Abstract
Backgrounds
A recent GWAS Study found a new locus (rs9810888 in CACNA1D) was associated with blood pressure (BP) in Chinese adults. But whether the association exists in children is unknown. Whether lifestyle behaviors could interact with rs9810888 on BP is not clear. This study aimed to identify the association between rs9810888 and BP in children, and also explore the gene-lifestyle interaction.
Methods
A case-control study was conducted among 2030 Chinese children aged 7 to 18 years. Genotyping was conducted by using the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Lifestyle behaviors were investigated with questionnaire.
Results
With adjustment for age, age square, sex, study group and body mass index (BMI), rs9810888 was significantly associated with diastolic BP (DBP) (b = 1.69, p = 0.021) and mean arterial BP (MAP) (b = 1.56, p = 0.010). Stratified analysis showed that the rs9810888 GG genotype carriers had higher DBP than GT/TT carriers (b = 3.78, p = 0.023) in the subgroup having protein intake (meat/fish/soybeans/egg) <twice/day, but not in the subgroup ≥twice/day. In addition, in the subgroup with screen time≥2h/day, the rs9810888 GG genotype carriers had higher DBP than GT/TT carriers (b = 5.49, p = 0.012), but not in the subgroup with screen time<2h/day. For MAP, in the subgroup having either fruits or vegetables < twice/day, the rs9810888 GG genotype carriers had higher MAP than GT/TT carriers (b = 2.64, p = 0.037), but not in the subgroup with both fruits and vegetables intake ≥twice/day. Additionally, in the subgroup with screen time≥2h/day, the rs9810888 GG genotype carriers had higher MAP than GT/TT carriers (b = 4.80, p = 0.017), but not in those with screen time<2h/day.
Conclusions
The CACNA1D rs9810888 polymorphism was significantly associated with DBP and MAP. In addition, unhealthy lifestyle behaviors and rs9810888 GG genotype had combined effects on BP level among Chinese children.
Backgrounds
Hypertension is the largest metabolic contributor to global disability-adjusted life-years of both men and women in 2016, which is a major public health problem all over the world[1]. Blood pressure (BP) is known as a complex phenotype affected by genetic and environmental factors. Genetically, the heredity of BP is estimated to be about 40~60%[2, 3]. But only a small part (2~3%) of the BP variance could be explained by the polymorphisms identified in Genome-Wide Association Study (GWAS)[4].
Environmental factors also play an important role in the etiology of hypertension. In addition, environmental risk factor could be modified, which mainly include physical activity, sedentary behaviors, and dietary behaviors[5].Thus, the gene-environmental interaction would be useful for developing practical prevention measures. Personalized lifestyle recommendation based on genetic background are being promoted in public health and precision medicine in recent years[6].
A recent GWAS found a polymorphism (rs9810888) in calcium voltage-gated channel subunit alpha1 D gene (CACNA1D) was associated with BP in Chinese adults[7]. CACNA1D is a member of the α-1 subunit family of voltage-dependent calcium channels, most of which play an important role in BP regulation and are targets of antihypertensive drugs calcium channel blockers. CACNA1D, together with CACNA1C, are widely expressed in (neuro)endocrine cells and electrically excitable cells of the cardiovascular system, especially vascular smooth muscle and cardiac muscle cells[8]. The polymorphisms of these genes could lead to channel activation at more hyperpolarized membrane potentials, resulting in increased Ca2+ influx in the pathogenesis of disease[9]. But whether the newly identified locus rs9810888 in CACNA1D was associated with BP in children is unknown. Therefore, we hypothesize that rs9810888 polymorphism is associated with BP in Chinese children, then the primary objective of the study was to explore the association in Chinese children.
Besides, gene-lifestyle interaction is now considered as an important explanation of high blood pressure[10]. Many studies have illustrated the gene-lifestyle interaction in the level of blood pressure or risk of hypertension[11, 12]. Recently, Xi’s study found the calcium channel gene ATP2B1’s polymorphism could interact with lifestyle factors to have an effect on BP among children[11]. However, whether lifestyle behaviors could interact with rs9810888 on BP phenotypes has not been explored or reported before. We hypothesize that rs9810888 polymorphism may have interactive effect with lifestyle behaviors on BP in children, so the second objective of the study was to explore the gene-lifestyle interaction between the CACNA1D rs9810888 polymorphism and lifestyle behaviors on BP.
Methods
Participants
A case-control study was conducted among 2030 children aged 7 to 18 years old from two independent study groups in Beijing, China. The first study group came from the study on Adolescent Lipids, Insulin Resistance, and candidate genes (ALIR) in nine middle schools of Dongcheng District of Beijing. The second study was from Comprehensive Prevention project for Overweight and Obese Adolescents (CPOOA) in five elementary and middle schools of Haidian District of Beijing. The sampling strategies for the two study groups were described in detail in the previous studies[13–15].The ALIR study recruited 937 children aged 14 to 17 years old. The CPOOA study recruited 1093 children aged 7 to 18 years old[14]. In the two study groups, nine participants were excluded because of absence of BP phenotype data, so totally, 2021 children were included in the current study.
Ethics approval and consent to participate
Both the ALIR study and CPOOA study were approved by the Ethics Committee of Peking University Health Science Center. There are two numbers of the approval of ethic committee, namely IRB00001052-06082 for the ALIR study and IRB00001052-06084 for the COOPA study. Written informed consent was provided by all participants and, in the case of minors, their parents. Studies were performed according to the Declaration of Helsinki.
Measurements
Height, weight and BP were measured by standard protocols, which is described in detail previously[16]. BP was calculated by averaging three measurements at one visit. It was measured three times with 5-minute time interval. BP was measured according to the recommendation of the National High Blood Pressure Education Program Working Group for Children and Adolescents[17]. Systolic BP (SBP) was defined as the onset of “tapping” Korotkoff sound (K1), and diastolic BP (DBP) was defined as the fifth Korotkoff sound (K5). Mean arterial pressure (MAP) and pulse pressure (PP) was calculated as follow: MAP = (SBP+2×DBP)/2 (mmHg), PP = SBP-DBP (mmHg)[18]. Systolic high blood pressure(SHBP) and diastolic high blood pressure (DHBP) were defined as SBP or DBP ≥ the age- and sex-specific 95th percentile of a representative Chinese children population, respectively[19]. HBP was defined as SHBP and/or DHBP.
Lifestyle behaviors
Lifestyle behaviors included dietary behaviors, physical activity and sedentary behaviors were measured by questionnaire. Dietary behaviors, including consumption of fruits, vegetables, high calorie foods (fried chips/cakes/cookies), protein intake (meat/fish/soy beans/egg), western food and soft drink were measured in the questionnaire. The frequencies of eating fruit, vegetable, high calorie foods (fried chips/cakes/cookies), soft drink were investigated with the options of “Never”, “1~3 times”, “4~6 times”, “daily”, “twice per day”, “3 times per day”, or “more than 3 times per day”. Physical activity and screen time were also measured by a questionnaire[14]. Time spent on physical activity each day was measured with the options of “Never”, “0~0.5 hour per day”, “0.5~1 hour per day”, “1~2 hours per day”, “2~3 hours per day”, “3~4 hours per day”, and “more than 4 hours per day”. The dietary behavior and physical activity variables were classified into two categories based on the national recommendation of nutrition and physical activity for Chinese children[20]. For sedentary behaviors, we investigated the screen time. Screen time included time spent on the television/video viewing and computer/video game playing, and were categorized into < 2 hours/day or ≥2 hours /day according to the American Academy of Pediatrics [21].
Genotyping
Genomic DNAs of participants were isolated from blood leukocytes by the phenol-chloroform extraction method. The CACNA1D rs9810888 polymorphism was assayed by using the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS, Agena). All the experiments were conducted by investigators who were blind to the phenotypes. The genotyping call rates of rs9810888 was 99.95%.
Statistical analyses
The Hardy-Weinberg equilibrium was tested with the Chi-square test for the genotype data of the control group (non-HBP group). The quantitative variables were described as mean and standard deviations (SD). The categorical variables were described as percentages. Differences between HBP group and non-HBP group were compared with Chi-square tests (categorical variables) and t-tests (quantitative variables). The effect of rs9810888 on BP phenotypes (quantitative variables) and interaction terms (genotype × lifestyle variables) were estimated with multivariate general linear model with age, age square, sex and BMI as covariates. Multivariate logistic regression model with age, age square, sex and BMI as covariates was conducted to test the significance of interaction terms (genotype × lifestyle variables) on risk of HBP. All analysis was conducted under a recessive genetic model (GG = 1, GT/TT = 0). For the genetic model selection, we calculated Akaike information criteria (AIC) and Bayesian information criteria (BIC), which are both useful in model comparison[22, 23], i.e. the smaller AIC and BIC were, the better the corresponding model fitted to the data. In our study, rs9810888 was significantly associated with DBP under a recessive model, AIC and BIC values all indicated the recessive genetic model are the optimal model after model comparison (S1 Table). The statistical analyses were performed with SPSS for Windows (version 20.0, SPSS Inc., Chicago, IL, USA). In the present study, we adjusted covariates of age, age square, sex and BMI as the GWAS study which identified this new locus. Considering age and age square may have a great weight to the analysis, we did sensitivity analysis to only adjust age without adjustment of age square.
Results
The general characteristics of the study population
In the present study, a total of 2021 children and adolescents were involved. The general characteristics of the participants were showed in Table 1. Mean age of the participants were 12.9 years old, 60.1% of them were boys, average BMI, SBP, DBP, MAP and PP were 23.8 kg/m2, 113.5 mmHg, 62.2 mmHg, 79.3 mmHg and 51.2 mmHg, respectively. The participants with HBP had significantly higher age, BMI, SBP, DBP, MAP and PP than the non-HBP participants (all p values<0.001). The frequency of TT, GT and GG genotype was 36.1%, 48.1% and 15.8%, respectively. The genotype distribution of CACNA1D polymorphism rs9810888 in the control group was in Hardy-Weinberg Equilibrium (p = 0.448), while the G allele frequency in the control group was 39.7%.
Table 1. General characteristics and behavior characteristics of the study population.
| Variables | Total(N = 2021) | Non HBP(n = 1319) | HBP(n = 702) | p* | |
|---|---|---|---|---|---|
| Sex | boys | 1215(60.1%) | 724(54.9%) | 491(69.9%) | <0.001 |
| girls | 806(39.9%) | 595(45.1%) | 211(30.1%) | ||
| Age(year) | 12.9±2.7 | 12.2±2.9 | 14.3±1.4 | <0.001 | |
| BMI(kg/m2) | 23.8±4.8 | 22.0±4.0 | 27.3±4.2 | <0.001 | |
| SBP(mmHg) | 113.5±16.5 | 104.4±10.9 | 130.5±10.9 | <0.001 | |
| DBP(mmHg) | 62.2±18.0 | 54.6±15.2 | 76.7±13.3 | <0.001 | |
| MAP(mmHg) | 79.3±16.0 | 71.2±12.1 | 94.6±10.1 | <0.001 | |
| PP(mmHg) | 51.2±15.0 | 49.8±14.3 | 53.8±16.0 | <0.001 | |
| rs9810888 | TT | 730(36.1%) | 475(36%) | 255(36.4%) | 0.491 |
| GT | 971(48.1%) | 644(48.8%) | 327(46.6%) | ||
| GG | 319(15.8%) | 200(15.2%) | 119(17.0%) | ||
*p value was calculated with t-test (quantitative variables) or Chi-square test (categorical variables). SBP: systolic blood pressure. DBP: diastolic blood pressure. MAP: mean arterial pressure. PP: pulse pressure. BMI: body mass index. HBP: high blood pressure.
Individual effect of rs9810888 on BP phenotypes
As presented in Table 2, under a recessive genetic model, with sex, age, age square, and study group as covariates, we found that rs9810888 was significantly associated with DBP (b = 1.78, SE = 0.74, p = 0.017) and MAP (b = 1.69, SE = 0.65, p = 0.009). With further adjustment for BMI, rs9810888 was still significantly associated with DBP (b = 1.69, SE = 0.73, p = 0.021) and MAP (b = 1.56, SE = 0.61, p = 0.010). No significant associations between rs9810888 and SBP or PP were detected (p>0.05). The association between rs9810888 and risk of HBP, SHBP or DHBP was also not significant (p>0.05, S2 Table).
Table 2. Association between the CACNA1D rs9810888 polymorphism and blood pressure phenotypes.
| BP phenotypes | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| SBP | 1.52 | 0.79 | 0.056 | 1.29 | 0.69 | 0.062 |
| DBP | 1.78 | 0.74 | 0.017 | 1.69 | 0.73 | 0.021 |
| MAP | 1.69 | 0.65 | 0.009 | 1.56 | 0.61 | 0.010 |
| PP | -0.26 | 0.84 | 0.759 | -0.40 | 0.81 | 0.622 |
Model 1 adjusted for sex, age, age square, and study group. Model 2 adjusted for sex, age, age square, study group, and BMI. SBP: systolic blood pressure. DBP: diastolic blood pressure. MAP: mean arterial pressure. PP: pulse pressure. BMI: body mass index. SE: standard error.
Combined effects between rs9810888 and lifestyle behaviors on DBP
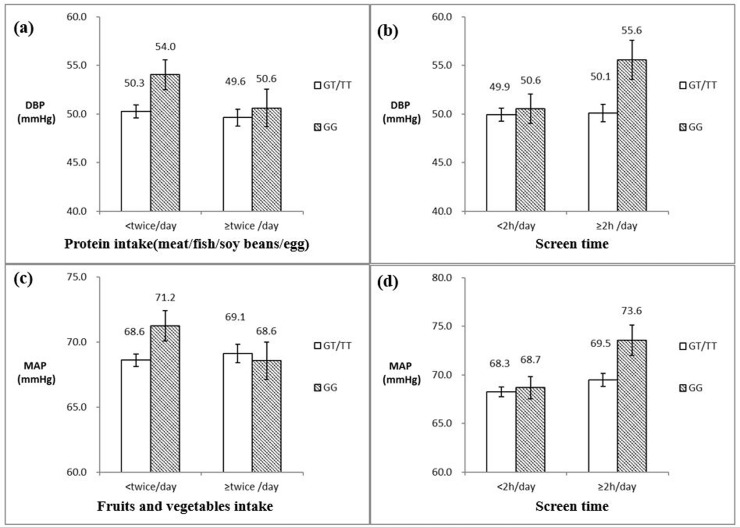
Table 3, Fig 1(A) and Fig 1(B) illustrated the associations between rs9810888 and DBP in different subgroups of lifestyle behaviors. In the participants who consumed protein (meat/fish/soy beans/egg) <twice/day, the rs9810888 GG genotype carriers had higher DBP than GT/TT genotype carriers (b = 3.78, SE = 1.66, p = 0.023), but in the participants who consumed protein (meat/fish/soy beans/egg) ≥twice/day, rs9810888 was not associated with DBP (p>0.05).
Table 3. Interaction between lifestyle behaviors and the CACNA1D rs9810888 polymorphism on DBP.
| Lifestyle behaviors | Category | Genotype | N | Mean | SE | b | SE | p | pinteraction |
|---|---|---|---|---|---|---|---|---|---|
| Protein intake (meat/fish/soy beans/egg) | <twice/day | GT/TT | 495 | 50.3 | 0.7 | 3.78 | 1.66 | 0.023 | 0.295 |
| GG | 90 | 54.0 | 1.5 | ||||||
| ≥twice/day | GT/TT | 292 | 49.6 | 0.9 | 0.98 | 2.12 | 0.645 | ||
| GG | 61 | 50.6 | 1.9 | ||||||
| Fruits and vegetables intakea | <twice/day | GT/TT | 583 | 49.8 | 0.6 | 3.16 | 1.65 | 0.055 | 0.257 |
| GG | 101 | 52.9 | 1.5 | ||||||
| ≥twice/day | GT/TT | 206 | 51.0 | 0.9 | 0.003 | 2.08 | 0.999 | ||
| GG | 51 | 51.0 | 1.9 | ||||||
| Fried chips/cakes/cookies | No | GT/TT | 183 | 50.4 | 1.2 | 0.21 | 2.7 | 0.937 | 0.478 |
| GG | 43 | 50.6 | 2.4 | ||||||
| Yes | GT/TT | 597 | 50.0 | 0.6 | 2.78 | 1.54 | 0.071 | ||
| GG | 107 | 52.8 | 1.4 | ||||||
| Western food | No | GT/TT | 528 | 50.0 | 0.6 | 1.25 | 1.61 | 0.439 | 0.249 |
| GG | 98 | 51.3 | 1.5 | ||||||
| Yes | GT/TT | 250 | 50.0 | 1.0 | 4.59 | 2.35 | 0.052 | ||
| GG | 51 | 54.6 | 2.1 | ||||||
| Soft drink | No | GT/TT | 337 | 50.0 | 0.8 | 1.92 | 2.04 | 0.347 | 0.844 |
| GG | 58 | 51.9 | 1.9 | ||||||
| Yes | GT/TT | 463 | 50.1 | 0.7 | 2.51 | 1.7 | 0.141 | ||
| GG | 95 | 52.6 | 1.6 | ||||||
| Physical activity | <1 hour/day | GT/TT | 358 | 50.9 | 0.8 | 2.44 | 2.02 | 0.227 | 0.987 |
| GG | 66 | 53.4 | 1.9 | ||||||
| ≥1 hour/day | GT/TT | 429 | 49.2 | 0.7 | 2.38 | 1.75 | 0.173 | ||
| GG | 85 | 51.6 | 1.6 | ||||||
| Screen time | <2 hours/day | GT/TT | 503 | 49.9 | 0.7 | 0.64 | 1.66 | 0.698 | 0.092 |
| GG | 95 | 50.6 | 1.5 | ||||||
| ≥2 hours/day | GT/TT | 292 | 50.1 | 0.9 | 5.49 | 2.18 | 0.012 | ||
| GG | 57 | 55.6 | 2.0 |
Adjusted for sex, age, age square and BMI.
a: for fruit and vegetable intake category, <twice/day means either fruits or vegetables intake <twice/day, and ≥twice/day means both fruits and vegetables ≥twice/day.
DBP: diastolic blood pressure. BMI: body mass index. SE: standard error. The mean of BP value was adjusted for sex, age, age square and BMI.
Fig 1. Adjusted means and standard errors of BP levels by CACNA1D rs9810888 polymorphism and different level of lifestyle behaviors.
DBP: diastolic blood pressure. MAP: mean arterial pressure. Adjusted means and standard errors were estimated under general linear regression models with age, age square, sex and BMI adjusted. For fruit and vegetable intake, <twice/day means either fruits or vegetables intake <twice/day, and ≥twice/day means both fruits and vegetables ≥twice/day.
In addition, in participants with screen time ≥2 hours/day, the rs9810888 GG genotype carriers had higher DBP than GT/TT genotype carriers (b = 5.49, SE = 2.18, p = 0.012), but in the participants with screen time <2 hours/day, rs9810888 was not associated with DBP (p>0.05).
Then we test the interaction for each lifestyle behavior and rs9810888 on DBP in general linear regression models with sex, age, age square, BMI, rs9810888, lifestyle behavior and rs9810888×lifestyle behavior as independent variables and DBP as dependent variable, but no significant interaction was identified (pinteraction>0.05).
Combined effects between rs9810888 and lifestyle behaviors on MAP
Table 4, Fig 1(C) and Fig 1(D) presented the associations between rs9810888 and MAP in different subgroups of lifestyle behaviors. In subgroups having either fruits or vegetables <twice/day, the rs9810888 GG genotype carriers had higher MAP than GT/TT genotype carriers (b = 2.64, SE = 1.27, p = 0.037), but in subgroups having both fruits or vegetables ≥twice/day, rs9810888 was not associated with MAP (p>0.05).
Table 4. Interaction between lifestyle behaviors and the CACNA1D rs9810888 polymorphism on MAP.
| Lifestyle behaviors | Category | Genotype | N | Mean | SE | b | SE | p | pinteraction |
|---|---|---|---|---|---|---|---|---|---|
| Protein intake (meat/fish/soy beans/egg) | <twice/day | GT/TT | 495 | 68.9 | 0.5 | 2.50 | 1.29 | 0.053 | 0.453 |
| GG | 90 | 71.4 | 1.2 | ||||||
| ≥twice/day | GT/TT | 292 | 68.5 | 0.7 | 0.95 | 1.62 | 0.557 | ||
| GG | 61 | 69.4 | 1.5 | ||||||
| Fruits and vegetables intakea | <twice/day | GT/TT | 583 | 68.6 | 0.5 | 2.64 | 1.27 | 0.037 | 0.132 |
| GG | 101 | 71.2 | 1.2 | ||||||
| ≥twice/day | GT/TT | 206 | 69.1 | 0.7 | -0.57 | 1.60 | 0.724 | ||
| GG | 51 | 68.6 | 1.4 | ||||||
| Fried chips/cakes/cookies | No | GT/TT | 183 | 69.6 | 0.9 | 1.27 | 2.00 | 0.524 | 0.892 |
| GG | 43 | 70.9 | 1.8 | ||||||
| Yes | GT/TT | 597 | 68.5 | 0.5 | 1.70 | 1.20 | 0.157 | ||
| GG | 107 | 70.2 | 1.1 | ||||||
| Western food | No | GT/TT | 528 | 68.9 | 0.5 | 1.09 | 1.23 | 0.375 | 0.390 |
| GG | 98 | 70.0 | 1.1 | ||||||
| Yes | GT/TT | 250 | 68.4 | 0.7 | 3.06 | 1.83 | 0.096 | ||
| GG | 51 | 71.5 | 1.7 | ||||||
| Soft drink | No | GT/TT | 337 | 68.6 | 0.6 | 0.79 | 1.55 | 0.610 | 0.534 |
| GG | 58 | 69.4 | 1.4 | ||||||
| Yes | GT/TT | 463 | 68.9 | 0.5 | 2.18 | 1.31 | 0.098 | ||
| GG | 95 | 71.0 | 1.2 | ||||||
| Physical activity | <1hour/day | GT/TT | 358 | 69.8 | 0.6 | 1.71 | 1.54 | 0.267 | 0.939 |
| GG | 66 | 71.5 | 1.4 | ||||||
| ≥1hour/day | GT/TT | 429 | 67.9 | 0.5 | 1.86 | 1.35 | 0.167 | ||
| GG | 85 | 69.7 | 1.2 | ||||||
| Screen time | <2 hours/day | GT/TT | 503 | 68.3 | 0.5 | 0.42 | 1.26 | 0.738 | 0.094 |
| GG | 95 | 68.7 | 1.2 | ||||||
| ≥2 hours/day | GT/TT | 292 | 69.5 | 0.7 | 4.08 | 1.70 | 0.017 | ||
| GG | 57 | 73.6 | 1.6 |
Note: Adjusted for sex, age, age square and BMI.
a: for fruit and vegetable intake category, <twice/day means either fruits or vegetables intake <twice/day, and ≥twice/day means both fruits and vegetables ≥twice/day.
MAP: mean arterial pressure. BMI: body mass index. SE: standard error. The mean of BP values was adjusted for sex, age, age square and BMI.
Additionally, in participants with screen time ≥2 hours/day, the rs9810888 GG genotype carriers had higher MAP than GT/TT genotype carriers (b = 4.80, SE = 1.70, p = 0.017), but in the participants with screen time <2 hours/day, rs9810888 was not associated with MAP (p>0.05).
Then we tested the interaction for each lifestyle behavior and rs9810888 on MAP in general linear regression models with sex, age, age square, BMI, rs9810888, lifestyle behavior and rs9810888×lifestyle behavior as independent variables and MAP as dependent variable, but no significant interaction was identified (p>0.05).
Combined effects between rs9810888 and lifestyle behaviors on SBP or HBP
S3 Table showed the association between rs9810888 and SBP level in different subgroups of lifestyle behaviors. No combined effects or interaction was identified for rs9810888 and lifestyle behaviors on SBP level (p>0.05). The associations between rs9810888 and the risk of HBP in different subgroups of lifestyle behaviors were demonstrated in S4 Table. No combined effects or interaction was identified for rs9810888 and lifestyle behaviors on the risk of HBP (p>0.05).
We did sensitivity analysis to only adjust age in the results. We got similar results in the sensitivity analysis (Data not shown).
Discussion
In the present study, we identified the individual genetic association between the CACNA1D rs9810888 polymorphism and DBP or MAP level among Chinese children, and there is potential difference of the genetic effect sizes across age groups (children vs adults). and for the first time we found the combined effects between CACNA1D and lifestyle behaviors on DBP or MAP level. We found only in the individuals who possess the unhealthy lifestyles (lower intake of protein “meat/fish/soy beans/eggs”, less fruits and vegetables intake or longer daily screen time), rs9810888 was associated with DBP or MAP level.
The CACNA1D rs9810888 polymorphism was firstly identified to be associated with BP among a hypertension GWAS in Chinese adults[7]. It demonstrated that rs9810888 polymorphism G allele was significantly associated with higher DBP (b = 0.39, SE = 0.06, p = 4.00×10−12). Similar to this GWAS study, we found the GG genotype of rs9810888 may be the risk genotype of higher DBP level among children in our study. The GG genotype carriers had significantly higher level of DBP than the GT/TT genotype carriers independent of BMI. But the genetic effect size of association between rs9810888 and DBP were much larger in children than adults, implying there is a potential difference across age groups.
CACNA1D is an important gene of calcium channel pathway and plays an important role in the regulation of the cellular calcium iron level, which is a key signal in the contraction and dilation of vascular smooth muscles[24]. As a subunit of L-type calcium channels which 4 main pore-forming α1 subunits, CACNA1D is widely expressed in the cells of cardiovascular system[8]. CACNA1D gene encodes a L-type calcium channel which could produce low-threshold, inactivating currents that resemble R-type current of the T-type current of sinoatrial node cells or many neurons, and control physiological processes, for example diastolic depolarization in these cells and neurons[25]. This could be the potential mechanism why polymorphism in CACNA1D is associated with DBP and MAP, since the neuroendocrine systems and the sinoatrial node cells plays important role in BP regulation. Nowadays calcium iron channel blocker is the first-line medication option for the treatment and control of hypertension. Kumite and colleagues demonstrated that two intron polymorphisms in CACNA1D (rs312481 and rs3774426, in very weak linkage disequilibrium with rs9810888, r2 = 0.06 and 0.07) is associated with individuals’ antihypertensive effects of L-type dihydropyridine calcium-channel blockers[26]. These findings also imply the importance of intron polymorphisms in CACNA1D in the BP regulation. In addition, another genetic study found several polymorphisms of CACNA1D were associated with aldosterone-producing adenomas and primary aldosteronism[9], which is the most common curable cause of secondary hypertension[27, 28]. Human CACNA1D, spanning >300kb, encodes Cav1.3, which involves extensive mRNA splicing. Although rs9810888 is an intron polymorphism of CACNA1D gene, it may modulate the mRNA splicing of CACNA1D to regulate its expression. But whether or how the rs9810888 polymorphism is linked with the splicing of Cav1.3’s mRNA needs further functional studies.
Furthermore, the combined effects of the CACNA1D rs9810888 polymorphism and lifestyle behaviors, such as screen time, protein intake (meat/fish/soy beans/egg) and fruit and vegetable intake, were found on DBP and MAP among Chinese children. To the best of our knowledge, there is no study on interaction or combined effect for CACNA1D polymorphism and lifestyle behaviors before. However, there are studies investigating the interaction between other genes and screen time on other phenotypes. For example, Smith and colleagues[29] found that in the subgroup with longer screen time, LIPG i24582 TT genotype carriers had lower high density lipoprotein cholesterol (HDL-C) than the CT/CC carriers (p<0.05), but the association is not significant in the subgroup with lower screen time. In an American adolescent study, Graff and colleagues also found the combined effects between obesity gene polymorphisms and longer screen time on BMI[30]. Number of studies showed that the unfavorable lifestyles, such as longer screen time, insufficient intake of fruit and vegetable, are associated with a worse BP profile[31–33]. At the same time, population genetic studies and functional studies have showed that CACNA1D gene is closely related with BP regulation [7, 34]. Therefore, it is highly likely that there are combined effects between these lifestyles and CACNA1D gene polymorphism rs9810888 on BP profile. But since the biological function of rs9810888 is still unknown now, the underlying mechanism of the combined effects between the risky lifestyles and the genetic predisposition to poorer BP profiles needs further functional studies.
Our findings of combined effects between the CACNA1D rs9810888 polymorphism and lifestyle behaviors on BP among children have public health implications. If the individuals who are genetically susceptible to higher BP and also have longer screen time or lower protein intake could change these unfavorable lifestyles, they may have a chance of getting the better BP profile, which has been reported to produce significant population-level change in cardiovascular diseases (CVDs) risk. According to a meta-analysis of randomized trials of antihypertensive treatment, for every 5-mmHg reduction of DBP or 10-mmHg of SBP, there would be a reduction of 22% reduction of coronary heart diseases (CHD) and 41% reduction of stroke[35].
In the present study, we analyzed the associations between rs981088 and multiple BP components (SBP, DBP, MAP, PP, HBP, SHBP and DHBP), and identified significant results in DBP and MAP. Franklin and colleagues emphasized the importance of combining multiple BP components for analysis, since combining multiple BP components was superior to predicting the CVD risk than the single BP components[36]. MAP, as an indicator of resistance, represents the average pressure during the cardiac cycle. It is more influenced by DBP according to the formula of MAP = (SBP+2×DBP)/3[18]. To some extent, the finding of associations between rs9810888 and MAP further verified results of DBP. It is reported that DBP was important indicators for CHD in younger people, while SBP was the predominant risk predictor in older subjects[37, 38]. Therefore, the individuals having the risk genotype of rs9810888 should be an important targeted population in the intervention to control the DBP and MAP level, which may also benefit for controlling the CHD risk in later life.
The present study had some strengths. Firstly, the measurement of BP was conducted by trained investigators according to a standard protocol to minimize the measurement error. Secondly, this study was conducted among children. Different from adult population, children have higher BP heritability[39, 40] and a majority of HBP children have simple HBP without other complications, which also make the pediatric population to be a more ideal population for genetic studies on BP. Thirdly, the utility of multiple BP components for analysis rather than single BP component provided more comprehensive results and insights into the genetic effect of CACNA1D on BP.
At the same time, there are several limitations of the present study that should be noted. Firstly, the sample size of this study provides enough power for the main effect of gene polymorphism (≥80%), but may not sufficient statistical power in interaction or combined effect analyses. Usually, interaction or combined effect studies need a relatively large sample size to produce reliable results in stratified analysis. The best method to ensure the reliability of our findings is further validation in a cohort study among children. Secondly, the behavior of physical activity could be more precisely measured by accelerometer other than a questionnaire. Thirdly, this is a case-control study, therefore the combined effects of gene and current lifestyle behaviors cannot be interpreted as causal associations. Lastly, for only one polymorphism of CACNA1D was studied in the present study, future studies should involve more polymorphisms.
In conclusion, our study confirmed that the CACNA1D rs9810888 polymorphism was significantly associated with DBP or MAP among Chinese children. In addition, we found combined effects of rs9810888 and lower protein (meat/fish/soy beans/egg) intake or longer screen time on DBP level, and also combined effects of rs9810888 and less fruit and vegetable intake or longer screen time on MAP were examined. This study is the first study investigating combined effect between the CACNA1D rs9810888 polymorphism and lifestyle behaviors on BP phenotypes. The findings provided new evidences for unhealthy lifestyle modification especially among children who are genetically predisposed to higher BP.
Supporting information
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank all the children and adolescents and their parents for their participation in this project.
Abbreviations
- CACNA1D
calcium voltage-gated channel subunit alpha1 D
- BP
blood pressure
- BMI
body mass index
- DBP
diastolic blood pressure
- MAP
mean arterial blood pressure
- GWAS
Genome-Wide Association Study
- SBP
Systolic blood pressure
- SHBP
Systolic high blood pressure
- DHBP
diastolic high blood pressure
- PP
pulse pressure
- SD
standard deviations
- MALDI-TOF MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- AIC
Akaike information criteria
- BIC
Bayesian information criteria
Data Availability
The datasets generated and/or analyzed during the present study are not publicly available, since ethics approval and participants’ consent does not allow public sharing of data, only available from the institute (Email: 121110127@bjmu.edu.cn) upon reasonable request.
Funding Statement
This study was supported by Grants from National Natural Science Foundation of China (81573170, HW, http://www.nsfc.gov.cn/), National Natural Science Foundation of China (81673192, JM, http://www.nsfc.gov.cn/), Hunan Provincial Natural Science Foundation of China (2019JJ50376, YDY) and a Project Supported by Scientific Research Fund of Hunan Provincial Education Department (18C0072, YDY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017;390(10100):1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hottenga JJ, Boomsma DI, Kupper N, Posthuma D, Snieder H, Willemsen G, et al. Heritability and stability of resting blood pressure. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2005;8(5):499–508. [DOI] [PubMed] [Google Scholar]
- 3.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension (Dallas, Tex: 1979). 2003;41(6):1196–201. [DOI] [PubMed] [Google Scholar]
- 4.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–9. 10.1038/nature10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Moraes AC, Carvalho HB, Rey-Lopez JP, Gracia-Marco L, Beghin L, Kafatos A, et al. Independent and combined effects of physical activity and sedentary behavior on blood pressure in adolescents: gender differences in two cross-sectional studies. PloS one. 2013;8(5):e62006 10.1371/journal.pone.0062006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempfle A, Scherag A, Hein R, Beckmann L, Chang-Claude J, Schafer H. Gene-environment interactions for complex traits: definitions, methodological requirements and challenges. European journal of human genetics: EJHG. 2008;16(10):1164–72. 10.1038/ejhg.2008.106 [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Wang L, Lin X, Huang J, Charles Gu C, He M, et al. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Human molecular genetics. 2015;24(3):865–74. 10.1093/hmg/ddu478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92(5):2633–41. 10.1152/jn.00486.2004 [DOI] [PubMed] [Google Scholar]
- 9.Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nature genetics. 2013;45(9):1050–4. 10.1038/ng.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pomeroy J, Soderberg AM, Franks PW. Gene-lifestyle interactions and their consequences on human health. Medicine and sport science. 2009;54:110–35. 10.1159/000235700 [DOI] [PubMed] [Google Scholar]
- 11.Xi B, Cheng H, Shen Y, Zhao X, Hou D, Wang X, et al. Physical activity modifies the associations between genetic variants and hypertension in the Chinese children. Atherosclerosis. 2012;225(2):376–80. 10.1016/j.atherosclerosis.2012.10.027 [DOI] [PubMed] [Google Scholar]
- 12.Montasser ME, Gu D, Chen J, Shimmin LC, Gu C, Kelly TN, et al. Interactions of genetic variants with physical activity are associated with blood pressure in Chinese: the GenSalt study. American journal of hypertension. 2011;24(9):1035–40. 10.1038/ajh.2011.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Ma J, Zhang S, Hinney A, Hebebrand J, Wang Y, et al. Association of the MC4R V103I polymorphism with obesity: a Chinese case-control study and meta-analysis in 55,195 individuals. Obesity (Silver Spring, Md). 2010;18(3):573–9. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Song J, Shang X, Chawla N, Yang Y, Meng X, et al. Physical activity and sedentary behavior can modulate the effect of the PNPLA3 variant on childhood NAFLD: a case-control study in a Chinese population. BMC medical genetics. 2016;17(1):90 10.1186/s12881-016-0352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JY, Song QY, Wang S, Ma J, Wang HJ. Physical Activity and Sedentary Behaviors Modify the Association between Melanocortin 4 Receptor Gene Variant and Obesity in Chinese Children and Adolescents. PloS one. 2017;12(1):e0170062 10.1371/journal.pone.0170062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YD, Song JY, Wang S, Liu FH, Zhang YN, Shang XR, et al. Genetic variations in sterol regulatory element binding protein cleavage-activating protein (SCAP) are associated with blood pressure in overweight/obese Chinese children. PloS one. 2017;12(5):e0177973 10.1371/journal.pone.0177973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 18.Tardieu, Bernard. The essentials in pressure monitoring: Martinus Nijhoff Medical Division; 1980.
- 19.Jie MI, Wang TY, Meng LH, Zhu GJ, Han SM, Zhong Y, et al. Development of blood pressure reference standards for Chinese children and adolescents. Chinese Journal of Evidence-Based Pediatrics. 2010;5(1):4–14. [Google Scholar]
- 20.Chen C. Prevention and control guidelines of overweight and obesity for Chinese school children and adolescents first ed: Beijng: People’s Medical Publishing House; 2008. p.36–47 p. [Google Scholar]
- 21.American Academy of Pediatrics: Children, adolescents, and television. Pediatrics. 2001;107(2):423–6. [DOI] [PubMed] [Google Scholar]
- 22.Hartman S, Widaman KF, Belsky J. Genetic moderation of effects of maternal sensitivity on girl's age of menarche: Replication of the Manuck et al. study. Dev Psychopathol. 2015;27(3):747–56. 10.1017/S0954579414000856 [DOI] [PubMed] [Google Scholar]
- 23.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists: Cambridge University Press; 2002. [Google Scholar]
- 24.Pande J, Mallhi KK, Sawh A, Szewczyk MM, Simpson F, Grover AK. Aortic smooth muscle and endothelial plasma membrane Ca2+ pump isoforms are inhibited differently by the extracellular inhibitor caloxin 1b1. American journal of physiology Cell physiology. 2006;290(5):C1341–9. 10.1152/ajpcell.00573.2005 [DOI] [PubMed] [Google Scholar]
- 25.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, et al. alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276(25):22100–6. 10.1074/jbc.M101469200 [DOI] [PubMed] [Google Scholar]
- 26.Kamide K, Yang J, Matayoshi T, Takiuchi S, Horio T, Yoshii M, et al. Genetic polymorphisms of L-type calcium channel alpha1C and alpha1D subunit genes are associated with sensitivity to the antihypertensive effects of L-type dihydropyridine calcium-channel blockers. Circ J. 2009;73(4):732–40. [DOI] [PubMed] [Google Scholar]
- 27.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. Journal of the American College of Cardiology. 2006;48(11):2293–300. 10.1016/j.jacc.2006.07.059 [DOI] [PubMed] [Google Scholar]
- 28.Rossi GP. A comprehensive review of the clinical aspects of primary aldosteronism. Nature reviews Endocrinology. 2011;7(8):485–95. 10.1038/nrendo.2011.76 [DOI] [PubMed] [Google Scholar]
- 29.Smith CE, Arnett DK, Tsai MY, Lai CQ, Parnell LD, Shen J, et al. Physical inactivity interacts with an endothelial lipase polymorphism to modulate high density lipoprotein cholesterol in the GOLDN study. Atherosclerosis. 2009;206(2):500–4. 10.1016/j.atherosclerosis.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graff M, North KE, Richardson AS, Young KM, Mohlke KL, Lange LA, et al. Screen time behaviours may interact with obesity genes, independent of physical activity, to influence adolescent BMI in an ethnically diverse cohort. Pediatric obesity. 2013;8(6):e74–9. 10.1111/j.2047-6310.2013.00195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heshmat R, Qorbani M, Shahr Babaki AE, Djalalinia S, Ataei-Jafari A, Motlagh ME, et al. Joint Association of Screen Time and Physical Activity with Cardiometabolic Risk Factors in a National Sample of Iranian Adolescents: The CASPIANIII Study. PloS one. 2016;11(5):e0154502 10.1371/journal.pone.0154502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hruby A, Jacques PF. Dietary protein and changes in markers of cardiometabolic health across 20 years of follow-up in middle-aged Americans. Public health nutrition. 2018;21(16):2998–3010. 10.1017/S1368980018001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Dong B, Zou Z, Wang S, Dong Y, Wang Z, et al. Association between Vegetable Consumption and Blood Pressure, Stratified by BMI, among Chinese Adolescents Aged 13–17 Years: A National Cross-Sectional Study. Nutrients. 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinbothe TM, Alkayyali S, Ahlqvist E, Tuomi T, Isomaa B, Lyssenko V, et al. The human L-type calcium channel Cav1.3 regulates insulin release and polymorphisms in CACNA1D associate with type 2 diabetes. Diabetologia. 2013;56(2):340–9. 10.1007/s00125-012-2758-z [DOI] [PubMed] [Google Scholar]
- 35.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ (Clinical research ed). 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin SS, Lopez VA, Wong ND, Mitchell GF, Larson MG, Vasan RS, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119(2):243–50. 10.1161/CIRCULATIONAHA.108.797936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Wei FF, Wang S, Cheng YB, Wang JG. Cardiovascular risks associated with diastolic blood pressure and isolated diastolic hypertension. Curr Hypertens Rep. 2014;16(11):489 10.1007/s11906-014-0489-x [DOI] [PubMed] [Google Scholar]
- 38.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103(9):1245–9. [DOI] [PubMed] [Google Scholar]
- 39.Lin HF, Boden-Albala B, Juo SH, Park N, Rundek T, Sacco RL. Heritabilities of the metabolic syndrome and its components in the Northern Manhattan Family Study. Diabetologia. 2005;48(10):2006–12. 10.1007/s00125-005-1892-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIlhany ML, Shaffer JW, Hines EA Jr. The heritability of blood pressure: an investigation of 200 pairs of twins using the cold pressor test. The Johns Hopkins medical journal. 1975;136(2):57–64. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
The datasets generated and/or analyzed during the present study are not publicly available, since ethics approval and participants’ consent does not allow public sharing of data, only available from the institute (Email: 121110127@bjmu.edu.cn) upon reasonable request.