Abstract
Retention in medication-assisted treatment (MAT) among opiate-dependent patients is associated with better outcomes. This systematic review (55 articles, 2010–2014) found wide variability in retention rates (i.e., 19%−94% at 3-month, 46%−92% at 4-month, 3%−88% at 6-month, and 37%−91% at 12-month follow-ups in randomized controlled trials), and identified medication and behavioral therapy factors associated with retention. As expected, patients who received naltrexone or buprenorphine had better retention rates than patients who received placebo or no medication. Consistent with prior research, methadone was associated with better retention than buprenorphine/naloxone. And, heroin-assisted treatment was associated with better retention than methadone among treatment-refractory patients. Only a single study examined retention in MAT for longer than one year, and studies of behavioral therapies may have lacked statistical power; thus, studies with longer-term follow-ups and larger samples are needed. Contingency Management showed promise to increase retention, but other behavioral therapies to increase retention, such as supervision of medication consumption, or additional counseling, education, or support, failed to find differences between intervention and control conditions. Promising behavioral therapies to increase retention have yet to be identified.
Keywords: opiate dependence, systematic review, treatment retention, behavioral therapies, medication-assisted treatment
Introduction
Opiate use and dependence are global problems.1 The US has seen a significant increase in the illicit use of prescription opiates and stable levels of heroin use.2,3 In 2007, there were approximately 1.2 million heroin users in the US, and 5.2 million people reporting inappropriate use of prescription opioids.4 Among people who use heroin or prescription opiates, 50% and 11%, respectively, meet addiction criteria.5 Opiate dependence in particular is viewed as a chronic, brain-based disorder with a high potential for relapse.6,7
The burden of opiate dependence is substantial, with high rates of morbidity and mortality, disease transmission, crime and law enforcement costs, family distress, lost productivity, and increased health care utilization.8 In the US, opiates are second only to alcohol as the primary reason for addiction treatment admission. From 1999 to 2009, annual treatment admissions for opiate misuse increased from approximately 280,000 to 421,000 individuals.9 A primary outcome in treating opiate dependence is retention in treatment because retention is associated with decreased drug use, improved social functioning and quality of life, and reduced mortality.8,10 Because of the benefits of retention for other outcomes, this systematic review examined factors associated with retention in medication-assisted treatment for opiate dependence.
Medication-Assisted Treatment
Medications approved by the FDA for the treatment of opiate dependence are methadone, buprenorphine, and naltrexone. The safety of methadone is well established.10 Methadone is used as a substitute for heroin or other opiates and, through mechanisms of tolerance and cross-tolerance, prevents opioid intoxication and withdrawal.8 Methadone is administered orally in liquid, tablet, or dispersible tablet formulation and is used for maintenance and for assisting in withdrawal.1,10 It is dispensed in specialized outpatient Methadone Maintenance clinics. Research has demonstrated methadone’s efficacy in reducing heroin use, morbidity and mortality, and illegal activities.11–14 Most patients require daily doses, and any “take-home” doses are strictly regulated to prevent diversion.15
Safety evaluations for buprenorphine are less developed than for methadone, but research suggests it is safe, with adverse effects equivalent to those of methadone and placebo.10 Buprenorphine, a partial opioid agonist, is administered sublingually in tablet or film formulations. It is also used in opiate detoxification and maintenance treatment.1 It is available both as a monotherapy and in combination with naloxone to reduce the harm associated with buprenorphine injection. Indeed, naloxone was combined with buprenorphine to decrease the potential for diversion and misuse of buprenorphine. Because buprenorphine is a partial agonist, associated physical dependence and withdrawal are less severe than with full agonists.1 Another advantage is its availability as a prescription medication outside of the highly regulated methadone clinic system; it can be taken once every two days, which makes it more appealing to many patients.16 However, buprenorphine is relatively more expensive than methadone, making it more readily available to individuals with adequate resources.1
Naltrexone’s safety is also well demonstrated, but evidence of its efficacy has not been strongly established.8,10,17,18 Naltrexone is administered orally in tablet formulation or intramuscularly in an extended-release formulation.10 Extended-release naltrexone is delivered by injection once per month. Subdermal implants for naltrexone are not currently FDA approved, although they are available at a limited number of treatment centers. Naltrexone completely blocks the effects of opioids and produces no euphoric effects.1
Present Study
The purpose of the present study is to identify factors associated with the outcome of retention in medication-assisted treatment (MAT) for opiate dependence; that is, treatment with methadone, buprenorphine, and naltrexone. We conducted a systematic review focused on comparisons of medications and behavioral therapies. Both sets of factors are modifiable and can be targeted for change to achieve better retention related to MAT. This review is intended to fill a critical gap in the literature in that identification of factors that promote higher rates of MAT retention will be useful to clinical providers and managers of addiction services seeking to achieve better outcomes among their opiate-dependent patients.
Method
To begin the systematic review, we entered the following search string in PubMed: (“opiate substitution treatment”[Mesh] OR “Opioid-Related Disorders/drug therapy”[Mesh] OR “Opioid-Related Disorders/rehabilitation”[Mesh]) AND (“naltrexone”[Mesh] OR “buprenorphine”[Mesh] OR “methadone”[Mesh]). The search (conducted on January 15, 2015) was limited to studies of humans reported in English language journal articles and published after December 31, 2009. This time frame was chosen to ensure that findings are relevant to current treatment programs, and also to constrain the studies to a manageable number for review. Excluded were case studies, abstracts, reviews, and commentaries. We also entered the same search string, publication date limits, and other constraints in CINAHL, using the option to exclude articles identified in MEDLINE (which is accessed within PubMed). From PubMed, a total of 289 unique citations were screened for inclusion. From CINAHL, only one additional citation was identified and screened, for a total of 290 unique citations.
Each citation (abstract) was reviewed by two authors; a full article review was conducted if one or both authors considered it to be indicated (the two authors agreed initially on the status of 285 of the 290 abstracts [98%]). Studies were eliminated at this stage of abstract review mainly because they focused on adults who were not opiate-dependent, on infants born to women maintained on opioid agonist medication, on short-term detoxification rather than medication-assisted treatment, or on biochemical effects of medications (e.g., hepatic safety). With this approach, 69 articles were retained for full text review because they possibly examined factors associated with retention in MAT for opiate dependence (Figure 1). Two authors conducted data extraction on each of the final 55 articles (elimination of 14 articles was also agreed upon by two authors’ reviews). Data collected from each study included study design, conditions, total number of participants (and percent male), type of medication, measure of retention, and retention rate. All articles retained reported studies of individuals with opiate dependence. When studies provided retention rates at more than one follow-up point, we coded the rate for the longest follow-up.
Figure 1.
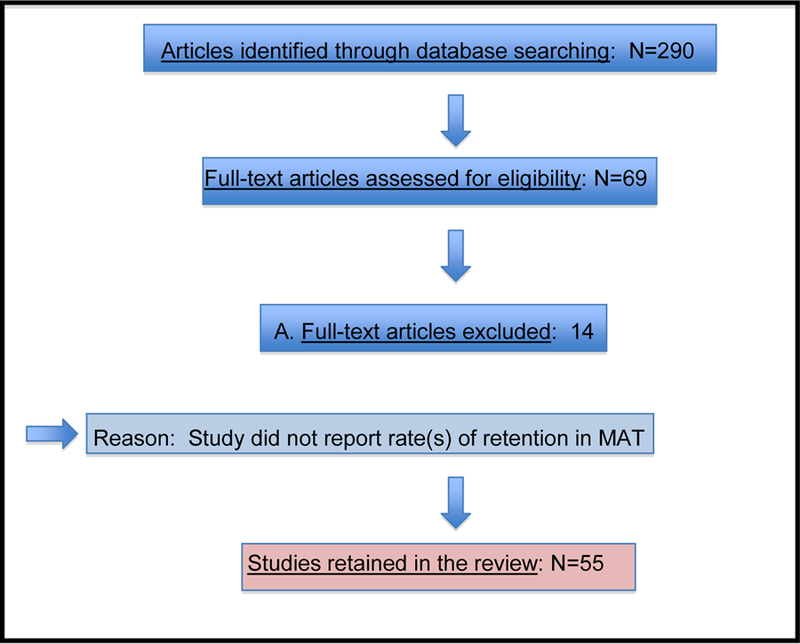
Article selection process for retention in medication-assisted treatment for opioid dependence. (Adapted from “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement”)
Regarding study design, the US Preventive Services Task Force’s quality rating criteria for individual studies rates randomized controlled trials (RCTs) the highest.19 Therefore, we separated RCTs from studies with other designs. In non-RCT designs, quasi-experimental studies are rated higher than cohort designs or case-control studies.19 More fine-grained criteria rate prospective cohort higher than retrospective cohort studies, and rate cohort studies higher than case-control studies.20
Results
MAT Retention Rates
The RCTs listed in Table 1 found a wide range of retention in MAT at follow-ups of 1 month (72.0%; N=1 RCT), 3 months (19.0% to 94.1%; N=9 RCTs), 4 months (45.9% to 91.9%; N=4 RCTs), 6 months (3.0% to 88.0%; N=13 RCTs), and 12 months (37.0% to 90.7%; N=6 RCTs). Studies with a design other than RCT, listed on Table 2, also found a wide range of retention in MAT at follow-ups of 3 (68.0% to 87.0%, N=1 study), 6 (21.4% to 78.1%; N=6 studies), or 12 (26.0% to 85.0%, N=6 studies) months.
Table 1.
Summary of published randomized controlled trials on medication and behavioral therapies potentially associated with retention in medication-assisted treatment for opiate dependence (N=38 studies)
| First author, year, country | Conditions | Sample N (% Male) | Medication | Retention outcome | Retention rate |
|---|---|---|---|---|---|
| Sigmon21, 2013, USA | 1 vs 2 vs 4 week taper; all received individual behavioral therapy (Community Reinforcement Approach) | Community, illicitly using prescription opioids; 70 (69.0) | BUP taper, then NTX | In MAT at 3 months | 50.0% 4-week taper 21.0% 2-week taper 21.0% 1-week taper |
| Tiihonen22, 2012 Russia | NTX implant vs placebo (P) implant | Polydrug dependent; 100 (not provided) | NTX | In MAT at 2.5 months | 52.0% NTX, 28.0% P |
| Krupitsky23, 2011, Russia | NTX vs P; all received counseling | <30 days of detox + abstinent at least 7 days; 250 (88.0) | NTX | Number of days in MAT up to 180 | Med=168 NTX Med=96 P |
| Krupitsky24, 2013, Russia | NTX+Guanfacine (G) vs NTX +GP vs NTXP+G vs NTXP+GP; all received counseling | Medically hospitalized; 301 (82.4) | NTX, G | In MAT at 6 months | 26.7% NTX+G 19.7% NTX+GP 10.7% NTXP+G 6.7% NTXP+G NTX+G, NTX+GP> NTXP+G, NTXP+GP |
| Ling25, 2010, USA | BUP vs P; all received counseling; 18 sites | In addiction treatment; 163 (68.7) | BUP | In MAT at 6 months | 65.7% BUP 30.9% P |
| Ruger26, 2012, Malaysia | P vs NTX vs BUP; all received counseling | Not provided; 126 (not provided) | BUP, NTX | In MAT at 6 months | 15.4% P 20.9% NTX 40.9% BUP BUP>P,NTX |
| Lucas27, 2010, USA | HIV clinical-based BUP vs case management plus referral to opioid treatment program | HIV+; 93 (72.0) | BUP, MET | In MAT at 12 Months | Rates not provided: BUP>referral |
| Saxon28, 2013, USA | BUP vs MET; 8 sites | MAT seekers; 1,269 (not provided) | BUP, MET | In MAT at 4 months | Significance not reported: 45.9% BUP 73.9% MET |
| Woody29, 2014, USA | MET vs BUP/NLX; 9 sites | MAT patients; 1,269 (67.8) | MET, BUP/NLX | In MAT at 6 months | 74.0% MET 46.0% BUP |
| Potter30, 2013, USA | MET vs BUP/NLX; 9 sites | MAT patients; 1,269 (67.8) | MET, BUP/NLX | In MAT at 6 months | Rates not provided: 57.6% overall; BUP<MET |
| Jones31, 2010, USA, Austria, Canada | BUP vs MET: all received comprehensive care and contingency management; 7 sites | Pregnant women; 187 (0.0) | BUP, MET | In MAT at end of pregnancy | 82.0% MET 67.0% BUP |
| Strang32, 2010, England | Supervised injectable (inj) MET vs supervised inj heroin vs optimized oral MET; all patients had a case worker | Receiving oral MET but still injecting heroin; 127 (73.0) | MET, heroin | In MAT at 6.5 months | Significance not reported: 81.5% inj MET 88.0% inj heroin 69.0% oral MET |
| Oviedo- Joekes33, 2010, Canada | Inj diacetylmorphine or inj hydromorphine vs oral MET | Treatment refractory; 192 (61.4) | Inj diacetelyl- morphine, inj hydromorphine, MET | In MAT at 12 months | Aboriginals: 84.4% Inj 57.1% MET Non- Aboriginals 90.7% Inj 50.9% MET |
| Nosyk34, 2010, Canada | Heroin-assisted treatment (HAT) vs MET | Treatment refractory; 251 (61.4) | HAT, MET | In MAT at 12 months | Rates not provided; Better in HAT vs MET |
| Eiroia- Orosa35, 2010, Germany | HAT vs MET; all received psychosocial services | Treatment refractory or treatment dropouts; 1,015 (73.3) | Heroin, MET | In MAT at 12 months | Significance not reported: 67.2% HAT 40.0% MET |
| Otiashvili36, 2013, Republic Of Georgia | MET vs BUP/NLX +dose taper+referral to treatment; all were offered individual and group counseling | Treatment settings; 80 (95.0) | MET, BUP/NLX | In MAT at 3 months | No difference: 85.0% overall |
| Neumann37, 2013, USA | MET vs BUP/NLX | Chronic non-malignant pain patients; 54 (53.7) | MET, BUP/NLX | In MAT at 6 months | 48.1% overall 46.4% MET 50.0% BUP/NLX |
| Oviedo-Joekes38, 2010, Canada | Diacetylmorphine vs hydromorphone | Treatment-refractory; 140 (not provided) | Diacetylmorphine, hydromorphine | In MAT at 12 months | No difference: 87.8% Diacetyl 88.0% Hydromor |
| Amass39, 2011, 10 European countries | Direct induction (DI) vs indirect induction (II) | In MAT; 187 (80.2) | BUP/NLX | In MAT at 1 month | No difference: 81.8% DI 80.8% II |
| Bisaga40, 2011, USA | Memantine (M) 60 mg/day vs M 30 mg/day vs P; all received therapy | New to MAT; 81 (81.5) | NTX | In MAT at 3 months | No difference: 22.0% M-60 19.0% M-30 26.0% P |
| Stein41, 2010, USA | Escitalopram (for depression) vs P; all received physician management | Depression symptoms; 147 (76.0) | BUP | In MAT at 3 months | No difference: 66.7% Escit 44.0% P |
| Oliveto42, 2011, USA | Disulfiram vs P; all received cognitive behavioral therapy (CBT) | Cocaine dependent; 161 (55.9) | MET | In MAT at 3.5 months | No difference: Rates not provided; 64.8% overall |
| Liebschutz43, 2014, USA | Detoxification (detox) vs facilitated linkage to treatment | Medically hospitalized, not seeking addiction treatment; 145 (71.2) | BUP/NLX | In MAT at 6 months | 3.0% Detox 16.7% Linkage |
| Chen44, 2013, China | MET+contingency management (CM) vs MET only | New to MAT; 246 (92.3) | MET | In MAT at 3 months | 81.7% MET+CM 67.5% MET |
| Hser45, 2011, China | CM vs usual care (UC) | MAT patients; 319 (76.2) | MET | In MAT at 3 months | 81.0% CM 67.0% UC |
| Dunn46, 2013, USA | CM vs prescription (take-home MAT) | Unemployed, in detox or on street; 67 (61.2) | NTX | In MAT at 6 months | 54.0% CM 16.0% Prescrip |
| Holland47, 2012, Scotland | Supervision: None vs 2 times per week vs daily | In MAT for 3 months at baseline; 60 (70.0) | MET | In MAT at 3 months | No difference: 94.1% None 85.7% 2x wk 72.7% Daily |
| Chawarski48, 2011, China | Daily medication (DM) vs DM plus weekly drug and HIV counseling (DM plus) | Heroin-dependent; 37 (81.0) | MET | In MAT at 6 months | No difference: 83.3% DM plus 76.2% DM |
| Jaffray49, 2014, Scotland | Pharmacies randomized to intervention (pharmacists received motivational Interviewing [MI] training & resources) vs UC | Mainly unemployed; 76 pharmacies; 542 patients (63.6) | MET | In MAT at 6 months | No difference: 88% MI 81% UC |
| Marsch50, 2013, USA | MAT+counseling vs MAT+reduced counseling+ web-based education | New to MAT; 160 (75.0) | MET | In MAT at 12 months | No difference: 38.7% overall |
| Schwartz51, 2011, USA | Interim (daily administration +emergency counseling; counseling; I) vs standard (take-home administration +regular counseling; S) vs restored (S+counselor reduced caseload; R); 2 sites | New to MAT; 230 (70.0) | MET | In MAT at 4 months | No difference: 91.9% I 80.8% S 88.9% R |
| Schwartz52, 2011, USA | I vs S vs R | On MAT program wait list; 230 (70.0) | MET | In MAT at 12 months | No difference: 60.6% I 54.8% S 37.0% R |
| Ling53, 2013, USA | CBT vs CM vs CBT+CM vs no behavioral treatment | Community and treatment settings; 202 (69.3) | BUP | In behavioral treatment at 4 months | No difference: 71.7% CBT 69.4% CM 73.5% CBT+CM 64.7% None |
| Mitchell54, 2013, USA | Outpatient (OP) counseling vs intensive outpatient (IOP) counseling | African-Americans starting MAT; 300 (62.3) | BUP | In MAT at 6 months | No difference: 58.7% OP 56.6% IOP |
| Ruetsch55, 2012, USA | Telephone support (TS) vs vs UC (324 provider sites) | New to MAT; 1426 (59.0) | BUP | In MAT at 12 months | No difference: 55.0% TS 56.1% UC |
| Tetrault56, 2012, USA | Physician management (PM) vs PM+enhanced medical management (EMM) | HIV+; 47 (83.0) | BUP/NLX | In MAT at 3 months | No difference: 80.0% PM 59% PI+EMM |
| Jones57, 2011, USA | Helping (motivational enhancement therapy, education, case and contingency management) vs control (support group) | Non-treatment seeking partners of opioid-dependent pregnant women; 62 (100) | Detox plus aftercare or MET | Days of treatment (most commonly MAT), past month, up to 30 | No difference: H; M=15.2 (SD=2.0) C; M=14.9 (SD=6.4) |
| Coviello58, 2010, USA | NTX plus psychosocial treatment only; all were under judicial supervision | Offenders; 111 (82.0) | NTX | In treatment (MAT or psychosocial) at 6 months | No difference: 32.0% NTX 29.0% psychosoc |
Note: Reported retention rates within studies are significantly different unless otherwise noted. BUP = buprenorphine, NTX = naltrexone, MET = methadone, NLX = naloxone; P = placebo, Med = median, inj = injectable, HAT = heroin-assisted treatment, CBT = cognitive behavioral therapy, I = interim, S= standard, R = restored, M = mean, SD = standard deviation
Table 2.
Summary of non-RCT published studies on treatment factors associated with retention in medication-assisted treatment for opiate dependence (N=17)
| First author, year, country | Design | Sample N (% Male) | Medication | Retention outcome | Retention rate |
|---|---|---|---|---|---|
| Gryczynski59, 2013, USA | Prospective cohort, MET vs BUP; secondary analysis of two RCTs | African-Americans entering MAT; 478 (65.2) | MET, BUP | In MAT at 6 months | 78.1% MET 57.7% BUP |
| Pinto60, 2010, England | Prospective cohort, MET vs BUP; all received care coordination | Requesting MAT; 361 (75.0) | MET, BUP | In MAT at 6 months | 69.6% MET 42.5% BUP |
| Bounes61, 2013, France | Prospective cohort | Treatment settings; 151 (74.0) | MET, BUP | In MAT at 12 months | 78.0% MET 26.0% BUP |
| Serpelloni62, 2013, Italy | Retrospective cohort (65 publicly-funded addiction treatment sites) | Patients in MAT in 2010; 8,145 (84.2) | MET, BUP | Days of stay during 2010 | MET: M=246.2 (SD=110.1) BUP: M=240.5 (SD=111.7) |
| Hao63, 2013, China | Prospective cohort, MET vs Jitai tablets; all received psychosocial counseling | Completed detox; 386 (84.4) | MET, Jitai tablets | In MAT at 12 months | 85.0% MET 74.3% Jitai |
| Gryczynski64, 2014, USA | Prospective cohort; secondary analysis of RCT studying counseling | African-Americans entering MAT; 297 (61.9) | BUP | In MAT at 6 months | 57.9% overall; retention associated with higher BUP dose, especially early in treatment |
| Curcio65, 2011, Italy | Quasi-experimental: MET vs BUP/NLX; 10 sites | Public outpatients; 3,812 (89.8) | MET, BUP/NLX | In MAT at 12 months | No difference: 92.5% MET 89.4% BUP/NLX |
| Miotto66, 2012, USA | Quasi-experimental: opioid treatment program (OTP), individual counseling vs primary care (PC), brief counseling vs psychosocial program (PP) with group CBT | Community sample; 94 (58.0) | BUP/NLX | In MAT at 6 months | 21.4% OTP 33.3% PC 54.5% PP |
| Gerra67, 2011, Italy | Quasi- experimental: supervised daily (SD) vs CM vs non-contingent take- home (NT); all received psychosocial treatment; 3 sites | Heroin dependent; 300 (82.0) | MET | In MAT at 12 months | 58.0% SD 74.0% CM 50.0% NT CM>SD, NT |
| Haddad68, 2012, USA | Retrospective cohort; 2 health care sites | Patients in MAT in 2007–08; 266 (69.2) | BUP | In MAT at 12 months | 61.6% overall; retention associated with during-MAT receipt of psychiatric medication and substance abuse counseling |
| Moore69, 2012, USA | Quasi-experimental: PM+weekly dispensing vs PM+3 times per week dispensing+CBT | Primary care patients; 58 (74.1) | BUP/NLX | In MAT at 3 months | Marginal difference: 68.0% PM+CBT 87.0% PM |
| Winklbaur- Hausknost70, 2012, Austria | Quasi-experimental: vouchers for attendance vs vouchers for attendance +drug free biological samples | Pregnant women in multidisciplinary care; 59 (0.0) | MET, BUP | In MAT at 1 month post-delivery | No difference: 22% attendance 10% attendance + samples |
| Coviello92, 2012, USA | Prospective cohort; 5 sites | Offenders; 61 (92.0) | Extended release injectable NTX | In MAT at 6 months | 40.0% |
| Fox93, 2012, USA | Prospective cohort | HIV+82 (72.0) | BUP | In MAT at 6 months | 56.0% |
| Fiellin94, 2011, USA | Prospective cohort | HIV+303 (67.7) | BUP/NLX | In MAT at 12 months | 49.0% |
| Apelt95, 2013, Germany | Prospective cohort | Patients who had received MAT then began BUP-NLX; 337 (76.6) | BUP/NLX | In MAT at 12 months | 57.1% overall |
| Blanken73, 2010, Netherlands | Prospective cohort; all received psychosocial, medical support | Positive responders to HAT; 149 (83.2) | MET plus heroin | In MAT at 4 years | 55.7% |
Note: Reported retention rates within studies are significantly different unless otherwise noted. BUP = buprenorphine, MET = methadone, NLX = naloxone,
NTX = naltrexone, HAT = heroin-assisted treatment
RCTs with a Medication Focus
Significant findings.
We focus first on RCTs that compared medication delivery conditions. (See Table 1, in which rates in the last column are significantly different within a given study unless otherwise noted in the table. Also, summaries of studies in this narrative follow the same order of studies in the table.) Patients receiving a 4-week rather than a briefer buprenorphine taper prior to naltrexone had higher MAT retention rates (50.0%) at 3-month follow-up.21 Receipt of a naltrexone implant rather than placebo was associated with a higher 3-month retention rate (52.0% vs 28.0%).22 Receipt of naltrexone rather than placebo was also associated with higher 6-month retention rates (≥20%), and a longer duration of MAT, but the additional receipt of guanfacine (used for ADHD and hypertension) did not increase retention rates.23,24 Receipt of buprenorphine rather than placebo was associated with a higher 6-month retention rate (65.7% vs 30.9%).25 When all patients received counseling, receipt of buprenorphine rather than placebo or naltrexone was again associated with a higher 6-month retention rate.26 Among patients who were HIV+, those receiving buprenorphine within the HIV clinic rather than a referral to an opioid treatment program were more likely to be in MAT at 12 months.27 Thus, as expected, receipt of naltrexone or buprenorphine was associated with better retention in MAT than placebo or no medication.
Three studies found that receipt of methadone rather than buprenorphine/naloxone was associated with higher retention in MAT at 4 months (73.9% vs. 45.9%) and at 6 months (74.0% vs 46.0%; 57.6% overall).28–30 Methadone receipt, compared to buprenorphine receipt, was also associated with higher end-of-pregnancy MAT retention rates among women receiving comprehensive pre-natal care and contingency management.31 However, among patients receiving oral methadone but still injecting heroin, 6-month retention rates were higher when patients were given injectable heroin (88.0%) or methadone (81.5%) than retained on oral methadone (69.0%); similar findings held at 12-month follow-up in another treatment-refractory sample.32,33 Similarly, among treatment-refractory patients, heroin-assisted treatment was associated with a higher 12-month retention rate than was methadone.34,35
Non-significant findings.
Contrary to the studies cited above that found an advantage for methadone relative to buprenorphine,28–31 one study found that patients had high 3-month retention rates (85.0%) whether they received methadone or buprenorphine/naloxone, perhaps because the latter group also received a dose taper and referral to treatment, i.e., weekly individual drug counseling and group therapy.36 Six-month MAT retention rates were also comparable (48%) for patients with chronic non-malignant pain who received either methadone or buprenorphine/naloxone.37 High 12-month retention rates (88%) were found among treatment-refractory patients treated with either diacetylmorphine (the active ingredient of heroin) or hydromorphone (a semisynthetic opioid analgesic).38 In another study, patients’ 1-month retention in MAT did not differ according to whether they received direct or indirect induction of buprenorphine/naloxone.39
Several studies failed to find significant effects on MAT retention for medications provided in addition to a primary opiate medication. In one, 3-month retention among patients receiving oral naltrexone did not differ according to whether they received varying doses of Memantine (a dementia drug) or a placebo (retention rates of ≥19%).40 And, 3-month retention among patients receiving buprenorphine did not differ according to whether they received escitalopram or placebo for depression.41 Finally, among cocaine-dependent patients maintained on methadone for dual opioid dependence, those receiving disulfiram instead of placebo did not have a significantly different likelihood of MAT retention at a 4-month follow-up.42
RCTs with a Behavioral Therapy Focus
Significant findings.
Among non-treatment-seeking hospitalized patients, a comparison of detoxification to facilitated linkage to buprenorphine treatment found higher rates of MAT retention among patients in the facilitated linkage condition; nevertheless, only 16.7% of linked patients were retained at 6 months.43 Compared to methadone-only patients, patients receiving methadone with contingency management were more likely to be retained at 3 months (67.5% vs 81.7%; 67.0% vs 81.0%).44,45 Similarly, compared to naltrexone-only patients, patients receiving naltrexone and contingency management were more likely to be retained at 6 months (16.0% vs 54.0%).46
Non-significant findings.
Several studies have examined MAT retention rates associated with different behavioral therapies among patients receiving methadone. Daily supervision of consumption was associated with a lower, but not significantly lower, 12-month retention rate (72.7%) compared to twice-weekly (85.7%) or no (94.1%) supervision.47 Adding counseling to receipt of daily methadone did not increase 6-month MAT retention rates, which were 76% or higher.48 The provision of pharmacist-delivered motivational interviewing did not improve 6-month retention rates compared to usual care (rates of ≥ 81%).49 Patients whose methadone was accompanied by web-based education but reduced (fewer sessions of) counseling had a comparable rate of MAT retention as patients receiving methadone plus more counseling (and no education); results suggest that less counselor staffing does not interfere with retention, but the overall retention rate at 12 months was only 38.7%.50 Varying counseling provision by whether it was routine or emergency only, in the context of different counselor caseloads and amounts of patient supervision, was not significantly associated with 4-month (89% to 92%) or 12-month (37% to 61%) retention rates.51,52
Another set of studies examined MAT retention rates associated with different behavioral therapies among patients receiving buprenorphine. The provision of cognitive behavioral treatment, contingency management, or both, did not significantly improve 4-month retention rates compared to no additional treatment (rates ≥ 65%).53 Similarly, the provision of intensive rather than standard outpatient counseling did not improve 6-month retention rates (≥ 57%).54 Furthermore, the provision of telephone support did not benefit 12-month retention rates (55.0%) above usual care (55.0%), although patients with at least three completed telephone support calls had higher retention rates than patients in usual care.55
The lack of significant findings related to behavioral therapies and MAT retention holds among samples of more complex opioid-dependent patients. Among patients diagnosed with HIV receiving buprenorphine/naloxone, patients provided with physician management only had an 80% 3-month retention rate compared to 59.0% with enhanced medical management (drug and medication adherence counseling) added on to physician management; this difference was not significant.56 Among partners of opioid-dependent pregnant women, participation in a support group, or receiving more comprehensive therapy, education, and case management, was not associated with number of days in MAT with methadone.57 Among individuals who were under judicial supervision, adding psychosocial counseling to naltrexone treatment was not associated with MAT retention rates at 6-month follow-up.58
Studies with non-RCT Designs with a Medication Focus
Significant findings.
Studies with a cohort design found methadone compared to buprenorphine was associated with better retention rates at 6 and 12 months, and compared to Jitai tablets (a traditional Chinese medicine used to treat neuropsychiatric disorders) at 12 months.59–63 Higher doses of buprenorphine, especially early in treatment, were associated with better retention in MAT at a 6-month follow-up.64
Non-significant findings.
In contrast to studies finding an advantage for methadone, a large study of public outpatient programs in Italy found high rates of MAT retention at 12 months for both methadone (93%) and buprenorphine/naloxone (89%).59,61,62,65
Studies with non-RCT Designs with a Behavioral Therapy Focus
Significant findings.
Among patients receiving buprenorphine/naloxone, a psychosocial program with group cognitive behavioral therapy yielded a higher MAT retention rate at 6 months (55%) than did brief counseling in primary care (33%) or individual counseling in opioid treatment (21%).66 Patients on methadone maintenance had a higher 12-month retention rate in a contingency management take-home condition (74%) than in a daily supervision (58%) or non-contingent take-home condition (50%).67 In a retrospective cohort study of patients receiving buprenorphine, MAT retention at 12-month follow-up was associated with the receipt of substance abuse counseling and psychiatric medication.68
Non-significant findings.
A study of primary care patients receiving buprenorphine/naloxone found physician management with weekly dispensing to be marginally significantly associated with better retention (87% at 3 months) than physician management with dispensing 3 times per week plus cognitive behavioral therapy (68%).69 Pregnant women who received vouchers for both MAT (methadone or buprenorphine) attendance and providing drug-free biological samples had a comparable retention rate at 1-month post-delivery to that of pregnant women who received vouchers for MAT attendance only.70
Discussion
This systematic review, summarizing 55 articles published during the past five years (from 2010 through 2014), found wide variability in the rates at which opiate-dependent patients are retained in medication-assisted treatment. As expected, retention rates are likely to decrease as the duration of follow-up lengthens.51,52 Retention in treatment represents the accomplishment of system and program goals that are important for patients’ attaining and sustaining substance-free and productive lives.71,72 This review identified medication factors and behavioral therapies associated with MAT retention to help clinical providers and managers of addiction services implement procedures linked to patients’ achieving better outcomes.
Notably, only a single study examined retention for longer than one year, even though the National Institute on Drug Abuse (NIDA) recommends a minimum of one year in methadone maintenance treatment for best outcomes.73 Indeed, despite extensive and prolonged use of methadone to treat heroin addiction since the mid-1960s, little is known about its effects over the long-term. The FDA issued in 2006 a physician safety alert regarding increased cardiac arrhythmias and deaths among methadone patients.74 More recent Norwegian animal studies suggest that methadone may negatively affect cognitive functioning, such as learning and memory.75 However, long-term studies of the effectiveness and consequences of MAT for opiate-dependence have yet to be conducted.
Medications and Retention
With regard to medications, this review found, as expected, that patients in RCTs who received naltrexone or buprenorphine had better 3-, 6-, or 12-month retention rates than patients who received placebo or no medication. RCTs and cohort studies also found that patients who received methadone rather than buprenorphine/naloxone were more likely to be retained in MAT at 4- and 6-month follow-ups and at the end of pregnancy. As reviewed by Whelan and Remski (2012), there is significant evidence that better MAT outcomes for opioid dependence are associated with high activity at the mμ receptor, for example, “the narcotic blockade” achieved with high doses of methadone; therefore, buprenorphine’s weaker mμ activity may account for its poorer performance compared to methadone in clinical trials.76 In addition, buprenorphine retains fewer people when doses are delivered flexibly or at low fixed doses, compared to fixed medium or high doses; however, fixed doses are rarely used in clinical practice.16 This is consistent with the finding cited here that higher doses of buprenorphine, especially early in treatment, were associated with better retention.64
The studies in this review finding benefits to retention of heroin-assisted treatment relative to methadone among treatment-refractory patients agree with earlier evidence in support of treatment with fully supervised, self-administered injectable heroin, when compared with oral methadone, for individuals with long-term refractory heroin dependence.77–81 However, heroin prescription is a controversial approach to treatment because of the question of whether giving users the drug they are addicted to constitutes treatment at all. Nevertheless, in the short term, heroin prescription may be considered as an effective way to retain users in treatment who have a history of failing in other treatment settings, with consequent benefits in terms of reduced drug use, HIV-risk behavior, and crime, and better social reintegration.82–84
Behavioral Therapies and Retention
In the RCTs reviewed, only the behavioral therapy of Contingency Management (CM) showed promise as an intervention to increase retention in MAT for opiate dependence. Similarly, Gerra et al.’s quasi-experimental study (2011) found a higher retention rate in a CM condition.67 In one of the RCTs, in China, participants in the CM condition drew for prizes on an escalating scale for having ingested methadone on each of the previous three days or having submitted a drug-free urine specimen; prizes ranged from very small to small monetary incentives (1 Yuan or 15 cents US, to 20 Yuan or $2.94 US).45 In another RCT conducted in China, in the CM intervention, participants drew for prizes for seven consecutive days of taking methadone, on an escalating scale; prizes were vouchers that could be redeemed only to pay for treatment.44 In the US, Dunn et al. (2012) found support for employment-based reinforcement of naltrexone adherence in terms of retention in MAT; in the CM condition, participants were required to ingest oral naltrexone under staff observation to gain access to a workplace where they could work and earn monetary vouchers.46
The efficacy of CM in terms of better retention and other treatment outcomes has also been established in studies of individuals dependent on stimulants or nicotine, with the benefits most apparent early in treatment.85–88 Indeed, CM has relatively strong empirical support in the treatment of addictions, but even so, CM has had weak and uneven adoption in clinical practice.89 The main barriers to use of CM are cost and ideology, such as beliefs by clinicians that CM does not address the underlying causes of addiction, or undermines a patient’s internal motivation for abstinence. These barriers have been addressed through the development of lower-cost adaptations as well as clinician trainings to address ideological concerns and make CM more community friendly.89
Other than Contingency Management, RCTs of behavioral therapies to increase retention failed to find differences between conditions. However, some of these studies may have been inadequately powered.47,56 Nevertheless, the lack of efficacy for behavioral therapies tested provides consistency with the body of studies that failed to find additional medications for psychological conditions, such as depression, to be efficacious in increasing MAT retention for opiate dependence.
Limitations
The major limitation of this study is that we relied on only two databases, PubMed and CINAHL, for the search of the literature, and did not review gray literature (e.g., technical reports, conference proceedings) or unpublished studies of retention in MAT, which may be more likely to show no effect for interventions intended to improve retention. However, PubMed, a service of the US National Library of Medicine, provides access to MEDLINE, the NLM database of indexed citations and abstracts to medical, nursing, dental, health care, and preclinical sciences journal articles, and includes additional life sciences journals not in MEDLINE. We also selected only English-language articles, although there may be publications relevant to this review that are not in English. We used study design to indicate the methodological rigor of the studies reviewed, but did not report attrition rates by condition, or effect sizes pertaining to the strength of interventions. Future systematic reviews are needed to address the additional limitation that this study focused on medication and behavioral therapy factors related to retention in MAT for opiate addiction, to the exclusion of other factors such as patient determinants, and other outcomes such as abstinence and psychosocial functioning.
Conclusion
This systematic review covering the past five years of research on MAT retention by opiate-dependent individuals suggests a continued advantage for methadone over buprenorphine, although the implementation of buprenorphine at higher doses may overcome this difference. In addition, offering MAT with contingency management may be associated with higher retention rates. The methodological quality of the body of research on retention is good given the large number of investigations using RCT designs. However, it is critical to address longer-term associations between medications and behavioral therapies and outcomes of MAT such as retention.90,91 Together, studies in this systematic review suggest that practices can be managed to increase the retention of opiate-dependent patients in medication-assisted treatments and ultimately improve their quality of life.
Acknowledgements
This research was supported by a Senior Research Career Scientist Award (RCS 00-001) to Dr. Timko by the Department of Veterans Affairs (VA) Health Services Research and Development (HSR&D) Service, and an Advanced Fellowship to Dr. Garrison-Diehn by the VA Office of Academic Affairs. The views expressed are the authors’ and do not necessarily reflect those of the VA. No conflicts of interest are reported by any of the authors listed on this manuscript.
References
- 1.Pecoraro A, Ma M, Woody GE. The science and practice of medication-assisted treatments for opioid dependence. Subst Use Misuse 2012. Jun-Jul; 47(8–9):1026–1040. DOI: 10.3109/10826084.2012.663292 [DOI] [PubMed] [Google Scholar]
- 2.UNODC. World drug report 2011 New York: United Nations Publications; 2011. 267 p. United Nations Publication Sales No. E.11.XI.10. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: summary of national findings Rockville (MD): Office of Applied Studies; 2011. 156 p. (DHHS Publication No. [SMA] 11–4658); (NSUDH Series H-41). [Google Scholar]
- 4.UNODC. World drug report 2007 New York: United Nations Publications; 2007. 275 p. United Nations Publication Sales No. S.07.XI.5 [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health, 2010. ICPSR 32722. [updated 2014 Sep 05; cited 2015 Mar 26]. Available from http://www.icpsr.umich.edu/icpsrweb/SAMHDA/studies/32722#cite.
- 6.Volkow ND, Li TK. Drug addiction: the Neurobiology of behavior gone awry. Nat Rev Neurosci 2004. December; 5(12):963–970. DOI: 10.1038/nrn1539 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Neuroscience of psychoactive substance use and dependence Geneva: World Health Organization; 2004. 264 p. [Google Scholar]
- 8.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother 2009. August; 10(11):1727–1740. DOI: 10.1517/14656560903037168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS). ICPSR 34876. [updated 2014 Sep 11; cited 2015 Mar 26]. Available from http://www.icpsr.umich.edu/icpsrweb/SAMHDA/studies/34876.
- 10.Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis, 2012; 31(3):207–225. DOI: 10.1080/10550887.2012.694598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009. July 8; Issue 3 Art. No.: CD002209 DOI: 10.1002/14651858.CD002209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanis DA, Woody GE. One-year mortality rates following methadone treatment discharge. Drug Alcohol Depend 1998. November; 52(3):257–260. DOI: 10.1016/S0376-8716(98)00097-0 [DOI] [PubMed] [Google Scholar]
- 13.Metzger DS, Woody GE, McLellan AT, O’Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. Human Immunodeficiency Virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr 1993. September; 6(9):1049–1056. [PubMed] [Google Scholar]
- 14.Ball JC, Ross A. The effectiveness of methadone maintenance treatment New York: Springer-Verlag; 1991. 283 p. DOI: 10.1007/978-1-4613-9089-3 [DOI] [Google Scholar]
- 15.Kleber HD. Methadone maintenance 4 decades later. JAMA 2008. November 19; 300(19):2303–2305. DOI: 10.1001/jama.2008.648 [DOI] [PubMed] [Google Scholar]
- 16.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014. February 6; Issue 2 Art. No.: CD002207 DOI: 10.1002/14651858.CD002207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction 2006. April; 101(4):491–503. DOI: 10.1111/j.1360-0443.2006.01369.x [DOI] [PubMed] [Google Scholar]
- 18.Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, Bayliss S, Roberts T, Burls A. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess 2007; 11(6). [DOI] [PubMed] [Google Scholar]
- 19.Harris R, Atkins D, Berg AO, Best D, Eden KB, Feightner JW. US Preventive Services Task Force procedure manual Rockville (MD): Agency for Healthcare Research and Quality; 2008. July AHRQ Publication No. 08–05118-EF. [Google Scholar]
- 20.Petticrew M, Roberts H. Evidence, hierarchies, and typologies: horses for courses. J Epidemiol Community Health 2003. July; 57(7):527–529. DOI: 10.1136/jech.57.7.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigmon SC, Dunn KE, Saulsgiver K, Patrick ME, Badger GJ, Heil SH, Brooklyn MD, Higgins ST. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry 2013. December; 70(12):1347–1354. DOI: 10.1001/jamapsychiatry.2013.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Föhr J, Tuomola P, Kuoppasalmi K, Kiviniemi V, Zwartau E. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry 2012. May; 169(5):531–536. DOI: 10.1176/appi.ajp.2011.11071121 [DOI] [PubMed] [Google Scholar]
- 23.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentere randomised trial. Lancet 2011. April 30; 377(9776):1506–1513. DOI: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 24.Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Tsoy M, Wahlgren V, Burakov A, Masalov D, Romanova TN, Palatkin V, Tyurina A, Yaroslavtseva T, Sinha R, Kosten TR. Naltrexone with or without guanfacine for preventing relapse to opiate addiction in St.-Petersburg, Russia. Drug Alcohol Depend 2013. October 1; 132(3):674–680. DOI: 10.1016/j.drugalcdep.2013.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, Bailey GL, Rosenthal RN, Beebe KL. Buprenorphine implants for treatment of opioid dependence. JAMA 2010. October 13; 304(14):1576–1583. DOI: 10.1001/jama.2010.1427 [DOI] [PubMed] [Google Scholar]
- 26.Ruger JP, Chawarski M, Mazlan M, Ng N, Schottenfeld R. Cost-effectiveness of buprenorphine and naltrexone for heroin dependence in Malaysia. PLoS ONE 2012; 7(12):e50673 DOI: 10.1371/journal.pone.0050673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, Keruly JC, Fielin DA, Finkelstein R, Barditch-Crovo P, Cook K, Moore RD. Clinic-based treatment for opioid-dependent HIV-infected patients versus referral to an opioid treatment program. Ann Intern Med 2010. June 1; 152(11):704–711. DOI: 10.1059/0003-4819-152-11-201006010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, Doraimani G, Tasissa G, Lokhnygina Y, Leimberger J, Bruce RD, McCarthy J, Wiest K, McLaughlin P, Bilangi R, Cohen A, Woody G, Jacobs P. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend 2013. February; 128(1–2):71–76. DOI: 10.1016/j.drugalcdep.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woody GE, Bruce D, Korthuis PT, Chhatre S, Poole S, Hillhouse M, Jacobs P, Sorensen J, Saxon AJ, Metzger D, Ling W. HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. J Acquir Immune Defic Syndr 2014. July 1; 66(3):288–293. DOI: 10.1097/QAI.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, Martin JA, Ang A, Baker R, Saxon AJ, Ling W. Buprenorphine/naltrexone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: Findings from starting treatment with agonist replacement therapies (START). J Stud Alcohol Drugs 2013. July; 74(4):605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 2010. December 9; 363(24):2320–2331. DOI: 1056/NEJMoa1005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, Williams H, Zador D, Evers R, Groshkova T, Charles V, Martin A, Forzisi L. Supervised injectable heroin or injectable methadone versus optimized oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet 2010. May 29; 375(9729):1885–1895. DOI: 10.1016/S0140-6736(10)60349-2 [DOI] [PubMed] [Google Scholar]
- 33.Oviedo-Joekes E, Guh D, Marsh DC, Brissette S, Nosyk B, Krausz M, Anis A, Christian WM, Spittal P, Schechter MT. Characteristics and response to treatment among Aboriginal people receiving heroin-assisted treatment. Can J Public Health 2010. May-Jun; 101(3):210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nosyk B, Geller J, Guh D, Oviedo-Joekes E, Brissette S, Marsh DC, Schechter MT, Anis AH. The effect of motivation status on treatment outcome in the North American Opiate Medication Initiative (NAOMI) study. Drug Alcohol Depend 2010. September 1; 111(1–2):161–165. DOI: 10.1016/j/drugalcdep.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 35.Eiroa-Orosa FJ, Verthein U, Kuhn S, Lindemann C, Karow A, Haasen C, Reimer J. Implication of gender differences in heroin-assisted treatment: Results from the German randomized controlled trial. Am J Addict 2010. Jul-Aug; 19(4):312–318. DOI: 10.1111/j.1521-0391.2010.00049.x [DOI] [PubMed] [Google Scholar]
- 36.Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, Woody GE. Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior—outcomes of a randomized trial. Drug Alcohol Depend 2013. December 1; 133(2):376–382. DOI: 1.1016/j.drugalcdep.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann AM, Blondell RD, Jaanimagi U, Giambrone AK, Homish GG, Lozano JR, Kowalik U, Azadfard M. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and co-existent opioid addiction. J Addict Dis 2013. January; 32(1):68–78. DOI: 10.1080/10550887.2012.759872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oviedo-Joekes E, Guh D, Brissette S, Marsh DC, Nosyk B, Krausz M, Anis A, Schechter MT. Double-blind injectable hydromorphone versus diacetylmorphine for the treatment of opioid dependence: a pilot study. J Subst Abuse Treat 2010. June; 38(4):408–411. DOI: 10.1016/j.jsat.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 39.Amass L, Pukeleviciene V, Subata E, Almeida AR, Pieri MC, D’Egidio P, Stankova Z, Costa A, Smyth BP, Sakoman S, Wei Y, Strang J. A prospective, randomized, multicenter acceptability and safety study of direct buprenorphine/naloxone induction in heroin-dependent individuals. Addiction 2011. January; 107(1):142–151. DOI: 10.1111/j.1360-0443.2011.03577.x [DOI] [PubMed] [Google Scholar]
- 40.Bisaga A, Sullivan MA, Chang WY, Carpenter KM, Mariani JJ, Levin FR, Raby WN, Nunes EV. A placebo controlled trial of memantine as an adjunct to oral naltrexone for opioid dependence. Drug Alcohol Depend 2011. December 1; 119(1–2):e23–e29. DOI: 10.1016/j.drugalcdep.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein MD, Herman DS, Kettavong M, Cioe PA, Friedmann PD, Tellioglu T, Anderson BJ. Antidepressant treatment does not improve buprenorphine retention among opioid-dependent persons. J Subst Abuse Treat 2010. September; 39(2):157–166. DOI: 10.1016/j.sat.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveto A, Poling J, Mancino MJ, Feldman Z, Cubells JF, Pruzinsky R, Gonsai K, Cargile C, Sofuoglu M, Chopra MP, Gonzalez-Haddad G, Carroll KM, Kosten TR. Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug Alcohol Depend 2011. January 15; 113(2–3):184–191. DOI: 10.1016.j.drugalcdep.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebschutz JM, Crooks D, Herman D, Anderson B, Tsui J, Meshesh LZ, Dossabhoy S, Stein M. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized controlled trial. JAMA Intern Med 2014. August; 174(8): 1369–1376. DOI: 10.1001/jamainternmed.2014.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Hong Y, Zou X, McLaughlin MM, Xia Y, Ling L. Effectiveness of prize-based contingency management in a methadone maintenance program in China. Drug Alcohol Depend 2013. November; 133(1):270–274. DOI: 10.1016/j.drugalcdep.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 45.Hser Y, Li J, Jiang H, Zhang R, Du J, Zhang C, Zhang B, Evans E, Wu F, Chang YJ, Peng C, Huang D, Stitzer ML, Roll J, Zhao M. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction 2011. October; 106(10):1801–1809. DOI: 10.1111/j.1360-0443.2011.03490.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn K, Defulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, Leoutsakos JM, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Exp Clin Psychopharmacol 2013; 21(1):74–83. DOI: 10.1037/a0030743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland R, Matheson C, Anthony G, Roberts K, Priyardarshi S, Macrae A, Whitelaw E, Appavoo S, Bond C. A pilot randomised controlled trial of brief versus twice weekly versus standard supervised consumption in patients on opiate maintenance treatment. Drug Alcohol Rev 2012. June; 31(4):483–491. DOI: 10.1111/j.1465-3362.2011.00394.x [DOI] [PubMed] [Google Scholar]
- 48.Chawarski MC, Zhou W, Schottenfeld RS. Behavioral drug and HIV risk reduction counseling (BDRC) in MMT programs in Wuhan, China: a pilot randomized clinical trial. Drug Alcohol Depend 2011. June 1; 115(3):237–239. DOI: 10.1016/j.drugalcdep.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaffray M, Matheson C, Bond CM, Lee AJ, McLernon DJ, Johnstone A, Skea L, Davidson B. Does training in motivational interviewing for community pharmacists improve outcomes from methadone patients? A cluster randomised controlled trial. Int J Pharm Pract 2014. February; 22(1):4–12. DOI: 10.1111/ijpp.12049 [DOI] [PubMed] [Google Scholar]
- 50.Marsch LA, Guarino H, Acosta M, Aponte-Melendez Y, Cleland C, Grabinski M, Brady R, Edwards J. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. J Subst Abuse Treat 2014. January; 46(1):43–51. DOI: 10.1016/j.jsat.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Interim methadone treatment compared to standard methadone treatment: 4-month findings. J Subst Abuse Treat 2011. July; 41(1):21–29. DOI: 10.1016/j.jsat.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction 2012. May; 107(5):943–952. DOI: 10.1111/j.1360-0443.2011.03700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction 2013. October; 108(10):1788–1798. DOI: 10.1111/add.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell SG, Gryczynski J, Schwartz RP, O’Grady KE, Olsen YK, Jaffe JH. A randomized trial of intensive outpatient (IOP) vs. standard outpatient (OP) buprenorphine treatment for African Americans. Drug Alcohol Depend 2013. March 1; 128(3), 222–229. DOI: 10.1016/j.drugalcdep.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruetsch C, Tkacz J, McPherson TL, Cacciola J. The effect of telephonic patient support on treatment for opioid dependence: outcomes at one year follow-up. Addict Behav 2012. May; 37(5):686–689. DOI: 10.1016/j.addbeh.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 56.Tetrault JM, Moore BA, Barry DT, O’Connor PG, Schottenfeld R, Fiellin DA, Fiellin LE. Brief versus extended counseling along with buprenorphine/naloxone for HIV-infected opioid dependent patients. J Subst Abuse Treat 2012. December; 43(4):433–439. DOI: 10.1016/j.jsat.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 57.Jones HE, Tuten M, O’Grady KE. Treating the partners of opioid-dependent pregnant patients: Feasibility and efficacy. Am J Drug Alcohol Abuse 2011; 37(3):170–178. DOI: 10.3109/00952990.2011.563336 [DOI] [PubMed] [Google Scholar]
- 58.Coviello DM, Cornish JW, Lynch KG, Alterman AI, O’Brien CP. A randomized trial of oral naltrexone for treating opioid-dependent offenders. Am J Addict 2010. Sep-Oct; 19(5):422–432. DOI: 10.1111/j.1521-0391.2010.00070.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gryczynski J, Mitchell SG, Jaffe JH, Kelly SM, Myers CP, O’Grady KE, Olsen YK, Schwartz RP. Retention in methadone and buprenorphine treatment among African Americans. J Subst Abuse Treat 2013. September; 45(3):287–292. DOI: 10.1016/j.jsat.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat 2010. December; 39(4):340–352. DOI: 10.1016/j.jsat.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 61.Bounes V, Palmaro A, Lapeyre-Mestre M, Roussin A. Long-term consequences of acute pain for patients under methadone or buprenorphine maintenance treatment. Pain Physician 2013; 16:E739–E747. [PubMed] [Google Scholar]
- 62.Serpelloni G, Gomma M, Genetti B, Zermiani M, Rimondo C, Mollica R, Gryczynski J, O’Grady KE, Schwartz RP. Italy’s electronic health record system for opioid agonist treatment. J Subst Abuse Treat 2013. August; 45(2):190–195. DOI: 10.1016/j.jsat.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hao SQ, Zhao M, Zhang RW, Zhang JC, Zhang J, Feng XS. The effectiveness comparison of Jitai tablets versus methadone in community-based drug treatment: a 1-year follow-up study. Addict Beh 2013. October; 38(10):2596–2600. DOI: 10.1016/j.addbeh.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 64.Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: patients’ reasons for cessation of care. J Subst Abuse Treat 2014. March; 46(3):356–361. DOI: 10.1016/j.jsat.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Curcio F, Franco T, Topa M, Baldassarre C. Buprenorphine/naloxone versus methadone in opioid dependence: a longitudinal survey. Eur Rev Med Pharmacol Sci 2011. August; 15(8):871–874. [PubMed] [Google Scholar]
- 66.Miotto K, Hillhouse M, Donovick R, Cunningham-Rathner J, Charuvastra C, Torrington M, Esagoff AE, Ling W. Comparison of buprenorphine treatment for opioid dependence in 3 settings. J Addict Med 2012. March; 6(1):68–76. DOI: 10.1097/ADM.0b013e318233d621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerra G, Saenz E, Busse A, Maremmani I, Ciccocioppo R, Zaimovic A, Gerra ML, Amore M, Manfredini M, Donnini C, Somaini L. Supervised daily consumption, contingent take-home incentive and non-contingent take-home in methadone maintenance. Prog Neuropsychopharmacol Biol Psychiatry 2011. March 30; 35(2):483–489. DOI: 10.1016/j.pnpbp.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 68.Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Dep 2013July; 1–2(1):127–135. DOI: 10.1016/j.drugalcdep.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore BA, Barry DT, Sullivan LE, O’Connor PG, Cutter CJ, Schottenfeld RS, Fiellin DA. Counseling and directly observed medication for primary care buprenorphine maintenance. J Addict Med September 2012; 6(3):205–211. DOI: 10.1097/ADM.0b013e3182596492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winklbaur-Hausknost B, Jagsch R, Graf-Rohrmeister K, Unger A, Baewert A, Langer M, Thau K, Fischer G. Lessons learned from a comparison of evidence-based research in pregnant opioid-dependent women. Hum Psychopharmacol 2013. January; 28(1):15–24. DOI: 10.1002/hup.2275 [DOI] [PubMed] [Google Scholar]
- 71.Knight DK, Logan SM, Simpson DD. Predictors of program completion for women in residential substance abuse treatment. Am J Drug Alcohol Abuse 2001; 27(1):1–18. [DOI] [PubMed] [Google Scholar]
- 72.Simpson DD, Joe GW, Dansereau DF, Chatham LR. Strategies for improving methadone treatment process and outcomes. J Drug Issues 1997. March; 27(2):239–260. [Google Scholar]
- 73.Blanken P, Hendricks VM, van Ree JM, van den Brink W. Outcome of long-term heroin-assisted treatment offered to chronic, treatment-resistant heroin addicts in the Netherlands. Addiction 2010. February; 105(2):300–308. DOI: 10.1111/j.1360-0443.2009.02754.x [DOI] [PubMed] [Google Scholar]
- 74.Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney CP. QTc interval screening in methadone treatment. Ann Int Med 2009. March 17; 150(6):387–395. DOI: 10.7326/0003-4819-150-6-200903170-00103 [DOI] [PubMed] [Google Scholar]
- 75.Andersen JM, Klykken C, Morland J. Long-term methadone treatment reduces phosphorylation of CaMKII in rat brain. J Pharm Pharmacol 2012. June; 64(6):843–847. DOI: 10.111/j.2042-7158.2012.01469.x [DOI] [PubMed] [Google Scholar]
- 76.Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract 2012. Jan-Apr; 3(1):45–50. DOI: 10.4103/0976-3147.91934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perneger TV, Giner F, del Rio M, Mino A. Randomised trial of heroin maintenance programme for addicts who fail in conventional drug treatments. BMJ 1998. July 4; 317(7150):13–18. DOI: 10.1136/bmj.317.7150.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van den Brink W, Hendriks VM, Blanken P, Koester MWJ, van Zwieten BJ, van Ree J. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ 2003; 327(724);310–315. DOI: 10.1136/bmj.327.7410.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid dependence. Br J Psychiatry 2007; 191(1):55–62. DOI: 10.1192/bjp.106.026112 [DOI] [PubMed] [Google Scholar]
- 80.Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid dependence. Br J Psychiatry 2007. July; 191(1):55–62. DOI: 10.1192/bjp.106.026112 [DOI] [PubMed] [Google Scholar]
- 81.Oviedo-Joekes E, Brissette S, Marsh DC, Lauzon P, Guh D, Anis A, Schechter MT. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 2009; 361:777–786. DOI: 10.1056/NEJMoa0810635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lloyd C, McKeganey N. Drugs research: an overview of evidence and questions for policy York: Joseph Rowntree Foundation; 2010. 70p. [Google Scholar]
- 83.Metrebian N, Shanahan W, Wells B, Stimson GV. Feasibility of prescribing injectable heroin and methadone to opiate-dependent drug users: associated health gains and harm reductions. Med J Aust 1998. June 15; 168(12):596–600. [DOI] [PubMed] [Google Scholar]
- 84.March JC, Oviedo-Joekes E, Perea-Milla E, Carrasco F. Controlled trial of prescribed heroin in the treatment of opioid addiction. J Subst Abuse Treat 2006. September; 31(2):203–201. DOI: 10.1016/j.jsat.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 85.Petitjean SA, Dursteler-MacFarland KM, Krokar MC, Strasser J, Mueller SE, Degen B, Trombini MV, Vogel M, Walter M, Wiesbeck GA, Farronato NS. A randomized, controlled trial of combined cognitive-behavioral therapy plus prize-based contingency management for cocaine dependence. Drug Alcohol Depend 2014. December 1; 145:94–100. DOI: 10.1016/j.drugalcdep.2014.09.785 [DOI] [PubMed] [Google Scholar]
- 86.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction 2006. February; 101(2):267–274. DOI: 10.1111/j.1360-0443.2006.01312.x [DOI] [PubMed] [Google Scholar]
- 87.Ledgerwood DM, Arfken CL, Petry NM, Alessi SM. Prize contingency management for smoking cessation: a randomized trial. Drug Alcohol Depend 2014. July 1; 140:208–212. DOI: 10.1016/j.drugalcdep.2014.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Fetinger DS. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction 2014. September; 109(9):1426–1436. DOI: 10.1111/add.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Ann N Y Acad Sci 2014; 1327:94–111. DOI: 10.1111/nyas.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guttinger F, Gschwend P, Schulte B, Rehm J, Uchtenhagen A. Evaluating long-term effects of heroin-assisted treatment: the results of a 6-year follow-up. Eur Addict Res 2003. April; 9(2):73–79. DOI: 10.1159/000068811 [DOI] [PubMed] [Google Scholar]
- 91.Skinner ML, Haggerty KP, Fleming CB, Catalano RF, Gainey RR. Opiate-addicted parents in methadone treatment: long-term recovery, health and family relationships. J Addict Dis 2011. January; 30(1):17–26. DOI: 10.1080/10550887.2010.531670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coviello DM, Cornish JW, Lynch KG, Boney TY, Clark CA, Lee JD, Friedmann PD, Nunes EV, Kinlock TW, Gordon MS, Schwartz RP, Nuwayser ED, O’Brien CP. A multisite pilot study of extended-release injectable naltrexone treatment for previously opioid-dependent parolees and probationers. Subst Abus 2012; 33(1):48–59. DOI: 10.1080/08897077.2011.609438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fox AD, Sohler NL, Starrels JL, Ning Y, Giovanniello A, Cunningham CO. Pain is not associated with worse office-based buprenorphine treatment outcomes. Subst Abus 2012; 33(4):361–365, DOI: 10.1080/08897077.2011.638734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, Chaudhry A, Cunningham CO, Gourevitch MN, Lum PJ, Sullivan LE, Schottenfeld RS, O’Connor PG. Drug treatment outcomes among HIV-infected opioid dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr 2011. March 1; 56(0–1):S33–S38. doi: 10.1097/QAI.0b013e3182097537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Apelt SM, Scherbaum N, Golz J, Backmund M, Soyka M. Safety, effectiveness and tolerance of buprenorphine-naloxone in the treatment of opioid dependence: results from a nationwide non-interventional study in routine care. Pharmacopsychiatry 2013. May; 46(3):94–107. DOI: 10.1055/s-0032-1330033 [DOI] [PubMed] [Google Scholar]


