. While motor performance showed a comparable improvement, long-term neuroplasticity could be detected only in tDCS group where white matter integrity in ipsilesional corticospinal tract and corpus callosum was increased, but sensorimotor cerebral blood flow was decreased especially in ipsilesional part, suggesting motor functional recovery and involvement of interhemispheric interaction.
Landsman et al., (2016) [14] demonstrate that locomotor and balance training can improve mobility and cognition in CVA patients affecting in activation of cerebral models. Due to training patients achieved increased activation in the right precentral gyrus, left/right superior gyrus and right frontal lobe, with bipedal ankle movement. Firstly patients showed increased activation in the contralesional hemisphere (Postcentral girus, parietal operculum, superior temporal gyrus and left cerebellum); decreased activation in the ipsilesional precentral gyrus and supplementary motor area. After training these differences were disappeared.
Can neuroplasticity protect cognitive functions?
Colcombe et al., (2004) [15] in their study demonstrate that cardiovascular fitness affects brain plasticity as people get older. Old adults demonstrated a higher activation in some regions related to attention control: Right MFG (Brodmann area 46), SFG (Brodmann area 8), SPL (Brodmann area 40) and in a significant manner less activity in ACC (anterior cingular cortex)/(Brodmann area 32). These data suggest that cardiovascular fitness can affect improvements in brain plasticity with age and can serve to reduce biologic and cognitive loss in humans.
Figure 2.
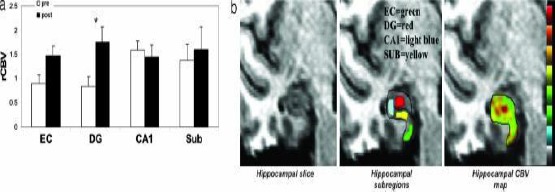
Exercise selectively increases dentate gyrus CBV in humans [16]. (a) Exercise had a selective effect on dentate gyrus CBV. The bar graph shows the mean relative CBV (rCBV) values for each hippocampal subregion before exercise (open bars) and after exercise (filled bars). As in mice, the dentate gyrus was the only hippocampal subregion that showed a significant exercise effect, whereas the entorhinal cortex showed a nonsignificant increase in CBV. (b) An individual example. (Left) High-resolution MRI slice that visualises the external morphology and internal architecture of the hippocampal formation. (Center) Parcellation of the hippocampal subregions (green, entorhinal cortex; red, dentate gyrus; blue, CA1 subfield; yellow, subiculum). (Right) Hippocampal CBV map (warmer colours reflect higher CBV)
Exercises can selectively raise CBF in dental girus and correlate with aerobic fitness and cognition according to Perreira et al., (2007) [16]. Data showed that dental girus was the only region of hippocampus whose CBF increased over time (F = 12, P = 0.0006). The other region which showed a raise was entorhinal cortex, although didn’t achieve statistical significance (F = 4.3, P = 0.064).
Erikson et al., (2011) [17] study demonstrates that training with aerobic fitness increases hippocampus volume. Aerobic exercises selectively raise the volume of the anterior hippocampus that involves dental gyrus but has a minimal effect in the section of the posterior volume. Thalamus volume raised in both groups Fig. 3c, Fig. 3but this raise was not significant [F(2, 114) = 0.65; P < 0.52]. The volume of the left and right caudate nucleus decreased Figure 3b, but only in the control group. The results demonstrate that the size of the hippocampus is modifiable in the stage of older adults and the moderate intensity of aerobic exercises is effective in the change of volume loss.
Figure 3.
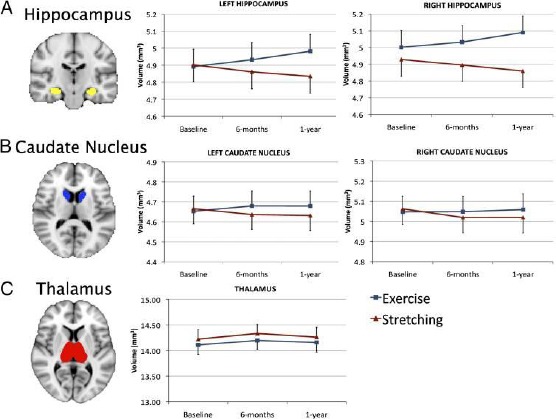
(A) Example of hippocampus segmentation and graphs demonstrating an increase in hippocampus volume for the aerobic exercise group and a decrease in volume for the stretching control group. The Time × Group interaction was significant (P < 0.001) for both the left and right regions. (B) Example of caudate nucleus segmentation and graphs demonstrating the changes in volume for both groups. Although the exercise group showed attenuation of decline, this did not reach significance (both P > 0.10). (C) Example of thalamus segmentation and graph demonstrating the change in volume for both groups. None of the changes was significant for the thalamus. Error bars represent SEM [17]
In the study of Colcombe et al., (2003) [18] it was seen that aerobic fitness reduces brain tissue loss in old adults. The effects of aerobic fitness in the grey matter are bigger in the prefrontal, superior parietal and temporal cortex. In white matter, the greatest effects of fitness are observed in anterior and transverse traces between frontal parietal and posterior lobe. Reported results suggest that potential benefits of aerobic exercises lay beyond the cardiovascular health markers and can effect in brain health reducing the brain volume tissue loss.
Can we use video games as cognitive training tools?
Training with 3D video games, but not with 2D results in significant improvement of cognition related to the hippocampus and in meaningful stimulation of human hippocampus according to Clemenson et al., (2015) [19]. Training with 3D video games improved memory discrimination and investigative evidence performance in the labyrinth (part of Super Mario video game) inducing an improvement of spatial memory in 3D game group comparing to control group, [F(1, 58)= 5.51, p < 0.05)].
Mayas et al., (2014) [20] in their study demostrate that training with video games raises concentracion and cognitive performance in attention functions. The results showed a significant reduce of distraction and a raise of vigilance in experimental group by 26 ms [F(1, 25) = 4.45, MSE = 1071.07, p = 0.04, d = 0.9] and no change in control group [F(1, 25) = 0.387, MSE = 1071.07, p= 0.54, d = 0.22]. These results suggest neurocognitive plasticity in old human brain as training raises cognitive performance in attention functions.
According to Connors et al., (2014) [21] video games affect the transfer of spatial information for navigation of new environments in blinds. High interactive exploration of virtual environment commits a blind user to develop similar skills for near positive transfer of learning, enabling them easy navigation in real-world environments.
If it is combined transcranial brain stimulation and video game it is enabled long term learning transfer and cognitive raise according to Looi et al., (2016) [22]. Those who received real tDCS performed significantly better in the game than the sham group and showed transfer effects to working memory. All effects associated with real tDCS remained 2 months post-training where tDCS group was 2.4 sec (20$) faster in reaction time than sham group [group: F(1,17) = 4.84, p< 0.04, η2 p= 0.22] and also showed a stable effect in verbal working memory capacity, but not in the visuospatial working memory [t(18) = 0.13, p= 0.89].
Discussion
Do pain and physical dysfunction play a role in a cortical reorganisation, thus enabling neuroplastic changes?
Reorganisation of the motor cortex in Recurrent Low Back Pain
Studies have shown that pain is an element that influences the inducement of cortical reorganisation. According to Tsao et al., (2008) [4] LBP relates to the reorganisation of the motor cortex network through TrA activation. Posterior and lateral displacement of GC and higher volume of representation were observed in individuals with LBP, unlike healthy individuals.
Relocation to specific muscle representation in the motor cortex is reported to people with recurrent pain. For example, studies of phantom pain patients as a result of limb amputation have demonstrated a shift in optimum localisation to induce facial muscle responses on the amputation side in terms of missing hand representation (Karl et al., 2001) [23]. These findings are similar to the extension to the somatosensory cortex representation area in patients with acute pain (Soros et al., 2001) [24] and chronic pain (Flor et al., 1997) [25]. However, increases in the volume of representation are not consistent in patients with complex regional syndromes (Pleger et al., 2004 [5], Krause et al., 2006 [26]), since limb immobilization for a long time reduces the area of representation rather than the presence of pain or injury (Liepert et al., 1995) [27]. These findings indicate that changes in the motor cortex contribute to the alteration of postural strategies.
’Pros’ and ’Cons’ the presence of pain in the reorganisation of S1 and M1
The reorganisation of S1 and M1 in the deafferentation area of the hand from the lip area was observed in patients who had phantom limb pain (Lotze et al., 2001) [7]. This was previously reported for somatosensory territory (Flor et al., 1995) [27] and has recently been extended to the motor cortex. In phantom limb pain patients, the imaginative movements of the amputated hand also activated the cortical representation of the mouth (Lotze et al., 2001) [7]. This co-activation is also reflected in the fact that stimulation of the mouth area usually activates the phantom sensations on the arm or the amputated hand (Ramachandran & Rogers-Ramachandran, 1996) [28]. However, no significant activation in the Brodmann 46/9 areas has been observed, which is considered to be an indicator of phantom pain.
Moreover, Flor et al., (1995) [27] demonstrate that besides the fact that the pain affects the cortical reorganisation, there is also a strong connection between the amount of cortical reorganisation and the intensity of the phantom pain. According to them, the presence of amputation does not affect reorganisation if phantom limb pain is not present. On the other hand, Pleger et al., (2004) [5] demonstrate a close relationship between the process of somatosensory cortex reorganisation and sustained increased inputs that progressively lower the pain-affected limb representation area.
Does physical therapy, stimulation techniques and motor skills training increase in adaptive plasticity and motor recovery after a cerebral vascular accident?
The role of physic training and neurostimulation techniques in motor function post CVA
According to Landsman et al., (2016) [14] locomotion and balance training can improve not only mobility but also cognition in patients with CVA and thereby affect brain activation patterns. In bipedal ankle movements, patients demonstrated increased activation of ipsi and contralesional frontal superior gyrus. Likewise, elevations were seen in the ipsilesional frontal lobe and in the precentral ipsilesional gyrus, which is considered as important areas for initiation and preparation of motor motions (Porro et al., 1996) [29]. Similar results are found in other studies such as Scholz et al., (2009) [30].
On the other hand, the use of tDCS during blocked practical (a single rolling motion) and random (some repeatable moves) programs are used to increase the motor function after CVA and premotor reaction time (Cauraugh and Kim, 2003) [10]. Other studies have demonstrated positive effects of tDCS (Fregni et al., 2005) [31], but also MI-BCI (Ang et al., 2009) [32] on motor recovery.
Hong et al., (2017) [13] showed that when combining tDCS with MI-BCI demonstrated improvements in motor performance even though there was no additional increase (behavioural improvement) as previously assumed. Interestingly, neuroimaging showed significant changes in the integrity of white matter and CBF by tDCS even with 4 weeks of rehabilitation training. The integrity of the white matter increased in the corticospinal tract and the corpus callosum, and there was a long-term decrease in CBF of the ipsilesional sensorimotor cortex correlating with the improvement of the motor function and suggesting an involvement of the interhemispherical intervention.
Can neuroplasticity protect cognitive functions?
Effects of cardiovascular fitness
A longitudinal study (Kramer et al., 1999) [33] has shown that upgrades in cardiovascular fitness may exhibit positive effects on cognitive abilities in humans. But, unlike (Kramer et al., 1999) [33]; Murdoch et al., (2016) [34] conclude that combining low-intensity aerobic exercises (30 min) with tDCS did not induce changes in corticomotoneuronal to overspread or neuroplasticity to subjects at chronic stage post-CVA.
Whereas, according to Colcombe et al., (2003) [18] aerobic fitness reduces the loss of brain tissue to older adults. The effects of the CBF in the grey matter are the largest in the prefrontal, superior parietal and temporal cortex that support executive functions. In white matter, the greatest effects of fitness have been observed in the front and transverse traces between the frontal and posterior lobes. Similarly, Colcombe et al., (2004) [15] have shown that increased cardiovascular fitness is associated with increased recruitment in frontal and parietal regions but a reduced amount of activity in ACC, a region associated with the presence of the conflict behaviour and the need to adapt the attention control processes.
On the other hand, aerobic fitness plays an important role in the hippocampus. Aerobic exercise training increases the volume of the hippocampus (Erickson et al., 2011) [17] as aerobic exercises selectively increase the volume of anterior hippocampus involving dentate gyrus. The cells in the anterior hippocampus mediate the absorption of spatial memory (Adlaf et al., 2017) [35]. Similar results are demonstrated in a study by Perreira et al., (2007) [17] which reveal that exercises selectively increase CBF in dentate gyrus and correlate with aerobic fitness as well as increase cognition by improving learning and memory.
Evidence shows that the potential benefits of aerobic exercise extend beyond cardiovascular health indicators and may also affect brain health by reducing the loss of tissue volume.
Can we use video-games as cognitive training tools?
Video games an important element in improving cognitive function
Most of the video games are accelerated, deep, violent, and may not be suitable for older adults. Interestingly, a study conducted with young adults showed that cognitive improvement is not limited to action games (Oei & Patterson, 2013) [36] and video-games training based on real-time strategies have been discovered to enhance executive control skills in older adults (Basak et al., 2008) [37].
On the other hand, video action games training increases concentration and enhances cognitive performance in attention functions (Looi et al., 2016) [22]. These results suggest neurocognitive plasticity in the old adult brain. One possibility is that gaming improves sustained attention and concentration in general. Or gaming practice could have helped participants to keep their attention and optimised efficiency (e.g., resistance to distraction in response to task information). One of the targeted video gaming regions is the hippocampus which plays an active role in navigation and spatial memory of virtual environments (Burgess, Maguire & O’Keefe, 2002) [38], which are much richer and more attractive than those used in typical lab tests (e.g., labyrinths in water). An interpretation of this result is that the long-term exploration of 3D worlds in video games can influence specific hippocampal processes, such as hippocampal neurogenesis which is detected in the dentate gyrus (Adlaf et al., 2017) [35]. In addition to the above advantages according to Connors et al., (2014) [21] video games affect the transfer of spatial information for navigation of new environments to the blind. Despite the role of the video game according to Looi et al., (2016) [22] combining transcranial stimulation with a video game, it is enabled a long-term learning and cognitive raise.
Evidence shows that in contrast to typical brain training, video games are not created with specific cognitive processes of the mind, but are more designed to fascinate the user within characters and adventures.
Conclusion
Based on our review, we conclude that rehabilitation plays an important role in neural plasticity being oriented toward pain, cognition and video games which enable expansion of many cortical changes. According to the observed evidence in scientific articles, we can identify that pain is one of the participatory elements in cortical reorganisation as well as in the volume of representation.
Rehabilitation role it is not only limited to pain, as it plays a role in adaptive plasticity and motor recovery in subjects after CVA where through locomotion training and neurostimulation techniques, improves mobility in these subjects through cortical reorganisation. Also through aerobic fitness and video games, cognitive functions get improved. In this manner, rehabilitation dedicated to promoting wellbeing and health stimulates beneficial neuroplastic changes in the brain thus corresponding with functional improvement.
Limitation
Studies focus on a small number of subjects, but despite this, we have observed consistent models of functional and structural changes. Therefore it is important that the results should be observed in a larger number of subjects for higher comparability, variability and credibility. Also, there are many studies that continue to remain in the use of animal models, and therefore, major financial support should be made to scientific research in humans in such a way as to invest in the growth of well-being and longevity.
Finally, studies with larger groups are mandatory to confirm and extend our findings in a respectful manner to the impaired brain impact. This should ultimately result in more refined knowledge regarding the effects of rehabilitation in neural plasticity.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4(11):1313. doi: 10.1038/3305. https://doi.org/10.1038/3305 PMid:9809557. [DOI] [PubMed] [Google Scholar]
- 2.Hebb DO. The organization of behavior;a neuropsycholocigal theory. A Wiley Book in Clinical Psychology. 1949:62–78. [Google Scholar]
- 3.Boudreau SA, Farina D, Falla D. The role of motor learning and neuroplasticity in designing rehabilitation approaches for musculoskeletal pain disorders. Manual therapy. 2010;15(5):410–4. doi: 10.1016/j.math.2010.05.008. https://doi.org/10.1016/j.math.2010.05.008 PMid:20615749. [DOI] [PubMed] [Google Scholar]
- 4.Tsao H, Galea MP, Hodges PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131(8):2161–71. doi: 10.1093/brain/awn154. https://doi.org/10.1093/brain/awn154 PMid:18669505. [DOI] [PubMed] [Google Scholar]
- 5.Pleger B, Tegenthoff M, Schwenkreis P, Janssen F, Ragert P, Dinse HR, Völker B, Zenz M, Maier C. Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the complex regional pain syndrome I. Experimental brain research. 2004;155(1):115–9. doi: 10.1007/s00221-003-1738-4. https://doi.org/10.1007/s00221-003-1738-4 PMid:15064892. [DOI] [PubMed] [Google Scholar]
- 6.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumers N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995 Jun;375(6531):482–484. doi: 10.1038/375482a0. https://doi.org/10.1038/375482a0 PMid:7777055. [DOI] [PubMed] [Google Scholar]
- 7.Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain An fMRI study in upper limb amputees. Brain. 2001;124(11):2268–77. doi: 10.1093/brain/124.11.2268. https://doi.org/10.1093/brain/124.11.2268 PMid:11673327. [DOI] [PubMed] [Google Scholar]
- 8.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. Journal of Neuroscience. 2011;31(20):7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. https://doi.org/10.1523/JNEUROSCI.5280-10.2011 PMid:21593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkins KB, Owen M, Ingo C, Carmona C, Dewald J, Yao J. Neural plasticity in moderate to severe chronic stroke following a device-assisted task-specific arm/hand intervention. Frontiers in neurology. 2017;8:284. doi: 10.3389/fneur.2017.00284. https://doi.org/10.3389/fneur.2017.00284 PMid:28659863 PMCid:PMC5469871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauraugh JH, Kim SB. Stroke motor recovery:active neuromuscular stimulation and repetitive practice schedules. Journal of Neurology, Neurosurgery &Psychiatry. 2003;74(11):1562–6. doi: 10.1136/jnnp.74.11.1562. https://doi.org/10.1136/jnnp.74.11.1562 PMid:14617717 PMCid:PMC1738214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfurtscheller G, Neuper C. Motor imagery and direct brain-computer communication. Proceedings of the IEEE. 2001;89(7):1123–34. https://doi.org/10.1109/5.939829. [Google Scholar]
- 12.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology. 2011;14(8):1133–45. doi: 10.1017/S1461145710001690. https://doi.org/10.1017/S1461145710001690 PMid:21320389. [DOI] [PubMed] [Google Scholar]
- 13.Hong X, Lu ZK, Teh I, Nasrallah FA, Teo WP, Ang KK, Phua KS, Guan C, Chew E, Chuang KH. Brain plasticity following MI-BCI training combined with tDCS in a randomized trial in chronic subcortical stroke subjects:a preliminary study. Scientific reports. 2017;7(1):9222. doi: 10.1038/s41598-017-08928-5. https://doi.org/10.1038/s41598-017-08928-5 PMid:28835651 PMCid:PMC5569072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landsmann B, Pinter D, Pirker E, Pichler G, Schippinger W, Weiss EM, Mathie G, Gattringer T, Fazekas F, Enzinger C. An exploratory intervention study suggests clinical benefits of training in chronic stroke to be paralleled by changes in brain activity using repeated fMRI. Clinical interventions in aging. 2016;11:97–103. doi: 10.2147/CIA.S95632. https://doi.org/10.2147/CIA.S95632 PMid:26869779 PMCid:PMC4734728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 2004;101(9):3316–21. doi: 10.1073/pnas.0400266101. https://doi.org/10.1073/pnas.0400266101 PMid:14978288 PMCid:PMC373255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. https://doi.org/10.1073/pnas.0611721104 PMid:17374720 PMCid:PMC1838482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. https://doi.org/10.1073/pnas.1015950108 PMid:21282661 PMCid:PMC3041121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. The Journals of Gerontology Series A:Biological Sciences and Medical Sciences. 2003;58(2):M176–80. doi: 10.1093/gerona/58.2.m176. https://doi.org/10.1093/gerona/58.2.M176. [DOI] [PubMed] [Google Scholar]
- 19.Clemenson GD, Stark CE. Virtual environmental enrichment through video games improves hippocampal-associated memory. Journal of Neuroscience. 2015;35(49):16116–25. doi: 10.1523/JNEUROSCI.2580-15.2015. https://doi.org/10.1523/JNEUROSCI.2580-15.2015 PMid:26658864 PMCid:PMC4682779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayas J, Parmentier FB, Andrés P, Ballesteros S. Plasticity of attentional functions in older adults after non-action video game training:a randomized controlled trial. PLoS One. 2014;9(3):e92269. doi: 10.1371/journal.pone.0092269. https://doi.org/10.1371/journal.pone.0092269 PMid:24647551 PMCid:PMC3960226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors EC, Chrastil ER, Sánchez J, Merabet LB. Virtual environments for the transfer of navigation skills in the blind:a comparison of directed instruction vs. video game based learning approaches. Frontiers in human neuroscience. 2014;8:223. doi: 10.3389/fnhum.2014.00223. https://doi.org/10.3389/fnhum.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looi CY, Duta M, Brem AK, Huber S, Nuerk HC, Kadosh RC. Combining brain stimulation and video game to promote long-term transfer of learning and cognitive enhancement. Scientific reports. 2016;6:22003. doi: 10.1038/srep22003. https://doi.org/10.1038/srep22003 PMid:26902664 PMCid:PMC4763231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. Journal of Neuroscience. 2001;21(10):3609–18. doi: 10.1523/JNEUROSCI.21-10-03609.2001. https://doi.org/10.1523/JNEUROSCI.21-10-03609.2001 PMid:11331390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soros P, Knecht S, Bantel C, Imai T, Wusten R, Pantev C. Functional reorganization of the human primary somatosensory cortex after acute pain demonstrated by magnetoencephalography. Neuroscience Letters. 2001;298(3):195–8. doi: 10.1016/s0304-3940(00)01752-3. https://doi.org/10.1016/S0304-3940(00)01752-3. [DOI] [PubMed] [Google Scholar]
- 25.Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neuroscience letters. 1997;224(1):5–8. doi: 10.1016/s0304-3940(97)13441-3. https://doi.org/10.1016/S0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- 26.Krause P, Förderreuther S, Straube A. TMS motor cortical brain mapping in patients with complex regional pain syndrome type I. Clinical neurophysiology. 2006;117(1):169–76. doi: 10.1016/j.clinph.2005.09.012. https://doi.org/10.1016/j.clinph.2005.09.012 PMid:16326140. [DOI] [PubMed] [Google Scholar]
- 27.Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalography and clinical neurophysiology/electromyography and motor control. 1995;97(6):382–6. doi: 10.1016/0924-980x(95)00194-p. https://doi.org/10.1016/0924-980X(95)00194-P. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proceedings of the Royal Society of London. Series B:Biological Sciences. 1996;263(1369):377–86. doi: 10.1098/rspb.1996.0058. https://doi.org/10.1098/rspb.1996.0058 PMid:8637922. [DOI] [PubMed] [Google Scholar]
- 29.Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, Di Prampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery:a functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16(23):7688–98. doi: 10.1523/JNEUROSCI.16-23-07688.1996. https://doi.org/10.1523/JNEUROSCI.16-23-07688.1996 PMid:8922425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nature neuroscience. 2009;12(11):1370. doi: 10.1038/nn.2412. https://doi.org/10.1038/nn.2412 PMid:19820707 PMCid:PMC2770457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–5. doi: 10.1097/01.wnr.0000177010.44602.5e. https://doi.org/10.1097/01.wnr.0000177010.44602.5e PMid:16148743. [DOI] [PubMed] [Google Scholar]
- 32.Ang KK, Guan C, Chua KS, Ang BT, Kuah C, Wang C, Phua KS, Chin ZY, Zhang H. A clinical study of motor imagery-based brain-computer interface for upper limb robotic rehabilitation. In 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2009:5981–5984. doi: 10.1109/IEMBS.2009.5335381. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. https://doi.org/10.1038/22682 PMid:10440369. [DOI] [PubMed] [Google Scholar]
- 34.Murdoch K, Buckley JD, McDonnell MN. The effect of aerobic exercise on neuroplasticity within the motor cortex following stroke. PloS one. 2016;11(3):e0152377. doi: 10.1371/journal.pone.0152377. https://doi.org/10.1371/journal.pone.0152377 PMid:27018862 PMCid:PMC4809484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adlaf EW, Vaden RJ, Niver AJ, Manuel AF, Onyilo VC, Araujo MT, Dieni CV, Vo HT, King GD, Wadiche JI, Overstreet-Wadiche L. Adult-born neurons modify excitatory synaptic transmission to existing neurons. Elife. 2017;6:e19886. doi: 10.7554/eLife.19886. https://doi.org/10.7554/eLife.19886 PMid:28135190 PMCid:PMC5279947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oei AC, Patterson MD. Enhancing cognition with video games:a multiple game training study. PLoS One. 2013;8(3):e58546. doi: 10.1371/journal.pone.0058546. https://doi.org/10.1371/journal.pone.0058546 PMid:23516504 PMCid:PMC3596277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychology and aging. 2008;23(4):765–77. doi: 10.1037/a0013494. https://doi.org/10.1037/a0013494 PMid:19140648 PMCid:PMC4041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–41. doi: 10.1016/s0896-6273(02)00830-9. https://doi.org/10.1016/S0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]


