Abstract
Human immunodeficiency virus (HIV) remains a significant source of morbidity and mortality worldwide. No effective vaccine is available to prevent HIV transmission, and although antiretroviral therapy can prevent disease progression, it does not cure HIV infection. Substantial effort is therefore currently directed toward basic research on HIV pathogenesis and persistence and developing methods to stop the spread of the HIV epidemic and cure those individuals already infected with HIV. Humanized mice are versatile tools for the study of HIV and its interaction with the human immune system. These models generally consist of immunodeficient mice transplanted with human cells or reconstituted with a near-complete human immune system. Here, we describe the major humanized mouse models currently in use, and some recent advances that have been made in HIV research/therapeutics using these models.
Keywords: HIV, AIDS, BLT, humanized mice, model
INTRODUCTION
Human immunodeficiency virus (HIV) infects and kills CD4+ T cells, causing a progressive debilitation of the immune system that almost invariably culminates in acquired immune deficiency syndrome (AIDS) (1). It is estimated that over 35 million people are currently infected with HIV, and a similar number have died of HIV/AIDS-related causes (2). The development of antiretroviral drugs that can inhibit HIV replication and prevent disease progression has significantly advanced over the past 30 years (3). However, at the end of 2015 only approximately half of the infected individuals worldwide had access to these drugs, and new infections were occurring at a rate of over 2 million per year (2). Importantly, antiretroviral therapy (ART) is not capable of curing HIV infection. Daily ART is therefore needed to continually prevent viral outgrowth from persistent HIV reservoirs, which requires life-long connection with specialized medical care and is associated with adherence issues, virologic drug resistance, drug-related side effects, and significant financial expense. To properly contain the epidemic and cure those individuals who have already become infected with HIV, additional scientific and medical advances are greatly needed.
In vitro experiments using isolated viral or cell components, transformed cell lines, or primary cell or tissue samples have produced many important insights into HIV biology. These systems provide contained experimental environments where direct interactions and effects can be studied in relative isolation. Yet these experiments do not reflect the complex collection of interactions that occur in an in vivo environment. This is particularly true of immunological studies, which often require highly orchestrated coordination between multiple cell types within an ordered tissue environment to produce effective and authentic immune responses. Clinical studies using samples from HIV-infected humans can complement in vitro approaches, but they are constrained by appropriate safety and ethical considerations and logistical issues including limited tissue accessibility. In vivo models, which involve the careful and judicious use of animals to study HIV, provide an important complementary approach that can validate and extend in vitro findings, generate new insights into HIV biology and pathogenesis, and connect promising in vitro therapeutic approaches to human studies.
Common in vivo models used to study HIV include rhesus (Macaca mulatta), pigtail (Macaca nemestrina), and cynomolgus (Macaca fascicularis) macaques infected with a simian immunodeficiency virus (SIV) or chimeric viruses containing a mixture of SIV and HIV genomes (SHIVs). These models recapitulate many aspects of HIV infection in humans and result in infection and depletion of CD4+ T cells, high viral loads in tissues and plasma, and development of simian AIDS (4, 5). However, the use of nonhuman primate models can be limited by significant expense and correspondingly small experimental animal group sizes, which often constrains the number of conditions and parameters that can be assessed when using these models. Interactions that are particular to HIV and human host cells also cannot be evaluated in nonhuman primates. A complementary approach to these nonhuman primate studies is the use of murine models to study HIV.
No lentiviruses related to HIV that might serve as direct models for HIV infection have been identified in mice, and unmodified mice cannot be infected with HIV owing to a lack of appropriate HIV entry receptors and multiple subsequent blocks to HIV replication in murine cells (6, 7). Advances have been made to genetically modify mice (8) or viruses (9) to allow some level of HIV replication directly in murine cells, or to transgenically express HIV proteins in mice to understand their effects in a whole-body system (10, 11), but the vast majority of HIV experiments in mice have been made possible by transplanting human cells or tissues into immunodeficient mice. Although no model can completely recapitulate all aspects of HIV infection in humans, the use of humanized mice has provided insights into many different aspects of HIV biology. The history of the different models, along with some of their major contributions to HIV research over the past three decades, has been reviewed elsewhere (12). In this review, we focus on humanized mouse models currently in common use and outline some of the recent advances in HIV research that have been realized through experiments using these models.
OVERVIEW OF COMMON HUMANIZED MOUSE MODELS
Mouse Strains Used as Hosts for Humanization
Mice with intact immune systems rapidly eliminate transplanted human cells through robust innate and adaptive immune responses. Durable reconstitution of mice with human cells therefore requires immunodeficient recipient mouse strains. Early immunodeficient mouse models included nude mice (13) and severe combined immunodeficiency (SCID) mice (14). Nude mice lack mature CD4+ and CD8+ T cells and consequently lack T-dependent B cell responses owing to a forkhead box protein N1 (Foxn1mu) mutation that leads to defective thymic stroma development and a resultant athymic phenotype (15) in addition to their characteristic abnormal hair growth. SCID mice harbor mutations in the protein kinase, DNA-activated, catalytic polypeptide gene (Prkdcscid). This mutation prevents the efficient DNA repair that is required for T cell and B cell receptor rearrangement, resulting in a lack of mature T and B cells. SCID mice have lower levels of residual immune function than nude mice and have thus been extensively used as recipients for human cell and tissue xenografts in vivo. An additional pathway to inhibit development of an adaptive immune response is to disrupt the recombination-activating genes Rag1 and Rag2, which are required in T cell and B cell receptor rearrangements (16, 17). Rag-knockout strains of mice are therefore also used as recipients for human cells. These immunodeficient mouse strains are extremely useful as human cell transplant recipients, but they do have several limitations, including some leakiness (unwanted production of T and B cells) in older SCID mice and an increased susceptibility of SCID mice to radiation due to inefficient DNA repair. However, the major limitation associated with each of the early models described above is that they retain relatively high levels of innate immune responses, including substantial natural killer (NK) cell function, which complicates their use in long-term, systemic reconstitution with human cells (18).
A further breakthrough in the development of more immunodeficient mice was the production of murine strains that have targeted disruption of the interleukin (IL)-2 receptor common γ chain (Il2rg, or γc), which is a component of receptors for the cytokines IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 (19-21). Mutation of γc interrupts critical cytokine signaling networks that are required by both adaptive and innate immune responses, with the absence of IL-15 in particular contributing to a complete lack of NK cells (22, 23). The combination of γc knockout with SCID or Rag-knockout mutations leads to highly immunodeficient strains that have no T cells, B cells, or NK cells, with severely debilitated monocyte/macrophage function. Strains that utilize disrupted γc include NSG (NOD-SCID-γc knockout; NOD.Cg-PrkdcscidIL2rgtm1Wjll/Sz), NOG (NOD.Cg-PrkdcscidIl2rgtm1Sug), NRG [e.g., NOD.129S7(B6)-Rag1tm1MomIL2rgtm1Wjll/Sz], and BRG [e.g., C.129(Cg)Rag2tm1FwaIL2rgtm1Sug/Jic] (reviewed in 12, 24–26). These strains can support more efficient, long-term, stable, and systemic engraftment with human cells and tissues than can strains with intact γc cytokine receptor genes, and these models are being further improved through additional genetic modifications to overcome remaining constrictions.
One potential limitation of currently available humanized mouse models is the development of graft-versus-host disease (GVHD) after reconstitution with human immune cells (27), which has led to efforts toward improving compatibility between murine host and human graft cells. For example, CD47 has been demonstrated as a marker of self (28), and interaction between CD47 and the inhibitory receptor signal regulatory protein α (SIRPα) on macrophages provides an antiphagocytic “don’t eat me” signal to prevent engulfment of self-cells by macrophages. However, C57BL/6 and BALB/c mouse SIRPα receptors do not recognize human CD47 (29, 30), which means that transplanted human hematopoietic stem cells (HSCs) in these models can be phagocytosed by murine macrophages (31). Alternatively, if phagocytic cells develop in an environment without CD47, then they become tolerized to cells that lack CD47 (32). Therefore, triple knockout (TKO) B6.129(Cg)-Rag2tm1FwaCd47tm1FplIl2rgtm1Wjl/J mice, which lack CD47 in addition to Rag 1 and IL2rg, have also been developed and used to produce humanized mice with excellent reconstitution and little GVHD (33). Additional efforts to improve humanized mouse platforms have included mouse major histocompatibility complex (MHC) class I, β2 microglobulin, and MHC class II-knockout strains; strains expressing human cytokines; and the use of clodronate liposomes to deplete endogenous macrophages (reviewed in 34). Many additional strains of immunodeficient mice have been generated through cross-breeding and targeted gene disruption for particular applications, resulting in fairly complex genetic backgrounds, common names, and technical nomenclatures, which are reviewed in more detail elsewhere (24-26). Although the details of individual, new recipient mouse strains for humanization are sometimes nuanced, the common goals are to reduce transplant rejection, prevent GVHD, enhance the levels of human immune cell reconstitution, and provide a more complete and authentic human immune response in the murine system.
Humanization Procedures
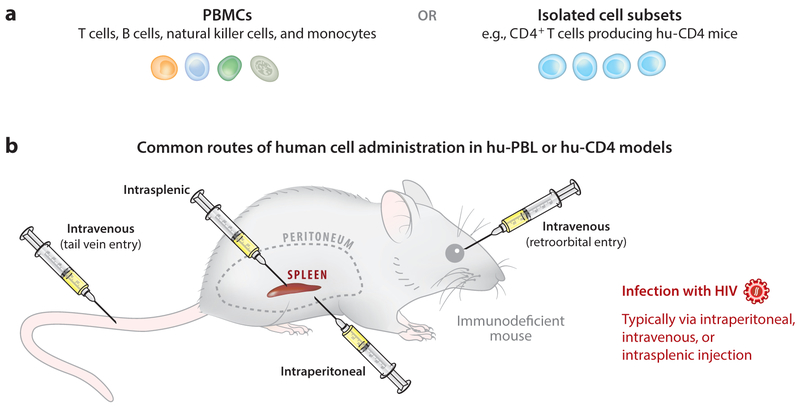
The procedures for creating humanized mice for HIV research can be condensed into four basic approaches, although the details of each approach can vary substantially depending on the goals of individual experiments, and several different names for broadly similar techniques are sometimes used in the literature. A human peripheral blood leukocyte (hu-PBL) approach involves injecting human peripheral blood mononuclear cells (PBMCs) into the peritoneal cavity of an immunodeficient mouse, resulting in the persistence of human cells in various murine organs and the development of some limited specific immune responses (Figure 1). Recipient SCID mice were originally used for this approach (SCID–hu-PBL) (35), but now more immunodeficient strains, including NSG, are commonly utilized (36-38). Administration routes for human cells have also been modified to include intravenous and intrasplenic injection. The hu-PBL approach is the fastest and most logistically simple method for mouse humanization; it can be performed with either total human PBMCs or sorted individual immune cell subsets. A caveat to this approach is that the introduced cells rapidly become activated and cause GVHD over time, which usually becomes evident 3 or 4 weeks after injection of PBMCs. This activation of human cells can also be beneficial in some experimental contexts, in addition to the obvious potential for studying GVHD and its prevention (39). For example, efficient HIV replication in CD4+ T cells requires them to be activated (40), and the rapid humanization with already mature immune cells coupled with in vivo activation of hu-PBL mice means that they can readily be infected with HIV soon after human cell injection (41). This in vivo activation is also useful in HIV latency studies in which patient-derived latently infected cells may become stimulated and express virus after implantation into immunodeficient mice (see the section titled Persistence and Latency).
Figure 1.
The human peripheral blood leukocyte (hu-PBL) and hu-CD4 models. (a) Implanted human primary cells are most often peripheral blood mononuclear cells (PBMCs) or isolated CD4+ T cells. These cells might be derived from healthy donors if the mouse is to be subsequently infected with virus, or from HIV-infected individuals if analysis of patient-derived cells or outgrowth of new primary virus isolates is desired. (b) Humanization of immunodeficient mice can be achieved by injecting mature cells through one or more of several different routes, but it is most commonly performed through intraperitoneal or intravenous injection. Infection with HIV is also generally performed through these same routes.
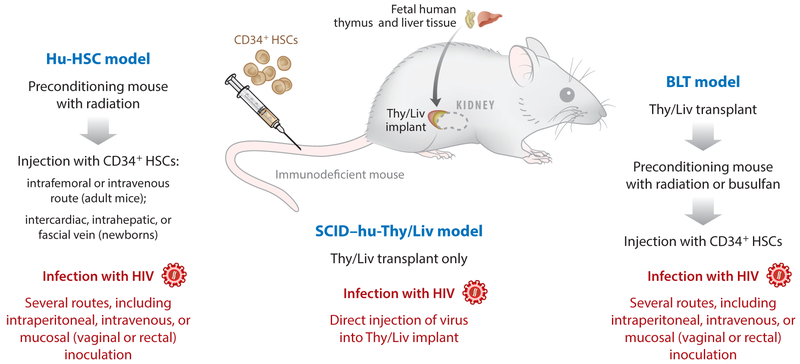
Models in which immature cells or stem cells are transplanted into immunodeficient mice for differentiation into mature cells in vivo include the SCID–human thymus/liver (SCID–hu-Thy/Liv) model, the human HSC (hu-HSC) model, and bone marrow/liver/thymus (BLT) model. The SCID–hu-Thy/Liv model involves implanting human fetal thymus and liver tissue under the kidney capsule of a SCID mouse (42). This produces a new vascularized organ that structurally and functionally resembles the human thymus (Figure 2) and allows CD34+ HSCs present in the fetal liver to differentiate through thymopoiesis into CD4+ CD8+ double-positive cells and then to naive CD4+ or CD8+ T cells. This provides a versatile model for HIV infection of the thymus, which has facilitated numerous advances in our understanding of HIV biology (reviewed in 12). However, systemic reconstitution with human immune cells is extremely limited in this model, with few human cells present outside of the Thy/Liv implant. Adaptive human immune responses are also not generated in the SCID–hu-Thy/Liv model. These limitations can be partially overcome by using NSG mice as recipient animals for transplant of Thy/Liv implants (43), which produces systemic reconstitution almost exclusively with human T cells (without human monocytes/macrophages, B cells, or dendritic cells), and the resultant T cell only mice (ToM) can be infected parenterally with HIV (43).
Figure 2.
The human hematopoietic stem cell (hu-HSC), severe combined immunodeficiency–human thymus/liver (SCID–hu-Thy/Liv), and bone marrow/liver/thymus (BLT) models. Reconstitution of a partial or near-complete human immune system in humanized mice can be achieved in several ways. The hu-HSC (or hu-CD34) model involves preconditioning mice with radiation and then injecting them with human CD34+ HSCs, which differentiate within the mice to produce systemic reconstitution with multiple immune lineages. In the SCID–hu-Thy/Liv model, implantation of human fetal liver and thymus tissues under the murine kidney capsule creates a conjoined organ (Thy/Liv implant) that resembles a human thymus and supports thymopoiesis and production of mature human CD4+ and CD8+ T cells. In SCID recipient mice, human cells are generally constrained to the Thy/Liv implant and are rare in the periphery; however, in NSG mice, mature T cells can also be found in the peripheral blood and lymphoid organs. In the BLT model, implantation of Thy/Liv is combined with preconditioning and HSC infusion.
Multilineage immune reconstitution with human immune cells can be achieved by preconditioning highly immunodeficient mice (e.g., NSG) with radiation followed by transfusion with HSCs derived from fetal liver, umbilical cord blood, or mobilized adult CD34+ cells from peripheral blood. This results in hu-HSC mice that harbor a wide variety of human cells including CD4+ and CD8+ T cells, NK cells, monocytes/macrophages, and dendritic cells, which are present in multiple organs, including peripheral blood, spleen, lung, liver, brain, gastrointestinal tract, vaginal and rectal mucosa, and bone marrow (44, 45). T cell maturation in hu-HSC mice occurs in the murine thymus, but if human leukocyte antigen restriction is desired (as is required, for example, in studies of transgenic human T cell receptor therapies), then hu-HSC procedures can be combined with Thy/Liv implantation to generate BLT mice that have human Thy/Liv implants, allowing more authentic human thymopoiesis (46, 47). Both hu-HSC and BLT models can generate some adaptive immune responses and can be infected by HIV through multiple routes. Therefore, these models are considered the most advanced and flexible approaches to studying HIV in humanized mice. Further modifications of these procedures have also been described. For example, HSC transplantation into NOD/SCID mice yields animals that have human myeloid cells and B cells but no T cells (48, 49), allowing HIV infection of myeloid cells in vivo to be studied in the absence of CD4+ T cells in myeloid only mice (MoM) (49).
RECENT HIV STUDIES IN HUMANIZED MICE
Type I Interferon
Recent advances in basic immunology have provided new insights into the roles of type I interferons (IFNs) in regulating immune responses in mice (50, 51). The use of humanized mice has helped translate these findings into a human system. This has included investigations into the multifactorial in vivo effects of IFNs and how they combine to affect HIV replication and antiviral immunity. The most well-defined type I IFNs are IFNα and IFNβ. IFNα (encoded in various isoforms by multiple genes) is produced predominantly by plasmacytoid dendritic cells and to a lesser extent by other immune cell types, whereas most cell types are capable of expressing the single gene that encodes IFNβ (52). IFNs are secreted after microbial products are sensed and result in autocrine IFN production loops and the induction of IFN-stimulated gene expression in infected and neighboring cells. Together, these responses help reduce the spread of viruses by inducing antiviral states in infected or potential host cells (53), and engage innate and adaptive immune responses through production of cytokines and chemokines, enhanced antigen presentation, and other pathways (52).
It is believed that primary infection with HIV is partially contained by these potent type I IFN responses. In support of this concept, the administration of IFNα2a before viral challenge initially prevented systemic viral infection in an SIV rhesus macaque model. Conversely, antibody-mediated blocking of type I IFN receptor led to accelerated CD4+ T cell depletion and more rapid progression to AIDS (54). However, continuous type I IFN signaling in chronic viral infections can also contribute to persistent immune activation and can have deleterious effects on antiviral responses (51, 55). This is particularly relevant for HIV, in which chronic immune activation is a common feature that may drive virus replication by providing a continuous supply of activated CD4+ T cells, which are optimal host cells for HIV. Type I IFNs may further exacerbate infection by recruiting new HIV host cells to active tissue sites of infection. In vivo studies involving type I IFNs therefore provide researchers the opportunity to develop a better understanding of antiviral immunity, and because the mechanism of action of IFN inhibition of HIV is completely different from that of ART, the potential for augmenting ART and reducing the reservoirs of HIV that persist during ART with combined approaches involving both ART and type I IFNs can also be explored.
Several recent studies have used humanized mouse models to specifically address the issue of type I IFN and HIV. Zhen et al. (56) used BLT mice to evaluate the effects of an anti–human IFN receptor 2 (IFNR2) antibody on chronic HIV infection. They found that blockade of type I IFNs reduced immune activation and HIV replication, and this was accompanied by a decrease in the expression of T cell exhaustion markers including PD-1 and TIM-3. Blockade of type I IFNs in conjunction with ART further decreased viral loads and reduced the frequency of HIV reservoir cells compared with using ART alone (56). Another study using hu-HSC NRG or BLT mice (57) showed similar results, with monoclonal antibody–mediated blockade of IFNα/β receptor signaling reducing immune hyperactivation that remained during ART and improving HIV-specific T cell immune responses. In this study, blockade of type I IFNs also reduced persistent HIV reservoirs in lymphoid tissue, resulting in a corresponding delay in viral rebound upon stopping ART (57). Therefore, further clinical studies on the use of type I IFN blockade to bolster anti-HIV immunity or deplete reservoirs of HIV that persist during ART are potentially promising avenues of investigation.
In addition, Lavender et al. (58) used C57BL/6 Rag2−/− γc −/−CD47−/− (TKO) BLT mice to investigate which IFNα subtypes can suppress HIV replication in vivo. Their study demonstrated IFNα14 has potent anti-HIV activity in vivo and can suppress HIV replication and proviral loads during postexposure prophylaxis and treatment of acute infection. Treatment with IFNα14 also reduced HIV-induced immune activation and activated innate immune pathways, inducing tetherin and MX2 upregulation. Importantly, IFNα14 produced more potent responses in these experiments than did IFNα2, which is the subtype that has been utilized in most prior clinical studies (59, 60), suggesting that clinical testing of IFNα14 for effects on HIV infection may be warranted. Another study examined endogenous production of IFNα by using different NSG mouse–based humanization procedures and found that IFNα is naturally produced in BLT mice but not detectable in hu-PBL mice (61), presumably because of a relative lack of plasmacytoid dendritic cells in the hu-PBL model. This study also showed that injecting plasmids expressing IFNα14 and IFNβ into hu-PBL mice prior to HIV challenge slowed CD4+ T cell decline and reduced viral loads to a greater extent than did several other IFNα subtypes, including IFNα2. Again, this finding suggests that IFNα 14 may better suppress HIV infection than more commonly used IFNα2 (61). Humanized mice have also allowed researchers to investigate how type I IFNs influence mucosal transmission of HIV and the early immunologic events preceding primary infection with HIV. Intravaginal application of type I IFNs to NSG-BLT mice before HIV infection led to reduced inflammation and reduced viral replication, and knockdown of the cytosolic exonuclease TREX1 (the absence of which allows IFN production by HIV-infected cells; 62) decreased viral replication for 3–4 weeks despite causing an increase in infiltrating immune cells (63).
Together, these new studies highlight the complex interplay between HIV and the immune system and demonstrate that type I IFNs may be either beneficial for or detrimental to controlling HIV depending on the timing and context of the intervention. Triggering type I IFN responses early in infection appears to help control virus replication, with IFNα14 in particular implicated as beneficial. However, the picture during chronic viral infection is more complex, with persistent IFN-mediated immune activation potentially damaging the immune system and allowing the virus to persist more efficiently during ART.
Antiretroviral Drug Testing
Over 30 antiretroviral drugs have been approved for the treatment of HIV. However, issues including the development of drug resistance, associated side effects and toxicities, and suboptimal penetration to all sites of HIV replication continue to drive the development of new antiretroviral drugs with improved properties. Humanized mice infected with HIV have historically been valuable in these efforts, because they allow researchers to directly compare in vivo different antiretroviral drugs and their ability to inhibit HIV replication or prevent transmission (reviewed in 12). This has continued in recent years with the more advanced humanization models that are now available. In particular, the more complete reconstitution of vaginal and rectal mucosa with human immune cells that is produced in hu-HSC and BLT mice has enabled researchers to model mucosal transmission of HIV in humanized mice more accurately than was previously possible.
Recent advances in this area include the use of BLT mice to evaluate the in vivo activity of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA), a nucleoside analog reverse transcriptase inhibitor (NRTI) that is under preclinical development and has much more potent activity against HIV in vitro than currently approved NRTIs, but with comparatively low cytotoxicity (64, 65). Monotherapy with EFdA administered orally could potently reduce HIV plasma viral loads and control HIV replication in both the gastrointestinal tract and the female reproductive tract of BLT mice (66). Preexposure prophylaxis with EFdA could also prevent vaginal and oral transmission of HIV in this same model (66). These data are consistent with a prior study showing that EFdA has favorable pharmacokinetic properties after oral dosing in mice and rhesus macaques, and can rapidly suppress HIV viremia in SCID–hu-Thy/Liv, NSG–hu-Thy/Liv, and NSG-BLT mice (67). Together, these new data suggest that EFdA might be further explored as a novel NRTI, which could be particularly useful in preventing mother-to-child transmission of HIV.
Other recent humanized mice studies have focused on another class of antiretroviral drug. For example, Veselinovic et al. (68) used RAG-hu (BALB/c-Rag1−/− γ c −/− and BALB/c-Reg2−/− γ c −/−) mice to test the pharmacokinetic properties of the HIV integrase strand transfer inhibitor raltegravir; the results indicated that raltegravir concentrations were two logs higher in intestinal mucosa and one log higher in vaginal and rectal tissues than in plasma (similar to the trends observed in human subjects). This finding indicates that humanized mice are suitable for preliminary pharmacokinetic preexposure prophylaxis studies, and that raltegravir appears to be a good candidate for HIV preexposure prophylaxis (68).
There is currently a substantial interest in long-acting forms of antiretroviral agents that do not require daily adherence to ART and could instead be administered once monthly or even less frequently (69). Adherence issues are particularly evident in HIV preexposure prophylaxis (PrEP) studies, in which the goal is to use ART to prevent infection with HIV in individuals who are particularly at risk of acquiring the infection. For example, in clinical studies involving tenofovir disoproxil fumarate (TDF) or emtricitabine (FTC) + TDF, only approximately 50–85% of participants have detectible tenofovir in blood (indicating a lack of adherence), and those participants without detectable concentrations of antiretroviral drugs in plasma were much more likely to become infected with HIV (70-72). In another PrEP study, less than 40% of individuals in the TDF + FTC group had evidence of recent pill use, which likely contributed to the lack of PrEP efficacy observed in that study (73).
Long-acting antiretroviral drugs, including the HIV integrase inhibitor cabotegravir (69) and a long-acting formulation of the non-nucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine (74), are currently being tested. Continuing this avenue of research, a recent PrEP study tested a long-acting form of raltegravir in humanized mice (75). This study showed that a single subcutaneous injection of long-acting raltegravir in NSG-BLT mice yielded raltegravir plasma concentrations that were comparable to human twice-daily 400-mg oral dosing, and that viral loads in HIV-infected BLT mice could be suppressed in plasma and cervicovaginal fluids using this approach. Moreover, a single injection of long-acting raltegravir also protected against vaginal HIV challenge at 1 week and 4 weeks after the drug was administered (75). Comparative evaluation of different approaches to inhibit mucosal transmission of HIV in humanized mice can readily be performed, but it may be easier to inhibit transmission in these models than in humans because of qualitative or quantitative differences in the human cells present in the murine mucosa of humanized mice versus normal human hosts. Given these data and the long history of successful testing of new antiretroviral drugs in humanized mice, murine models for HIV are likely to continue to be utilized in preclinical testing as new PrEP and long-acting ART regimens are developed.
Antibody Therapies
The contribution of antibodies to the immune response against HIV has long been acknowledged, with elicitation of strong mucosal antibodies against HIV a central goal of prophylactic vaccine studies. However, the power of antibody-mediated mechanisms to suppress viral loads and facilitate killing of HIV-infected cells during established HIV infection has become fully appreciated only recently. Key developments in this field include the identification of elite neutralizers, the approximately 1% of HIV-infected individuals who generate highly potent neutralizing antibodies with cross-clade activity (76), and the development of single-cell antibody cloning techniques (77). These studies have yielded broadly neutralizing antibodies (bNAbs) that can bind to and neutralize a remarkably diverse array of primary HIV isolates (78). Passive transfer of these highly potent bNAbs can suppress plasma viral loads to below the level of detection in a hu-HSC model based on NOD Rag1−/− Il2rgnull mice (79), and reductions in viral loads by passive antibody administration in macaque models or in infected humans have also been achieved (80, 81). Moreover, the longer half-life of antibodies compared with those of standard ART drugs allowed control of viremia in humanized mice for an average of 60 days after bNAb therapy was stopped (79). As is the case with natural humoral immunity, the antiviral effects of these transfused antibodies are likely due to multiple mechanisms, including blocking the interaction between virus and host cell receptors, killing of infected cells by antibody-dependent cell-mediated cytotoxicity, and increasing in vivo viral decay rates (78, 82). The potential benefits of using antibody therapy to treat established HIV infections include its use in conjunction with ART to intensify therapy and help further suppress any residual virus replication that might occur (83). bNAbs might also be used to suppress viral rebound if ART is stopped for a period to avoid toxicity and virologic drug resistance issues related to long-term chemoexposure, potentially in conjunction with HIV reservoir depletion agents that help reduce the frequency of latently infected cells (84, 85). Finally, bNAbs may serve as an alternative to ART in individuals harboring multidrug-resistant virus or those who experience ART-related side effects.
One mechanism by which bNAbs can function is through engagement of activating but not inhibitory FcγR on effector cells (86). Recent advances using antibody therapies in humanized mouse models include the demonstration that passive transfusion of the bNAb 3BNC117 decreases the half-life of CD4+ T cells infected with HIV through a mechanism that requires engagement of FcγR (87). This study describes experiments in ART-treated NRG (Rag1−/− Il2rgnull) mice, in which adoptively transferred HIV-expressing cells (including those infected with patient-derived HIV viral isolates) were cleared more rapidly in mice treated with the bNAbs than in mice treated with an isotype control. However, when bNAbs that carry mutations that specifically abrogate mouse FcγR binding were used, accelerated clearance of HIV-infected cells was not observed. Similar results were reported when Fc-FCγRIV interactions were blocked with antibodies, implicating FCγRIV in the bNAb-mediated clearance of infected cells (87).
Various methods for administering antibodies to prevent transmission of HIV, including direct topical vaginal application (88), adeno-associated virus vector–mediated immunoprophylaxis (89, 90), HSC-based transgenic approaches (91), and passive immunization (92), have been tested in humanized mice. This work is ongoing, as researchers apply different humanized mouse models to test antibody-mediated methods to inhibit HIV transmission. A recent study in this area demonstrated that NSG-BLT mice can be used to evaluate bNAb activity against intravaginal HIV infection, and showed that preexposure treatment with the second-generation bNAb PGT126 produced sterilizing protection against up to eight low-dose challenges with HIV strain JR-CSF (92).
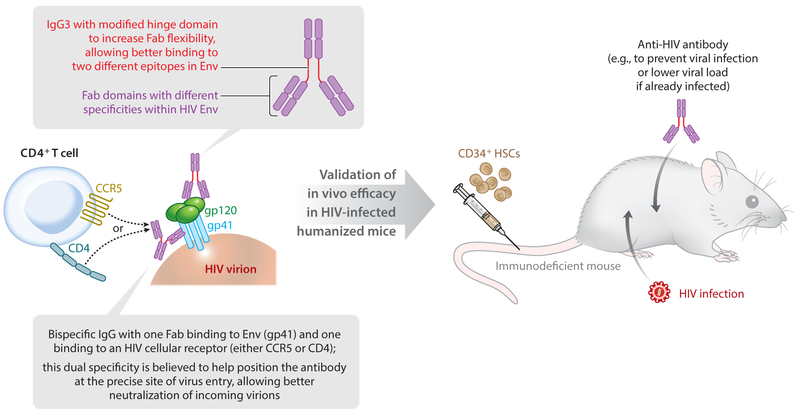
Further advances in the field of HIV bNAbs have involved engineering bispecific antibodies, in which the Fab domains of a single immunoglobulin G (IgG) have different specificities. This approach may be used to create antibodies with specificity for both HIV Env proteins and effector cells, for example, to draw together HIV-infected cells and CD3+ T cells (93, 94), leading to enhanced CD8+ T cell–mediated cytolysis of HIV-expressing cells. Two recent studies (95, 96) have further developed bispecific antibodies against HIV by creating versions with markedly improved potency and breadth over the parental monoclonal versions and validating their in vivo activity in humanized mice (Figure 3). One study (95) modified the IgG3 hinge domain to increase Fab flexibility and incorporated Fab fragments derived from two bNAbs that target different epitopes within HIV Env. This resulted in a bispecific antibody exhibiting synergistic activity compared with the two parental bNAbs, with broad capacity to neutralize different HIV isolates and high potency. An HIV-infected NRG (NOD Rag1−/− Il2rgnull)–hu-HSC mouse model was used to evaluate the in vivo activity of this bispecific antibody during passive immunization, and the bispecific version reduced viral load more effectively than did mixtures of the parental antibodies (95).
Figure 3.
Testing bispecific antibodies in humanized mice. Two recent studies describing the development of bispecific antibodies against HIV provide examples of in vivo testing and validation of promising therapeutics using humanized mice. A new, highly effective bispecific antibody that binds to two different epitopes within HIV Env was developed (upper gray box and text) (95). Bispecific antibodies that can bind to both HIV Env and one of the indicated HIV cellular receptors are described (lower gray box and text) (96). Each of these antibodies was tested in HIV-infected humanized mice to demonstrate in vivo efficacy.
The other, similar study (96) developed bispecific antibodies reported to be the most broad and potent HIV-neutralizing antibodies ever described. The bispecific antibodies generated in this study consisted of one antibody arm targeting either the human CD4 or the human CCR5 receptor and the other arm targeting HIV Env. Prepositioning the antibody on one of these HIV receptors is thought to concentrate it at the precise site of virus entry and thus enhance virus neutralization mediated through the anti-HIV Env arm (96). One of these CD4-Env bispecific antibodies (10E8v2.0/iMab) neutralized 118 HIV-1 pseudotyped viruses with a mean IC50 of 2 ng/mL and also neutralized 99% of isolates in a second virus panel consisting of over 200 HIV-1 subtype C strains. This antibody could also suppress HIV viral loads in infected NSG–hu-HSC mice more effectively than could mixtures of the parental antibodies, and prevent infection with HIV strain JR-CSF when administered as preexposure prophylaxis prior to virus challenge (96) (Figure 3).
As antibody therapies against HIV are further advanced, humanized mice will likely continue to be valuable translational research tools for identifying the most effective agents and approaches to protect against HIV acquisition or to treat established HIV infection, and serve as important intermediaries between tissue culture–based approaches and approaches in large animals or human subjects.
Species Specificity and Lentiviral Strain Differences
Humanized mice provide an in vivo environment populated with human cells, which allows researchers to ask basic science questions that are difficult or impossible to address with other systems. HIV-1 is responsible for well over 95% of global HIV infections and has thus been the most intensively studied virus strain in humanized mouse studies, including almost all the studies described herein. However, HIV-2 has infected up to 1–2 million people in West Africa. Although HIV-2 is associated with lower viral loads and slower disease progression compared with HIV-1, significant numbers of HIV-2-infected individuals also progress to AIDS (97, 98). Studying HIV-2 infection in humanized mice might allow researchers to better understand important differences in the in vivo replicative capacity and pathogenicity of HIV-1 and HIV-2, and to test in vivo therapies directed toward HIV-2. To this end, a recent study tested HIV-2 infection in a hu-HSC model based on BRG (BALB/c Rag1−/− γ c −/− Rag2−/− γ c −/−) mice (99). HIV-2 replicated efficiently in this model after intraperitoneal injection, leading to plasma viral loads of over 10,000 RNA copies/mL between weeks 7 and 14 postinfection. This was accompanied by corresponding declines in CD4+ T cell numbers. HIV-2 viral loads could also be suppressed by a triple-drug ART combination consisting of the NRTIs abacavir and lamivudine and the integrase inhibitor dolutegravir, validating this model system for the in vivo study of agents that inhibit HIV-2 replication (99).
Rare natural events such as zoonoses of lentiviruses into humans can be modeled with mouse models. A recent example of this includes the first in vivo study of SIV cross-species transmission into human cells, which utilized NSG-BLT mice (100). This study demonstrated that all studied strains of SIVcpz are capable of infecting NSG-BLT mice, which not only provides direct evidence for the crossing of the presumed HIV-1 groups M and N ancestral SIVcpz strains into humans but also provides clear evidence that other, more distantly related SIVcpz strains are also capable of zoonosis into humans and could give rise to new outbreaks if given the right circumstances (100).
Cell and Tissue Tropism of HIV
For over 30 years HIV has been studied in a variety of in vitro and in vivo models; however, our understanding of the cellular and tissue tropism in infected humans is in some respects incomplete and the subject of debate. One particular question in the field relates to the in vivo relevancy of monocyte/macrophage infection by HIV. CD4+ T cells have long been understood to be the major cell target for HIV in vivo (101). Nonetheless, macrophages can also be infected with HIV both in vitro and in vivo, particularly in the brain, where infection of perivascular monocyte-derived macrophages and parenchymal microglial cells can result in HIV encephalitis and contribute to HIV-associated dementia in untreated individuals (102). Nevertheless, the relative frequency and role of HIV-infected monocyte/macrophage cells in the peripheral blood and lymphoid system and their potential for maintaining HIV infection during ART have been the subject of some debate. In vivo study of these questions has been complicated because both SIV and HIV studies have shown that macrophages become positive for viral DNA by phagocytosing infected CD4+ T cells (103, 104), which may result in tissue macrophages appearing infected when they have instead engulfed an infected T cell. A recent study overcame this confounding influence of T cells by infecting the NOD/SCID–hu-HSC myeloid-only mice (MoM) described above with HIV, demonstrating that in the absence of T cells macrophages can support HIV replication in vivo in multiple compartments, although only following infection with certain macrophage-tropic HIV strains such as HIVADA (49).
Another recent exploration (105) into the use of humanized mice to study in vivo tissue distribution of infected cells also found evidence for HIV-infected CD14+CD16+ monocyte/macrophage lineage cells and other cell types harboring integrated HIV proviruses in NSG–hu-HSC mice. This finding further supports the concept that tissue-resident cells such as differentiated macrophages, which are difficult to study in human subjects, can be much more readily evaluated in humanized mice (105). Humanized mice will likely serve more frequently in the future as models to study HIV infection in tissues and cell types that are difficult to thoroughly analyze in infected human subjects, including, for example, microglia and other cell types within the central nervous system (106).
The ability to infect humanized mice with different HIV genotypes under controlled conditions, followed by extensive tissue recovery and analysis, permits a high-resolution evaluation of HIV spread in vivo. This is exemplified in a study in NSG–hu-PBL and NSG–hu-HSC mice (107) showing that interactions between infected and uninfected cells in vivo form virological synapses that allow the simultaneous transmission of multiple copies of HIV to new T cells, similar to the synapses identified during in vitro HIV infection (108). This study also identified other features of in vivo infection, including local clustering of infected cells harboring the same virus genotype, suggesting microcompartmentalized HIV spread in tissues (107).
Persistence and Latency
ART efficiently suppresses HIV replication and can prevent disease progression, but it is not capable of curing HIV infection. This is because replication-competent HIV persists in cellular and tissue reservoirs throughout many years of ART, and rapidly reemerges if therapy is stopped (109). Latently infected CD4+ T cells represent one of the best-studied and likely the largest reservoir of persistent virus during years of ART. These cells are long lived and harbor nonexpressing integrated copies of HIV that can nevertheless be induced with appropriate stimulation to produce infectious virus (110-112). Studying latently or persistently infected cells during ART in infected patients is challenging because they are extremely rare (approximately 1 per million resting CD4+ T cells based on single-round stimulation estimates, translating to approximately 1 million per patient) and are most abundant in inaccessible tissues (reviewed in 113). Historically, humanized mice have proven useful for studying HIV latency and testing experimental methods for eliminating persistent HIV reservoirs, with HIV-infected SCID–hu-Thy/Liv mice providing a relatively abundant source of latently infected cells for ex vivo study (114-117) and newer NSG-BLT or BRG (Rag2−/− γ c −/−)–hu-HSC mouse models treated with ART also supporting the formation of latency (118-120).
One potential pathway to eliminate latent HIV is to activate viral protein expression with a latency-reversing agent (LRA), which would make the (now productively infected) host cell susceptible to viral cytopathic effects and visible to immune effector cells, including cytotoxic T lymphocytes and NK cells. There is intense interest in identifying or creating LRAs that are safe and effective in vivo and capable of depleting latently infected cells. This approach was recently tested in ART-suppressed NSG-BLT mice using the histone deacetylase inhibitor LRA panobinostat (121). The authors found that panobinostat induced systemic histone acetylation in human cells within the mice in the bone marrow, liver, lung, lymph node, spleen, and Thy/Liv implant, but that it did not produce observable changes in HIV DNA, HIV RNA, or latently infected cells. These findings indicate that the NSG-BLT mouse model is suitable for in vivo evaluation of LRAs, but that panobinostat might be most effective when used in a combination of LRA approaches rather than as a single agent to deplete the reservoir.
NSG-BLT mouse models were also employed in a study investigating how the molecular chaperone heat shock protein 90 (Hsp90) can influence HIV latency and rebound upon cessation of ART (122). In this study, in vitro heat shock (raising temperature to 39.5°C) mediated by Hsp90 upregulated HIV expression by activating key HIV transcription factors including NF-κB, STAT5, and NFAT. In HIV-infected BLT mice that were treated with ART, Hsp90 inhibitors in clinical development prevented viral rebound for up to 11 weeks after stopping ART, even as cells harboring replication-competent virus persisted in tissues and virus could be induced to express ex vivo (122). These data suggest that Hsp90 plays a critical role in HIV rebound in vivo, and that inhibiting the activity of this protein might prevent HIV outgrowth from persistent viral reservoirs present in infected patients. Therefore, Hsp90 could be used as a component of approaches to cure HIV.
Another approach for studying HIV latency in mouse models is to take advantage of the GVHD and immune activation that occurs upon transplantation of mature immune cells into immunodeficient mice. This activation can be used to provide a sensitive outgrowth assay to identify and potentially quantify latently infected cells derived from HIV-positive patients. This approach is particularly important because quantitative viral outgrowth assays (QVOAs), which are typically based on a single round of limiting dilution stimulation of latently infected cells and the addition of feeder cells, can underestimate the size of the reservoir (123). This approach has been used (124) in the form of a murine viral outgrowth assay, in which PBMCs or isolated CD4+ T cells derived from SIV-infected pigtail macaques or HIV-infected human subjects with undetectable viral loads were transplanted into NSG mice. The SIV samples were derived from animals with viral loads suppressed through ART, and the human samples were from either ART-treated individuals or elite suppressors, individuals who naturally control HIV viral loads.
Human CD8+ T cells were also depleted in vivo with a monoclonal antibody to enhance viral outgrowth, and in some cases cells were stimulated in vivo with an anti-CD3 antibody. Resultant macaquized or humanized mice were evaluated for viral load and the relative frequency of CD4+ T cells. Xenografting in this manner resulted in amplification of virus in all the tested samples, including from one elite suppressor whose cells did not produce quantifiable virus in an ex vivo QVOA assay. Therefore, this murine viral outgrowth assay, which can be performed with relatively large numbers of CD4+ T cells (10–50 million cells per mouse), might represent a more sensitive diagnostic assay than QVOA for identifying latently infected CD4+ T cells in some situations (124).
CONCLUDING REMARKS
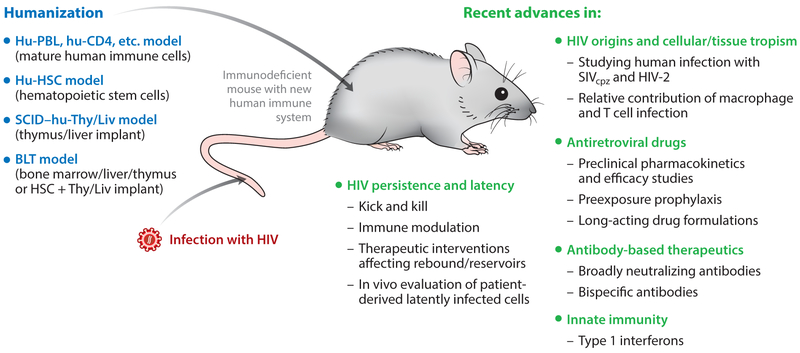
As with any model system, data generated with humanized mice should be interpreted with care when translating the findings into human studies, with a full consideration of potential limitations and caveats. Nevertheless, humanized mouse models for HIV are highly effective tools for studying a wide range of current research areas, including in vivo testing of novel therapeutic antibodies and drugs and developing a more complete picture of HIV species tropism, tissue/cellular tropism, and persistence/latency (Figure 4). Further advances in humanized mouse models should allow researchers to more effectively apply such models to these and other exciting and important areas of HIV research, including vaccine studies and gene- or cell-based therapeutic approaches for eliminating HIV.
Figure 4.
Overview of common humanized mouse models and examples of their recent use in HIV research.
ACKNOWLEDGMENTS
The authors’ laboratories have been supported by grants from the National Institutes of Health, including AI70010 and AI124743 to J.A.Z. and AI124763 to M.D.M., and by the University of California, Los Angeles, Center for AIDS Research (AI28697).
Glossary
- HIV
human immunodeficiency virus
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- SCID
severe combined immunodeficiency
- NSG
NOD-SCID-γc knockout
- GVHD
graft-versus-host disease
- Thy/Liv
thymus/liver
- BLT
bone marrow/liver/thymus
- bNAb
broadly neutralizing antibody
- QVOA
quantitative viral outgrowth assay
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, et al. 1981. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N. Engl. J. Med 305:1425–31 [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. 2016. UNAIDS Fact Sheet November 2016. Global HIV Statistics. Geneva: UNAIDS; http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf [Google Scholar]
- 3.Marsden MD, Zack JA. 2013. HIV/AIDS eradication. Bioorg. Med. Chem. Lett 23:4003–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner MB, Luciw PA. 2008. Macaque models of human infectious disease. ILAR J. 49:220–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat. Rev. Microbiol 10:852–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrow WJ, Wharton M, Lau D, Levy JA. 1987. Small animals are not susceptible to human immunodeficiency virus infection. J. Gen. Virol 68(Pt. 8):2253–57 [DOI] [PubMed] [Google Scholar]
- 7.Bieniasz PD, Cullen BR. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol 74:9868–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning J, Horner JW, Pettoello-Mantovani M, Raker C, Yurasov S, et al. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. PNAS 94:14637–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potash MJ, Chao W, Bentsman G, Paris N, Saini M, et al. 2005. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. PNAS 102:3760–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonard JM, Abramczuk JW, Pezen DS, Rutledge R, Belcher JH, et al. 1988. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242:1665–70 [DOI] [PubMed] [Google Scholar]
- 11.Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163–75 [DOI] [PubMed] [Google Scholar]
- 12.Marsden MD, Zack JA. 2015. Studies of retroviral infection in humanized mice. Virology 479–80:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan SP. 1966. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res 8:295–309 [DOI] [PubMed] [Google Scholar]
- 14.Bosma GC, Custer RP, Bosma MJ. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527–30 [DOI] [PubMed] [Google Scholar]
- 15.Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. 1994. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372:103–7 [DOI] [PubMed] [Google Scholar]
- 16.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–77 [DOI] [PubMed] [Google Scholar]
- 17.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–67 [DOI] [PubMed] [Google Scholar]
- 18.Murphy WJ, Kumar V, Bennett M. 1987. Rejection of bone marrow allografts by mice with severe combined immune deficiency (SCID). Evidence that natural killer cells can mediate the specificity of marrow graft rejection. J. Exp. Med 165:1212–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2:223–38 [DOI] [PubMed] [Google Scholar]
- 20.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. PNAS 92:377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, et al. 1996. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood 87:956–67 [PubMed] [Google Scholar]
- 22.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. 2003. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 101:4887–93 [DOI] [PubMed] [Google Scholar]
- 24.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol 12:786–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat. Rev. Immunol 7:118–30 [DOI] [PubMed] [Google Scholar]
- 26.Akkina R, Allam A, Balazs AB, Blankson JN, Burnett JC, et al. 2016. Improvements and limitations of humanized mouse models for HIV research: NIH/NIAID “Meet the Experts” 2015 Workshop Summary. AIDS Res. Hum. Retrovir 32:109–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenblatt MB, Vrbanac V, Tivey T, Tsang K, Tager AM, Aliprantis AO. 2012. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLOS ONE 7:e44664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. 2000. Role of CD47 as a marker of self on red blood cells. Science 288:2051–54 [DOI] [PubMed] [Google Scholar]
- 29.Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, et al. 2011. Functional CD47/signal regulatory protein alpha (SIRPα) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. PNAS 108:13224–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, et al. 2007. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol 8:1313–23 [DOI] [PubMed] [Google Scholar]
- 31.Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, et al. 2001. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J. Exp. Med 194:541–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, Yang YG. 2007. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. PNAS 104:13744–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavender KJ, Pang WW, Messer RJ, Duley AK, Race B, et al. 2013. BLT-humanized C57BL/6 Rag2−/− γ c −/−CD47−/− mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood 122:4013–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brehm MA, Shultz LD, Luban J, Greiner DL. 2013. Overcoming current limitations in humanized mouse research. J. Infect. Dis. 208(Suppl. 2):S125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. 1988. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335:256–59 [DOI] [PubMed] [Google Scholar]
- 36.King MA, Covassin L, Brehm MA, Racki W, Pearson T, et al. 2009. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin. Exp. Immunol 157:104–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harui A, Kiertscher SM, Roth MD. 2011. Reconstitution of huPBL-NSG mice with donor-matched dendritic cells enables antigen-specific T-cell activation. J. Neuroimmune Pharmacol 6:148–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas T, Seay K, Zheng JH, Zhang C, Ochsenbauer C, et al. 2016. High-throughput humanized mouse models for evaluation of HIV-1 therapeutics and pathogenesis. Methods Mol. Biol 1354:221–35 [DOI] [PubMed] [Google Scholar]
- 39.Hatano R, Ohnuma K, Yamamoto J, Dang NH, Yamada T, Morimoto C. 2013. Prevention of acute graft-versus-host disease by humanized anti-CD26 monoclonal antibody. Br. J. Haematol 162:263–77 [DOI] [PubMed] [Google Scholar]
- 40.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. 1990. HIV-1 entry into quiescent primary lymphocytes: Molecular analysis reveals a labile, latent viral structure. Cell 61:213–22 [DOI] [PubMed] [Google Scholar]
- 41.Rizza P, Santini SM, Logozzi MA, Lapenta C, Sestili P, et al. 1996. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J. Virol 70:7958–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632–39 [DOI] [PubMed] [Google Scholar]
- 43.Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D, Garcia JV. 2013. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology 10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, et al. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104–7 [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, et al. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 106:1565–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, et al. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12:1316–22 [DOI] [PubMed] [Google Scholar]
- 47.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. 2006. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108:487–92 [DOI] [PubMed] [Google Scholar]
- 48.Islas-Ohlmayer M, Padgett-Thomas A, Domiati-Saad R, Melkus MW, Cravens PD, et al. 2004. Experimental infection of NOD/SCID mice reconstituted with human CD34+ cells with Epstein-Barr virus. J. Virol 78:13891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, et al. 2016. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig 126:1353–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osokine I, Snell LM, Cunningham CR, Yamada DH, Wilson EB, et al. 2014. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. PNAS 111:7409–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, et al. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340:202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat. Rev. Immunol 14:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacMicking JD. 2012. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol 12:367–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, et al. 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, et al. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340:207–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhen A, Rezek V, Youn C, Lam B, Chang N, et al. 2017. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Investig 127:260–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng L, Ma J, Li J, Li D, Li G, et al. 2017. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Investig 127:269–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lavender KJ, Gibbert K, Peterson KE, Van Dis E, Francois S, et al. 2016. Interferon alpha subtype-specific suppression of HIV-1 infection in vivo. J. Virol 90:6001–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, et al. 2013. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J. Infect. Dis 207:213–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, et al. 2010. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J. Infect. Dis 201:1686–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abraham S, Choi JG, Ortega NM, Zhang J, Shankar P, Manjunath N. 2016. Gene therapy with plasmids encoding IFN-β or IFN-α14 confers long-term resistance to HIV-1 in humanized mice. Oncotarget 7:78412–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. 2010. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol 11:1005–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wheeler LA, Trifonova RT, Vrbanac V, Barteneva NS, Liu X, et al. 2016. TREX1 knockdown induces an interferon response to HIV that delays viral infection in humanized mice. Cell Rep. 15:1715–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawamoto A, Kodama E, Sarafianos SG, Sakagami Y, Kohgo S, et al. 2008. 2′-deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int. J. Biochem. Cell Biol 40:2410–20 [DOI] [PubMed] [Google Scholar]
- 65.Sohl CD, Singh K, Kasiviswanathan R, Copeland WC, Mitsuya H, et al. 2012. Mechanism of interaction of human mitochondrial DNA polymerase gamma with the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine indicates a low potential for host toxicity. Antimicrob. Agents Chemother 56:1630–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shanmugasundaram U, Kovarova M, Ho PT, Schramm N, Wahl A, et al. 2016. Efficient inhibition of HIV replication in the gastrointestinal and female reproductive tracts of humanized BLT mice by EFdA. PLOS ONE 11:e0159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoddart CA, Galkina SA, Joshi P, Kosikova G, Moreno ME, et al. 2015. Oral administration of the nucleoside EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque. Antimicrob. Agents Chemother 59:4190–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veselinovic M, Yang KH, Sykes C, Remling-Mulder L, Kashuba AD, Akkina R. 2016. Mucosal tissue pharmacokinetics of the integrase inhibitor raltegravir in a humanized mouse model: implications for HIV pre-exposure prophylaxis. Virology 489:173–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andrews CD, Heneine W. 2015. Cabotegravir long-acting for HIV-1 prevention. Curr. Opin. HIV AIDS 10:258–63 [DOI] [PubMed] [Google Scholar]
- 70.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med 363:2587–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, et al. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med 367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, et al. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med 367:423–34 [DOI] [PubMed] [Google Scholar]
- 73.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, et al. 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med 367:411–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson AG, Else LJ, Mesquita PM, Egan D, Back DJ,et al. 2014. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin. Pharmacol. Ther 96:314–23 [DOI] [PubMed] [Google Scholar]
- 75.Kovarova M, Swanson MD, Sanchez RI, Baker CE, Steve J, et al. 2016. A long-acting formulation of the integrase inhibitor raltegravir protects humanized BLT mice from repeated high-dose vaginal HIV challenges. J. Antimicrob. Chemother 71:1586–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, et al. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol 83:7337–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301:1374–77 [DOI] [PubMed] [Google Scholar]
- 78.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, et al. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., et al. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, et al. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Igarashi T, Brown C, Azadegan A, Haigwood N, Dimitrov D, et al. 1999. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat. Med 5:211–16 [DOI] [PubMed] [Google Scholar]
- 83.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, et al. 2016. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, et al. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marsden MD, Zack JA. 2014. Neutralizing the HIV reservoir. Cell 158:971–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, et al. 2016. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352:1001–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veselinovic M, Neff CP, Mulder LR, Akkina R. 2012. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology 432:505–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, et al. 2014. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med 20:296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hur EM, Patel SN, Shimizu S, Rao DS, Gnanapragasam PN, et al. 2012. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood 120:4571–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deruaz M, Moldt B, Le KM, Power KA, Vrbanac VD, et al. 2016. Protection of humanized mice from repeated intravaginal HIV challenge by passive immunization: a model for studying the efficacy of neutralizing antibodies in vivo. J. Infect. Dis 214:612–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sung JA, Pickeral J, Liu L, Stanfield-Oakley SA, Lam CY, et al. 2015. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J. Clin. Investig 125:4077–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pegu A, Asokan M, Wu L, Wang K, Hataye J, et al. 2015. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat. Commun 6:8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. 2016. Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell 165:1609–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, et al. 2016. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell 165:1621–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gottlieb GS, Eholie SP, Nkengasong JN, Jallow S, Rowland-Jones S, et al. 2008. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS 22:2069–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Campbell-Yesufu OT, Gandhi RT. 2011. Update on human immunodeficiency virus (HIV)-2 infection. Clin. Infect. Dis 52:780–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu S, Neff CP, Kumar DM, Habu Y, Akkina SR, et al. 2017. A humanized mouse model for HIV-2 infection and efficacy testing of a single-pill triple-drug combination anti-retroviral therapy. Virology 501:115–18 [DOI] [PubMed] [Google Scholar]
- 100.Yuan Z, Kang G, Ma F, Lu W, Fan W, et al. 2016. Recapitulating cross-species transmission of simian immunodeficiency virus SIVcpz to humans by using humanized BLT mice. J. Virol 90:7728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123–26 [DOI] [PubMed] [Google Scholar]
- 102.Anthony IC, Bell JE. 2008. The neuropathology of HIV/AIDS. Int. Rev. Psychiatry 20:15–24 [DOI] [PubMed] [Google Scholar]
- 103.Calantone N, Wu F, Klase Z, Deleage C, Perkins M, et al. 2014. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity 41:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baxter AE, Russell RA, Duncan CJ, Moore MD, Willberg CB, et al. 2014. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe 16:711–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arainga M, Su H, Poluektova LY, Gorantla S, Gendelman HE. 2016. HIV-1 cellular and tissue replication patterns in infected humanized mice. Sci. Rep 6:23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Honeycutt JB, Sheridan PA, Matsushima GK, Garcia JV. 2014. Humanized mouse models for HIV-1 infection of the CNS. J. Neurovirol 21:301–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Law KM, Komarova NL, Yewdall AW, Lee RK, Herrera OL, et al. 2016. In vivo HIV-1 cell-to-cell transmission promotes multicopy micro-compartmentalized infection. Cell Rep. 15:2771–83 [DOI] [PubMed] [Google Scholar]
- 108.Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK. 2011. Multiploid inheritance of HIV-1 during cell-to-cell infection. J. Virol 85:7169–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marsden MD, Zack JA. 2010. Establishment and maintenance of HIV latency: model systems and opportunities for intervention. Future Virol. 5:97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, et al. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. PNAS 94:13193–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, et al. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–300 [DOI] [PubMed] [Google Scholar]
- 112.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, et al. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–95 [DOI] [PubMed] [Google Scholar]
- 113.Marsden MD, Zack JA. 2015. Experimental approaches for eliminating latent HIV. For. Immunopathol. Dis. Ther. 6:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brooks DG, Kitchen SG, Kitchen CM, Scripture-Adams DD, Zack JA. 2001. Generation of HIV latency during thymopoiesis. Nat. Med 7:459–64 [DOI] [PubMed] [Google Scholar]
- 115.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. 2002. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol 76:8118–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, et al. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413–23 [DOI] [PubMed] [Google Scholar]
- 117.Brooks DG, Arlen PA, Gao L, Kitchen CM, Zack JA. 2003. Identification of T cell-signaling pathways that stimulate latent HIV in primary cells. PNAS 100:12955–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, et al. 2012. HIV latency in the humanized BLT mouse. J. Virol 86:339–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, et al. 2012. Generation of HIV latency in humanized BLT mice. J. Virol 86:630–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. 2012. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/− γc−/− mouse. J. Virol 86:114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsai P, Wu G, Baker CE, Thayer WO, Spagnuolo RA, et al. 2016. In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection. Retrovirology 13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joshi P, Maidji E, Stoddart CA. 2016. Inhibition of heat shock protein 90 prevents HIV rebound. J. Biol. Chem 291:10332–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, et al. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Metcalf Pate KA, Pohlmeyer CW, Walker-Sperling VE, Foote JB, Najarro KM, et al. 2015. A murine viral outgrowth assay to detect residual HIV type 1 in patients with undetectable viral loads. J. Infect. Dis 212:1387–96 [DOI] [PMC free article] [PubMed] [Google Scholar]