Abstract
Extravillous trophoblast (EVT) uterine artery remodeling (UAR) promotes placental blood flow, but UAR regulation is unproven. Elevating estradiol (E2) in early baboon pregnancy suppressed UAR and EVT vascular endothelial growth factor (VEGF) expression, but this did not prove that VEGF mediated this process. Therefore, our primate model of prematurely elevating E2 and contrast-enhanced ultrasound cavitation of microbubble (MB) carriers was used to deliver VEGF DNA to the placental basal plate (PBP) to establish the role of VEGF in UAR. Baboons were treated on days 25 to 59 of gestation (term, 184 days) with E2 alone or with E2 plus VEGF DNA-conjugated MBs briefly infused via a maternal peripheral vein on days 25, 35, 45, and 55. At each of these times an ultrasound beam was directed to the PBP to collapse the MBs and release VEGF DNA. VEGF DNA-labeled MBs per contrast agent was localized in the PBP but not the fetus. Remodeling of uterine arteries >25 µm in diameter on day 60 was 75% lower (P < 0.001) in E2-treated (7% ± 2%) than in untreated baboons (30% ± 4%) and was restored to normal by E2/VEGF. VEGF protein levels (signals/nuclear area) within the PBP were twofold lower (P < 0.01) in E2-treated (4.2 ± 0.9) than in untreated (9.8 ± 2.8) baboons and restored to normal by E2/VEGF (11.9 ± 1.6), substantiating VEGF transfection. Thus, VEGF gene delivery selectively to the PBP prevented the decrease in UAR elicited by prematurely elevating E2 levels, establishing the role of VEGF in regulating UAR in vivo during primate pregnancy.
During early human and nonhuman primate pregnancy, placental extravillous trophoblasts (EVTs) migrate to, invade, and replace the smooth muscle and vascular endothelial cells within the uterine spiral arteries, thereby remodeling and increasing lumenal area of these vessels (1–5). Consequently, spiral arteries change from high-resistance/low-capacity to low-resistance/high-capacity vessels, which increases uterine artery blood flow and promotes fetal development. Defective uterine artery remodeling (UAR) underpins the etiology of pregnancy disorders that comprise the syndrome of ischemic placental disease (6–10)—for example, preeclampsia, fetal growth restriction, and preterm birth (11–18)—which is associated with uterine artery blood flow impedance, maternal systemic vascular dysfunction, and maternal and neonatal morbidity/mortality (19–26). However, despite the absolute importance of UAR to successful pregnancy, the regulation of UAR has not been clearly established.
In vitro studies have shown that numerous growth factors, including vascular endothelial growth factor (VEGF)-A, IGF-II, epidermal growth factor (EGF), heparin-binding EGF, TNF-α, and others, can stimulate primary, immortalized, and malignant trophoblasts to migrate across synthetic membrane or form endothelial-like tubes (27–34). However, in other in vitro studies several of these factors, including VEGF (35–37), did not stimulate EVT migration or tube formation. Thus, which, if any, of these growth factors has a physiologically essential role in vivo in regulating UAR is unknown. Most importantly, considering the highly complex interplay of different cell types and molecular events that occur in vivo during UAR, it is unclear whether EVT migration and tube formation as assessed in vitro validly mirrors the process of UAR as it occurs in vivo. This represents a fundamental gap in knowledge, considering the essential role that UAR has in normal and abnormal pregnancy.
Clinical studies have shown that maternal serum levels of the truncated receptor sFlt-1 that binds to and suppresses bioavailability of VEGF were increased, and those of placental growth factor decreased preceding onset of the complications, for example, maternal vascular dysfunction, of preeclampsia (38–47). However, the clinical utility of screening for the latter and other biomarkers to predict preeclampsia has met with limited success (44). Moreover, placental expression and serum levels of sFlt-1, VEGF, IGF-I, EGF, heparin-binding EGF, and other peptides are either decreased, increased, or unaltered at term in pregnancies compromised by preeclampsia and fetal growth restriction (27, 30, 38, 39, 41, 45–51), which may simply be a consequence, not cause, of the pathophysiology elicited by these conditions. Importantly, because UAR was not simultaneously examined in these studies, the regulatory role of these factors on UAR has not been established in human pregnancy.
Systemic adenoviral delivery of sFlt-1 induced (41, 52), and adenoviral delivery of VEGF121 in BPH/5 (53), sFlt-1 (54–56), and placental ischemia (57–59) rodent and sheep models prevented, maternal vascular dysfunction and fetal demise typical of preeclampsia, but UAR was not determined. Systemic administration to mice of an antibody that paradoxically neutralized both Flt-1 and sFlt-1 decreased uterine artery length and blood flow but not fetal weight (60). Induction of uteroplacental ischemia by aortic or uterine artery ligation increased maternal sFlt-1 levels and elicited renal vascular dysfunction in rodents (61) and baboons (62), but UAR was not examined. Thus, animal studies have focused on the clinical manifestations of adverse pregnancy but not UAR (63–65). Moreover, there are significant differences in placental morphology, the process of UAR, uterine vascular anatomy, and the maternal–placental–fetal endocrine interrelationships between rodents and humans (33, 66–69), making translation to the human uncertain. In sum, the regulation of UAR, particularly the role of VEGF, has not been established.
Our laboratory has been instrumental in establishing the baboon as a primate model for the study of human placental and fetal development. Humans and baboons exhibit similar anatomy, physiology, and ontogeny of the fetal–placental unit (67, 70) and share >96% DNA/genetic homology (71, 72). Using the baboon as a translational model, we have shown that the low level of ovarian estradiol (E2) during the first trimester of normal pregnancy is essential for promoting UAR (73, 74). Thus, simply shifting the normal rise in E2 from the second third to the first third of pregnancy suppressed UAR, and this was associated with a decrease in EVT VEGF expression, although this did not prove that VEGF mediated this process. Therefore, the early E2-treated baboon provides a novel primate experimental model in which the temporal rise in levels of an endogenous hormone fundamental to pregnancy is simply advanced to investigate the regulation of UAR. Contrast-enhanced ultrasound (CEU)–mediated cavitation of acoustically active microbubble (MB) carriers (CEU/MB) has emerged as an innovative strategy to target delivery of genes in vivo to specific tissues (75–79). In the current study, therefore, our nonhuman primate model of prematurely elevating E2 and CEU/MB was used to selectively deliver VEGF DNA to the placental basal plate (PBP) during early baboon pregnancy to establish the role of VEGF in regulating UAR.
Materials and Methods
Animals
Female baboons (Papio anubis) were obtained from the Southwest National Primate Research Center (San Antonio, TX), housed individually in large primate cages, and received standard primate chow (Teklad Primate Diet 2050; Envigo, Frederick, MD) and fresh fruit twice daily, and water ad libitum. Females were paired with male baboons for 5 days at the anticipated time of ovulation as estimated by menstrual cycle history and the pattern of external sex skin turgescence. Day 1 of pregnancy was designated as the day preceding perineal deturgescence. Baboons were cared for and used strictly in accordance with the United States Department of Agriculture regulations and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed.). The present experimental protocol was approved by the Institutional Animal Care and Use Committees of the University of Maryland School of Medicine and Eastern Virginia Medical School.
Maternal baboons were untreated (n = 11), treated daily on days 25 to 59 of gestation (term, 184 days) with E2 benzoate [25 µg/kg body weight/d subcutaneously (SC) in 1.0 mL of sesame oil, n = 11], or treated daily on days 25 to 59 with E2 benzoate (25 µg/kg body weight/d SC) plus VEGF DNA and enhanced green fluorescent protein (GFP) DNA (n = 6) delivered to the PBP via CEU/MB as detailed below. At least eight randomly selected areas (5 mm3) of PBP tissue (which includes anchoring villi, trophoblastic shell, and decidua basalis) were collected following cesarean section performed under isoflurane anesthetization on day 60 of gestation. Blood samples (2 to 4 mL) were obtained from a maternal peripheral saphenous vein at 1- to 2-day intervals throughout the study period after sedation with ketamine HCl (10 mg/kg body weight, intramuscularly) and from the uterine veins at cesarean section for E2, progesterone, cortisol, and VEGF assay.
CEU/MB VEGF delivery
Cationic lipid-encapsulated/decafluorobutane-filled MBs (∼2 µm diameter, zeta potential of 40 to 60 mV in 1 mM KCl) were charge conjugated with a bicistronic plasmid vector encoding VEGF121 (Fig. 1) and GFP (synthesized by Nature Technology, Lincoln, NE) at a saturation ratio of 20 µg/108 MBs (79) and 0.5, 2.4, or 4.5 × 109 MBs with attached VEGF/GFP DNA/kg body weight/h infused (1.0 mL saline/min) into a maternal saphenous vein of ketamine-propofol–sedated baboons for 10 minutes on days 25, 35, 45, and 55 of gestation as illustrated in Fig. 2. VEGF121 is a VEGF-A isoform that is abundantly expressed in the placenta, lacks a membrane-bound motif, and thus is readily secreted/freely diffusible and induces association of the type 2 (Flk-1) VEGF receptor with heparin sulfate, which potentiates the magnitude and duration of VEGF signaling (80). A 2.0- to 6.0-MHz 6C2 transducer (Siemens Acuson Sequoia 512; Siemens, Malvern, PATeklad Primate Diet 2050; Envigo, Frederick, MD) with high B-mode frequency and a resting mechanical index (that is, negative acoustic pressure/square root of frequency) of 0.2 was positioned on the abdomen during MB/VEGF DNA infusion, and the ultrasound beam was directed to the PBP, identified by two-dimensional images of the placental chorionic villi, gestational sac, and decidua. The mechanical index was then increased to 1.9 with repeated 5-second burst pulses (three bursts per minute) during the 10-minute delivery period to collapse the MBs and thus detach/release the VEGF DNA within the PBP region (Fig. 2). The ultrasound waves used to cavitate the MBs induce sonoporation (i.e., transient pores in the cell membrane and active cell uptake), thereby facilitating DNA uptake into cells (81–83). Between days 25 and 55 of baboon pregnancy the placental disc/basal plate diameter increases from ∼2.0 to 3.5 cm, which corresponds to the ultrasound sector width at the acoustic focus.
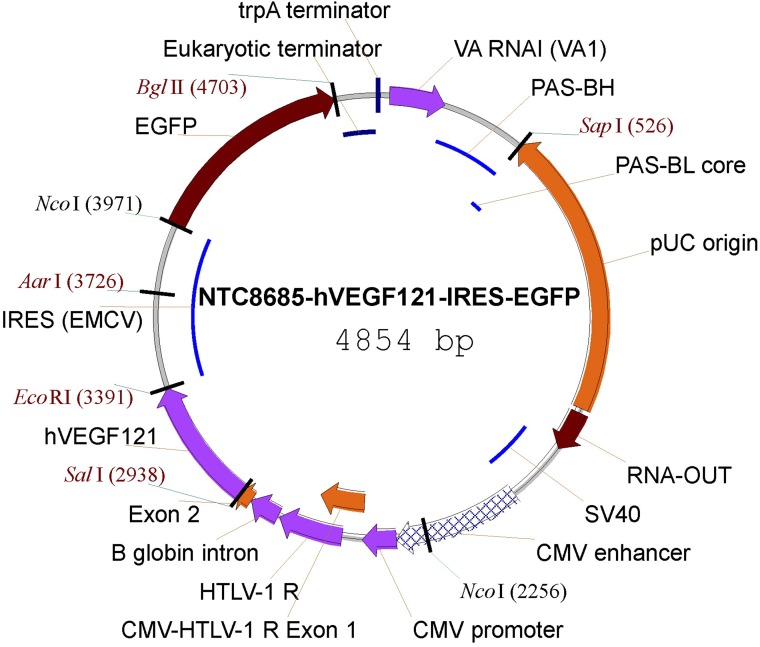
Figure 1.
Vector map of the NTC8685–human (h)VEGF121–IRES–EGFP. CMV, cytomegalovirus; EGFP, enhanced GFP; HTLV-1, human T-cell leukemia virus type 1; IRES (ECMV), internal ribosomal entry site (from the encephalomyocarditis virus); PAS, primosomal assembly site; PAS-BH, primosomal assembly site sequence on the pBR322 H strand; PAS-BL, primosomal assembly site sequence on the pBR322 L strand; pUC, plasmid University of California; SV, simian virus; trpA, tryptophan A; VA RNAI, virus-associated RNA I, a type of noncoding RNA that plays a role in regulating translation.
Figure 2.
Illustration of CEU/MB-targeted delivery of the VEGF gene to the PBP of E2-treated baboons during early pregnancy.
Quantification of UAR
PBP samples were fixed in 10% formalin, embedded in paraffin, sectioned (5 µm), and processed for hematoxylin and eosin histology and cytotrophoblast/epithelial cell–specific cytokeratin immunocytochemistry. Light microscopy (Nikon Eclipse E 1000 M; Nikon, Tokyo, Japan) and an image analysis system (IP Laboratory, version 3.63; Scanalytics, Fairfax, VA) were used to analyze all arteries in each tissue sample and categorize them into groups with diameters of <25, 26 to 50, 51 to 100, and >100 µm as performed in our laboratory (73). Arterial diameter was measured via an eyepiece micrometer as the smallest distance across the center of the vessel lumen from the inside edge of the surrounding smooth muscle (not invaded) or cytotrophoblast (invaded) layers. Each observed artery/arteriole was considered a separate vessel, although any single vessel may have appeared more than once as it traversed a given tissue section. The number of arterioles/arteries exhibiting EVT invasion in hematoxylin and eosin sections was quantified by identifying spiral arterioles/arteries in which the vessel wall was extensively occupied by trophoblasts. Vessel invasion was confirmed by demonstration of positive cytokeratin immunostaining in corresponding serial sections. The level of UAR is expressed as the number of vessels exhibiting EVT invasion divided by the total number of vessels counted.
Cytokeratin immunocytochemistry
PBP tissue sections were microwaved in 0.01 M sodium citrate buffer (pH 6.0) for antigen retrieval, incubated in H2O2 to inhibit endogenous peroxidase, and blocked with 5% normal horse serum (Millipore Sigma, St. Louis, MO). Tissues were incubated overnight at 4°C with mouse monoclonal antibody to cytokeratin [12.5 mg/mL dilution, 345779, CAM 5.2; BD Biosciences, San Jose, CA (84)] and then incubated for 1 hour at room temperature with biotinylated anti-mouse IgG [Vector Laboratories, Burlingame, CA (85)] and for 1 hour at room temperature with avidin–biotin–peroxidase complex (Vectastain Elite ABC horseradish peroxidase, Vector Laboratories). Tissue sections were developed using diaminobenzidine and Harris hematoxylin. Negative control for immunocytochemistry was the substitution of nonimmune mouse serum (Invitrogen/Thermo Fisher Scientific, Waltham, MA) for primary antibody.
GFP immunofluorescence
Placental plate tissue sections were microwaved in 0.01 M sodium citrate buffer (pH 6.0), incubated overnight at 4°C with polyclonal rabbit antibody to GFP [1:1000 dilution; A11122; Invitrogen/Thermo Fisher Scientific (86)], and then incubated for 1 hour at room temperature with Alexa Fluor donkey anti-rabbit secondary antibody [Invitrogen/Thermo Fisher Scientific (87)]. The sections were coverslipped with mounting media containing 4′,6-diamidino-2-phenylindole. Negative controls for immunofluorescence included tissue sections from animals not infused with plasmid VEGF/GFP and substitution of rabbit IgG isotype [Invitrogen/Thermo Fisher Scientific (88)] for primary antibody.
Proximity ligation assay of VEGF
Paraffin-embedded PBP tissue sections (5 µm thick) were subjected to 0.01 M sodium citrate and serum block and incubated overnight at 4°C with rabbit polyclonal primary VEGF antibody [1:250 dilution, RB 9031, P1611F; Thermo Fisher (89)]. Tissue was then incubated for 90 minutes at 37°C in a humidity chamber with proximity ligation assay (PLA) probes consisting of two secondary anti-rabbit antibodies each tagged with an oligonucleotide [diluted 1:5 in antibody diluent supplied in the Duolink In Situ PLA kit (Millipore Sigma) as described previously (74, 90)]. A ligation solution (supplied in the PLA kit and diluted 1:5 in water) consisting of two oligonucleotide linkers, complementary to each PLA probe and ligase (diluted 1:40 in the ligation solution), was added to tissue and incubated for 30 minutes at 37°C in a humidity chamber. Tissue sections were washed and incubated for 100 minutes with amplification solution (supplied in the PLA kit and diluted 1:5 in H2O) consisting of nucleotides and fluorescently labeled oligonucleotides and polymerase (diluted 1:80 in the amplification solution). The ligation step connects the hybridization linkers to the PLA probe oligonucleotides, forming a complete circle connecting both antibodies, and the polymerization step uses rolling circle DNA amplification to generate a concatemeric oligonucleotide product linked to the antibody complex. The fluorescently labeled oligonucleotides hybridize to the rolling circle amplification product, and the resultant signal is visible as a fluorescent dot. The tissues were then washed in decreasing concentrations of Tris-based buffers (PLA kit wash buffers A and B). Following the last wash, the PLA sections were incubated overnight at 4°C with cytokeratin (for cell-specific localization of VEGF) followed the next day by a 1-hour incubation with Alexa Fluor 488 donkey anti-mouse secondary antibody (Invitrogen/Thermo Fisher Scientific). The sections were then washed, air dried, and coverslipped with mounting media containing 4′,6-diamidino-2-phenylindole for detection of the nuclei. VEGF protein red PLA signals were visualized by fluorescence microscopy (Nikon Eclipse E 1000) and image analysis software (IP Laboratory). PLA signals were quantified and expressed per pixelated nuclear area using MetaMorph software (version 7.8.0.0; Molecular Devices, San Jose, CA) in a minimum of five randomly selected areas (32,350 µm2) of the distal region of anchoring villi, trophoblastic shell, and trophoblasts within spiral arteries. Negative controls for PLA included substitution of rabbit IgG isotype for the primary antibody or omission of one PLA probe.
Serum steroids and VEGF
Serum E2, progesterone, and cortisol levels were quantified by an Immulite competitive chemiluminescent enzyme immunoassay (91–93) and VEGF (free, bioavailable) levels by Quantikine ELISA [R&D Systems, Minneapolis, MN (94)]. For the Immulite immunoassays, the intra-assay and interassay CVs were 6.9% and 7.3%, respectively, for E2, 7.6% and 7.9% for progesterone, and 6.3% and 10.5% for cortisol. For the Quantikine assay, the intra-assay CV was 5.4% for VEGF.
Statistical analysis
Data were expressed as the mean ± SE and analyzed by ANOVA with post hoc comparison of the means by a Newman–Keuls multiple comparison test or the Kruskal–Wallis test and a Dunn multiple comparison test.
Results
Serum steroid and VEGF levels and weights
Mean (±SE) maternal peripheral serum E2 levels on days 25 to 60 of gestation were fivefold greater (P < 0.01) in E2-treated (0.75 ± 0.06 ng/mL) and E2 plus VEGF DNA–treated (0.81 ± 0.07 ng/mL) baboons than in untreated animals (0.15 ± 0.01 ng/mL). Maternal saphenous vein and uterine vein serum E2 levels on day 60 were approximately fourfold and twofold greater (P < 0.01), respectively, in E2-treated and E2 plus VEGF–treated baboons than in untreated animals (Table 1). However, serum E2 levels in the first trimester in E2-treated baboons were twofold lower than in the second trimester of normal pregnancy (∼2.0 ng/mL).
Table 1.
Maternal Serum E2 levels (ng/mL) and Placental, Maternal, and Fetal Body Weights in Baboons
| Treatment | N | Saphenous Vein | Uterine Vein | Placental Weight, g | Fetal Weight, g | Maternal Weight, kg |
|---|---|---|---|---|---|---|
| Untreated | 11 | 0.21 ± 0.03a | 0.59 ± 0.08a | 32.0 ± 1.8 | 12.6 ± 0.5 | 16.5 ± 0.7 |
| E2 | 11 | 0.82 ± 0.05b | 1.15 ± 0.05b | 29.0 ± 1.1 | 11.3 ± 0.5 | 15.7 ± 0.5 |
| E2 + VEGF | 6 | 0.87 ± 0.07b | 0.98 ± 0.06b | 30.4 ± 2.1 | 13.6 ± 0.9 | 15.8 ± 0.5 |
Mean ± SE maternal saphenous and uterine vein serum E2 levels and placental, maternal, and fetal body weights on day 60 of gestation in baboons untreated or treated daily on days 25 to 59 (term, 184 d) with E2 (25 µg/kg body weight/d SC) or on days 25 to 59 with E2 (25 µg/kg body weight/d) plus VEGF DNA delivered by CEU/MB to PBP on days 25, 35, 45, and 55.
Values marked with different letters are statistically different at P < 0.01.
Mean (±SE) maternal serum progesterone levels in untreated and E2-treated baboons were 12.2 ± 0.7 and 14.5 ± 1.2 ng/mL, respectively, in the saphenous vein on days 25 to 60 and 48.2 ± 3.9 and 47.6 ± 2.7 ng/mL, respectively, in the uterine vein on day 60. Maternal peripheral serum cortisol levels on days 25 to 60 were 32.8 ± 2.0 and 33.4 ± 1.8 µg/dL, respectively, in untreated and E2-treated animals. VEGF was not detectable (limit of detection ≤2 pg/mL) in either maternal saphenous or uterine vein serum or plasma in untreated, E2-treated, or E2 plus VEGF DNA–treated baboons.
Placental and maternal and fetal body weights on day 60 of gestation were similar in untreated, E2-treated, and E2 plus VEGF–treated baboons (Table 1).
CEU/MB imaging and UAR
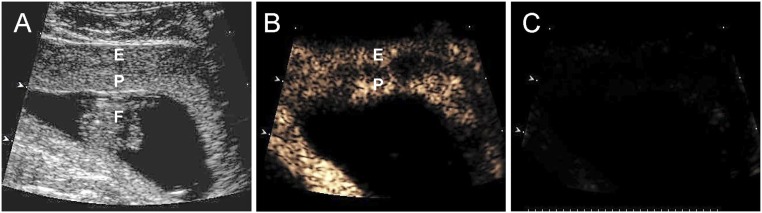
Figure 3 includes a grayscale Doppler image of the endometrium, placenta, and fetus (Fig. 3A) and CEU images showing the presence of VEGF DNA–labeled MBs/contrast agent in the endometrium and PBP but not the fetus before (Fig. 3B) and absence at the end (Fig. 3C) of a 5-second burst and collapse of the MBs to release VEGF DNA on day 45 of gestation. This visually confirms MB cavitation, which was repeated every 20 seconds to allow fresh MB wash-in.
Figure 3.
(A) Grayscale Doppler image of the endometrium (E), placenta (P), and fetus (F), and CEU images (B) before and (C) after collapse of the MBs/uncoupling of VEGF DNA on day 45 of gestation in an E2-treated baboon.
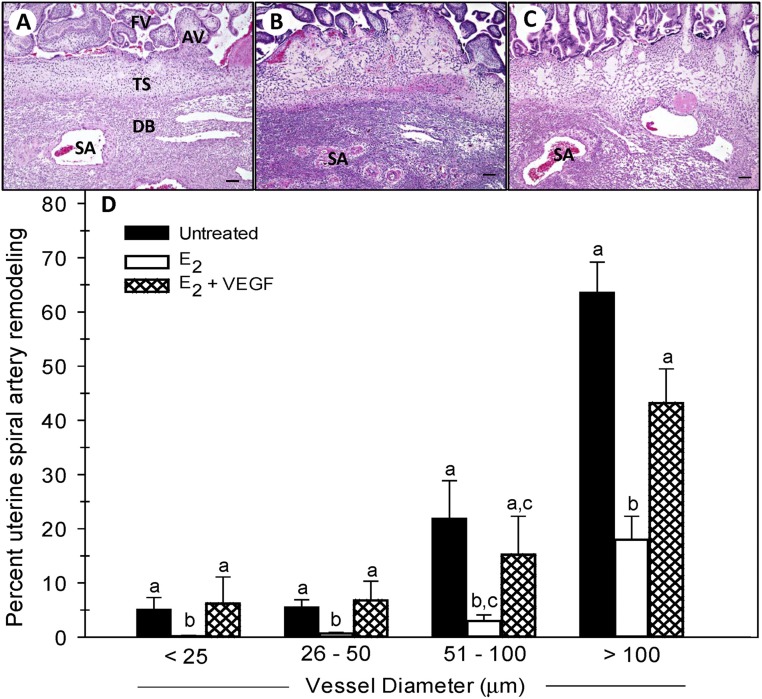
Figure 4 shows representative histology of the chorionic floating villi, anchoring villi (which are the principal source of EVTs), trophoblastic shell, and decidua basalis. Illustrated in this figure are spiral arteries invaded by EVTs in an untreated baboon (Fig. 4A), arteries not remodeled in an E2-treated baboon (Fig. 4B), and arteries invaded in an E2 and VEGF DNA–treated baboon (Fig. 4C). The marked structural modification of the vessel wall after EVT invasion in the untreated baboon contrasts with the highly coiled structure of the noninvaded arteries in the E2-treated animal. Importantly, spiral artery remodeling appeared to be restored by VEGF DNA delivery to E2-treated animals.
Figure 4.
(A–C) Photomicrographs of hematoxylin and eosin histology of the basal plate (scale bars, 100 µm) and (D) percentage remodeling of uterine spiral arteries and arterioles (i.e., the number of vessels exhibiting EVT invasion divided by total number of vessels counted) classified by vessel diameter in baboons untreated or treated with E2 daily on days 25 to 59 of gestation or with E2 daily on days 25 to 59 plus VEGF DNA targeted by CEU to the PBP on days 25, 35, 45, and 55. Values with different lettering are statistically different (P < 0.05 to P < 0.01) from one other. AV, anchoring villi; DB, decidua basalis; FV, floating villi; SA, spiral artery; TS, trophoblastic shell.
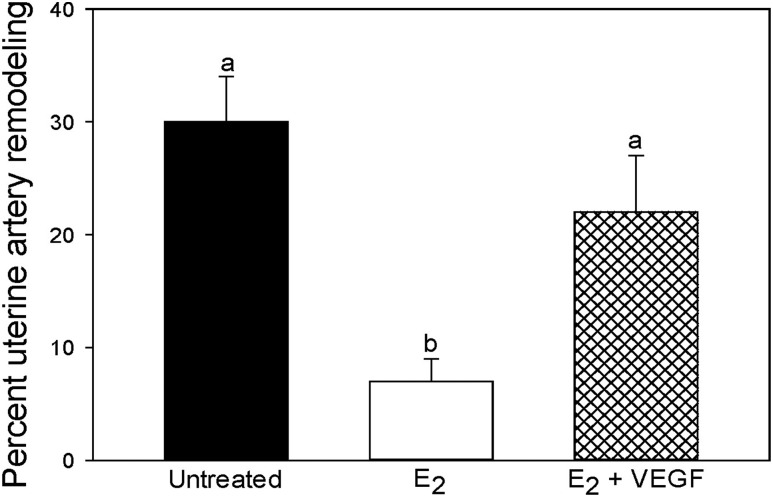
When quantified by image analysis as shown in Fig. 4D, the mean (±SE) number of uterine spiral arteries invaded and remodeled by EVTs, expressed as a ratio of the total number of arteries counted, in untreated baboons for vessels with diameters of <25 µm (5.0% ± 2.3%), 26 to 50 µm (5.5% ± 1.4%), 51 to 100 µm (21.8% ± 7.1%), and >100 µm (63.5% ± 5.7%) was lower (P < 0.05 to P < 0.001) in E2-treated animals (0.2% ± 0.1%, 0.7% ± 0.2%, 3.0% ± 1.1%, and 18.0% ± 4.3%, respectively). Importantly, the concomitant administration of E2 and targeted delivery of VEGF DNA to the PBP by CEU/MB restored the level of UAR to values (6.2% ± 4.9%, 6.8% ± 3.5%, 15.2% ± 7.1%, and 43.2% ± 6.3%, respectively; Fig. 4D) greater (P < 0.05 to P < 0.01) than in animals treated with E2 alone and similar to those in the untreated baboons. Thus, as shown in Fig. 5 the overall level of remodeling collectively of uterine arteries >25 µm in diameter was ∼75% lower (P < 0.001) in E2-treated (7% ± 2%) than in untreated baboons (30% ± 4%) and restored by E2 plus VEGF treatment to a level (22% ± 5%) greater (P < 0.05) than in animals treated only with E2 and similar to that exhibited in the untreated animals. The overall level of vessel remodeling appeared to be similar when 0.5, 2.4, or 4.5 × 109 VEGF-conjugated MBs/kg body weight/h were infused in E2-treated baboons, indicating a maximal response at the lowest dose of VEGF administered. In contrast to the changes in vessel remodeling, we have previously shown that the total number of spiral arteries of different sizes within the decidua basalis was similar in untreated and E2-treated baboons (95).
Figure 5.
Percentage remodeling of uterine spiral arteries collectively >25 µm in diameter on day 60 of gestation for the baboons in which artery invasion is shown in Fig. 4. Values with different lettering are statistically different (P < 0.05 to P < 0.001) from each other.
VEGF quantification by PLA
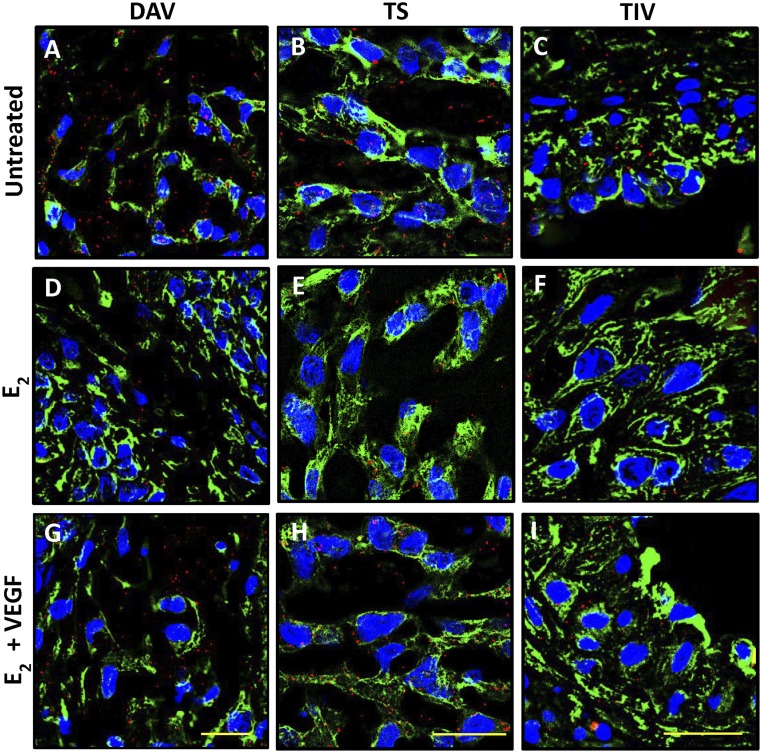
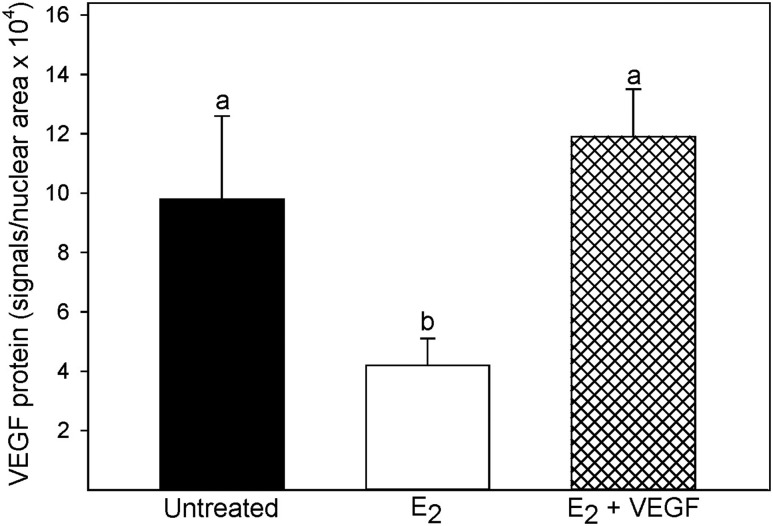
GFP was localized as green fluorescent dots in trophoblasts within uterine spiral arteries after VEGF/GFP delivery to E2-treated baboons (Fig. 6A), but they were not present in uterine arteries of untreated baboons that did not receive VEGF/GFP (Fig. 6B). To confirm the efficacy of in vivo transfection and treatment effects, VEGF protein levels were quantified by PLA in the PBP. VEGF protein was detected by PLA as small red immunofluorescent signals in cells of the distal anchoring villi (Fig. 7A), trophoblastic shell (Fig. 7B), and trophoblasts within vessels, that is, uterine spiral arteries (Fig. 7C). In contrast to the abundant expression of VEGF in untreated baboons, VEGF expression in E2-treated animals appeared to be lower in the distal region of the anchoring villi (Fig. 7D), trophoblastic shell (Fig. 7E), and trophoblasts that have migrated to and invaded the spiral arteries (Fig. 7F). Importantly, VEGF protein appeared to be restored in each of these locations in E2 plus VEGF–treated animals (Fig. 7G–7I). Thus, when quantified by PLA as the number of immunofluorescent signals per pixelated nuclear area × 104 collectively within the distal anchoring villi, trophoblastic shell, and trophoblasts within vessels, VEGF protein levels were more than twofold lower (P < 0.01) in E2-treated (4.2 ± 0.9) than in untreated baboons (9.8 ± 2.8) and restored to normal after VEGF gene delivery to E2-treated animals (11.9 ± 1.6, Fig. 8). When rabbit IgG isotype was substituted for the primary VEGF antibody, the mean (±SE) number of PLA signals per nuclear area × 104 was 0.1 ± 0.0 in the PBP tissue, confirming specificity of the PLA method and VEGF antibody.
Figure 6.
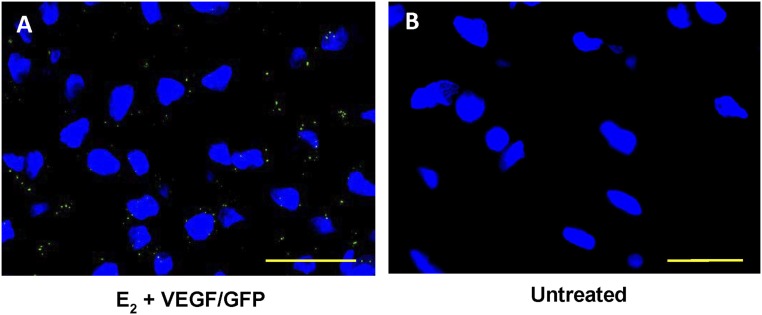
Photomicrographs of GFP localization (green dots) in trophoblasts within uterine spiral arteries on day 60 of gestation in (A) baboons treated with E2 plus VEGF/GFP DNA and (B) untreated animals. Nuclei are labeled blue. Scale bars, 25 µm.
Figure 7.
Photomicrographs of the localization of red PLA VEGF immunofluorescent signals in the distal anchoring villi (DAV), trophoblastic shell (TS), and trophoblasts in vessels (TIV) on day 60 of gestation in (A–C) untreated baboons and baboons (D–F) treated with E2 or (G–I) treated with E2 plus VEGF DNA. Each red PLA signal represents a single VEGF protein molecule. EVTs are labeled green (cytokeratin). Nuclei are labeled blue. Scale bars, 25 µm.
Figure 8.
VEGF protein levels quantified by PLA as analyzed collectively in the distal anchoring villi, trophoblastic shell, and trophoblast within uterine spiral arteries of the PBP on day 60 of gestation in baboons untreated, treated with E2, or treated with E2 plus VEGF DNA. Values with different lettering are statistically different (P < 0.05 to P < 0.01) from each other.
Discussion
The current study shows that delivery of the VEGF gene selectively to the PBP prevented the decrease in UAR induced by prematurely elevating E2 in early baboon pregnancy. Moreover, the current study showed that levels of VEGF were reduced in the PBP of baboons in which UAR was suppressed by early E2 treatment when compared with untreated animals in which endogenous E2 levels are very low, whereas VEGF levels were restored to normal in baboons concomitantly treated with E2 and the VEGF gene. Therefore, the current study establishes that VEGF has a pivotal role in vivo in regulating EVT invasion and remodeling of the uterine spiral arteries in nonhuman primate pregnancy.
A multitude of growth and related factors have been shown in cell culture to stimulate primary or immortalized trophoblasts or cancerous chorionic villous cells to migrate across semipermeable synthetic membrane or aggregate into tube-like structures (27–34). However, because of the difficulty in replicating in vitro the complex physiological, paracrine, and stereospecific interplay of different cell types and cellular remodeling processes that occur in vivo, it has not been established which, if any, of the many factors studied in vitro has a physiologically relevant role in vivo in early pregnancy in regulating EVT migration, invasion, and remodeling of the uterine spiral arteries. In the current study, VEGF delivery alone prevented the striking decline in uterine spiral artery transformation experimentally induced in early baboon pregnancy, thereby establishing its fundamentally important role in the latter process. Because VEGF promotes cell migration, remodeling, and proliferation mechanisms, including integrin expression (96), it is likely that the observed impact of VEGF on vessel transformation is mediated by integrin, matrix metalloproteinase, cell adhesion, and related cell remodeling molecules. Indeed, we have shown that expression of α1β1 and α5β1 integrins (74) and metalloproteinase-9 (97) within the PBP of E2-treated baboons was reduced, coinciding with a decrease in VEGF expression.
Demonstration of a regulatory role for VEGF on UAR in vivo in a well-established nonhuman primate model is highly significant from a basic science perspective considering the importance of UAR in promoting uterine artery blood flow and fetal development. Thus, we have shown that compared with untreated controls, uterine artery and fetal umbilical artery downstream flow impedance was increased, uterine artery volume flow was reduced (98), and fetal body weight was ∼10% lower (97) near term in vasochallenged baboons in which UAR had been suppressed by prematurely elevating E2 in early pregnancy. The results of the current study are also significant from a clinical perspective considering the impact that defective spiral artery remodeling has in underpinning the devastating consequences of adverse conditions of human pregnancy, notably preeclampsia, fetal growth restriction, and preterm birth (19–26). The current study is foundational for future investigation of the potential ability of VEGF delivery to prevent the appearance of the maternal vascular impairments associated with defective UAR and placental perfusion.
A limitation of the current study is the absence of administration of CEU/blank plasmid–labeled MBs to E2-treated baboons to assess potential effects of CEU acoustic pressure on spiral artery remodeling. However, because CEU/VEGF-conjugated MB delivery reversed and did not further alter the reduction in UAR observed in E2-treated baboons, it seems unlikely that negative acoustic pressure alone elicited by CEU accounts for the restoration of vessel remodeling achieved by CEU VEGF delivery. Moreover, the CEU procedure to collapse the MBs and deliver VEGF was performed for a short period of time and on only 4 days of gestation. Additionally, CEU/MB burst at the same level of mechanical index/acoustic pressure employed in baboons of the current study did not alter the constant level of maternal blood flow/flux rate into the intervillous space between 6 and 12 weeks of human pregnancy (99) or the level of spiral artery perfusion/flux rate induced by maternal protein restriction in rhesus macaques (100). Ultrasound acoustic pressure bursts as employed in the current study also did not cause maternal complement activation or placental apoptosis, inflammation, or oxidative stress during middle and late macaque gestation or placental microvascular hemorrhage or syncytiotrophoblast microvilli disruption during the first trimester of human pregnancy (101). In addition, extensive data showed no evidence of petechiae, fibrosis, or proangiogenic effects in rodents exposed to MB reporter control plasmid delivery and CEU acoustic pressure bursts (76, 78, 79, 102, 103). We propose that early noninvasive real-time detection of defective UAR in early abnormal pregnancy when combined with CEU/MB VEGF delivery specifically to the PBP point to the potential therapeutic value of CEU/MB gene delivery in adverse conditions of human pregnancy.
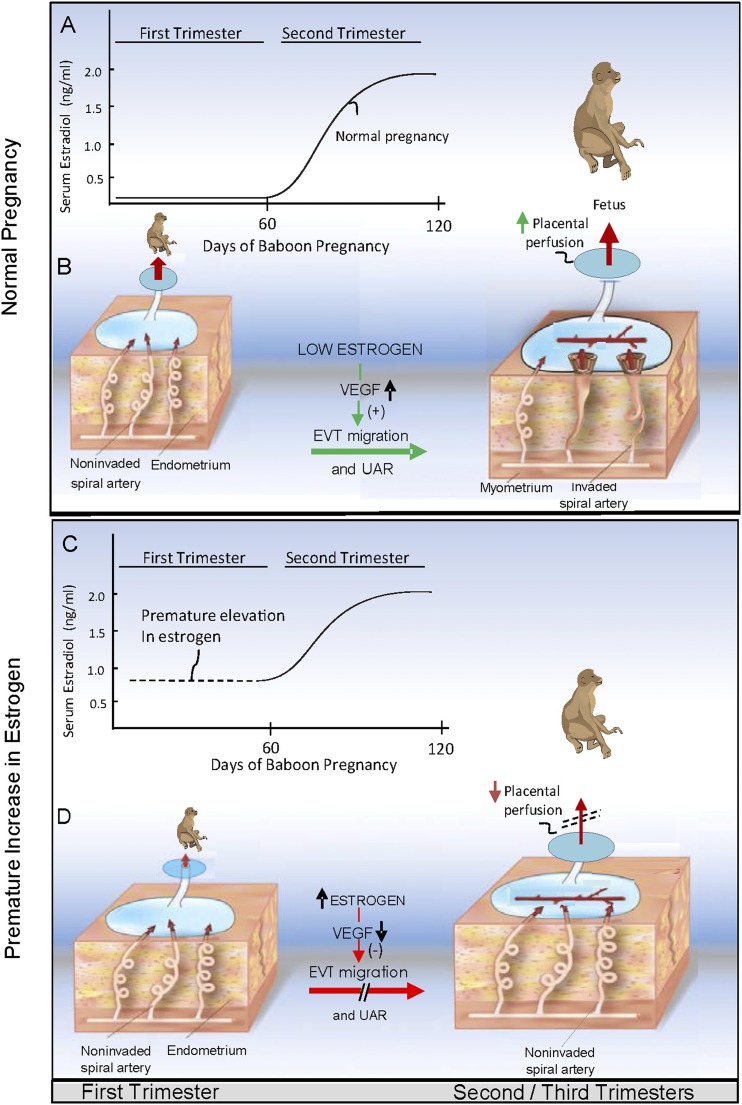
UAR is initiated early in gestation and proceeds rapidly during the first trimester of pregnancy, coinciding with low levels of E2 that are of ovarian origin. The rate of UAR then diminishes thereafter, coinciding with a progressive increase in E2 levels, which are now synthesized by the placenta. In the current study, shifting the rise in E2 from the second to the first trimester of baboon pregnancy repressed PBP VEGF expression and UAR, effects reversed by VEGF gene delivery. We further propose, therefore, that UAR is temporally coordinated by E2 and that: (i) the low level of E2 in the first trimester of pregnancy promotes placental EVT VEGF expression and thus a rapid rate of spiral artery remodeling (Fig. 9A and 9B), and (ii) the increase in E2 with advancing pregnancy, as experimentally elicited by prematurely elevating E2 in the first trimester, has a physiologically important role in repressing EVT VEGF formation and thus the extent to which the uterine arteries are remodeled (Fig. 9C and 9D). A restraint in the rate of UAR is physiologically important because extensive invasion and remodeling of the uterine arteries upstream, for example, in placenta acreta (104–106), impairs uterine artery vasomotor tone after delivery, a pathophysiological condition that causes excessive bleeding at the time of delivery.
Figure 9.
Proposed role of VEGF (A and B) in promoting EVT migration and UAR during normal pregnancy and (C and D) in mediating the repression of EVT migration and UAR induced by prematurely elevating estrogen in early primate pregnancy.
The experimental paradigm of prematurely elevating maternal serum E2 levels to ∼1 ng/mL in early baboon pregnancy was employed as an approach to investigate the regulation of UAR. However, maternal serum E2 levels are elevated to a similar nanogram range (i.e., ∼3 ng/mL) by superovulation during human in vitro fertilization (107–109), a procedure associated with an increased incidence of preeclampsia and fetal growth restriction (107–112), adverse conditions arising from improper placental perfusion. Freezing and implanting superovulated ova in a subsequent normal menstrual cycle in the presence of much lower levels of E2 diminished the risk of abnormal pregnancy (108). Moreover, maternal exposure to endocrine disruptors that mimic E2 action at the estrogen receptor site (113) and that are present in maternal serum in high levels during human pregnancy (114, 115) are thought to disrupt reproductive function and cause fetal growth restriction (116). Indeed, treatment of mice in early gestation with the endocrine disruptor bisphenol A at 50 µg/kg body weight, a level comparable to the daily human intake exposure (117), impaired spiral artery remodeling (118). Although an increase, decrease, or no change in E2 levels has been shown at mid-gestation and term in human preeclampsia (119–121), this may secondarily reflect aberrant placental function elicited by this adverse condition of pregnancy.
VEGF DNA arriving in the uterine arteries during infusion of baboons was expected to be detached from the MBs by the increase in acoustic pressure and transcribed/translated into RNA/protein within tissues fed by the arterial effluent. The abundant expression of VEGF protein observed in trophoblasts within the spiral arteries, distal ends of the anchoring villi, and trophoblastic shell of E2 plus VEGF DNA–treated baboons is consistent with the latter expectation and confirms VEGF transfection. VEGF transfection in tissues other than within the PBP is unlikely because MBs do not cross the syncytial border into the villous placenta or fetus [Fig. 3 and Hua et al. (122) and Arthuis et al. (123)], cell sonoporation was targeted to the PBP by the CEU beam, and free plasmid DNA is rapidly degraded by DNAase within the bloodstream (124, 125). Although cells in addition to EVTs may well have been transfected by VEGF—for example, fibroblasts and immune cells within the PBP and endothelial cells lining the spiral arteries—this would not confound the results because endogenous VEGF expressed by such cells as well as by EVTs may have a role in UAR.
We have previously shown that the volumes (i.e., mass) of the placental villous cytotrophoblasts and syncytiotrophoblast, as well as the level of placental villous cytotrophoblast 5-bromo-2′-deoxyuridine and Ki67 immunostaining reflecting proliferation, were unaltered by E2 treatment in early baboon pregnancy (126). Additionally, maternal serum levels of progesterone, which is produced primarily by the villous placenta after days 20 to 25 of baboon pregnancy (127, 128), and cortisol produced by the adrenal (129) were similar in untreated and E2/VEGF-treated baboons of the current study. Because the E2 receptor is also expressed in the latter tissues, these findings suggest that the regulation of uterine spiral artery remodeling by E2 reflects a direct action of E2 on VEGF expression within the PBP and does not secondarily involve changes in placental villous trophoblast or adrenal structure or function.
A major advantage of CEU/MB gene delivery is that the VEGF-conjugated MBs did not cross the syncytia border and enter the villous placenta and fetus [Fig. 3B and Hua et al. (122) and Arthuis et al. (123)]. However, because the syncytiotrophoblast is bathed in maternal blood, once detached from the collapsed MBs, VEGF may enter the villous placenta. This would not confound the results of the current study, as we have previously shown that in contrast to the E2-induced decline in VEGF expression by the EVTs, expression of VEGF is increased in placental villous trophoblasts by prematurely elevating E2 levels in early baboon pregnancy (130, 131). The divergent effects of E2 on VEGF expression by the extravillous and villous compartments are also exhibited in other estrogen-responsive tissues, including the breast and prostate (132). The differential regulation of VEGF by E2 in the extravillous and villous placenta may reflect the cell-specific presence of estrogen receptor subtypes, as well as functional enhancers, coactivators and/or corepressors that modulate E2-regulated target gene transcription (133–135).
In summary, VEGF gene delivery selectively to the PBP by CEU/MB prevented the decrease in UAR elicited by prematurely elevating E2 levels in early baboon pregnancy, indicating that VEGF has a major role in vivo in regulating EVT invasion and remodeling of the uterine spiral arteries during nonhuman primate pregnancy. We propose that the low level of ovarian E2 in the first trimester of pregnancy promotes placental EVT VEGF expression and consequently a rapid rate of UAR and the progressive rise in placental E2 levels during the second and third trimesters, as experimentally induced by prematurely elevating E2 in the first trimester, and has an important role in repressing EVT VEGF formation and thus the extent of UAR to promote a physiologically normal level of uteroplacental perfusion.
Acknowledgments
The authors thank Irene Baranyk for computer preparation of the manuscript, Terrie Lynch for the assay of serum steroids, and Marcia Burch for the assay of serum VEGF.
Financial Support: This work was supported by National Institutes of Health Research Grant R01 HD093070.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CEU
contrast-enhanced ultrasound
- CEU/MB
contrast-enhanced ultrasound–mediated cavitation of acoustically active microbubble carriers
- E2
estradiol
- EGF
epidermal growth factor
- EVT
extravillous trophoblast
- GFP
green fluorescent protein
- MB
microbubble
- PBP
placental basal plate
- PLA
proximity ligation assay
- SC
subcutaneously
- UAR
uterine artery remodeling
- VEGF
vascular endothelial growth factor
References and Notes
- 1. Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat. 1960;94:297–328. [PMC free article] [PubMed] [Google Scholar]
- 2. Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124(6):647–652. [DOI] [PubMed] [Google Scholar]
- 3. Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. [DOI] [PubMed] [Google Scholar]
- 4. Enders AC, King BF. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am J Anat. 1991;192(4):329–346. [DOI] [PubMed] [Google Scholar]
- 5. Frank HG, Kaufmann P. Nonvillous parts and trophoblast invasion. In: Benirschke K, Kaufmann P, Baergen RN, eds. Pathology of the Human Placenta. 5th ed. New York, NY: Springer; 2006:191–312. [Google Scholar]
- 6. Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol. 2014;38(3):131–132. [DOI] [PubMed] [Google Scholar]
- 7. Friedman AM, Cleary KL. Prediction and prevention of ischemic placental disease. Semin Perinatol. 2014;38(3):177–182. [DOI] [PubMed] [Google Scholar]
- 8. Heazell AE, Worton SA, Higgins LE, Ingram E, Johnstone ED, Jones RL, Sibley CP. IFPA Gábor Than Award Lecture: recognition of placental failure is key to saving babies’ lives. Placenta. 2015;36(Suppl 1):S20–S28. [DOI] [PubMed] [Google Scholar]
- 9. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36(1):56–59. [DOI] [PubMed] [Google Scholar]
- 10. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2Suppl):S745–S761. [DOI] [PubMed] [Google Scholar]
- 11. Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J Obstet Gynaecol Br Commonw. 1964;71(2):222–230. [DOI] [PubMed] [Google Scholar]
- 12. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. [DOI] [PubMed] [Google Scholar]
- 13. Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101(8):669–674. [DOI] [PubMed] [Google Scholar]
- 14. Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046–1054. [DOI] [PubMed] [Google Scholar]
- 15. Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–1142. [DOI] [PubMed] [Google Scholar]
- 16. Myatt L, Roberts JM. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17(11):83. [DOI] [PubMed] [Google Scholar]
- 17. Veerbeek JH, Brouwers L, Koster MP, Koenen SV, van Vliet EO, Nikkels PG, Franx A, van Rijn BB. Spiral artery remodeling and maternal cardiovascular risk: the spiral artery remodeling (SPAR) study. J Hypertens. 2016;34(8):1570–1577. [DOI] [PubMed] [Google Scholar]
- 18. Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96(4):1509–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):93–104. [DOI] [PubMed] [Google Scholar]
- 20. Simmons LA, Hennessy A, Gillin AG, Jeremy RW. Uteroplacental blood flow and placental vascular endothelial growth factor in normotensive and pre-eclamptic pregnancy. BJOG. 2000;107(5):678–685. [DOI] [PubMed] [Google Scholar]
- 21. Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med. 2015;21(2):88–97. [DOI] [PubMed] [Google Scholar]
- 22. Aardema MW, Oosterhof H, Timmer A, van Rooy I, Aarnoudse JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta. 2001;22(5):405–411. [DOI] [PubMed] [Google Scholar]
- 23. Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–384. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(Pt 1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19(1):103–111. [DOI] [PubMed] [Google Scholar]
- 26. Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1Suppl):S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lala N, Girish GV, Cloutier-Bosworth A, Lala PK. Mechanisms in decorin regulation of vascular endothelial growth factor-induced human trophoblast migration and acquisition of endothelial phenotype. Biol Reprod. 2012;87(3):59. [DOI] [PubMed] [Google Scholar]
- 29. Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S. Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod. 2004;10(4):229–235. [DOI] [PubMed] [Google Scholar]
- 30. Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;266(2):223–237. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Zhu H, Klausen C, Peng B, Leung PC. Vascular endothelial growth factor-A (VEGF-A) mediates activin A-induced human trophoblast endothelial-like tube formation. Endocrinology. 2015;156(11):4257–4268. [DOI] [PubMed] [Google Scholar]
- 32. Knöfler M, Pollheimer J. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta. 2012;33(Suppl):S55–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albrecht ED, Pepe GJ. Placental endocrine function and hormone action. In: Plant T, Zeleznik A, eds. Knobil and Neill’s Physiology of Reproduction. 4th ed.New York, NY: Academic Press; 2015:1783–1834. [Google Scholar]
- 34. McNally R, Alqudah A, Obradovic D, McClements L. Elucidating the pathogenesis of pre-eclampsia using in vitro models of spiral uterine artery remodelling. Curr Hypertens Rep. 2017;19(11):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Athanassiades A, Hamilton GS, Lala PK. Vascular endothelial growth factor stimulates proliferation but not migration or invasiveness in human extravillous trophoblast. Biol Reprod. 1998;59(3):643–654. [DOI] [PubMed] [Google Scholar]
- 36. Fitzpatrick TE, Lash GE, Yanaihara A, Charnock-Jones DS, Macdonald-Goodfellow SK, Graham CH. Inhibition of breast carcinoma and trophoblast cell invasiveness by vascular endothelial growth factor. Exp Cell Res. 2003;283(2):247–255. [DOI] [PubMed] [Google Scholar]
- 37. Dubinsky V, Poehlmann TG, Suman P, Gentile T, Markert UR, Gutierrez G. Role of regulatory and angiogenic cytokines in invasion of trophoblastic cells. Am J Reprod Immunol. 2010;63(3):193–199. [DOI] [PubMed] [Google Scholar]
- 38. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. [DOI] [PubMed] [Google Scholar]
- 39. Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188(1):177–182. [DOI] [PubMed] [Google Scholar]
- 40. Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, Uzan S, Rondeau E. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50(9):1702–1703. [DOI] [PubMed] [Google Scholar]
- 41. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wathén KA, Tuutti E, Stenman UH, Alfthan H, Halmesmäki E, Finne P, Ylikorkala O, Vuorela P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91(1):180–184. [DOI] [PubMed] [Google Scholar]
- 43. Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, Simpson NA, North RA; SCOPE consortium. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG. 2013;120(10):1215–1223. [DOI] [PubMed] [Google Scholar]
- 44. Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, Thorp JM Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Iams JD, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y, Anderson GD; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119(6):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baker PN, Krasnow J, Roberts JM, Yeo KT. Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet Gynecol. 1995;86(5):815–821. [DOI] [PubMed] [Google Scholar]
- 46. Cooper JC, Sharkey AM, Charnock-Jones DS, Palmer CR, Smith SK. VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1996;103(12):1191–1196. [DOI] [PubMed] [Google Scholar]
- 47. Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179(6 Pt 1):1539–1544. [DOI] [PubMed] [Google Scholar]
- 48. Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89(5):2484–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Armant DR, Fritz R, Kilburn BA, Kim YM, Nien JK, Maihle NJ, Romero R, Leach RE. Reduced expression of the epidermal growth factor signaling system in preeclampsia. Placenta. 2015;36(3):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, Ness RB. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am J Obstet Gynecol. 2005;193(1):185–191. [DOI] [PubMed] [Google Scholar]
- 51. Powers RW, Roberts JM, Plymire DA, Pucci D, Datwyler SA, Laird DM, Sogin DC, Jeyabalan A, Hubel CA, Gandley RE. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60(1):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196(4):396.e1–396.e7, discussion 396.e7. [DOI] [PubMed] [Google Scholar]
- 53. Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, Davisson RL. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50(4):686–692. [DOI] [PubMed] [Google Scholar]
- 55. Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves AC, Gröne HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14(6B):1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mateus J, Bytautiene E, Lu F, Tamayo EH, Betancourt A, Hankins GD, Longo M, Saade GR. Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am J Physiol Heart Circ Physiol. 2011;301(5):H1781–H1787. [DOI] [PubMed] [Google Scholar]
- 57. Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55(2):380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):H541–H550. [DOI] [PubMed] [Google Scholar]
- 59. Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, Peebles DM, David AL. Peri- and postnatal effects of prenatal adenoviral VEGF gene therapy in growth-restricted sheep. Biol Reprod. 2016;94(6):142. [DOI] [PubMed] [Google Scholar]
- 60. Khankin EV, Mandala M, Colton I, Karumanchi SA, Osol G. Hemodynamic, vascular, and reproductive impact of FMS-like tyrosine kinase 1 (FLT1) blockade on the uteroplacental circulation during normal mouse pregnancy. Biol Reprod. 2012;86(2):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. [DOI] [PubMed] [Google Scholar]
- 62. Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71(10):977–984. [DOI] [PubMed] [Google Scholar]
- 63. McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta. 2011;32(6):413–419. [DOI] [PubMed] [Google Scholar]
- 64. Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol. 2004;24(6):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. LaMarca B, Amaral LM, Harmon AC, Cornelius DC, Faulkner JL, Cunningham MW Jr. Placental ischemia and resultant phenotype in animal models of preeclampsia. Curr Hypertens Rep. 2016;18(5):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23(1):3–19. [DOI] [PubMed] [Google Scholar]
- 67. Albrecht ED, Pepe GJ. Endocrinology of pregnancy. In: Brans YW, Kuehl TJ, eds. Non-Human Primates in Perinatal Research. New York, NY: John Wiley & Sons; 1988:13–78. [Google Scholar]
- 68. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda). 2009;24:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Silva JF, Serakides R. Intrauterine trophoblast migration: a comparative view of humans and rodents. Cell Adhes Migr. 2016;10(1–2):88–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Enders AC, Blankenship TN, Fazleabas AT, Jones CJ. Structure of anchoring villi and the trophoblastic shell in the human, baboon and macaque placenta. Placenta. 2001;22(4):284–303. [DOI] [PubMed] [Google Scholar]
- 71. VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol. 1997;26(3):113–119. [DOI] [PubMed] [Google Scholar]
- 72. Cox LA, Mahaney MC, Vandeberg JL, Rogers J. A second-generation genetic linkage map of the baboon (Papio hamadryas) genome. Genomics. 2006;88(3):274–281. [DOI] [PubMed] [Google Scholar]
- 73. Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology. 2008;149(10):5078–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bonagura TW, Babischkin JS, Aberdeen GW, Pepe GJ, Albrecht ED. Prematurely elevating estradiol in early baboon pregnancy suppresses uterine artery remodeling and expression of extravillous placental vascular endothelial growth factor and α1β1 and α5β1 integrins. Endocrinology. 2012;153(6):2897–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108(8):1022–1026. [DOI] [PubMed] [Google Scholar]
- 76. Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101(3):295–303. [DOI] [PubMed] [Google Scholar]
- 77. Belcik JT, Qi Y, Kaufmann BA, Xie A, Bullens S, Morgan TK, Bagby SP, Kolumam G, Kowalski J, Oyer JA, Bunting S, Lindner JR. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol. 2012;60(7):618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xie A, Belcik T, Qi Y, Morgan TK, Champaneri SA, Taylor S, Davidson BP, Zhao Y, Klibanov AL, Kuliszewski MA, Leong-Poi H, Ammi A, Lindner JR. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc Imaging. 2012;5(12):1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xie A, Wu MD, Cigarroa G, Belcik JT, Ammi A, Moccetti F, Lindner JR. Influence of DNA-microbubble coupling on contrast ultrasound-mediated gene transfection in muscle and liver. J Am Soc Echocardiogr. 2016;29(8):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu D, Fuster MM, Lawrence R, Esko JD. Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J Biol Chem. 2011;286(1):737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Meijering BD, Juffermans LJ, van Wamel A, Henning RH, Zuhorn IS, Emmer M, Versteilen AM, Paulus WJ, van Gilst WH, Kooiman K, de Jong N, Musters RJ, Deelman LE, Kamp O. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res. 2009;104(5):679–687. [DOI] [PubMed] [Google Scholar]
- 82. Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J Control Release. 2006;114(1):89–99. [DOI] [PubMed] [Google Scholar]
- 83. Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics. 2012;2(12):1208–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.RRID:AB_2800363, https://scicrunch.org/resolver/AB_2800363.
- 85.RRID:AB_2336171, https://scicrunch.org/resolver/AB_2336171.
- 86.RRID:AB_221569, https://scicrunch.org/resolver/AB_221569.
- 87.RRID:AB_2535792, https://scicrunch.org/resolver/AB_2535792.
- 88.RRID:AB_2532938, https://scicrunch.org/resolver/AB_2532938.
- 89.RRID:AB_149828, https://scicrunch.org/resolver/AB_149828.
- 90. Bonagura TW, Babischkin JS, Pepe GJ, Albrecht ED. Assessment of protein expression by proximity ligation assay in the nonhuman primate endometrium, placenta, and fetal adrenal in response to estrogen. In: Eyster KM, ed. Methods in Molecular Biology: Estrogen Receptors. Vol 1366.New York, NY: Humana Press/Springer; 2016:149–161. [DOI] [PubMed] [Google Scholar]
- 91.RRID:AB_2800400, https://scicrunch.org/resolver/AB_2800400.
- 92.RRID:AB_2800399, https://scicrunch.org/resolver/AB_2800399.
- 93.RRID:AB_2800398, https://scicrunch.org/resolver/AB_2800398.
- 94.RRID:AB_2800364, https://scicrunch.org/resolver/AB_2800364.
- 95. Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta. 2006;27(4-5):483–490. [DOI] [PubMed] [Google Scholar]
- 96. Fukushima K, Miyamoto S, Tsukimori K, Kobayashi H, Seki H, Takeda S, Kensuke E, Ohtani K, Shibuya M, Nakano H. Tumor necrosis factor and vascular endothelial growth factor induce endothelial integrin repertories, regulating endovascular differentiation and apoptosis in a human extravillous trophoblast cell line. Biol Reprod. 2005;73(1):172–179. [DOI] [PubMed] [Google Scholar]
- 97. Babischkin JS, Aberdeen GW, Pepe GJ, Albrecht ED, unpublished observation.
- 98. Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: impact on uterine and fetal blood flow dynamics. Am J Physiol Heart Circ Physiol. 2012;302(10):H1936–H1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Roberts VH, Morgan TK, Bednarek P, Morita M, Burton GJ, Lo JO, Frias AE. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: new insights from contrast-enhanced ultrasound and tissue histopathology. Hum Reprod. 2017;32(12):2382–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roberts VH, Lo JO, Lewandowski KS, Blundell P, Grove KL, Kroenke CD, Sullivan EL, Roberts CT Jr, Frias AE. Adverse placental perfusion and pregnancy outcomes in a new nonhuman primate model of gestational protein restriction. Reprod Sci. 2018;25(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Roberts VH, Lo JO, Salati JA, Lewandowski KS, Lindner JR, Morgan TK, Frias AE. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am J Obstet Gynecol. 2016;214(3):369.e1–369.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Front Neurol Neurosci. 2015;36:11–22. [DOI] [PubMed] [Google Scholar]
- 103. Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19(5):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639–645. [DOI] [PubMed] [Google Scholar]
- 105. Snegovskikh D, Clebone A, Norwitz E. Anesthetic management of patients with placenta accreta and resuscitation strategies for associated massive hemorrhage. Curr Opin Anaesthesiol. 2011;24(3):274–281. [DOI] [PubMed] [Google Scholar]
- 106. Hannon T, Innes BA, Lash GE, Bulmer JN, Robson SC. Effects of local decidua on trophoblast invasion and spiral artery remodeling in focal placenta creta—an immunohistochemical study. Placenta. 2012;33(12):998–1004. [DOI] [PubMed] [Google Scholar]
- 107. Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–1379. [DOI] [PubMed] [Google Scholar]
- 108. Imudia AN, Awonuga AO, Kaimal AJ, Wright DL, Styer AK, Toth TL. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertil Steril. 2013;99(1):168–173. [DOI] [PubMed] [Google Scholar]
- 109. Wei D, Yu Y, Sun M, Shi Y, Sun Y, Deng X, Li J, Wang Z, Zhao S, Zhang H, Legro RS, Chen ZJ. The effect of supraphysiological estradiol on pregnancy outcomes differs between women with PCOS and ovulatory women. J Clin Endocrinol Metab. 2018;103(7):2735–2742. [DOI] [PubMed] [Google Scholar]
- 110. Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103(3):551–563. [DOI] [PubMed] [Google Scholar]
- 111. Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, Hankins GD, Eddleman K, Dolan S, Dugoff L, Craigo S, Timor IE, Carr SR, Wolfe HM, Bianchi DW, D’Alton ME. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106(5 Pt 1):1039–1045. [DOI] [PubMed] [Google Scholar]
- 112. Calhoun KC, Barnhart KT, Elovitz MA, Srinivas SK. Evaluating the association between assisted conception and the severity of preeclampsia. ISRN Obstet Gynecol. 2011;2011:928592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab. 2006;20(1):63–75. [DOI] [PubMed] [Google Scholar]
- 114. Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16(6):735–739. [DOI] [PubMed] [Google Scholar]
- 116. Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chapin RE, Adams J, Boekelheide K, Gray LE Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395. [DOI] [PubMed] [Google Scholar]
- 118. Müller JE, Meyer N, Santamaria CG, Schumacher A, Luque EH, Zenclussen ML, Rodriguez HA, Zenclussen AC. Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci Rep. 2018;8(1):9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zeisler H, Jirecek S, Hohlagschwandtner M, Knöfler M, Tempfer C, Livingston JC. Concentrations of estrogens in patients with preeclampsia. Wien Klin Wochenschr. 2002;114(12):458–461. [PubMed] [Google Scholar]
- 120. Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G, Uzan S, Rondeau E, Rozenberg P, Rafestin-Oblin ME. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203(5):477.e1–477.e9. [DOI] [PubMed] [Google Scholar]
- 121. Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. 2013;61(2):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hua X, Zhu LP, Li R, Zhong H, Xue YF, Chen ZH. Effects of diagnostic contrast-enhanced ultrasound on permeability of placental barrier: a primary study. Placenta. 2009;30(9):780–784. [DOI] [PubMed] [Google Scholar]
- 123. Arthuis CJ, Novell A, Escoffre JM, Patat F, Bouakaz A, Perrotin F. New insights into uteroplacental perfusion: quantitative analysis using Doppler and contrast-enhanced ultrasound imaging. Placenta. 2013;34(5):424–431. [DOI] [PubMed] [Google Scholar]
- 124. Emlen W, Mannik M. Kinetics and mechanisms for removal of circulating single-stranded DNA in mice. J Exp Med. 1978;147(3):684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Emlen W, Mannik M. Effect of DNA size and strandedness on the in vivo clearance and organ localization of DNA. Clin Exp Immunol. 1984;56(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- 126. Babischkin JS, Burleigh DW, Mayhew TM, Pepe GJ, Albrecht ED. Developmental regulation of morphological differentiation of placental villous trophoblast in the baboon. Placenta. 2001;22(4):276–283. [DOI] [PubMed] [Google Scholar]
- 127. Castracane VD, Goldzieher JW. Timing of the luteal-placental shift in the baboon (Papio cynocephalus). Endocrinology. 1986;118(2):506–512. [DOI] [PubMed] [Google Scholar]
- 128. Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev. 1990;11(1):124–150. [DOI] [PubMed] [Google Scholar]
- 129. Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16(5):608–648. [DOI] [PubMed] [Google Scholar]
- 130. Albrecht ED, Robb VA, Pepe GJ. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab. 2004;89(11):5803–5809. [DOI] [PubMed] [Google Scholar]
- 131. Robb VA, Pepe GJ, Albrecht ED. Acute temporal regulation of placental vascular endothelial growth/permeability factor expression in baboons by estrogen. Biol Reprod. 2004;71(5):1694–1698. [DOI] [PubMed] [Google Scholar]
- 132. Lee JE, Chung KW, Han W, Kim SW, Kim SW, Shin HJ, Bae JY, Noh DY. Effect of estrogen, tamoxifen and epidermal growth factor on the transcriptional regulation of vascular endothelial growth factor in breast cancer cells. Anticancer Res. 2004;24(6):3961–3964. [PubMed] [Google Scholar]
- 133. Hu X, Lazar MA. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab. 2000;11(1):6–10. [DOI] [PubMed] [Google Scholar]
- 134. Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol. 2006;20(5):1048–1060. [DOI] [PubMed] [Google Scholar]
- 135. Hewitt SC, Korach KS. Estrogen receptors: new directions in the new millennium. Endocr Rev. 2018;39(5):664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]