Abstract
Background
The Los Angeles (LA) grade of reflux esophagitis (A to D) is assumed to reflect severity of the underlying GERD. Thus, LA-D esophagitis patients might be expected to have the most conditions predisposing to GERD (e.g. obesity, hiatal hernia), and the highest frequency of GERD symptoms.
Goals
To compare clinical features of patients with the most severe (LA-D) and mildest (LA-A) grades of esophagitis.
Study
For this comparative study, we searched our endoscopy database for patients diagnosed with LA-D or LA-A esophagitis, reviewed their endoscopic images, and reviewed medical records of the first 100 we confirmed to have LA-D or LA-A esophagitis.
Results
Compared to LA-A patients, LA-D patients were older (mean age 65±13.4 vs. 56±13.4 years, p<0.001), had lower BMIs (25.9±5.6 vs. 29.4±5.3, p<0.001), were more frequently hospitalized (70% vs. 3%, p<0.001) and in the ICU (15% vs. 0%, p<0.001), and had significantly more serious cardiopulmonary disorders and gastrointestinal bleeding. Conversely, a GERD history was more common in LA-A than LA-D patients (67% vs. 45%, p=0.002). Hiatal hernia was more frequent in LA-A patients than LA-D patients, but not significantly (48% vs. 36%, p=0.09).
Conclusions
LA-D esophagitis primarily affects hospitalized, older, non-obese patients who often have serious comorbidities, and no history of GERD or hiatal hernia. In contrast, LA-A patients are generally younger, obese outpatients who often have a history of GERD and hiatal hernia without serious comorbidities. These profound differences between LA-A and LA-D patients suggest that factors other than typical GERD contribute to LA-D esophagitis pathogenesis.
Keywords: Gastroesophageal reflux disease, reflux esophagitis, hiatal hernia, endoscopy
INTRODUCTION
Gastroesophageal reflux disease (GERD) is an extremely common condition that affects up to 20% of the American adult population.1,2 Although GERD is primarily a clinical diagnosis, endoscopy is used to look for GERD complications such as Barrett’s esophagus, to rule out other upper gastrointestinal diseases in equivocal cases, and to determine if GERD has caused esophageal mucosal injury, i.e. reflux esophagitis. Only approximately 30% of patients with typical GERD symptoms have endoscopic evidence of erosive reflux esophagitis, a finding that has important diagnostic and therapeutic implications.3
Presently, the Los Angeles (LA) classification is the endoscopic scoring system most commonly used to grade the severity of reflux esophagitis.4 The LA system divides reflux esophagitis into four categories (A-D) based on the extent of esophageal mucosal breaks. LA grade A (LA-A) esophagitis is defined as one or more mucosal breaks not longer than 5 mm, and not extending between the tops of two mucosal folds (Figure 1). In contrast, LA-D esophagitis is defined as one or more mucosal breaks involving 75% or more of the esophageal circumference (Figure 2). LA-D is the most infrequently encountered grade of reflux esophagitis.5
Figure 1.
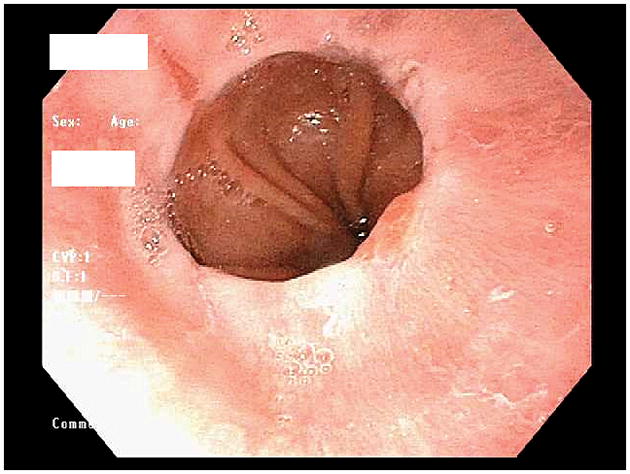
Endoscopic photo of the distal esophagus showing LA-A esophagitis. Note the small mucosal break (<5 mm in length, not extending between the tops of two mucosal folds) in the 10 o’clock position just proximal to the squamo-columnar junction.
Figure 2.
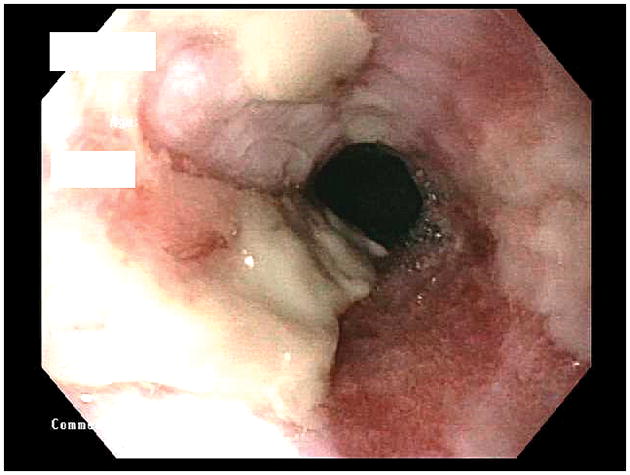
Endoscopic photo of the distal esophagus showing LA-D esophagitis. The extensive mucosal breaks involve most (>75%) of the circumference of the distal esophagus. Much of the broken mucosal surface is covered by dense, whitish exudate.
Implicit in the LA classification system is the assumption that there is a spectrum of GERD severity ranging from mild to severe, and that the LA grade of esophagitis reflects the severity of the underlying GERD. If LA-D esophagitis represents the most severe form of GERD, then LA-D patients would be expected to have a greater frequency and severity of conditions that contribute to GERD (e.g. obesity, hiatal hernia) than patients with reflux esophagitis of lesser severity. It might also be anticipated that LA-D patients would have a long history of progressively increasing GERD symptoms. However, over the years, we have observed that our LA-D patients frequently have clinical features that would not be expected if typical GERD were the sole factor underlying this esophagitis. We hypothesized that patients with LA-D esophagitis have clinical features that differ significantly from those of patients with LA-A reflux esophagitis, and that factors other than typical GERD contribute to the pathogenesis of LA-D esophagitis. To explore these hypotheses, we conducted a retrospective, comparative study on the clinical features of patients with LA-D and LA-A esophagitis.
MATERIALS AND METHODS
This study was approved by the institutional review board of the Dallas VA Medical Center. We reviewed our endoscopy database to identify patients who had an upper gastrointestinal endoscopy performed between March 2003 and August 2014 that showed either LA-D or LA-A erosive esophagitis. We reviewed all available endoscopic images to confirm the endoscopic diagnoses, and we included the first 100 consecutive patients confirmed to have LA-D and the first 100 consecutive patients with LA-A esophagitis. Subjects were excluded if they had a history of esophageal surgery, if no endoscopic images of the esophagus were available for review, or if esophagitis was either suspected or confirmed to be due to a cause other than GERD (e.g. candida esophagitis, eosinophilic esophagitis). We also recorded the indication for endoscopy and other pertinent endoscopic findings including the presence of hiatal hernia, gastric outlet obstruction, and esophageal strictures.
For each study subject, we reviewed the electronic medical record to extract demographic data including age, race and gender. We recorded conditions potentially related to the development of reflux esophagitis including obesity (determined by body mass index [BMI]), history of GERD symptoms, gastroparesis, diabetes, and use of tobacco and alcohol. We also recorded other potentially pertinent data including a history of malignancy, immunosuppression, chest irradiation or treatment with a nasogastric tube. We recorded any history of cardiopulmonary diseases including coronary artery disease, congestive heart failure, peripheral vascular disease, obstructive sleep apnea and chronic obstructive pulmonary disease. We determined the hospitalization status of each study subject at the time of the diagnosis of LA-A or LA-D reflux esophagitis (outpatient, inpatient, or in the intensive care unit [ICU]). Finally, we reviewed pharmacy records to identify medications that might contribute to esophagitis, and we recorded the use of acid suppressive medications including proton pump inhibitors (PPIs) and histamine H2-receptor antagonists. Outpatients were considered to be using an acid-suppressive medication if the pharmacy record listed it as ‘active’ within the thirty days prior to endoscopy, whereas inpatients were considered to be using an acid-suppressive medication if the pharmacy record listed it as ‘active’ within the thirty days prior to hospital admission.
Categorical variables were analyzed by chi-square testing and continuous variables by t-tests. Logistic regression was performed to identify independent factors associated with LA-D esophagitis. P-values of <0.05 were considered statistically significant. Statistical analysis was performed using SAS, version 9.3.
RESULTS
Study subjects included 100 patients with LA-D esophagitis and 100 patients with LA-A esophagitis. LA-D patients were significantly older than LA-A patients (mean age 65±13.4 years vs. 56±13.4 years, p<0.001) (Table 1). The two groups did not differ significantly in gender or race. The BMI of LA-D patients was significantly lower than that of LA-A patients (25.9±5.6 vs. 29.4±5.3, p<0.001). LA-D patients were significantly more likely to have never consumed alcohol than LA-A patients (38%vs. 17%, p <0.001), and LA-D patients were significantly less likely to be current users of alcohol than LA-A patients (25% vs. 55%, p<0.001). There were no significant differences between the groups in their history of tobacco use.
Table 1.
Baseline Clinical Characteristics of Patients with LA-D and LA-A Esophagitis
| Grade D n=100 |
Grade A n=100 |
p-value | ||
|---|---|---|---|---|
| N | N | |||
| Age (mean) | 65.0 ±13.4 | 56.5 ±13.4 | <0.001 | |
| Gender (male) | 97 | 93 | 0.19 | |
| Race | Caucasian | 68 | 78 | 0.28 |
| African American | 26 | 18 | ||
| Other1 | 6 | 4 | ||
| Body Mass Index (mean) | 25.9 ± 5.6 | 29.4 ± 5.3 | <0.001 | |
| Tobacco Use | Current | 29 | 37 | 0.16 |
| Former | 44 | 31 | ||
| Never | 27 | 32 | ||
| Alcohol Use | Current | 25 | 55 | <0.001 |
| Former | 37 | 28 | ||
| Never | 38 | 17 | ||
American Indian, Hispanic, Unknown
Regarding endoscopic findings other than reflux esophagitis, there were no significant differences between LA-D and LA-A patients in the frequency of hiatal hernia (36% LA-D vs. 48% LA-A, p=0.09), gastric outlet obstruction (6% LA-D vs. 2% LA-A, p=0.15), and esophageal stricture (11% LA-D vs. 4% LA-A, p=0.06). Nevertheless, it is notable that hiatal hernia was seen less frequently in LA-D patients than in LA-A patients.
Co-morbid conditions and acute illnesses were significantly more common in LA-D patients than in LA-A patients (Table 2). Regarding hospitalization status, 70% of patients with LA-D esophagitis were hospitalized at the time of diagnosis (either on the medical ward or in the ICU), compared to only 3% of patients with LA-A esophagitis (p<0.001). Furthermore, no LA-A patient was in the ICU, while 15% of LA-D patients were in the ICU. Further review of medical records revealed that 13 of the 30 outpatients found to have LA D esophagitis had been hospitalized within six months prior to their outpatient endoscopy. Conversely, none of the outpatient LA-A patients had been hospitalized within six months prior to their endoscopy. Patients with LA-D esophagitis had a significantly higher prevalence of cardiopulmonary disorders such as coronary artery disease, congestive heart failure, and chronic obstructive pulmonary disease (COPD). LA-D patients were also more likely to have had a history of chest irradiation (11% vs. 0%, p=0.001) or treatment with a nasogastric tube (10% vs. 1%, p=0.005). Malignancy, diabetes, peripheral vascular disease and obstructive sleep apnea all were seen more frequently in patients with LA-D esophagitis than in LA-A patients, but the differences were not statistically significant. In contrast, patients with LA-A esophagitis were significantly more likely to have no major co-morbid conditions documented than LA-D patients (45% vs. 23%, p=0.001), and a history of GERD was significantly more common in patients with LA-A than with LA-D esophagitis (67% vs. 45%, p=0.002).
Table 2.
Hospitalization Status and Co-Morbid Conditions of Patients With LA-D and LA-A Esophagitis
| Grade D n=100 |
Grade A n=100 |
p-value | ||
|---|---|---|---|---|
| N | N | |||
| Hospitalization Status when Endoscopy Showed Esophagitis | Inpatient | 55 | 3 | <0.001 |
| ICU | 15 | 0 | ||
| Outpatient | 30 | 97 | ||
| No Co-Morbidity | 23 | 45 | 0.001 | |
| GERD | 45 | 67 | 0.002 | |
| CAD | 32 | 9 | <0.001 | |
| CHF | 16 | 3 | 0.001 | |
| COPD | 27 | 7 | <0.001 | |
| PVD | 9 | 4 | 0.15 | |
| OSA | 7 | 12 | 0.26 | |
| Gastroparesis | 0 | 1 | 0.32 | |
| Diabetes | 28 | 18 | 0.09 | |
| Immunosuppression1 | 3 | 3 | 1.00 | |
| Malignancy | 22 | 16 | 0.28 | |
| Chest Radiation | 11 | 0 | 0.001 | |
| NG tube | 10 | 1 | 0.005 | |
HIV or organ transplant recipient
GERD=gastroesophageal reflux disease, CAD=coronary artery disease, CHF=congestive heart failure, COPD=chronic obstructive pulmonary disease, PVD=peripheral vascular disease, OSA=obstructive sleep apnea, NG=nasogastric
We performed a sub-analysis to explore differences between outpatients and inpatients with LA-D esophagitis. As described above, 70 of our 100 LA-D patients were acutely ill and hospitalized, and 30 were outpatients. Among the 30 outpatients, 13 had been hospitalized in the previous 6 months for various serious medical issues, and 17 had no recent hospitalizations. We divided our LA-D esophagitis patients into two subgroups: 1) a subgroup of 83 patients found to have LA-D esophagitis while hospitalized or recently hospitalized, and 2) a subgroup of 17 outpatients with no recent hospitalizations found to have LA-D esophagitis. Supplementary Tables 1 and 2 list clinical features of these two subgroups, showing that ‘true’ outpatients with LA-D esophagitis resemble LA-A patients more than they resemble hospitalized LA-D patients, and accentuating the differences between hospitalized LA-D patients and LA-A patients.
The indications for endoscopy also differed between patients with LA-D and LA-A esophagitis (Table 3). Gastrointestinal bleeding was the most common indication for endoscopy in patients found to have LA-D esophagitis; it was the indication in 42% of LA-D patients, all of whom were hospitalized at the time of endoscopy. In contrast, only 2% of patients with LA-A esophagitis had endoscopy for evaluation of GI bleeding (p<.0001). GERD was the indication for endoscopy in only 7% of LA-D patients, while it was the most common indication for endoscopy in patients with LA-A esophagitis (30%, p<.0001). Other endoscopic indications such as dysphagia, abdominal pain, and iron deficiency anemia were similar between groups. Thirty-six of our 100 LA-D patients had prior endoscopies; these showed evidence of reflux esophagitis in 20 of the 36 cases (56%) and no erosive esophagitis in 16 cases (44%). Among the 20 LA-D patients with esophagitis found on prior endoscopy, that endoscopy was performed during a hospitalization in 12 cases.
Table 3.
Indications for Endoscopy in Patients with LA-D and LA-A Esophagitis
| Grade D n=100 |
Grade A n=100 |
p-value | |
|---|---|---|---|
| Gastrointestinal bleeding1 | 42 | 2 | <0.0001 |
| Dysphagia/Odynophagia | 20 | 24 | 0.5 |
| GERD | 7 | 30 | <0.0001 |
| Nausea/vomiting | 5 | 4 | 0.73 |
| Iron deficiency anemia | 6 | 12 | 0.14 |
| Abdominal pain | 3 | 4 | 0.70 |
| Surveillance2 | 8 | 15 | 0.12 |
| Abnormal Imaging | 4 | 3 | 0.70 |
| Other3 | 5 | 6 | 0.76 |
Hematemesis, hematochezia, melena, fecal occult blood test positivity, acute on chronic anemia
Esophagitis, gastric ulcers, Barrett’s esophagus, varices
Crohn’s disease, duodenal polyps, diarrhea, assess tears after ERCP, hiccups, weight loss, Helicobacter Pylori eradication, globus
Medication usage is shown in Table 4. There were no significant differences between the groups in the use of any medication recorded. Notably, proton pump inhibitor (PPI) use was almost equivalent in both groups (used by 55% of LA-D patients, 53% of LA-A patients). Use of medications that could potentially contribute to esophagitis such as bisphosphonates, potassium, iron and tetracycline was more common in LA-D patients than in LA-A patients, but this difference was not statistically significant (26% vs. 12%, p=0.23).
Table 4.
Medication Use in Patients with LA-D and LA-A Esophagitis
| Grade D (n=100) | Grade A (n=100) | p-value | |
|---|---|---|---|
| N | N | ||
| Proton pump inhibitors (PPI) | 55 | 53 | 0.78 |
| Histamine-2 receptor antagonists (H2RA) | 8 | 7 | 0.79 |
| PPI+H2RA | 5 | 4 | 0.73 |
| Aspirin | 37 | 28 | 0.17 |
| Clopidogrel | 8 | 3 | 0.12 |
| NSAIDs | 19 | 16 | 0.58 |
| Corticosteroids | 7 | 1 | 0.24 |
| Other medication associated with pill esophagitis1 | 26 | 12 | 0.23 |
Iron, Potassium, Clarithromycin, Erythromycin, Bisphosphonates, Tetracycline
Multivariate logistic regression was performed to identify independent risk factors for LA-D erosive esophagitis (Table 5). Inpatient hospitalization was by far the single strongest predictor of LA-D esophagitis (OR 61.00, 95% CI 16.8 – 220.97). Other significant risk factors included the presence of an esophageal stricture and lower BMI.
Table 5.
Multivariate Logistic Regression Analysis of LA-D vs. LA-A Esophagitis Patient Characteristics
| OR | 95% CI | P value | ||
|---|---|---|---|---|
| BMI | 0.91 | 0.83 | 0.98 | 0.02 |
| Inpatient Hospitalization Status | 61.00 | 16.80 | 220.97 | <0.0001 |
| Esophageal Stricture | 9.34 | 2.26 | 38.54 | 0.002 |
DISCUSSION
We have identified a number of significant differences between patients with LA-D esophagitis and patients with LA-A esophagitis in regard to their clinical features. Compared to LA-A patients, our patients with LA-D esophagitis were significantly older (mean age 65±13.4 vs. 56±13.4 years), had significantly lower BMIs (25.9±5.6 vs. 29.4±5.3), and were significantly more likely to have never consumed alcohol (38% vs. 17%). Serious cardiopulmonary disorders including coronary artery disease, congestive heart failure and COPD were significantly more common in LA-D patients than in LA-A patients. Most of our patients with LA-D esophagitis were hospitalized (70%, including 15% in the ICU), and 13 of the 30 outpatients found to have LA-D esophagitis had been hospitalized within 3 months of the outpatient endoscopy. In contrast, the large majority of our patients with LA-A esophagitis were outpatients (97%), none of whom had been hospitalized within the prior 3 months. A history of GERD was significantly more common in LA-A than in LA-D patients (67% vs. 45%), and GERD was also more commonly the indication for endoscopy in patients with LA-A esophagitis (30%) than in patients with LA-D esophagitis (7%). In contrast, gastrointestinal bleeding was the most common indication for the endoscopy revealing LA-D esophagitis (42%), while gastrointestinal bleeding was an infrequent indication for endoscopies showing LA-A esophagitis (2%). In addition, hiatal hernia was more common in LA-A than in LA-D patients (48% vs. 36%). Thus, LA-D esophagitis appears to be a disorder primarily of older, non-obese, hospitalized patients who have serious co-morbid conditions, often including gastrointestinal bleeding, and who usually have no prior history of GERD or of hiatal hernia. These features suggest that factors other than typical gastroesophageal reflux contribute to the development of LA-D esophagitis.
If LA-D esophagitis were merely the far end of the spectrum of GERD severity, then LA-D patients should have more factors that predispose to gastroesophageal reflux than patients with lesser grades of esophagitis. Obesity is thought to predispose to gastroesophageal reflux, perhaps because excessive abdominal fat can increase intra-abdominal pressure, and because overeating can cause gastric distention that results in reflux. Indeed, most of our patients with LA-A esophagitis were overweight or obese, with a mean BMI of 29.4. In contrast, the mean BMI of our LA-D patients was only 25.9. Hiatal hernia can be associated with low pressure at the gastroesophageal junction that predisposes to gastroesophageal reflux, and with adverse effects on esophageal clearance of refluxed material that might contribute to esophagitis. However, hiatal hernia was more common in our LA-A patients than in our LA-D patients. Furthermore, If GERD were the only factor underlying LA-D esophagitis, then it might be anticipated that LA-D patients would have a prior history of GERD symptoms more often than patients with lower grades of esophagitis. This also was not the case, as a prior history of GERD symptoms was more common in our LA-A patients than in our LA-D patients. GERD was also a less frequent indication for endoscopy in patients found to have LA-D esophagitis than LA-A patients. Thus, patients with LA-D esophagitis often do not have clinical features typically associated with GERD.
Some reports have described a positive association between obesity and reflux esophagitis,6–8 but few studies have attempted specifically to correlate obesity with the endoscopic grade of esophagitis. Our finding that obesity is associated with LA-A but not LA-D esophagitis contradicts the results of a study by El-Serag, who found that obesity was an independent risk factor for severe esophagitis.5 Those contradictory findings might be explained by a number of major differences between these studies in patient population and design. El-Serag’s study included 6,709 patients who had varying grades of reflux esophagitis documented during endoscopies performed as screening for clinical trials comparing different PPIs. Those study subjects were primarily healthy, younger (mean age 46 years) outpatients who had volunteered to participate in pharmaceutical studies. In contrast, we specifically identified consecutive patients with LA-D esophagitis in our endoscopy database, and our LA-D patients were considerably older (mean age 65), hospitalized patients who had substantial co-morbidities. Indeed, our sub-analysis of LA-D patients who were ‘true’ outpatients suggests that this small subgroup of the LA-D esophagitis population resembles LA-A patients more than hospitalized LA-D patients. Furthermore, since LA-D patients comprised only a small minority (7%) of all the patients with esophagitis in El-Serag’s study, the investigators combined their LA-D patients with LA-C patients to make one “severe esophagitis” group for comparisons. There were almost three-times as many LA-C patients as LA-D patients in that one group, which might have obscured any distinctive features of the LA-D patients.
We found that inpatient hospitalization was the single strongest risk factor for LA-D esophagitis (odds ratio 61 compared to LA-A esophagitis), and 15% of our LA-D patients were in the ICU. Among the 30 patients who had LA-D esophagitis diagnosed during outpatient endoscopies, furthermore, 13 had been hospitalized in the preceding three months. These data suggest that severe, acute illness plays a role in the development of LA-D esophagitis. In contrast, no patient who had LA-A esophagitis diagnosed on an outpatient endoscopy had been hospitalized in the prior three months.
Illness requiring hospitalization, especially ICU treatment, can have physiologic effects that might contribute to esophagitis. For example, Kölbel et al performed 24-hour esophageal manometry in ICU patients treated with sedatives and found that, irrespective of the underlying primary disease process, esophageal motility was significantly impaired to the point that it could affect esophageal acid clearance.9 Impaired esophageal motility is especially likely to result in prolonged esophageal acid exposure in patients who are supine, a position assumed for prolonged periods by hospitalized patients.10 Acute illness also can delay gastric emptying, resulting in gastric distention that predisposes to reflux, and acutely ill patients might be treated with medications that promote reflux.11 Another potential contributor is transient esophageal hypo-perfusion that leads to regional esophageal ischemia, a situation similar to that causing gastric stress ulcers in acutely ill patients.12 Thus, although gastroesophageal reflux and prolonged esophageal exposure to acid and bile might well contribute to the development of LA-D esophagitis in acutely ill patients, the mechanisms involved might not apply to otherwise healthy individuals with GERD. In hospitalized patients, it may be more appropriate to consider LA-D esophagitis a manifestation of acute illness rather than just the far end of the GERD spectrum. Conversely, our subgroup analysis showing that ‘true’ outpatients with LA-D esophagitis resemble LA-A patients more than hospitalized LA-D patients suggests that GERD might be the primary factor contributing to the pathogenesis of outpatient LA-D esophagitis. Thus, the popular notion that LA-D esophagitis merely represents the severe end of the GERD spectrum might be correct only in the small minority of cases found in outpatients.
We found that cardiopulmonary disorders including coronary artery disease, congestive heart failure, and COPD were risk factors for LA-D esophagitis. In contrast, other studies that involved outpatients primarily and that included relatively few patients with LA-D esophagitis found no significant association of erosive esophagitis with coronary artery disease or congestive heart failure, although one study did identify COPD as a risk factor for LA-D esophagitis.13–14 As discussed above, it is likely that differences in study design and patient population underlie the disparities between these reports and ours. COPD exacerbations are often treated with theophylline and beta-2 receptor agonists that might promote gastroesophageal reflux. COPD exacerbations also might cause pressure changes in the chest and abdomen that promote reflux. However, the association between cardiopulmonary disorders and LA-D esophagitis also raises the possibility that alterations in esophageal mucosal blood flow contribute to the pathogenesis of LA-D esophagitis. It is conceivable that this altered blood supply might render the esophagus especially susceptible to injury by refluxed gastric contents.
LA-D esophagitis in hospitalized patients appears to have more in common with a rare condition called acute esophageal necrosis (also known as “black esophagus” or necrotizing esophagitis) than with typical GERD. In acute esophageal necrosis, the distal esophagus appears diffusely blackened, and the black appearance terminates abruptly at the esophago-gastric junction.15 Similar to our patients with LA-D esophagitis, those with acute esophageal necrosis typically have severe comorbid conditions, and the large majority of cases are identified during endoscopies performed for upper gastrointestinal bleeding.15 The pathogenesis of acute esophageal necrosis has been attributed to a combination of factors including tissue hypo-perfusion, gastroesophageal reflux, and diminished esophageal mucosal defenses.15,16 It may be that the black esophagus is just the most severe form of LA-D esophagitis. Rather than thinking of LA-D esophagitis in hospitalized patients as the far end of the GERD spectrum, it might be more appropriate to consider it the beginning of acute esophageal necrosis.
Our study has a number of limitations. It is a retrospective study, limiting our ability to control for confounding factors that might have contributed to differences between patient groups. Study subjects were identified by review of our endoscopy database and, since LA-D is far less common than LA-A esophagitis, a longer timeframe was needed to identify the 100 LA-D patients. This temporal discrepancy between the groups might have resulted in bias. The endoscopies were performed by a number of different endoscopists whose interpretation of the LA grading system might have differed. To minimize this problem, we reviewed available endoscopic photographs and included only patients for whom we could confirm the diagnoses of LA-D and LA-A esophagitis. Nevertheless, we may well have excluded a number of appropriate patients whose diagnosis could not be confirmed. Data on GERD symptoms were obtained by review of the medical record, not through direct patient interview. In addition, our veteran patient population is predominantly male, and our findings might not be applicable to female patients with LA-D esophagitis.
In summary, our study shows that the clinical features of LA-D esophagitis differ significantly from those of LA-A esophagitis. LA-D esophagitis appears to be a disorder primarily of older, non-obese, hospitalized or recently hospitalized patients who often have serious cardiopulmonary co-morbidities and gastrointestinal bleeding, and who usually have no prior history of GERD or of hiatal hernia. In contrast, patients with LA-A esophagitis are generally younger, overweight outpatients, who often have a history of GERD and hiatal hernia but without comorbid conditions. These findings suggest that LA-D esophagitis in hospitalized patients is not merely the far end of the GERD spectrum, and that factors other than typical gastroesophageal reflux contribute to its pathogenesis. Further, prospective studies are needed to clarify the mechanisms underlying our findings.
Supplementary Material
Acknowledgments
This work was supported by Merit Review Award #BX002666 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research Program (SJS), and the National Institutes of Health (R01 DK103598 to RFS and SJS).
Footnotes
VA/US Government disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflicts of Interest and Source of Funding: Stuart J Spechler, M.D. has served as a consultant for Interpace Diagnostics, Ironwood Pharmaceuticals and Takeda Pharmaceuticals. Rhonda F. Souza, M.D. has served as a consultant for Interpace Diagnostics and Ironwood Pharmaceuticals. Anh D. Nguyen, M.D. has no conflicts of interest to declare. Monique N. Shuler, M.S. has no conflicts of interest to declare. Kerry B. Dunbar, M.D., Ph.D. has no conflicts of interest to declare.
References
- 1.Locke GR, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krugmann J, Neumann H, Vieth M, et al. What is the role of endoscopy and oesophageal biopsies in the management of GERD? Best Pract Res ClinGastroenterol. 2013;27:373–85. doi: 10.1016/j.bpg.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Johanson JF. Risk factors for the severity of erosive esophagitis in Helicobacter pylori-negative patients with gastroesophageal reflux disease. Scand J Gastroenterol. 2002;37:899–904. doi: 10.1080/003655202760230847. [DOI] [PubMed] [Google Scholar]
- 6.Lee HL, Eun CS, Lee OY, et al. Association between GERD-related erosive esophagitis and obesity. J ClinGastroenterol. 2008;42:672–5. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Graham DY, Satia JA, et al. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–50. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 8.Chua CS, Lin YM, Yu FC, et al. Metabolic risk factors associated with erosive esophagitis. J GastroenterolHepatol. 2009;24:1375–9. doi: 10.1111/j.1440-1746.2009.05858.x. [DOI] [PubMed] [Google Scholar]
- 9.Kölbel CB, Rippel K, Klar H, et al. Esophageal motility disorders in critically ill patients: a 24-hour manometric study. Intensive Care Med. 2000;26:1421–7. doi: 10.1007/s001340000630. [DOI] [PubMed] [Google Scholar]
- 10.Ribolsi M, Balestrieri P, Emerenziani S, et al. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46–51. doi: 10.1038/ajg.2013.373. [DOI] [PubMed] [Google Scholar]
- 11.Chapman M, Fraser R, Matthew G, et al. Glucose absorption and gastric emptying in critical illness. CritCare. 2009;13:R140. doi: 10.1186/cc8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Labenz J, Jaspersen D, Kulig M, et al. Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am J Gastroenterol. 2004;99:1652–6. doi: 10.1111/j.1572-0241.2004.30390.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen TS, Chang FY. The prevalence and risk factors of reflux esophagitis among adult Chinese population in Taiwan. J ClinGastroenterol. 2007;41:819–22. doi: 10.1097/01.mcg.0000225658.30803.79. [DOI] [PubMed] [Google Scholar]
- 15.Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: new insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60:444–53. doi: 10.1007/s10620-014-3382-1. [DOI] [PubMed] [Google Scholar]
- 16.Gurvits GE, Shapsis A, Lau N, et al. Acute esophageal necrosis: a rare syndrome. J Gastroenterol. 2007;42:29–38. doi: 10.1007/s00535-006-1974-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


