Abstract
Aim
To investigate whether patient-specific instrumentation (PSI) and single-use instrumentation (SUI) improve operating room efficiency in terms of time and cost to the healthcare provider over conventional/reusable instrumentation (CVR) when performing total knee arthroplasty (TKA).
Patients and methods
Patients requiring TKA were randomised into one of four surgical groups: CVR, CVS (conventional/SUI), PSR (PSI/reusable) and PSS (PSI/SUI). All surgical procedures were video recorded to determine specific surgical time intervals. Other variables reported included the number of instrument trays used, missing equipment, direct instrument costs and the weight of the instruments the staff had to handle. Oxford Knee Score (OKS), estimated blood loss and lengths of hospital stay were also recorded as markers of patient experience.
Results
PSR was significantly quicker in all the recorded time intervals, used less trays, experienced less missing equipment and resulted in lower blood loss and shorter hospital stays. SUI reported significantly slower operating room times and resulted in higher blood loss, but SUI was 88% lighter and 20% cheaper on average when compared with their reusable counterparts. Despite the economic advantages of PSI and SUI, the patients who reported greatest improvements in OKS were those allocated to the CVR group, but no clinically meaningful difference in OKS was found at any time point.
Conclusions
PSI and SUI for TKA have the potential of reducing operating room times over conventional, reusable sets. This reduction will benefit theatre personnel ergonomically, while presenting the healthcare provider with potential cost-saving benefits in terms of reduced sterilisation costs and surgical times.
Keywords: cost-effectiveness, qualitative research, randomised controlled trial, surgery
Introduction
Total knee arthroplasty (TKA) is currently the most effective and successful treatment for advanced osteoarthritis and its related pain in the knee.1–3 In 2017, over 110 000 TKA procedures were performed in the UK.4 Worldwide ageing populations and growing obesity rates are causing substantial increases in the volume of TKA procedures carried out annually.5 One recent study predicted a growth in the procedure of 673% in the USA by 2030.6
The economic efficiency of TKA must be optimised for healthcare services to be able to respond to the growing demand in TKA without sacrificing the level of care provided to patients. This could be attained by reducing operative costs and times. Achieving these goals would allow for greater number of surgical cases to be completed daily without having to extend operating hours or increase the number of theatres used per day; both of which imply additional cost to the institution.
Over recent years, several orthopaedic manufacturers have introduced patient-specific instrumentation (PSI) and single-use instrumentation (SUI) for TKA. PSI is a bespoke surgical approach which aims to provide increased implant accuracy and surgical efficiency over conventional techniques.7 8 PSI for TKA is designed using MRI or CT scans of the patient’s preoperative knee.7–9 Using the 3D image of the knee, a plan of the intended procedure can be created by the surgeon, which includes recommended implant size and alignment.7 10 From the agreed plan, bespoke cutting blocks are created to guide the saw intraoperatively for accurate placement of the implant.8 Given the detailed preoperative plan, PSI reduces the number of TKA instruments and intraoperative surgical steps. PSI could therefore reduce the length of each procedure and save on sterilisation costs of the reusable equipment.
Single-use instruments have the potential to further reduce the costs of sterilisation, as the instruments are disposed of postoperatively. Disposable instruments are commonly used in hospitals due to their appealing traits in favour of sterility, safety, efficiency and, when scaled sufficiently, cost.11 TKA SUI is provided by the manufacturer in sealed presterilised size-specific kits containing all the required tools. The published literatures on SUI have investigated their effect on surgical efficiency, institutional costs and their impact on risk of infections.12–17
However, current data on the use of both SUI and PSI in TKA are limited, with conflicting views on whether the technologies are appropriate and cost-effective for routine use.
The aim of this study was to perform a comparative investigation of the efficiency of conventional instrumentation, PSI and SUI. The research question was: Do PSI and SUI present improved efficiency in terms of time and cost savings to the institution over the conventional reusable instrumentation sets when performing TKA? The objective was to assess (1) instrument-related surgical efficiency, (2) instrument-related costs, and (3) whether patient-related outcomes were affected by the instrumentation used. We hypothesised that the use of PSI would improve surgical efficiency at the expense of increased cost, and that SUI would present similar efficiency while improving cost.
Materials and methods
Patient recruitment
A sample size calculation was used to predict group size, where the level of significance was 5% (α=0.05) and the power was 80% (β=0.2).18 The outcome variable used was the minimum clinically important difference in surgical time. Fifteen minutes was deemed clinically important, as this would allow for the addition of one extra TKA procedure a day. The SD for this variable used was 9 min, which was calculated from 50 consecutive conventional TKA operations carried out by the surgeon. Using these variables, it was determined that six patients were required per patient group.
Eligible patients were those diagnosed with osteoarthritis of the knee which was sufficiently symptomatic to require TKA. Patients were excluded if they showed signs of inflammatory arthritis, ligament problems, significant knee deformities, or if they required complex bone augmentation procedures. Written and verbal informed consent was provided by all patients.
The patients were randomised into four separate groups by block stratification (figure 1). The four instrument groups were: conventional/reusable (CVR), patient-specific/reusable (PSR), conventional/single-use (CVS) and patient-specific/single-use (PSS) instrumentation. Recruitment ended when at least six patients had undergone TKA in each surgical group.
Figure 1.
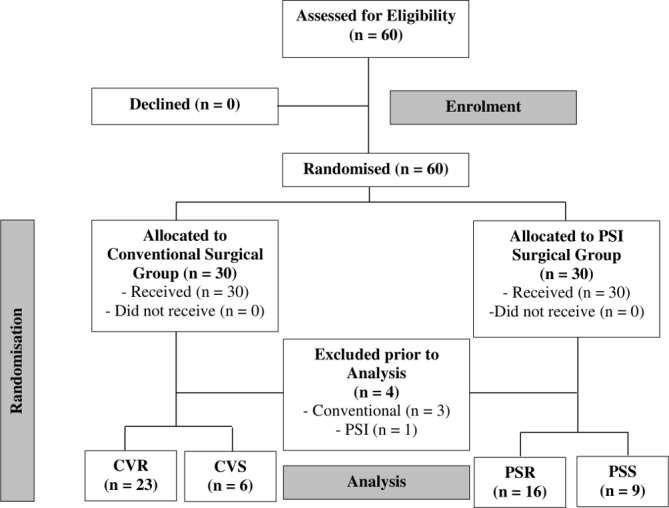
A Consolidated Standards of Reporting Trials (CONSORT) diagram of patient recruitment and involvement in this study. CVR, conventional/reusable; CVS, conventional/single use; PSI, patient-specific instrumentation; PSR, patientspecific/reusable; PSS, patient specific/single use.
This study is registered with ClinicalTrials.gov.
Surgical technique and instrumentation
One surgeon (LCB) performed all procedures between 2015 and 2016 at the Royal Infirmary of Edinburgh using a fixed-bearing prosthesis (GMK Sphere, Medacta International, Switzerland).
Conventional instrumentation
Patients randomised into the conventional group received standard care. Routine radiographs were taken of the preoperative knee during elective clinics and used to guide the operative plan. Bone cuts were performed using standard GMK Sphere instrumentation, using an intramedullary guidance rod for the femur and an extramedullary guidance rod for the tibia. The instruments were sterilised using an in-house standard autoclave procedure.
Patient-specific instrumentation
MyKnee PSI manufactured by Medacta International was used for this study protocol (Medacta International, Castel San Pietro, Switzerland). Patients randomised into the PSI group underwent a preoperative brief low radiation CT scan in addition to routine radiographs, as per the standardised MyKnee protocol. 3D plans of the preoperative knee showing the virtual positioning of the implant were uploaded onto an online case database so that the surgeon and technician could liaise on the operative plans. Bespoke cutting blocks were then designed, based on the plans to fit securely onto the osteophytes of the tibial and femoral bones (figure 2A). Once the plans were finalised, the PSI was created by 3D printing technology and shipped to the hospital. Intraoperatively, the cutting jigs were placed in their preplanned positions, checked by the surgeon and then pinned in place. The bone cuts were then performed according to the preoperative plan. Prosthetic fixation was performed using antibiotic laden cement. Reusable instruments were sterilised using an in-house standard autoclave procedure.
Figure 2.
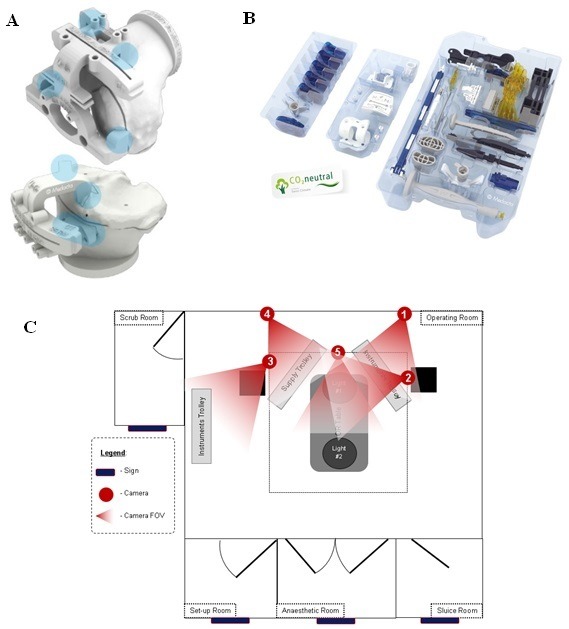
(A) MyKnee patient-specific cutting blocks secured onto the distal end of a femur (above) and proximal end of a tibia (below) using distinct anatomical landmarks (blue highlights). (B) The single-use GMK Efficiency instruments used for total knee arthroplasty (TKA). (C) Camera placement in theatre. FOV, field of view.
Single-use instrumentation
The SUI used for this study was the GMK Efficiency, designed specifically to implant the GMK Sphere TKA (Medacta International). Patients randomised into the SUI group were not required to undergo any additional procedures. The SUI arrived in the hospital presterilised and in a presealed sterilised package (figure 2B).
Data collection
Intraoperatively, each surgery was video recorded using five cameras (figure 2C). Bespoke software synchronised the five recordings then extracted data on surgical time intervals and the number of trays opened per case (MATLAB Release 2016b, The MathWorks, Massachusetts, USA).
Complications, and the number of missing equipment reported per case, were documented intraoperatively by the surgeon. This information was recorded for the study by a member of the research team and then verified by the surgeon. Complications or delays experienced during surgeries were classified as ‘instrument-related’ or ‘general’. The time spent waiting intraoperatively as a result of a general issue (verified by an orthopaedic surgeon) was deducted from the corresponding surgical interval, while instrument-related issues and delays were included in the analysis.
The time taken for the instruments to be taken for sterilisation and returned back to the theatre instrument stores was recorded using an in-house barcode tracking system. The weights of the conventional and SUI trays were averaged over three different instances throughout the length of the study.
Reusable instrument costs for the institution were calculated as the sterilisation cost (as quoted by the in-house sterilisation unit) for sterilising a single instrument tray. This price was subsequently multiplied by the average number of conventional trays used during the surgery. SUI prices were obtained from the company issuing the instruments, as the price the institution paid for each individual SUI set. The company also supplied the price for the patient-specific cutting jig and their corresponding 3D-printed bone models. CT scan cost was obtained from the institution radiology centre.
Additional variables were also reported to investigate the effect of PSI and SUI on patient outcome. The Oxford Knee Score (OKS) was completed by patients at preadmission clinics, then 6 weeks and 1 year following surgery. The OKS was scored by one member of the trial team and verified by another at a later date. The lengths of hospital stay for all patients were also reported. Finally, haematocrit levels were assessed at preadmission clinics and 24 hours following surgery. These variables were used to estimate blood loss during surgery (Equation 1).19
The estimated blood volume (EBV) was calculated by multiplying the patient weight (in kilograms) by a constant (mL/kg) which varied depending on body habitus.19
Patient and public involvement
Patients and members of the public were not involved in the design, recruitment or conduct of this study. There were lay members on the trial steering committee, however. The results of this study will be communicated to study participants on request.
Results
Patient demographics
Sixty patients agreed to participate in this investigation. Of those, four were excluded from the analysis after randomisation, due to drained camera batteries which led to incomplete recordings (figure 1). There were no significant differences in patient demographics between groups (table 1).
Table 1.
Study group demographics
| CVR | CVS | PSR | PSS | Total | |
| n | 23 | 6 | 18 | 9 | 56 |
| Male/female* | 10:13 | 3:3 | 11:7 | 5:4 | 29:27 |
| Left/right knee* | 13:10 | 4:2 | 9:9 | 6:3 | 32:24 |
| Age (years)† | 70.1 (35) | 71.2 (24) | 71.1 (39) | 70.7 (21) | 70.6 (40) |
| BMI (kg/m2)† | 30.8 (15.2) | 31 (21.51) | 30.6 (17.52) | 27.19 (14.66) | 30.3 (21.92) |
*Values are presented as ratios.
†Values are presented as mean (range).
BMI, body mass index; CVR, conventional/reusable; CVS, conventional/single use; PSR, patient specific/reusable; PSS, patient specific/single use.
Complications
Instrument-related issues occurred intraoperatively. In one case, a new tray was opened in a CVR procedure to replace a femoral extractor which showed remains of cement from previous use. In one CVS case, an SUI femoral extractor failed, and so a new reusable instrument tray was opened to replace it. In another CVS case, the tibia required recutting due to inaccuracies of SUI equipment. One complication arose in the PSR group, where the surgeon felt the tibial cutting jig was one size too small for the patient’s knee, and so CVR instrumentation was used for the tibial cuts.
These complications were clearly the results of the original instrumentation trays containing inadequate equipment for the procedure, or missing crucial parts. Table 2 shows that for each conventional procedure carried out with reusable equipment, 1.48 parts were missing. This was lower when the PSI instrumentation was used (0.22).
Table 2.
Results for all instrumentation sets
| Variables | CVR | CVS | PSR | PSS | P value | ||
| CVR versus PSR | CVR versus CVS | PSR versus PSS | |||||
| IDT* | 00:02:29 (00:04:58) | 00:03:54 (00:06:49) | 00:01:14 (00:01:56) | 00:07:50 (00:19:52) | 0.006† | 0.433† | 0.021‡ |
| IAT* | 00:09:22 (00:13:31) | 00:12:35 (00:06:59) | 00:07:25 (00:12:31) | 00:15:01 (00:21:03) | 0.133‡ | 0.067‡ | 0.013‡ |
| IST* | 00:11:52 (00:17:55) | 00:16:30 (00:10:41) | 00:08:49 (00:23:26) | 00:22:51 (00:36:29) | 0.022† | 0.025‡ | 0.0005† |
| SST* | 00:30:10 (00:36:44) | 00:34:51 (00:28:25) | 00:25:43 (00:19:30) | 00:31:54 (00:25:05) | 0.054‡ | 0.258‡ | 0.017‡ |
| PT* | 01:03:10 (00:38:02) | 01:05:28 (00:23:22) | 00:58:10 (00:23:04) | 00:58:46 (00:19:35) | 0.054‡ | 0.57‡ | 0.814‡ |
| ICT* | 00:05:13 (00:09:14) | 00:02:39 (00:03:35) | 00:02:50 (00:04:18) | 00:02:39 (00:05:02) | 0.002† | 0.025† | 0.754‡ |
| IPT* | 00:03:37 (00:06:00) | 00:02:24 (00:05:57) | 00:03:14 (00:07:26) | 00:01:39 (00:03:05) | 0.18† | 0.109‡ | 0.041† |
| ICuT* | 00:09:15 (00:15:05) | 00:05:03 (00:09:32) | 00:06:04 (00:10:11) | 00:04:18 (00:06:22) | 0.004‡ | 0.015‡ | 0.117‡ |
| TIT* | 01:25:12 (00:28:12) | 01:23:26 (00:48:03) | 01:15:48 (00:41:34) | 01:17:59 (00:23:28) | 0.004‡ | 0.199† | 0.585‡ |
| IST* | 74:16:29 (216:19:45) | N/A | 85:11:48 (451:58:40) | N/A | 0.536† | N/A | N/A |
| nTrays | 3.74 (2) | N/A | 2.89 (1) | N/A | <0.0001† | N/A | N/A |
| Missing equipment | 1.48 (5) | N/A | 0.22 (1) | N/A | 0.009† | N/A | N/A |
| Blood loss (mL) | 898.87 (1135.83) | 936.82 (502.90) | 785.24 (1784.97) | 811.96 (1112.08) | 0.358‡ | 0.773‡ | 0.888‡ |
| OKS (preoperative) | 20.55 (29) | 25 (11) | 20.53 (25) | 24.38 (14) | 0.994‡ | 0.143‡ | 0.183‡ |
| OKS (6 weeks) | 32.44 (25) | 30.33 (7) | 33.07 (20) | 25.8 (18) | 0.782‡ | 0.608‡ | 0.031‡ |
| OKS (1 year) | 40.87 (17) | 35.67 (22) | 36.85 (19) | 36.67 (28) | 0.05‡ | 0.205‡ | 0.544† |
| Length of hospital stay | 5 (11) | 5.17 (5) | 4.56 (7) | 4.13 (3) | 0.988† | 0.507† | 0.862† |
Data expressed as mean (range).
nTrays denotes number of conventional instrument trays used per surgery.
Bold values denote statistical significance.
*Data presented in the format of (hours):(minutes):(seconds).
†Mann-Whitney test.
‡Independent samples t-test.
CVR, conventional/reusable; CVS, conventional/single use; IAT, instrument assembly time=scrub nurse started decanting first tray—scrub nurse finished decanting instruments; ICT, instrument count time=scrub nurse started counting instruments—scrub nurse finished counting instruments; ICuT, instrument clean-up time=scrub nurse started counting instruments—instrument trays closed and taken to sluice room; IDT, instrument decant time=instrument trays entered operating room—scrub nurse opened first tray; IPT, instrument packing time=scrub nurse started packing instruments—instrument trays closed and taken to sluice room; IST, instrument sterilisation time=time instruments were picked up by sterilisation unit (SU)—time instruments were dispatched from SU; IST, instrument set-up time=scrub nurse started assembling instruments—scrub nurse finished assembling instruments; N/A, not applicable; OKS, Oxford Knee Score; PSR, patient specific/reusable; PSS, patient specific/single use; PT, procedure time=knife to skin—closure with last clip; SST, surgical set-up time=scrub nurse performs first task—knife to skin; TIT, total instrument time=scrub nurse opened first tray—instrument trays closed and taken to sluice room.
In addition to instrument-related complications, three conventional instrumentation surgeries were delayed by approximately an hour each, and a further two surgeries were cancelled. Finally, one serious adverse event occurred in a CVR case when a sterilised instrument was found to be missing a filter, potentially affecting its sterility. This led to staff questioning whether the entire content of the tray was sterile, causing a delay of 20 min in initiating the procedure while a new sterile tray was sourced.
Time analysis
When comparing CVR and PSR surgical times, PSR instruments were quicker in all the surgical time intervals recorded (table 2). In particular, PSR total instrument time showed a statistically significant reduction of 9 min and 24 s over CVR (p=0.004). This was echoed in the procedure time (PT), which was 5 min shorter and borderline significant (p=0.054).
The single-use instruments used in conventional procedures took longer to set up in theatre than the conventional reusable instrumentation (table 1). The procedure was also longer, with differences near significance (p=0.054). Conversely, all variables which were recorded after set-up of the instruments were quicker with the SUI. ‘Instrument Count Time’ and ‘Instrument Clean-up Time’ were statistically significantly shorter when SUI was used. Findings were similar during PSI procedures. The only stage where SUI was significantly faster than reusable instrumentation was during packing of the instruments (p=0.041).
Weight analysis
The conventional and PSI instrumentation were shown to weigh similar amounts; however, the reusable instrumentation was significantly heavier than the SUI for both surgical approaches (table 3). On average, the differences between SUI and reusable equipment were approximately 30 kg. The mean number of trays used in conventional procedures using reusable equipment was 3.74 (table 3). This was one tray lower in PSI-guided procedures (2.89).
Table 3.
Instrument weights and costs per surgery for all four groups
| Instrument type | Weight breakdown (kg) | Total mean weight (kg) | Cost breakdown (£) | Total cost (£) | |
| CVR | Sphere femoral finishing tray | 9.9 | 35.9 | 424.12* | 424.12 |
| Sphere conventional tray | 7.2 | ||||
| Sphere trial components | 10.6 | ||||
| General instruments and accessories tray | 8.2 | ||||
| CVS | Conventional Efficiency instrument set | 1.4 | 4.6 | 110.00 | 320.00 |
| General Efficiency instrument set | 2.2 | 110.00 | |||
| Efficiency femoral set | 0.5 | 50.00 | |||
| Efficiency insert set | 0.5 | 50.00 | |||
| PSR | MyKnee instruments | 12.5 | 33.7 | 327.73† | 727.73 |
| Conventional MyKnee instrument set | 10.1 | ||||
| Sphere trial components | 10.6 | ||||
| MyKnee cutting blocks | 0.5 | 250.00 (+150.00‡) | |||
| PSS | General Efficiency instrument set | 2.2 | 3.7 | 110.00 | 610.00 |
| Efficiency femoral set | 0.5 | 50.00 | |||
| Efficiency insert set | 0.5 | 50.00 | |||
| MyKnee cutting blocks | 0.5 | 150.00 | |||
*Sterilisation cost for a single tray × 3.74 trays.
†Sterilisation cost for a single tray × 2.89 trays.
‡Preoperative CT scan cost.
CVR, conventional/reusable; CVS, conventional/single use; PSR, patient specific/reusable; PSS, patient specific/single use.
Cost analysis
The PSI variants were more costly than conventional instrumentation (table 3) due to the costs of a preoperative scan and manufacturing of the patient-specific cutting blocks.
Clinical outcomes
Patients who underwent TKA with reusable PSI were estimated to have lost the least amount of blood (table 2). Those allocated to the conventional group with SUI lost the greatest volume (936.82 mL). The PSI variants reported lower blood loss in patients than the conventional surgery, but this did not reach statistical significance.
Similar patterns were observed in the length of hospital stay for the patient groups (table 2). The patients who had lost the greatest EBV were also those who remained in hospital for longest (5 days). Those who were discharged earliest were in the PSS group. These patients left the hospital a day earlier than those undergoing conventional surgery (4.13 days).
OKS improved in all groups with each visit (table 2). Preoperatively, there were no statistically significant differences in the OKS between groups. Six weeks postoperatively, patients who had undergone the surgery with PSI and reusable equipment had the best scores, whereas the PSI group who had been allocated to the SUI had the worst (p=0.031). One year postoperatively, these differences had narrowed. Patients who were allocated to the conventional group with reusable equipment had the greatest average OKS at 1 year. This score was significantly greater than that reported in patients in the PSI group allocated to the reusable equipment (p=0.05). The lowest average score at 1 year was reported in the conventional group who had undergone TKA with SUI.
According to a study by Beard and colleagues, a meaningful change in OKS at the group level is 9 points.20 Additionally, the between-group minimal important difference for clinical trials was estimated to be 5 points. All groups in this study showed clinically meaningful improvement in their Oxford scores, but no group (when compared with another) showed a clinically meaningful difference in their Oxford score preoperatively, at 6 weeks, or at 1 year.
Discussion
PSI and SUI are increasing in use in TKA to address the growing volume of patients undergoing TKA. These technologies aim to reduce surgical times, simplify the surgical process to improve TKA efficiency, and improve implantation accuracy and maintain or improve clinical outcomes. The main objective of this study was to assess instrument-related surgical efficiency with Medacta MyKnee and Efficiency instrumentation.
The simplest way to improve surgical efficiency is to reduce the length of each procedure. PSI should improve surgical efficiency, as it simplifies the surgical procedure by reducing the surgical steps required. Although some authors have reported a reduction in surgical times with PSI,21–28 others have found no differences29–31 or longer surgical times.32 In this study, the shortest procedures on average were reported in the PSR group (table 2).
The PSI groups were discharged from hospital on average a day earlier than the conventional groups, representing an efficiency saving in operative time and inpatient stay.
Sterilisation delays directly affected surgeries as they led to long delays in initiating the procedures. Sterilisation times for the instruments turned out to be very unreliable, as can be noted from the ranges of instrument sterilisation time (IST) (table 2). Sterilisation-related issues are considered as one of the major pitfalls of reusable instrumentation, and this resonated in our study. Although each hospital has its own individual sterilisation arrangements, it is a familiar and common scenario that there can be breaches in the sterilisation wrapping around trays, cement on instruments and missing items from trays; all of which cause surgical delays. Using SUI reduces these risks, as sterilisation units are involved in the process to a much lesser extent.15 33 Another factor which could be considered an additional risk of using reusable equipment is the transport of equipment off-site for sterilisation. This is commonplace for many hospitals, and can bring with it further complications and potential delays.34 These complications could be reduced with the use of SUI.
Some surgeries were prolonged due to missing equipment in the instrument trays or incomplete sterilisation of certain equipment. These problems affect efficiency and add to the risk of surgical field contamination leading to infections, which are known to drastically affect patient recovery and institutional costs.
PSR showed a significant reduction in missing equipment compared with conventional reusable instruments. This may be associated with the fact that PSR uses fewer conventional trays.24 32 This reduction in equipment reduces the likelihood of sterilisation-related problems arising during surgery. Quicker time intervals coupled with a reduction in trays and missing equipment correlate with an improved efficiency, while also reducing the risk of infection.35
When comparing efficiency of SUI with their corresponding reusable counterparts, the most prominent results identified a limitation in our study. Due to the fact that the SUI was relatively new to the surgeon and the theatre personnel, the results may have been affected by a learning curve. This may explain why CVS and PSS results do not agree with previous studies for SUI which reported notable time reductions during set-up time, operative time, clean-up time and turnover time.13 14 In light of this, the results for CVS and PSS should be considered with caution. CVS and PSS both resulted in a significant increase in instrument set-up time and surgical set-up time. PT was also longer for both cases, although only by a narrow margin. On the contrary, clean-up times were significantly shorter, which ultimately compensated for the longer set-up times, leading to a marginally shorter total instrument time for CVS, while total instrument time for PSS was slightly longer. Clean-up times were predicted to be shorter as single-use instruments do not need to be placed in certain locations within the conventional trays, as they are disposed postoperatively. Moreover, CVS does not require any sterilisation resulting in significant cost saving while avoiding the intraoperative complications which arise with missing equipment and sterilisation issues. This results in significant turnarounds in terms of time and costs.
CVS was 87.2% lighter than CVR, and PSS was 89% lighter than PSR (table 3), making SUI very ergonomic and beneficial for the well-being of theatre personnel who have to repeatedly handle the trays. SUI is easier on the theatre personnel, which subsequently improves efficiency while also avoiding work-related injuries.
The second objective of this study was to investigate instrument-related costs. PSI was clearly more expensive than conventional instrumentation (table 3). This is mainly due to the cost of the preoperative CT scan and the manufacturing costs of the cutting jig. In this study we did not include costs saved as a result of shorter operating times, as they were not considered to be directly related instrument costs. Cendán and Good reported that even small deductions in surgical times (mean of 16 min) can open the opportunity for an increase in number of cases performed per day.36 These values are consistent with the total instrument time reductions recorded for PSR in our study, which suggest that if operating room running costs were considered PSI costs have the possibility of approximating their conventional counterparts. However, the PSI patients left hospital on average a day earlier in our study which may offset the costs of the preoperative scan.
Alternatively, SUI offers a cheaper alternative, with CVS being 24.6% cheaper than CVR and PSS being 16.2% cheaper than PSR. This agrees with previous studies on the cost of SUI in TKA.13 15 These benefits are an addition to the aforementioned advantages, since SUI is lighter and is unaffected by sterilisation delays. Use of SUI also reduces the cost of sterilisation. Further cost savings can be made in hospitals where equipment is usually transported off-site for sterilisation.34
Furthermore, it should be noted that a study performed on the SUI used in this study found that 435 L of water can be saved for each TKA surgery (otherwise used in sterilisation), thus reducing their environmental impact while further adding to their indirect cost savings.37
The third objective of this study was to investigate patient outcomes. The results reported in this study agree with previously published research that have concluded that PSI results in comparable outcomes to conventional TKA.20 38 39 However, the PSI groups had lower blood loss and left hospital a day earlier on average. SUI resulted in a slight increase in blood lost in comparison to their reusable counterparts, which is attributed to the longer PT (table 2). Those who were estimated to have lost the greatest volume of blood (CVS) were found to remain in hospital longest. Although differences were not statistically or clinically significant, they may be economically important, as earlier discharge of patients is a way to substantially save on TKA costs.
This study has limitations that should be acknowledged in light of the results obtained. First, we only investigated surgeries performed using instrumentation and prosthesis from a single manufacturer. Thus, it is unknown whether the results obtained in this study can be extrapolated to instrumentation produced by other manufacturers. Second, all the surgeries were performed by a single, high-volume surgeon who was accustomed to performing TKA surgeries using both CVR and PSR, but not using SUI. This may have influenced the results in terms of a reduction in time saved due to inexperience using specific single-use equipment. This was also noted with the theatre personnel who showed a clear learning curve when working with SUI. If the learning curve effects are surpassed, SUI has the potential of improving surgical efficiency and of presenting the healthcare provider with definite cost savings.13–15 36 37 Finally, an uneven number of patients were randomised into the SUI and reusable groups. Due to a problem with dispatching the SUI at the beginning of the trial, patients were initially randomised into either the standard or the PSI group using reusable instrumentation. When the issue was resolved, the patients were randomised to one of the four intended groups, as originally planned. This was deemed necessary to ensure that those who would have been randomised into an SUI group did not have to wait longer than is necessary for their operation. A greater sample size for the SUI groups may have increased the reliability of the results.
This study formed one part of a larger investigation comparing the outcomes of conventional TKA to patient-specific TKA. This explains why we had access to data from significantly more patients in the non-SUI groups. Once we had completed collecting the data for this investigation, we deemed it appropriate to include all available results in the data analysis to improve the robustness of the research and to ensure we were being transparent about the data we had collected.
Conclusion
Surgical efficiency was significantly improved by PSI at the expense of increased cost. Surgical efficiency was not improved through use of SUI—nevertheless, instrument-related costs were cheaper and avoided sterilisation complications.
PSI presents the healthcare provider with potential cost savings should indirect instrument costs be considered. Conversely, SUI was less efficient due to learning curve effects.
Footnotes
Contributors: AA, GFT, MS, PRi, PRo and LCB made substantial contributions to the concept and design of the work. AA, GFT, MS and LCB acquired the data. Analysis and interpretation of the results were done by AA, GFT, MS, PRi, PRo and LCB. AA and GFT were responsible for writing the manuscript and MS, PRi, PRo and LCB critically revised it. AA, GFT, MS, PRi, PRo and LCB agreed on the final version of this manuscript. AA, GFT, MS, PRi, PRo and LCB agree to be accountable for all aspects of the work.
Funding: The study was supported by the University of Strathclyde and Medacta International through a PhD studentship.
Competing interests: LCB is on the speakers bureau at Medacta International.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was obtained for this study by a local research ethics committee (REC reference: 15/SS/0058; IRAS ID: 177817).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1. Dowsey MM, Nikpour M, Dieppe P, et al. Associations between pre-operative radiographic changes and outcomes after total knee joint replacement for osteoarthritis. Osteoarthritis Cartilage 2012;20:1095–102. 10.1016/j.joca.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 2. Klit J, Jacobsen S, Rosenlund S, et al. Total knee arthroplasty in younger patients evaluated by alternative outcome measures. J Arthroplasty 2014;29:912–7. 10.1016/j.arth.2013.09.035 [DOI] [PubMed] [Google Scholar]
- 3. Lewis S, Price M, Dwyer KA, et al. Development of a scale to assess performance following primary total knee arthroplasty. Value in Health 2014;17:350–9. 10.1016/j.jval.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 4. National Joint Registry National joint Registry: 13th annual report 2017. Available: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/13th%20Annual%20Report/07950%20NJR%20Annual%20Report%202016%20ONLINE%20REPORT.pdf> [Accessed 19th Jun 2017].
- 5. Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM&R 2012;4:S10–S19. 10.1016/j.pmrj.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- 7. Abdel MP, Parratte S, Blanc G, et al. No benefit of patient-specific instrumentation in TKA on functional and gait outcomes: a randomized clinical trial. Clin Orthop Relat Res 2014;472:2468–76. 10.1007/s11999-014-3544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodrigues AST, Gutierres MAP. Patient-specific instrumentation in total knee arthroplasty. Should we adopt it? Revista Brasileira de Ortopedica 2016;52:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okada Y, Teramoto A, Suzuki T, et al. Preoperative corrections are required for planning of patient-specific instrumentation in total knee arthroplasty. The Knee 2017;24:1492–7. 10.1016/j.knee.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 10. Chen JY, Chin PL, Tay DKJ, et al. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty 2015;30:1724–8. 10.1016/j.arth.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 11. Haas SB, Carli A. Disposable instruments in total knee arthroplasty. Minim. Invasive Surg. Orthop. Cham: Springer International Publishing 2016:1349–56. [Google Scholar]
- 12. Mont MA, McElroy MJ, Johnson AJ, et al. Single-use instruments, cutting blocks, and trials increase efficiency in the operating room during total knee arthroplasty. The Journal of Arthroplasty 2013;28:1135–40. 10.1016/j.arth.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 13. Siegel GW, Patel NN, Milshteyn MA, et al. Cost analysis and surgical site infection rates in total knee arthroplasty comparing traditional vs. single-use instrumentation. J Arthroplasty 2015;30:2271–4. 10.1016/j.arth.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 14. Mont MA, Pivec R, Johnson AJ, et al. Single-use cutting blocks and trials lower costs in primary total knee arthroplasty. Surg Technol Int 2012;22:331–5. [PubMed] [Google Scholar]
- 15. Bonutti PM, Zywiel MG, Johnson AJ, et al. The use of disposable cutting blocks and trials for primary total knee arthroplasty. Techniques in Knee Surgery 2010;9:249–55. 10.1097/BTK.0b013e3181ef5246 [DOI] [Google Scholar]
- 16. Siddiqi A, Hardaker WM, Eachempati KK, et al. Advances in computer-aided technology for total knee arthroplasty. Orthopedics 2017;40:338–52. 10.3928/01477447-20170831-02 [DOI] [PubMed] [Google Scholar]
- 17. DeHaan AM, Adams JR, DeHart ML, et al. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty 2014;29:2065–9. 10.1016/j.arth.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 18. Noordzij M, Tripepi G, Dekker FW, et al. Sample size calculations: basic principles and common pitfalls. Nephrology Dialysis Transplantation 2010;28:1388–93. [DOI] [PubMed] [Google Scholar]
- 19. Gross JB. Estimating allowable blood loss. Anesthesiology 1983;58:277–80. 10.1097/00000542-198303000-00016 [DOI] [PubMed] [Google Scholar]
- 20. Beard DJ, Harris K, Dawson J, et al. Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. J Clin Epidemiol 2015;68:73–9. 10.1016/j.jclinepi.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abane L, Anract P, Boisgard S, et al. A comparison of patient-specific and conventional instrumentation for total knee arthroplasty: a multicentre randomised controlled trial. Bone Joint J 2015;97-B:56–63. 10.1302/0301-620X.97B1.34440 [DOI] [PubMed] [Google Scholar]
- 22. Barrack RL, Ruh EL, Williams BM, et al. Patient specific cutting blocks are currently of NO proven value. The Journal of Bone and Joint Surgery. British volume 2012;94-B(11_Supple_A):95–9. 10.1302/0301-620X.94B11.30834 [DOI] [PubMed] [Google Scholar]
- 23. Boonen B, Schotanus MGM, Kort NP. Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop 2012;83:387–93. 10.3109/17453674.2012.711700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J 2013;95-B:354–9. 10.1302/0301-620X.95B3.29903 [DOI] [PubMed] [Google Scholar]
- 25. Noble JW, Moore CA, Liu N. The value of Patient-Matched instrumentation in total knee arthroplasty. The Journal of Arthroplasty 2012;27:153–5. 10.1016/j.arth.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 26. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res 2012;470:889–94. 10.1007/s11999-011-2221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfitzner T, Abdel MP, von Roth P, et al. Small improvements in mechanical axis alignment achieved with MRI versus CT-based patient-specific instruments in TKA: a randomized clinical trial. Clin Orthop Relat Res 2014;472:2913–22. 10.1007/s11999-014-3784-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietsch M, Djahani O, Zweiger C, et al. Custom-fit minimally invasive total knee arthroplasty: effect on blood loss and early clinical outcomes. Knee Surg Sports Traumatol Arthrosc 2013;21:2234–40. 10.1007/s00167-012-2284-z [DOI] [PubMed] [Google Scholar]
- 29. Barke S, Musanhu E, Busch C, et al. Patient-matched total knee arthroplasty : Does it offer any clinical advantages. Acta Orthop Belg 2013;79:307–11. [PubMed] [Google Scholar]
- 30. Barrett W, Hoeffel D, Dalury D, et al. In-vivo alignment comparing patient specific instrumentation with both conventional and computer assisted surgery (Cas) instrumentation in total knee arthroplasty. J Arthroplasty 2014;29:343–7. 10.1016/j.arth.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 31. Stronach BM, Pelt CE, Erickson J, et al. Patient-specific total knee arthroplasty required frequent Surgeon-directed changes. Clin Orthop Relat Res 2013;471:169–74. 10.1007/s11999-012-2573-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamilton WG, Parks NL, Saxena A. Patient-specific instrumentation does not shorten surgical time: a prospective, randomized trial. J Arthroplasty 2013;28(8 Suppl):96–100. 10.1016/j.arth.2013.04.049 [DOI] [PubMed] [Google Scholar]
- 33. Dell'Osso G, Celli F, Bottai V, et al. Single-use instrumentation technologies in knee arthroplasty: state of the art. Surg Technol Int 2016;28:243–6. [PubMed] [Google Scholar]
- 34. Dehnavieh R, Mirshekari N, Ghasemi S, et al. Health Technology assessment: Off-site sterilization. Med J Islam Repub Iran 2016;30. [PMC free article] [PubMed] [Google Scholar]
- 35. Peersman G, Laskin R, Davis J, et al. Prolonged operative time correlates with increased infection rate after total knee arthroplasty. HSS Jrnl 2006;2:70–2. 10.1007/s11420-005-0130-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cendán JC, Good M. Interdisciplinary work flow assessment and redesign decreases operating room turnover time and allows for additional caseload. Archives of Surgery 2006;141:65–9. 10.1001/archsurg.141.1.65 [DOI] [PubMed] [Google Scholar]
- 37. Medacta & Solvay Carbon footprint of single-use and reusable surgical instruments. Alpharetta, GA 2016;3. [Google Scholar]
- 38. Huijbregts HJTAM, Khan RJK, Sorensen E, et al. Patient-specific instrumentation does not improve radiographic alignment or clinical outcomes after total knee arthroplasty. Acta Orthop 2016;87:386–94. 10.1080/17453674.2016.1193799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Voleti PB, Hamula MJ, Baldwin KD, et al. Current data do not support routine use of patient-specific instrumentation in total knee arthroplasty. J Arthroplasty 2014;29:1709–12. 10.1016/j.arth.2014.01.039 [DOI] [PubMed] [Google Scholar]


