Abstract
Sierra Leone has a high tuberculosis (TB) burden with a prevalence of 441 cases per 100 000 population. As a result of the Global Fund, some facilities in the country have access to improved diagnostics, including Xpert MTB/RIF testing, of particular use in diagnosing those at risk of drug resistance, in the form of rifampicin-resistant (RR) TB. This quality improvement project describes how a small, rural district general hospital in Masanga village improved the diagnosis of TB and RR-TB by creating a formal link with the regional hospital in Makeni city. In an effort to improve diagnosis, all patients with a suspicion of TB and one of the following would have a sample sent for Xpert MTB/RIF testing: previous TB treatment (of any course length), HIV positive or known contact of a RR-TB case. The samples were transported by the logistics team, who already drove weekly from Masanga to Makeni for supplies, and the results were texted to the clinician in charge of the medical ward. Over the course of the first 4 months of this intervention, 34 samples had Xpert MTB/RIF testing performed compared with two samples in the previous 12 months since the machine had been installed. This yielded nine additional diagnoses of TB (in patients with negative or unavailable smear results) and five diagnoses of RR-TB with subsequent appropriate isolation and transfer to the central tertiary centre. This study shows that it is feasible to centralise Xpert MTB/RIF testing in low-resource settings using creative methods for sample transfer and results dissemination, leading to both improved diagnostics and infection control.
Keywords: quality improvement, laboratory medicine, infection control
Problem
Masanga Hospital is a non-governmental, rural district general hospital in the Tonkolili District of Sierra Leone, West Africa, serving a population of around 400 000. It is a 100-bedded hospital which provides paediatric, obstetric, adult internal medical and general surgical care. There is also a busy outpatient department (OPD) which treats around 500 patients per month. Care is co-ordinated by local community health officers and supervised by a multidisciplinary team of international volunteers.
Masanga Hospital has a well-resourced laboratory for the area with three full-time laboratory technicians able to provide basic haematological, biochemical and microbiological testing as well as a 24 hours transfusion service. However, the microbiological diagnosis of tuberculosis (TB) is limited to Acid Fast Bacilli (AFB) microscopy alone. Microscopy has limited sensitivity, especially in HIV-positive patients, and cannot diagnose drug resistance.
Sierra Leone has a high TB burden with a prevalence of 441 cases per 100 000 population.1 As a result of the Global Fund, some facilities in the country have access to improved diagnostics, including Xpert MTB/RIF testing, of particular use in diagnosing those at risk of rifampicin-resistant (RR) TB.2 3
The WHO recognises that fast and accurate diagnosis of TB and RR-TB is essential for disease control and that Xpert MTB/RIF has a substantial role in this. However, it also recognises that this testing is not commonly available in low-resource areas and it recommends that drug sensitivity testing is centralised in specialised laboratories.3
Our quality improvement project involved improving the microbiological diagnostics for both TB and RR-TB by testing samples with Xpert MTB/RIF, a technology not available at our hospital. In order to do this, a formal link with the regional hospital in Makeni City was established and samples were taken from Masanga to Makeni once or twice per week for testing.
Our SMART (Specific, Measurable, Attainable, Relevant, Time-based) objective mirrored the national guidelines in that 100% of patients with a suspicion of TB and one of the following would have a sample sent for Xpert MTB/RIF testing: previous TB treatment (of any course length); HIV positive or known contact of a RR-TB case.
The target date for completion was 4 months following implementation as this was the date the international internal medical volunteer was to leave the country. We planned to record the number of samples sent, the reasons why they were sent and the results of the Xpert MTB/RIF test. We used these findings to improve our intervention using a Plan-Do-Study-Act (PDSA) model.
Background
RR-TB is a major global public health concern and requires rapid diagnosis and treatment with second-line drugs. Around 150 000 cases of drug-resistant TB were reported in 2017, around 2.4% of all TB diagnoses,4 with a significant estimated number of missed diagnoses or patients never initiated on treatment.
Xpert MTB/RIF (also known as GeneXpert) is an automated molecular test with a sensitivity and specificity of over 97%2 for both the presence of Mycobacterium tuberculosis (MTB) and genes transferring rifampicin resistance. It should diagnose 23% more cases than microscopy alone5 and additionally, it can be used to improve both the accuracy and the efficiency of TB and RR-TB diagnosis in specific subgroups of patients such as those who are AFB smear-negative or HIV positive,2 3 5 although the sensitivity does drop to around 70% in both groups.5
Rifampicin resistance is often used as a proxy for isoniazid resistance, thereby fulfilling multidrug-resistant criteria, especially in lower-resourced TB programmes with limited phenotypic drug-sensitivity testing. Globally, isoniazid sensitivity in RR isolates varies from 0.5% to 11.6% but may be as high as approximately 40%.6 However, this is often not clinically relevant where drug options are restricted and patients receive the same second-line regiment regardless.
Xpert MTB/RIF was recommended for use by the WHO in high-incidence countries in 20105 and a large number of units have been installed in such places. However, it is not a technology found in every hospital due to drawbacks including: high costs (a typical four-module machine costing around $18 000 with a single-use cartridge cost of almost $107); the need for reliable power supply; specialised staff3 and an operating temperature of less than 30°C.8 Therefore, the WHO recommends specialised centralised laboratories be developed to perform Xpert MTB/RIF testing. Unfortunately, this has its own challenges, chiefly in ensuring safe and rapid transfer of samples from peripheral sites to central ones.3 Other African countries have had some success with transport systems independent of healthcare systems such as a national bus system in Malawi9 or the Ugandan postal service.10
Xpert MTB/RIF is a crucial tool that can be used in case-finding as it the most widely available drug-sensitivity test globally and much faster than mycobacterial culture. Timely diagnosis of RR-TB improves individual patient prognosis and protects public health as community exposure to resistant organisms is reduced, assuming there are facilities for treating such patients. It is therefore essential that high-incidence countries use this technology effectively and that there are systems in place for transferring samples to sites where testing is available, which is what we have tried to do in our project.
Measurement
The Xpert MTB/RIF machine was installed in Makeni Hospital on 28 Dec 2016. Our retrospective data start from December 2016 as this is the first time where Xpert MTB/RIF testing would be possible in the region and finishes in November 2017, when the project was conceived. From December 2017, data collection is prospective until March 2018 when the authors left country.
In these first 12 months, 442 patients had had samples tested locally with microscopy for AFBs with 100 patients positive (94 new diagnoses, 6 follow-up cases). During this time, special measures had been taken by the doctors of Masanga for two patients to have Xpert MTB/RIF testing as they were highly clinically suspicious for drug resistant TB, being smear-positive after months of treatment. While both of these were positive for TB on Xpert MTB/RIF testing, one also had RR-TB and was transferred to the newly opened national centre for RR-TB near the capital, Freetown. However, as the technology was new to that area, there was not a robust referral system in place for routine testing and results dissemination at the time our project started, thereby missing opportunities for RR-TB diagnosis.
Design
During handover from a previous international medical volunteer to the authors, the case of the above patient with RR-TB and the availability of Xpert MTB/RIF testing was discussed. Some weeks later, when a patient presented with a chronic, productive cough despite ongoing TB treatment having previously defaulted some months prior, an agreement was made with the regional head of TB services to perform Xpert MTB/RIF testing. The sample was couriered by the regional head on a motorbike 45 minutes away to Makeni City. This sample was also positive for RR-TB and the patient was isolated and transferred to the RR-TB centre for treatment, which was the protocol for RR-TB cases given from the Department of Health of Sierra Leone.
This second case acted as proof of concept and the idea of regular samples to Makeni was conceived to improve the diagnostic capabilities of our hospital. We consulted with the medical superintendent and head of logistics about the best way to transport samples as it was not sustainable to rely on the regional director to hand-deliver each sample.
National guidelines at the time were that Xpert MTB/RIF should be performed on all patients with a chronic cough and any of the following: exposure to RR-TB; previously defaulted or completed TB treatment or HIV positive. It was suggested by the authors that samples from such patients would be transported weekly with the ‘logistics car’ as it already went from Masanga to Makeni each Wednesday for food and medical supplies. The driver of this car was informed of what they were transporting and agreed to make the deliveries.
It was agreed with the regional director that any samples that fit the national guidelines be tested in Makeni at the expense of the national TB programme as he was enthusiastic about the new venture at improving case-finding.
The laboratory staff at Makeni already had ample stock of testing disposables (such as Xpert MTB/RIF cartridges) provided by the national programme, which is funded by international donors, and were happy to take samples from elsewhere.
Strategy
Plan-Do-Study-Act (PDSA) cycle 1
In the first month (December 2017) of the new agreement, five samples were transported with the logistics car in labelled sputum pots sealed in sample bags and placed in protective cardboard boxes. Three of these proved positive for TB and two of those for RR-TB. Interestingly, two of the patients who proved positive on Xpert MTB/RIF had also had three negative AFB samples at our laboratory.
Initially, the results were given to the regional director of the TB programme who would eventually contact Masanga Hospital. However, we found that process added a delay in getting the results to the clinicians treating the patients so we began adding contact numbers for the doctors on the wards to the sample forms and asking the laboratory staff at Makeni to text them the results directly.
PDSA cycle 2
After this first month, it was clear that this agreement was effective at both improving diagnostics of TB and RR-TB in a timely manner and it was continued. The use of Xpert MTB/RIF testing was highlighted to the community health officers at medical handover to increase awareness of the new process.
One problem with the process was that samples only went on a Wednesday, thereby leaving a potential of a patient waiting up to 6 days for this test. We discussed this with the other medical staff who volunteered to deliver samples to the laboratory in Makeni when passing through the city on their way to other parts of the country either for national meetings or weekend breaks, which typically happened at least each Friday. This reduced the amount of time required to wait between presentation of the patient and diagnostic testing. Twelve samples were sent this month (January 2018) with five positive for TB, including four AFB-negative samples. Samples were broadened from sputum only to include ascites and pus from surgical sites.
PDSA cycle 3
In order to improve the sustainability of the project, so that it would not rely on the presence and activity of an international volunteer, it was handed over to the national laboratory staff after month three (February 2018) to co-ordinate the collection and transfer of samples as well as receive and disseminate the results. They were given adequate explanation on the process and the regional team were informed of the transition. Handover was done a month before the international volunteer left the post in order to assist with any problems encountered with the new responsibilities.
A small amount of phone credit was also given to the laboratory staff in Makeni who were using personal mobiles to transmit results. This unexpected but necessary cost was covered by the medical volunteers but in future could come from the running costs of the hospital or from the national TB programme budget.
Results
In total, 34 samples were sent for Xpert MTB/RIF testing in the 4-month period between the start of the intervention in December 2017 and the date the first author left Sierra Leone in March 2018 compared with 2 in the previous 12 months from December 2016 when the machine was installed until November 2017.
Sixteen of these 34 (47%) were positive with 5 diagnoses (15%) of RR-TB and 9 new diagnoses (26%) of TB that had been missed on microscopy. These figures are presented in table 1.
Table 1.
A table displaying the changes in diagnosis of tuberculosis during the project
| Testing period | Months | Microscopy samples performed | Microscopy samples positive | Xpert samples performed | Xpert samples positive | Rifampicin Resistant positive | Additionaldiagnoses by Xpert |
| December 2016–November 2017 | 12 | 442 | 100 | 2 | 2 | 1 | 0 |
| December 2017–March 2018 | 4 | 219 | 47 | 34 | 16 | 5 | 9 |
By the end of PDSA 3, 100% of eligible inpatients had a sample sent for Xpert MTB/RIF. The figure of eligible outpatients who underwent Xpert MTB/RIF testing is unknown as the records from the OPD were not comprehensive enough to allow for this data extraction.
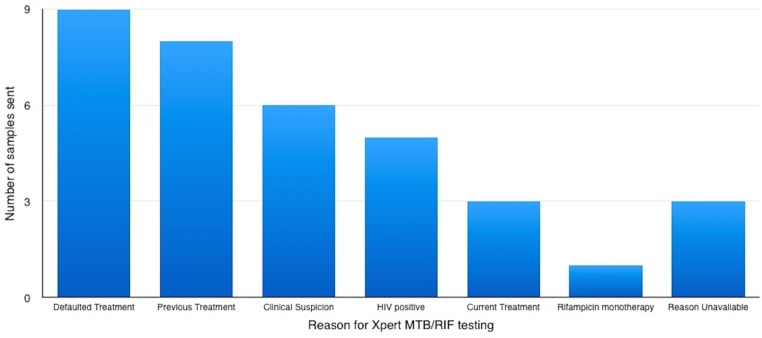
The reasons why samples were sent for Xpert MTB/RIF are presented (figure 1). The most common reasons were either previous treatment or loss-to-follow-up, and given the lack of reliable treatment completion data, it is likely these two groups overlap. It should also be noted that some samples were sent due to high clinical suspicion of TB with negative AFBs and that in one case a patient had both had previous treatment and was HIV positive.
Figure 1.
Reasons for Xpert MTB/RIF testing by number.
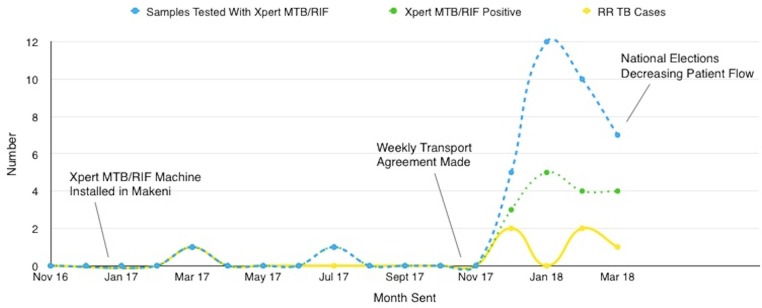
A run-chart is shown in figure 2 demonstrating the number of Xpert MTB/RIF samples sent for testing with their results. Each month from December 2017 through to March 2018 corresponds to a new PDSA cycle.
Figure 2.
Run chart of the number of Xpert MTB/RIF tests and results, including RR by month. RR, rifampicin resistance; TB, tuberculosis.
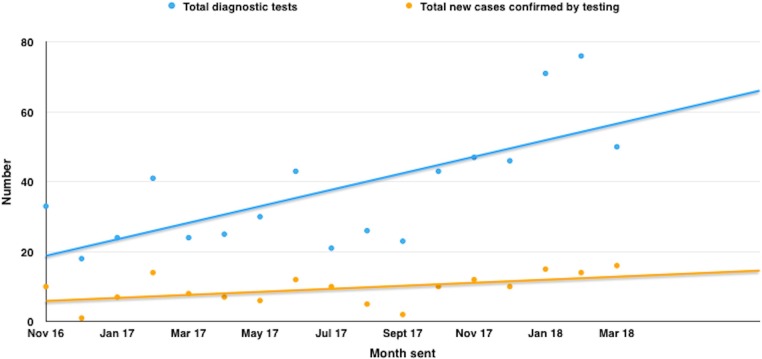
During the time period studied, the total number of TB diagnoses increased as demonstrated in figure 3. This is in part because of our intervention; however, it is also to be noted that the number of diagnostic tests increased, which may be a reflection of other factors discussed later.
Figure 3.
Number of diagnostic tests and their results for TB by monthly statements. TB, tuberculosis.
Lessons and limitations
In the brief period this project was performed, we demonstrated an increase of patients undergoing advanced diagnostics from 2 in 12 months to 34 in 4 months by innovative utilisation of the infrastructure already in place. While this timeframe is short, this project demonstrates the feasibility of centralising testing in this low-resource area and is in line with the literature that Xpert MTB/RIF improves diagnostics in high-incidence countries.2 3 5
We have also demonstrated an increased number of TB diagnoses compared with a similar period of time from the previous year. This will in part be due to the use of Xpert MTB/RIF testing, which found nine diagnoses missed on microscopy, although there are likely other factors involved such as increased patient flow to the hospital and more diagnostic tests performed. It is also unknown if the incidence of the disease is increasing in the area or whether increased numbers are secondary to increased testing. It is clear that the increased number of RR-TB diagnoses can only be from Xpert MTB/RIF testing.
An essential part of our project was to use both transport links already in place and advances of mobile technology in the area to improve the efficiency of communication between hospitals in an area where travel is difficult. This part of the project should be generalisable to areas facing similar challenges and is another example of using creative methods for specimen transfer in Africa.9 10
A crucial part of quality improvement by international staff in low-income and middle-income countries is ensuring local involvement in order to maintain sustainability. We involved the local laboratory staff, logistics co-ordinator and regional TB director early on in the project and ensured handover was timed properly to allow the international volunteers time to help with ‘teething problems’.
An important limitation is that all these data come from the time where there was an international medical volunteer in post who was passionate about the project. It is unknown whether it is still 100% of eligible patients have a sample sent for Xpert MTB/RIF, although communication with other doctors in post suggests that this link between Masanga and Makeni remains strong and that samples are still transported weekly for testing, although it would be useful to examine this formally in the future.
Another limitation is that the data come almost exclusively from an inpatient population, where the authors were predominantly working. The OPD is largely run by community health officers and while we are aware of a few samples being sent for Xpert MTB/RIF on outpatients, we suspect it was not 100% of those who met criteria. However, as the community health officers tended to admit most patients with clinical suspicion of RR-TB, the number of missed patients is unlikely to be large.
Available guidance from international TB programmes supports the safety of transferring high-risk samples in tightly-sealed containers inside air-tight sample bags, cushioned from difficult terrain in other containers.11 12 Given that we followed this advice and that drivers and clinicians voluntarily transported these samples, we do not feel there was an undue risk to staff and that the risk of missed diagnosis to the community at large was much greater if the samples had not been sent.
The project increased the resources required by both the hospital and the national TB programme. Additional resources such as sputum containers, sample bags, referral forms were provided by Masanga Hospital. As stated above, phone credit for the laboratory technicians to text results was donated by one of the authors. Fuel costs for transporting the samples did not increase as samples were only taken by drivers already travelling between Masanga and Makeni.
The largest increase in resources was provided by the national programme who supplied all the cartridges for the Xpert MTB/RIF testing. This project would have been considerably harder if we had to convince the hospital to pay for these and could be a substantial limiting factor in other places trying to replicate it.
Increasing the diagnostics of RR-TB also required the national programme to become responsible for more patients. These patients were isolated in Masanga Hospital before being transported to the tertiary centre for RR-TB for treatment, as per the policy of the Department of Health. This also increased the burden on patients and their families who had to support themselves in isolation until transfer, which could occasionally be a couple of weeks due to shortages of beds in the tertiary hospital. Once at the tertiary hospital however, admission, food and medication were all provided free of charge by the national programme.
Conclusion
Xpert MTB/RIF is an essential part of a TB programme, especially in a low-resource country, and its use is endorsed by the WHO. However, its high cost limits access to testing to only a few sites.
This quality improvement project shows that it is feasible to centralise Xpert MTB/RIF testing in Sierra Leone using creative methods for sample transfer and results in dissemination, leading to both improved diagnostics and infection control and that this should be imitated across the region.
Acknowledgments
We would like to thank Maxwell Sesay, Kelfala Kamara and John Sesay for their assistance in country as well as Dr Rashidatu Kamara and Dr Lynda Foray of the National TB programme of Sierra Leone.
Footnotes
Contributors: DH and JVN designed the study and helped collate data. DH, JVN and MPG were all extensively involved with drafting the work and gave final approval of the of the version published. DH is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Anderson and Hamblion Epidemiological review of TB disease in Sierra Leone. [ONLINE], 2015. Available: http://www.afro.who.int/sites/default/files/2017-06/octoberr%202025.pdf [Accessed 21 June 2018].
- 2. Boehme CC, Nabeta P, Hillemann D, et al. . Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med Overseas Ed 2010;363:1005–15. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organisation Companion Handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2014 Geneva: World Health Organization, 2014. [PubMed] [Google Scholar]
- 4. WHO Global tuberculosis report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 5. Steingart KR, Schiller I, Horne DJ, et al. . Xpert®MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurbatova EV, Cavanaugh JS, Shah NS, et al. . Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int J Tuberc Lung Dis 2012;16:355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parternship STB. TB REACH Xpert Budget Estimation Tool. [ONLINE], 2018. Available: http://www.stoptb.org/global/awards/tbreach/bet.asp [Accessed 21 June 2018].
- 8. Bernardo J. UpToDate: Diagnosis of pulmonary tuberculosis in adults. [ONLINE], 2018. Available: https://www.uptodate.com/contents/diagnosis-of-pulmonary-tuberculosis-in-adults [Accessed 21 June 2018].
- 9. Harries AD, Michongwe J, Nyirenda TE, et al. . Using a bus service for transporting sputum specimens to the central reference laboratory: effect on the routine TB culture service in Malawi. Int J Tuberc Lung Dis 2004;2004:204–10. [PubMed] [Google Scholar]
- 10. Joloba M, Mwangi C, Alexander H, et al. . Strengthening the tuberculosis specimen referral network in Uganda: the role of public-private partnerships. J Infect Dis. 2016;213:S41–S46. 10.1093/infdis/jiw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Global Health Delivery Project and Fischer-Mackey Transporting Suspected and Confirmed MDR-TB Patients and Sputum Samples. [ONLINE], 2010. Available: https://www.ghdonline.org/ic/discussion/transporting-suspected-and-confirmed-mdr-patients/brief/ [Accessed 29 Sep 2018].
- 12. World Health Organisation Division of emerging and other communicable diseases surveillance control. Guidelines for the safe transport of infectious substances and diagnostic specimens. 1997 Geneva: World Health Organization, 1997. [Google Scholar]