Abstract
There are no Federal Drug Administration approved drugs for the treatment of systemic sclerosis vascular digital ulcers (DU) in the United States, which are thought to be an endstage result of prolonged ischaemia due to severe, prolonged Raynaud’s phenomenon. Most therapeutics for vasodilation used in SSc work different pathways to target the smooth muscle to induce vessel relaxation. Longitudinal studies of vascular function allow insight into the effects of medications used for Raynaud’s phenomenon in the SSc patient population. In this review, we discuss vascular tone, the function of the endothelium in SSc, and provide the rationale for longitudinal studies of vascular function and therapeutics that target the endothelial shear stress in addition to vasodilation for treatment and prevention of DU. This review provides the rationale for vasodilatory medication use for treatment of SSc-related DU and justifies access to non-FDA approved medications for this indication.
Keywords: systemic sclerosis, digital ulcer, vascular function
Introduction
Systemic sclerosis (SSc) is a chronic autoimmune disease with heterogeneous multi-organ microvascular manifestations (vasculopathy) and fibrosis. Among the autoimmune diseases, SSc has a high mortality and morbidity and a lack of effective therapeutic options (1). Immunosuppressive agents that are the standard of care for other rheumatic diseases have little efficacy for SSc, and efforts to treat end-stage vasculopathy, such as ace-inhibitors for scleroderma renal crisis and vasodilators for pulmonary arterial hypertension have made the largest impact on survival in this patient population and are well-established treatments (2). Of note, the aforementioned aspects of end-stage vasculopathy have clear clinical definitions. In contrast, other aspects of SSc-related vasculopathy, such as digital ulcers (DU) have a less clear clinical definition (3–6). Another challenge for SSc-related DU management is there are no Federal Drug Administration (FDA) approved drugs for the treatment, which are thought to be an end-stage result of prolonged ischemia due to severe, prolonged Raynaud’s phenomenon. Our previous work has suggested that a novel and important source of vascular dysfunction is at the endothelial cell level (6, 7), and is accompanied by elevated oxidative stress and attenuated antioxidant capacity (8). We have also found that this endothelial dysfunction (particularly patients with DU) may respond to an endothelial based therapeutic approach (9, 10). In this review, we discuss the rationale for vasodilators in SSc, measurement of therapeutic effectiveness of vascular-based therapeutics with non-invasive vascular imaging (e.g. brachial artery flow mediated dilation [FMD]), and implications of endothelial targeted treatment in SSc.
Vascular tone in systemic sclerosis
The role of enhanced vascular tone in the natural history of SSc is perhaps best demonstrated by the near universal presence of Raynaud’s phenomenon in this patient population. This tri-phasic color change of the fingers in response to cold or stress, usually precedes the development of puffy hands and skin fibrosis, and is commonly associated with microvascular abnormalities on capillaroscopy, making these findings critical for SSc classification (11). Raynaud’s phenomenon is in most cases the first symptom of SSc (12). Repeat episodes of Raynaud’s phenomenon leads to prolonged digital ischemia that may progress to digital ulceration (DU) or in extreme cases to critical digital ischemia with gangrene. While SSc is considered a fibrosing disorder, the role of vascular tone in dysregulated endothelium is unclear. Nonetheless, end stage fibrosis via endothelial to mesenchymal transition (Endo-MT) is gaining traction (13). Endo-MT occurs when the endothelial cells delaminate from the cell monolayer, reduce cell-cell contacts, lose endothelial markers such as vascular endothelial-cadherin (VE-cadherin), gain mesenchymal markers like alphasmooth muscle actin (α-SMA), and acquire mesenchymal cell-like properties. Whether vascular tone promotes this transition is unclear, however, fibrosis occurs at varying rates in different organs. This differential fibrosis highlights that while improvement of vascular function in all SSc patients represents a potential therapeutic goal, the disease duration and degree of organ fibrosis complicates the study and treatment of SSc vasculopathy, particularly in cross-sectional clinical studies. Evaluation of the skin microvasculature in SSc reveals absence of inflammatory cells and presence of features of oxidative stress including, swollen endothelial cells with a duplicated, lamellated appearance of the basement membrane (14–16), and regardless of limited or diffuse cutaneous subsets, disease duration, or internal organ clinical features (16). Endothelial cells are the only mesenchymal cell type that undergo apoptosis in early SSc, whereas vascular smooth-muscle cells and pericytes proliferate vigorously (17, 18). These features suggest that endothelial homeostasis is disrupted, which under conditions of stress, dysregulates the synthesis, degradation and recycling of cellular components. Endothelial cells control vascular tone by vasoactive molecules, of which nitric oxide (NO) produced by endothelial NO synthase is one of the most important for endothelium dependent dilatation (19). NO is the primary vasodilatory molecule released from the vascular endothelium in response to stimulation by agonists, such as catecholamines; platelet products, including serotonin; autacoids formed in or near the vascular wall (including bradykinin and adiponectin), and physical factors at the vessel surface (hemodynamic shear stress) (20). NO enhances vasodilatation, reduces platelet aggression and adhesion (anti-thrombotic), prevents smooth muscle proliferation, inhibits adhesion of leukocytes and expression of pro-inflammatory cytokines genes (anti-inflammatory), and counters the oxidation of low density lipoprotein (LDL) cholesterol (21). Endothelial dysfunction is induced by a shift in the equilibrium that favors NO deficiency and enhanced reactive oxygen species (ROS) formation. Endothelial dysfunction therefore can be influenced by reduced activity of endothelial NO synthase, which is modulated by the calcium concentration and phosphorylation, or failure of NO diffuse to vascular smooth muscle (22). When NO reaches the vascular smooth muscle, it interacts with soluble guanylyl cyclase to produce cyclic guanosine monophosphate (cGMP). Under homeostasis, NO bioavailability is evidenced by an intact, robust endothelium-dependent dilation (23–26) and is, in part, responsible for mediating the angiogenic capacity, peripheral permeability, and anti-inflammatory properties of a healthy vascular endothelium (26, 27). Endothelial dysfunction, characterized by reduced NO and impaired vasodilator capacity, results in diminished peripheral tissue blood flow (28).
Functions of the endothelium in SSc
Glycocalyx
The endothelial glycocalyx is a gel-like, thin polysaccharide layer that projects from the endothelial cell wall toward the vessel lumen, protecting the vessel and playing a role in mechanotransduction of shear stress (29). The glycocalyx coats the healthy vascular endothelium, and modifies the interaction between the blood and vessel wall and helps to prevent adhesion of leukocytes and platelets. The glycocalyx can dictate the migration pattern of immune cells, which protects against inflammation, thrombosis, abnormal perfusion and subsequent fibrosis (29). Leukocyte adhesion and infiltration into the vessel wall is an important part of the tissue inflammatory process that leads to oxidative stress and the augmented production of free radicals. As such, penetration into the glycocalyx and the perfused barrier region (PBR) is important in vascular health, particularly where immune cell extravasation is involved. Our previous work using intravital microscopy has demonstrated that mean PBR across all microvessel segments was significantly higher in patients with SSc compared with healthy age-matched controls (2.1±0.0 vs. 1.9±0.0 μm, respectively; p=0.012). We found glycocalyx thickness was significantly lower in patients with SSc compared with controls (p<0.001), with PBR was significantly, inversely associated other measures of glycocalyx thickness (r=−0.41, p=0.003). This implicates that endothelial dysfunction is not only associated with enhanced vascular tone, but also blunted glycocalyx, which could allow for greater immune cell adhesion and infiltration into critical tissues to promote fibrosis.
Leukocyte adhesion/infiltration
While SSc vasculopathy is not classically inflammatory, increased infiltration of immune cells in the perivascular tissue is implicated in the pathogenesis of SSc (30–32). Immune cells are increased in peripheral tissues of patients with SSc (33) and likely result from greater adhesion of immune cells to the vascular endothelium (34). While a healthy endothelium can serve as a barrier to the movement of immune cells from the circulation into tissues, the unhealthy endothelium in SSc can augment the immune dysregulatory process (16), thereby stimulating greater infiltration of immune cells into the peripheral tissues. Oxidative stress also increases vascular endothelial permeability, which is coupled with alterations in endothelial cell signal transduction.
Oxidative stress
Transforming growth factor-β signaling, which is widely considered one of the most important pro-fibrotic factors in SSc, causes a pro-oxidant shift in redox homeostasis and a concomitant decrease in nitric oxide (NO) signaling (35). Oxidative stress, defined as an excess production of free radicals relative to antioxidant defenses, has been documented in SSc (36). Serum and urinary markers of systemic oxidative stress are greater in SSc compared with healthy age matched controls (37–40). The functional consequences of oxidative stress are widespread, but the vascular endothelium is particularly vulnerable to oxidative damage from ROS (41). NO produced by the endothelium reacts with superoxide to form the ROS peroxynitrite (ONOO−), (42) resulting in reduced NO available to signal vasodilatation. ROS production, including superoxide and ONOO− formation, is increased in the circulation and skin of patients with SSc (43, 44). Thus, oxidative stress is implicated as a major contributor to the reduced NO bioavailability and endothelial dysfunction, and leads to the deleterious endothelial phenotype characterized by enhanced permeability, reduced peripheral blood flow, increased immune cell adhesion and infiltration, and increased local vascular inflammation (41, 42). ROS are also considered transducers of fibroblast proliferation, collagen-gene expression, and myofibroblast phenotype conversion in SSc, which leads to pathological fibrosis (45). However, independent of markers of oxidative stress, there is still evidence of universal endothelial dysfunction in SSc (8), which may reflect the importance of disease duration and vascular adaption.
Angiogenesis
Angiogenesis, i.e., new vessel growth, is required for the appropriate expansion of the tissue during growth or in times of sustained or frequent tissue hypoxia (46, 47). The angiopoietin(Ang)/Tie2 system is a key regulator of vascular biology and has been reported as an important aspect of SSc vasculopathy (48). A dysregulation of membrane bound (mb) Tie2 and Ang-1, which ensures vessel stability, and Ang-2, which is inducible by vascular endothelial growth factor (VEGF), inflammation, and hypoxia is proposed (48). The role of shear-stress on this dysregulation is unclear, however, there is clear clinical evidence of a decrease of new blood vessel growth in SSc, despite elevation of potent angiogenic growth factors (49). In healthy tissue, hypoxic stress stimulates the pro-angiogenic transcription factor hypoxia inducible factor 1α (HIF1α), leading to increased angiogenic factors, such as VEGF (50) and subsequent angiogenesis. SSc is thought to induce an hypoxic environment in tissues, which increases HIF1α (51) and VEGF (52–54), but subsequent angiogenesis is blunted because of enhanced angiogenic inhibitors such as endostatin (55). While initially VEGF may be of benefit in reducing damage to small blood vessels, chronic overexpression may be deleterious and result in overproduction of angiostatic molecules including soluble VEGF receptors, endostatin, angiostatin, and soluble endoglin (49). Interestingly, higher levels of endostatin is associated with reduced eNOS activation (56), reduced NO production and apoptosis (57). Furthermore, the angiogenic actions of VEGF signaling are dependent on a functional vascular endothelium and presence of NO (58). Increased serum levels of endothelin-1 (ET-1), asymmetric dimethylarginine (ADMA, which induces oxidative stress), and VEGF are strong predictors of DU (59).
Vascular biomarkers
While vascular biomarkers of progressive vascular injury are reported in SSc (60), the ideal vascular biomarkers can measures pathways fundamental to disease pathogenesis, predicts future development of relevant outcomes, is easily measurable, and changes with effective therapy (49). Autoantibodies are reported at the first diagnosis in more than 95% of SSc patients and have been associated with distinct vascular disease subtypes and with differences in disease severity, and as such, have prognostic value for DU (61). In SSc, where compensatory angiogenesis does not occur normally in spite of an important increase in many angiogenic factors, a clearer understanding of the role of endothelial progenitors (EPCs) homing ability to a site of ischemia to contribute to de novo vessel formation is critical to understanding pathogenesis (62). While significant advances have been made in understanding the biology of EPCs and molecular mechanisms regulating EPC function, the detailed events that contribute to shear stress-induced protection in EPCs, particularly the mechanisms of signal transduction to repair injured vascular endothelial cells are insufficiently understood (63). In addition, to laboratory vascular biomarkers, clinical vascular biomarkers such as nailfold capillaroscopy (NC) provide evidence that progressive vascular abnormalities (particularly capillary loss) are associated with disease severity (such as DU), however, the role of this important vascular biomarker in the interpretation of progressive vascular shear stress is not clear (64).
In summary, while vascular biomarkers (laboratory and clinical) exist in SSc, it is critical to longitudinally characterize the dysfunctional endothelium in SSc patients. SSc endothelium is characterized by a reduced glycocalyx, impaired vasodilator capacity due to reduced NO bioavailability, and abnormal angiogenesis response, perhaps due to oxidative stress. Targeting SSc vascular pathology may require additive therapeutic approaches, which include reduction of vascular damage and loss of capillaries not compensated by new vessel formation, correction of the imbalance of vasoconstrictive/vasodilatory factors, and reduction of proliferative vasculopathy characterized by prominent intimal proliferation (65). The accurate measurement of endothelial function in response to these therapeutics is critical, particularly with regards to DU treatment.
Measuring endothelial function in systemic sclerosis by flow-mediated dilation
The noninvasive study of the natural history of SSc endothelial function can be assessed noninvasively in humans using duplex ultrasound and techniques related to the brachial artery the flowmediated dilation (FMD) technique, which has historically been used to measure subclinical atherosclerosis (7, 66–70). FMD is an indirect measure of endothelial function. This approach involves inflating a cuff on a limb (typically the upper forearm) to a supra-systolic external pressure for several minutes and measuring change in diameter and blood flow in a segment of an artery (typically the brachial artery) proximal to the occlusion following rapid deflation of the cuff. The ischaemia-evoked dilation of resistance vessels distal to the occlusion produces a marked temporary increase in blood flow (reactive hyperemia, RH) in the proximal conduit arteries that can be quantified and, in turn, causes dilation (FMD) of those proximal conduit arteries. Thus, this procedure not only assesses the ability of peripheral conduit arteries to dilate in response to the physiological stimulus of increases in intravascular shear, but also the vasodilatory ability of the peripheral resistance arteries to a brief bout of ischemia. Thus, utilizing duplex ultrasound and established FMD protocols provides information on endothelial function (brachial artery flow mediated dilation), perfusion (resting forearm blood flow), and vasodilator ability (reactive hyperemia) and has been demonstrated as a potential early clinical marker of DU risk in RP patients and in SSc patients by our group and others (7, 67). Of note FMD, is different from peripheral endothelial function measured by forearm blood dilatation response to brachial artery occlusion using noninvasive plethysmography in that FMD assesses shear rate. Nonetheless, noninvasive plethysmography provides evidence of complex pathological progression of SSc vasculopathy (71).
FMD is a procedure that requires subject preparation and standardization (7), and is valuable as a proof-of-concept procedure for identifying aspects of endothelial dysfunction that may be valuable for future research. In our SSc cohort, we have examined all the aforementioned FMD variables at the time of routine care, in order to determine which features are most helpful for understanding DU. Of the 123 SSc patients with baseline FMD, 70 had at least two standardized FMD measurements with clinical characteristics available at the time of the assessment (Table I) as previously described by our group (7). Among these patients, DU was present at baseline in 22 and 10 developed a new DU in up to 56 months of follow-up. The timeline between FMD measures for complete healing for initial DU patients ranged from 3.9 to 24.9 months (mean 6.7 months). Of the 10 patients that developed a new DU at the time of a repeat FMD measure: 2 patients developed one within 6 months; 2 between 6 and 12 months; and 3 over one year after initial measurement. We examined serial FMD values by whether a patient had ever had a DU, and adjusted the analysis for vasodilator use, days between measurements, SSc duration, and age (Table II). We found we found lower baseline flow in those with DU than those without DU (p=0.01). When we examined differences between FMD measures over time between those with and without DU, we found significantly lower change in baseline flow (p=0.002) and change in shear rate among those with DU than without DU (p<0.001). Thus, in this early analysis, the implications of FMD applied to routine clinical care of SSc patients support that vasodilators are acting on vascular smooth muscle to improve blood flow (perfusion) to digits and perhaps reduce DU. The identification of vascular shear rate as an important variable in DU occurrence implies that therapeutics that effect shear at the vessel wall may be an important future target.
Table I.
Clinical features of systemic sclerosis patients with flow mediated dilation.
| Patient characteristics (n=70) | Total number or mean |
|---|---|
| Limited cutaneous SSc | 49 |
| Diffuse cutaneous SSc | 21 |
| Female | 61 |
| Male | 9 |
| White | 58 |
| Hispanic | S |
| Other race | 4 |
| Systolic blood pressure (mmHg) | 114.6 ± 15 |
| Systolic blood pressure (mmHg) | 70 ± 9 |
| Mean arterial pressure (mmHg) | 85 ± 9 |
| Heart rate (beats/min) | 76 ± 1 |
| Duration of SSc at first FMD visit | 10 ± 9.8 |
| (years) | |
| Smoking | |
| Never | 59 |
| Former | 8 |
| Current | 3 |
| Age | 55.4 ± 11.8 |
| Calcium channel blocker | 68 |
| Ace inhibitor | 1 |
| Angiotensin receptor blocker | 2 |
| Prostacyclin analog | 1 |
| Endothelin receptor agonist | 0 |
| Phosphodiesterase 5 inhibitor | 1 |
| Anti-centromere | 58 |
| Anti-topoi somerase | 9 |
| Anti-RNA polymerase III | 3 |
| Anti-Th/To | 1 |
Table II.
Flow mediated dilation in systemic sclerosis patients stratified by presence of digital ulcerations.
| Vascular function variables in 70 SSc patients at follow-up FMD | Difference between SSc patients with DU (n=32) and those without DU (n=38) | p value |
|---|---|---|
| Baseline flow | −17.2 ± 6.9 | 0.01 |
| Absolute FMD | −.03 ± .04 | 0.50 |
| Normalised absolute FMD | 0.00007 ± .00006 | 0.25 |
| Relative FMD | −0.7 ± 1.3 | 0.58 |
| Normalised relative FMD | −0.002 ± 0.002 | 0.33 |
| Shear Rate | −5013 ± 3238 | 0.12 |
| Peak Hyperemia | 31.0 ± 36 | 0.39 |
| Vascular resistance | 0.57 ± 0.34 | 0.09 |
| Change in Baseline flow* | −40.8 ± 12.9 | 0.002 |
| Normalised Absolute FMD change* | −0.00007 | .30 |
| Absolute FMD change* | −0.002 ± 0.05 | 0.96 |
| Relative FMD change* | −0.3 ± 1.53 | 0.84 |
| Normalised Relative FMD change* | −0.002 ± .002 | 0.3 |
| FMD Shear Rate change* | −14374 ± 4037 | 0.0001 |
| FMD Peak Hyperaemia change* | −33.8 ± 37.7 | 0.36 |
| Normalised FMD change* | −0.00002 ± 0.00004 | 0.57 |
Change between measures at baseline FMD adjusted for days between measures and duration of SSc.
Shear stress effect on the endothelium
Vessel wall shear stress induces biologic effects in endothelial cells that can affect the crucial balance between matrix synthesis and breakdown (72). Specifically, high laminar shear stress stimulates ECs to produce NO that might suppress synthetic smoothmuscle proliferation (matrix synthesis) (73). Pertinent to the SSc population, the distribution of laminar shear stress can be significantly affected by disrupted blood flow as well as the velocity of flow in vessels with abnormal shapes (74). Importantly, abnormal laminar and oscillatory shear stress can induce pro-inflammatory/matrixremodeling genes levels, contributing to vascular smooth muscle cells phenotypic switching from a contractile to a synthetic phenotype, and can markedly induce autophagy (75). Impaired endothelial cell autophagy in SSc (18) can further compromise compensatory shear stress-induced NO generation (76). Thus, life-style interventions and therapeutics that can elevate the endogenous endothelial repair response to vascular injury through modulation of vessel shear stress with improved endothelial function have potential value for SSc vasculopathy.
Therapeutics that effect vessel shear stress and improve endothelial cell function
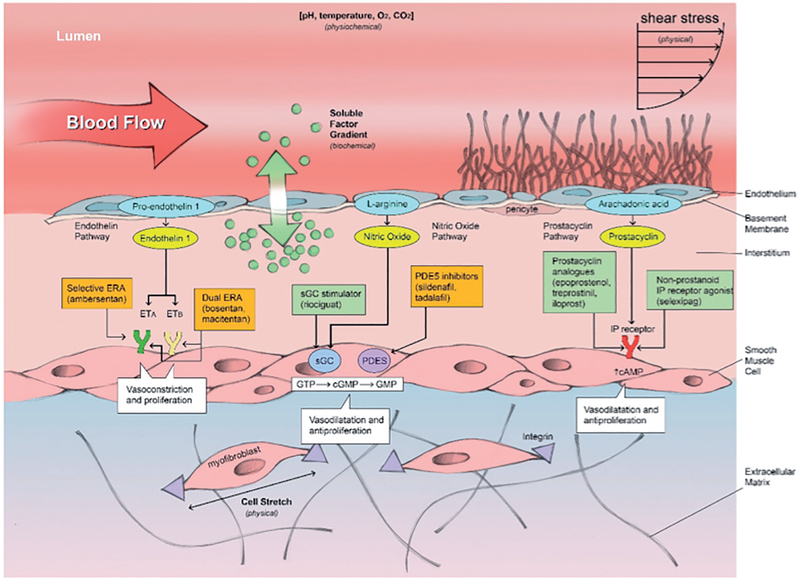
Most therapeutics for vasodilation used in SSc work different pathways to target the smooth muscle to induce relaxation. Dihydropyridine-type calcium antagonist (such as, nifedipine, felodipine, and amlodipine) inhibit influx of calcium across the smooth muscle membrane to prevent vessel contraction. Endothelin receptor antagonists can be selective (ambrisentan) or dual (bosentan, macitentan) and work through the endothelin pathway. The prostacyclin pathway, which includes prostacyclin analogs (epoprostenol, treprostenol, iloprost) and non-prostanoid IP receptor agonist (selixipag), work through the arachindonic acid pathway. Phosphodiesterase type 5 inhibitors (PDE5I) (including sildenafil and tadalafil), and soluble guanylate cyclase stimulator (riociguat) act through the nitric oxide signaling pathway to induce smooth muscle vasodilation. Soluble guanylate cyclase stimulators might target both vascular remodeling and tissue fibrosis (77). The use of these classes of medications is supported by data, but access to vasodilator therapeutics is often limited by cost (78). Of note, these medications primarily work at the smooth muscle level and do not target endothelial function (Fig. 1).
Fig. 1.
Therapeutics targeting vasodilatation in systemic sclerosis.
To target endothelial function, the glycocalyx and activity of nitric oxide synthase may be important targets. While pathological shedding of the glycocalyx in response to mechanical shear stress, inflammatory mediators, endotoxins, ischemia-reperfusion injury, and free radicals is recognized (79), the ability of therapeutics to affect the glycocalyx has been inadequately studied, but warrants attention in SSc. Tetrahydrobiopterin (BH4) is an essential cofactor for nitric oxide synthase, and inadequate BH4 leads to uncoupling of nitric oxide synthase and production of highly oxidative radicals. Importantly, the guanosine triphosphate cyclohydrolase/tetrahydrobiopterin (GTPCH)/(BH4) pathway has been proved to regulate the function of endothelial progenitor cells (EPCs) in response to vessel shear stress (80).
Numerous human studies have reported the beneficial effect of BH4 supplementation on endothelial dysfunction caused by a variety of vascular diseases, including hypercholesterolemia (81), diabetes (82), hypertension (83), chronic heart failure (84), and tobacco use (85). Acute oral BH4 administration improves vascular phenotypes in patients with cardiovascular disease as well as healthy older adults.(82, 85–87). In these studies adverse effects were mild, occurred in less than 5% of participants, and included headache, runny nose, nasal congestion, and sore throat. Important for SSc, BH4 has been studied in animal models of pulmonary hypertension (88) and renal ischemia/reperfusion injury in an animal model of aortic cross-clamping (89). Mechanistically, BH4 has shown a positive effect on in vivo endothelial repair capacity of early EPC in hypertensive hyperaldosteronism patients (90). Oral BH4 supplementation for 4 days was sufficient to improve endothelium dependent dilation measured by FMD in patients with hypercholesterolemia (91). We have recently shown that acute administration of oral BH4 improves endothelial function in the brachial artery in patients with SSc who had a history of DU and can be used safely with other vasodilators (9). BH4 has not been studied in other rheumatic diseases.
Conclusions
Understanding SSc-related vasculopathy requires longitudinal studies of endothelial function which capture vascular shear stress in the context of multi-organ disease severity. There is rationale for vasodilatory medication use for treatment of SSc-related DU and patients should have access to non-FDA approved medications for this indication. The importance of inadequate endothelial cell response to shear stress and development of DU highlights the value of longitudinal functional studies such as FMD in studies of DU. Targets that have been inadequately studied but may influence shear stress include the glycocalyx and BH4. Importantly, endothelial targeted treatments in SSc can help all patients with Raynaud’s phenomenon, and can safely supplement vasodilator therapeutics for DU providing a sound rationale for further study.
Fundings:
The research reported in this publication was supported by the National Institute of Arthritis And Musculoskeletal And Skin Diseases of the National Institutes of Health under Award Number K23AR067889. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project described was supported by Award Number I01CX001183 from the Clinical Science Research & Development Service of the VA Office of Research and Development.
Footnotes
Competing interests: none declared.
References
- 1.RUBIO-RIVAS M, ROYO C, SIMEON CP, CORBELLA X, FONOLLOSA V: Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014; 44: 208–19. [DOI] [PubMed] [Google Scholar]
- 2.KOWAL-BIELECKA O, LANDEWÉ R, AVOUAC J et al. : EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis 2009; 68: 620–8. [DOI] [PubMed] [Google Scholar]
- 3.MULLER-LADNER U, DISTLER O, IBBAMANNESCHI L, NEUMANN E, GAY S: Mechanisms of vascular damage in systemic sclerosis. Autoimmunity 2009; 42: 587–95. [DOI] [PubMed] [Google Scholar]
- 4.BARON M, POPE J, ROBINSON D et al. : Calcinosis is associated with digital ischaemia in systemic sclerosis-a longitudinal study. Rheumatology (Oxford). 2016; 55: 2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SULIMAN YABC, JOHNSON SR, PRAINO E et al. : Defining skin ulcers in systemic sclerosis: systematic literature review and proposed World Scleroderma Foundation (WSF) definition. J Scleroderma Relat Disord 2017; 2: 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MACHIN DR, GATES PE, VINK H, FRECH TM, DONATO AJ: Automated Measurement of Microvascular Function Reveals Dysfunction in Systemic Sclerosis: A Cross-sectional Study. J Rheumatol 2017; 44: 1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FRECH T, WALKER AE, BARRETT-O’KEEFE Z et al. : Systemic sclerosis induces pronounced peripheral vascular dysfunction characterized by blunted peripheral vasoreactivity and endothelial dysfunction. Clin Rheumatol 2015; 34: 905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MACHIN DR, CLIFTON HL, GARTEN RS et al. : Exercise-induced brachial artery blood flow and vascular function is impaired in systemic sclerosis. Am J Physiol Heart Circ Physiol 2016; 311: H1375–H81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MACHIN DR, CLIFTON HL, RICHARDSON RS, WRAY DW, DONATO AJ, FRECH TM: Acute oral tetrahydrobiopterin administration ameliorates endothelial dysfunction in systemic sclerosis. Clin Exp Rheumatol 2017; 35 (Suppl. 106): S167–72. [PMC free article] [PubMed] [Google Scholar]
- 10.SILVA I, ALMEIDA J, VASCONCELOS C: A PRISMA-driven systematic review for predictive risk factors of digital ulcers in systemic sclerosis patients. Autoimmun Rev 2015; 14: 140–52. [DOI] [PubMed] [Google Scholar]
- 11.VAN DEN HOOGEN F, KHANNA D, FRANSEN J et al. : 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65: 2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BELLANDO-RANDONE S, MATUCCI-CERINIC M: Very Early Systemic Sclerosis and Pre-systemic Sclerosis: Definition, Recognition, Clinical Relevance and Future Directions. Curr Rheumatol Rep 2017; 19: 65. [DOI] [PubMed] [Google Scholar]
- 13.PIERA-VELAZQUEZ S, LI Z, JIMENEZ SA: Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 2011; 179: 1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PRESCOTT RJ, FREEMONT AJ, JONES CJ, HOYLAND J, FIELDING P: Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol 1992; 166: 255–63. [DOI] [PubMed] [Google Scholar]
- 15.NORTON WL, HURD ER, LEWIS DC, ZIFF M: Evidence of microvascular injury in scleroderma and systemic lupus erythematosus: quantitative study of the microvascular bed. J Lab Clin Med 1968; 71: 919–33. [PubMed] [Google Scholar]
- 16.FRECH TM, REVELO MP, DRAKOS SG et al. : Vascular leak is a central feature in the pathogenesis of systemic sclerosis. J Rheumatol 2012; 39: 1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GABRIELLI A, AVVEDIMENTO EV, KRIEG T: Scleroderma. N Engl J Med 2009; 360: 19892003. [DOI] [PubMed] [Google Scholar]
- 18.FRECH T, DE DOMENICO I, MURTAUGH MA et al. : Autophagy is a key feature in the pathogenesis of systemic sclerosis. Rheumatol Int 2014; 34: 435–9. [DOI] [PubMed] [Google Scholar]
- 19.VANHOUTTE PM, ZHAO Y, XU A, LEUNG SW: THIRTY YEARS OF SAYING NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ Res 2016; 119: 375–96. [DOI] [PubMed] [Google Scholar]
- 20.SMITS P, WILLIAMS SB, LIPSON DE, BANITT P, RONGEN GA, CREAGER MA: Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation 1995; 92: 2135–41. [DOI] [PubMed] [Google Scholar]
- 21.YUYUN MF, NG LL, NG GA: Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc Res 2018; 119: 7–12. [DOI] [PubMed] [Google Scholar]
- 22.FORSTERMANN U, CLOSS EI, POLLOCK JS et al. : Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994; 23: 1121–31. [DOI] [PubMed] [Google Scholar]
- 23.COHEN RA, PLANE F, NAJIBI S, HUK I, MALINSKI T, GARLAND CJ: Nitric oxide is the mediator of both endothelium-dependent relaxation and hyperpolarization of the rabbit carotid artery. Proc Natl Acad Sci USA 1997; 94: 4193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PERSSON MG, GUSTAFSSON LE, WIKLUND NP, HEDQVIST P, MONCADA S: Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. Br J Pharmacol 1990; 100: 463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MONCADA S, PALMER RM, HIGGS EA: Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991; 43: 109–42. [PubMed] [Google Scholar]
- 26.LUSCHER TF, BARTON M: Biology of the endothelium. Clin Cardiol 1997; 20 (Suppl. 2): II-3–10. [PubMed] [Google Scholar]
- 27.MONCADA S: The L-arginine: nitric oxide pathway, cellular transduction and immunological roles. Adv Second Messenger Phosphoprotein Res 1993; 28: 97–9. [PubMed] [Google Scholar]
- 28.GARDINER SM, COMPTON AM, BENNETT T, PALMER RM, MONCADA S: Control of regional blood flow by endothelium-derived nitric oxide. Hypertension 1990; 15: 486–92. [DOI] [PubMed] [Google Scholar]
- 29.LEE DH, DANE MJ, VAN DEN BERG BM et al. : Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One 2014; 9: e96477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.KRÄLING BM, MAUL GG, JIMENEZ SA: Mononuclear Cellular Infiltrates in Clinically Involved Skin from Patients with Systemic Sclerosis of Recent Onset Predominantly Consist of Monocytes/Macrophages. Pathobiology 1995; 63: 48–56. [DOI] [PubMed] [Google Scholar]
- 31.LIU X, GAO N, LI M et al. : Elevated levels of CD4(+)CD25(+)FoxP3(+) T cells in systemic sclerosis patients contribute to the secretion of IL-17 and immunosuppression dysfunction. PLoS One 2013; 8: e64531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BREMBILLA NC, CHIZZOLINI C: T cell abnormalities in systemic sclerosis with a focus on Th17 cells. Eur Cytokine Netw 2012; 23: 128–39. [DOI] [PubMed] [Google Scholar]
- 33.ISHIKAWA O, ISHIKAWA H: Macrophage infiltration in the skin of patients with systemic sclerosis. J Rheumatol 1992; 19: 1202–6. [PubMed] [Google Scholar]
- 34.CARVALHO D, SAVAGE C, BLACK CM, PEARSON JD: IgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines. J Clin Invest 1996; 97: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LAFYATIS R: Transforming growth factor beta--at the centre of systemic sclerosis. Nat Rev Rheumatol 2014; 10: 706–19. [DOI] [PubMed] [Google Scholar]
- 36.GABRIELLI A, SVEGLIATI S, MORONCINI G, AMICO D: New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol J 2012; 6: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.OGAWA F, SHIMIZU K, MUROI E et al. : Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology (Oxford) 2006; 45: 815–8. [DOI] [PubMed] [Google Scholar]
- 38.SHIMIZU K, OGAWA F, AKIYAMA Y et al. : Increased serum levels of N(epsilon)(hexanoyl)lysine, a new marker of oxidative stress, in systemic sclerosis. J Rheumatol 2008; 35: 2214–9. [DOI] [PubMed] [Google Scholar]
- 39.STEIN CM, TANNER SB, AWAD JA, ROBERTS LJ 2ND, MORROW JD: Evidence of free radical-mediated injury (isoprostane overproduction) in scleroderma. Arthritis Rheum 1996; 39: 1146–50. [DOI] [PubMed] [Google Scholar]
- 40.CRACOWSKI JL, CARPENTIER PH, IMBERT B et al. : Increased urinary F2-isoprostanes in systemic sclerosis, but not in primary Raynaud’s phenomenon: effect of cold exposure. Arthritis Rheum 2002; 46: 1319–23. [DOI] [PubMed] [Google Scholar]
- 41.CAI H, HARRISON DG: Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840–4. [DOI] [PubMed] [Google Scholar]
- 42.WIDLANSKY ME, GOKCE N, KEANEY JF,JR, VITA JA: The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003; 42: 1149–60. [DOI] [PubMed] [Google Scholar]
- 43.SAMBO P, JANNINO L, CANDELA M et al. : Monocytes of patients wiht systemic sclerosis (scleroderma spontaneously release in vitro increased amounts of superoxide anion. J Invest Dermatol 1999; 112: 78–84. [DOI] [PubMed] [Google Scholar]
- 44.SAMBO P, BARONI SS, LUCHETTI M et al. : Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum 2001; 44: 2653–64. [DOI] [PubMed] [Google Scholar]
- 45.HECKER L, VITTAL R, JONES T et al. : NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 2009; 15: 1077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MANALO DJ, ROWAN A, LAVOIE T et al. : Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005; 105: 659–69. [DOI] [PubMed] [Google Scholar]
- 47.CARMELIET P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000; 6: 389–95. [DOI] [PubMed] [Google Scholar]
- 48.MORITZ F, SCHNIERING J, DISTLER JHW et al. : Tie2 as a novel key factor of microangiopathy in systemic sclerosis. Arthritis Res Ther 2017; 19: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HUMMERS LK: The current state of biomarkers in systemic sclerosis. Curr Rheumatol Rep 2010; 12: 34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DISTLER O, DISTLER JH, SCHEID A et al. : Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res 2004; 95: 109–16. [DOI] [PubMed] [Google Scholar]
- 51.HONG KH, YOO SA, KANG SS, CHOI JJ, KIM WU, CHO CS: Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol 2006; 146: 362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IOANNOU M, PYRPASOPOULOU A, SIMOS G et al. : Upregulation of VEGF expression is associated with accumulation of HIF-1alpha in the skin of naive scleroderma patients. Mod Rheumatol 2013; 23: 1245–8. [DOI] [PubMed] [Google Scholar]
- 53.CHOI JJ, MIN DJ, CHO ML et al. : Elevated vascular endothelial growth factor in systemic sclerosis. J Rheumatol 2003; 30: 1529–33. [PubMed] [Google Scholar]
- 54.JINNIN M, MAKINO T, KAJIHARA I et al. : Serum levels of soluble vascular endothelial growth factor receptor-2 in patients with systemic sclerosis. Br J Dermatol 2010; 162: 751–8. [DOI] [PubMed] [Google Scholar]
- 55.HEBBAR M, PEYRAT JP, HORNEZ L, HATRON PY, HACHULLA E, DEVULDER B: Increased concentrations of the circulating angiogenesis inhibitor endostatin in patients with systemic sclerosis. Arthritis Rheum 2000; 43: 889–93. [DOI] [PubMed] [Google Scholar]
- 56.URBICH C, REISSNER A, CHAVAKIS E et al. : Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J 2002; 16: 706–8. [DOI] [PubMed] [Google Scholar]
- 57.DHANABAL M, RAMCHANDRAN R, WATERMAN MJF et al. : Endostatin Induces Endothelial Cell Apoptosis. J Biol Chem 1999; 274: 11721–6. [DOI] [PubMed] [Google Scholar]
- 58.PAPAPETROPOULOS A, GARCÍA-CARDEÑA G, MADRI JA, SESSA WC: Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997; 100: 3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.SILVA I, TEIXEIRA A, OLIVEIRA J, ALMEIDA I, ALMEIDA R, VASCONCELOS C: Predictive value of vascular disease biomarkers for digital ulcers in systemic sclerosis patients. Clin Exp Rheumatol 2015; 33 (Suppl. 91): S127–30. [PubMed] [Google Scholar]
- 60.CHORA I, GUIDUCCI S, MANETTI M et al. : Vascular biomarkers and correlation with peripheral vasculopathy in systemic sclerosis. Autoimmun Rev 2015; 14: 314–22. [DOI] [PubMed] [Google Scholar]
- 61.AFFANDI AJ, RADSTAKE TR, MARUT W: Update on biomarkers in systemic sclerosis: tools for diagnosis and treatment. Semin Immunopathol 2015; 37: 475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DEL PAPA N, PIGNATARO F: The Role of Endothelial Progenitors in the Repair of Vascular Damage in Systemic Sclerosis. Front Immunol 2018; 9: 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.CUI X, ZHANG X, BU H et al. : Shear stressmediated changes in the expression of complement regulatory protein CD59 on human endothelial progenitor cells by ECM-integrinalphaVbeta3-F-actin pathway in vitro. Biochem Biophys Res Commun 2017; 494: 416–21. [DOI] [PubMed] [Google Scholar]
- 64.PAXTON D, PAULING JD: Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum 2018. [DOI] [PubMed] [Google Scholar]
- 65.GUIDUCCI S, DISTLER O, DISTLER JH, MATUCCI-CERINIC M: Mechanisms of vascular damage in SSc--implications for vascular treatment strategies. Rheumatology (Oxford) 2008; 47 (Suppl. 5): v18–20. [DOI] [PubMed] [Google Scholar]
- 66.HARRIS RA, NISHIYAMA SK, WRAY DW, RICHARDSON RS: Ultrasound assessment of flow-mediated dilation. Hypertension 2010; 55: 1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.SILVA I, LOUREIRO T, TEIXEIRA A et al. : Digital ulcers in systemic sclerosis: role of flow-mediated dilatation and capillaroscopy as risk assessment tools. Eur J Dermatol 2015; 25: 444–51. [DOI] [PubMed] [Google Scholar]
- 68.TAKAHASHI T, ASANO Y, AMIYA E et al. : Clinical correlation of brachial artery flowmediated dilation in patients with systemic sclerosis. Mod Rheumatol 2014; 24: 106–11. [DOI] [PubMed] [Google Scholar]
- 69.ROLLANDO D, BEZANTE GP, SULLI A et al. : Brachial artery endothelial-dependent flowmediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 2010; 37: 1168–73. [DOI] [PubMed] [Google Scholar]
- 70.DOMSIC RT, DEZFULIAN C, SHOUSHTARI A et al. : Endothelial dysfunction is present only in the microvasculature and microcirculation of early diffuse systemic sclerosis patients. Clin Exp Rheumatol 2014; 32 (Suppl. 86): S-154–60. [PMC free article] [PubMed] [Google Scholar]
- 71.KAWASHIRI SY, NISHINO A, IGAWA T et al. : Prediction of organ involvement in systemic sclerosis by serum biomarkers and peripheral endothelial function. Clin Exp Rheumatol 2018; 36 (Suppl. 113): SYY–YY. [PubMed] [Google Scholar]
- 72.KWAK BR, BACK M, BOCHATON-PIALLAT ML et al. : Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 2014; 35: 3013–20, 20a20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.GIJSEN FJ, WENTZEL JJ, THURY A et al. : Strain distribution over plaques in human coronary arteries relates to shear stress. Am J Physiol Heart Circ Physiol 2008; 295: H1608–14. [DOI] [PubMed] [Google Scholar]
- 74.HONG H, YEOM E, JI HS, KIM HD, KIM KC: Characteristics of pulsatile flows in curved stenosed channels. PLoS One 2017; 12: e0186300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.SUN L, ZHAO M, LIU A et al. : Shear Stress Induces Phenotypic Modulation of Vascular Smooth Muscle Cells via AMPK/mTOR/ULK1-Mediated Autophagy. Cell Mol Neurobiol 2018; 38: 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.BHARATH LP, CHO JM, PARK SK et al. : Endothelial Cell Autophagy Maintains Shear Stress-Induced Nitric Oxide Generation via Glycolysis-Dependent Purinergic Signaling to Endothelial Nitric Oxide Synthase. Arterioscler Thromb Vasc Biol 2017; 37: 1646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DISTLER O, COZZIO A: Systemic sclerosis and localized scleroderma--current concepts and novel targets for therapy. Semin Immunopathol 2016; 38: 87–95. [DOI] [PubMed] [Google Scholar]
- 78.KOWAL-BIELECKA O, FRANSEN J, AVOUAC J et al. : Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017; 76: 1327–39. [DOI] [PubMed] [Google Scholar]
- 79.PILLINGER NL, KAM P: Endothelial glycocalyx: basic science and clinical implications. Anaesth Intensive Care 2017; 45: 295–307. [DOI] [PubMed] [Google Scholar]
- 80.BAI YP, XIAO S, TANG YB et al. : Shear stress-mediated upregulation of GTP cyclohydrolase/tetrahydrobiopterin pathway ameliorates hypertension-related decline in reendothelialization capacity of endothelial progenitor cells. J Hypertens 2017; 35: 784–97. [DOI] [PubMed] [Google Scholar]
- 81.STROES E, KASTELEIN J, COSENTINO F et al. : Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest 1997; 99: 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.HEITZER T, BROCKHOFF C, MAYER B et al. : Tetrahydrobiopterin improves endotheliumdependent vasodilation in chronic smokers : evidence for a dysfunctional nitric oxide synthase. Circ Res 2000; 86: E36–41. [DOI] [PubMed] [Google Scholar]
- 83.HIGASHI Y, SASAKI S, NAKAGAWA K et al. : Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens 2002; 15: 326–32. [DOI] [PubMed] [Google Scholar]
- 84.SETOGUCHI S, HIROOKA Y, ESHIMA K, SHIMOKAWA H, TAKESHITA A: Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J Cardiovasc Pharmacol 2002; 39: 363–8. [DOI] [PubMed] [Google Scholar]
- 85.HEITZER T, KROHN K, ALBERS S, MEINERTZ T: Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 2000; 43: 1435–8. [DOI] [PubMed] [Google Scholar]
- 86.MAIER W, COSENTINO F, LUTOLF RB et al. : Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol 2000; 35: 173–8. [DOI] [PubMed] [Google Scholar]
- 87.ESKURZA I, MYERBURGH LA, KAHN ZD, SEALS DR: Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 2005; 568: 1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.FRANCIS BN, SALAMEH M, KHAMISY-FAR-AH R, FARAH R: Tetrahydrobiopterin (BH4): Targeting endothelial nitric oxide synthase as a potential therapy for pulmonary hypertension. Cardiovasc Ther 2018; 36(1). [DOI] [PubMed] [Google Scholar]
- 89.RAHMANIA L, ORBEGOZO D, SU F, TACCONE FS, VINCENT JL, DE BACKER D: Administration of Tetrahydrobiopterin (BH4) Protects the Renal Microcirculation From Ischemia and Reperfusion Injury. Anesth Analg 2017; 125: 1253–60. [DOI] [PubMed] [Google Scholar]
- 90.CHEN L, DING ML, WU F et al. : Impaired Endothelial Repair Capacity of Early Endothelial Progenitor Cells in Hypertensive Patients With Primary Hyperaldosteronemia: Role of 5,6,7,8-Tetrahydrobiopterin Oxidation and Endothelial Nitric Oxide Synthase Uncoupling. Hypertension 2016; 67: 430–9. [DOI] [PubMed] [Google Scholar]
- 91.HURLIMANN D, NOLL G, DELLI GATTI C et al. : Oral treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolemia. Circulation 2005; 112: 138. [DOI] [PubMed] [Google Scholar]