Abstract
Introduction:
Adjunctive driver-guided ablation in addition to pulmonary vein isolation has been proposed as a strategy to improve procedural success and outcomes for various populations with atrial fibrillation (AF). First, this study aimed to evaluate the different mapping techniques for driver/rotor identification and second to evaluate the benefits of driver/rotor-guided ablation in patients with paroxysmal and persistent AF (PerAF).
Methods:
We searched the electronic database in PubMed using the keywords “atrial fibrillation,” “rotor,” “rotational driver,” “atrial fibrillation source,” and “drivers” for both randomized controlled trials and observational controlled trials. Clinical studies reporting efficacy or safety outcomes of driver-guided ablation for paroxysmal AF or (PerAF) were identified. We performed subgroup analyses comparing different driver mapping methods in patients with PerAF. The odds ratios (ORs) with random effects were analyzed.
Results:
Out of 175 published articles, seven met the inclusion criteria, of which two were randomized controlled trials, one was quasiexperimental study, and four observational studies (three case-controlled studies and one cross-sectional study). Overall, adjunctive driver-guided ablation was associated with higher rates of acute AF termination (OR: 4.62, 95% confidence interval [CI]: 2.12–10.08; P < 0.001), lower recurrence of any atrial arrhythmia (OR: 0.44, 95% CI: 0.30–0.065; P < 0.001), and comparable complication incidence.
Conclusions:
Adjunctive driver-guided catheter ablation suggested an increased freedom from AF/AT relative to conventional strategies, irrespective of the mapping techniques. Furthermore, phase mapping appears to be superior to electrogram-based driver mapping in PerAF ablation.
Keywords: atrial fibrillation, catheter ablation, driver, meta-analysis, phase-mapping
1 |. INTRODUCTION
Atrial fibrillation (AF) is the most frequently occurring sustained arrhythmia, which causes significant morbidity and mortality.1 Has-saïguerre et al2 and Chen et al3 first reported the dominant and pathologic role of pulmonary vein (PV) triggers in the arrhythmogenesis of AF. Owing to the advancement of mapping techniques and broader knowledge on pathogenesis, catheter ablation has been considered as an effective and alternative treatment option for AF patients. Electrical isolation of PV has become the cornerstone for catheter ablation in AF with achievement of rhythm control in approximately 70%−75% cases of paroxysmal AF (PAF).4 However, it is less effective in patients with persistent AF (PerAF), and repeat procedures are often required.5 Additional targeting of signals with high frequencies for catheter ablation during AF has been previously proposed as a treatment strategy.6–10 However, randomized controlled trials (RCTs) did not show any benefit of performing additional linear ablation or of complex fractionated atrial electrograms ablation in addition to PV isolation (PVI) in patients with PerAF.11
Because of the advancement of signal processing systems, mapping systems, and mapping catheters, several recent studies have demonstrated successful driver identification during AF ablation. Lin et al, Haïssaguerre et al, and Narayan et al used phase-mapping-based strategy to identify drivers during procedure,9,12,13 whereas Atienza et al, Jadidi et al, and Seitz et al used electrogram-based driver mapping strategy to identify small radius reentry responsible for the maintenance of AF.14–16 Owing to the advances in mapping techniques and understanding of the pathogenesis, driver-guided ablation has emerged as a potential therapeutic target for PerAF ablation.
Several meta-analyses have been conducted by different groups to evaluate the benefit of driver-guided ablation in addition to the standard approach, and the results are controversial. These meta-analyses did not focus on PerAF ablation only but also included PAF and PerAF patients. Additionally, the driver mapping methodology (phase mapping or electrogram-based mapping) was not investigated. We, therefore, systematically reviewed the published literature to compare the reported efficacy and safety of phase mapping and electrogram-based driver mapping for AF and PerAF patients.
2 |. MATERIALS AND METHODS
2.1 |. Search strategy and study eligibility
We searched the electronic database in PubMed for both experimental and observational studies published before 1 September 2017. The search strategy included “atrial fibrillation,” “rotor,” “rotational driver,” “atrial fibrillation source,” and “drivers” as the medical subject headings and text keywords. We aimed to systematically review the literature for evidence of clinical effectiveness of driver/rotor ablation of AF in RCT, quasiexperimental studies, and observational studies with a comparison group following the recommendations for the reporting of meta-analysis of observational studies.17 For the subgroup analyses in subjects with persistent AF, we conducted an individual patient meta-analysis, which focused on patients with PerAF. The trials investigating only patients with PAF were excluded from the subgroup analysis. There is currently no consensus or guidelines for driver/rotor identification, and thus, we classified mapping strategy for drivers during AF using the two predominant methods as1 phase-mapping and2 electrogram-based driver mapping strategies. Four studies used phase-mapping-based driver identification, and three studies used electrogram-based driver identification strategy. These studies were included in the pooled analyses along with a discussion about any impact they may have had on the results.
2.2 |. Definitions of driver mapping
The studies were classified into phase mapping and electrogram-based driver mapping based on the methodology used in each study. Several studies demonstrated successful driver identification by phase mapping of simultaneous recordings using a basket catheter,13 noninvasive array of body surface electrodes,12 or nonlinear processing technique to identify the morphological repetitiveness of waveform patterns by using double spiral catheters.9 Some studies also revealed the successful driver identification by recognizing the localized high-frequency source,14 electrogram dispersion during AF,16 and local rotational activity15 by using the electrogram-based driver mapping strategy.
2.3 |. Study end points
We grouped studies according to the following analysis areas for patients with AF who underwent driver-guided ablation:
-
Analysis 1:
Efficacy (AF termination and 1-year freedom from AF/atrial tachycardia [AT] recurrence) of driver-guided ablation compared to conventional ablation therapy.
-
Analysis 2:
Subgroup analysis of efficacy (1-year freedom from AF/AT recurrence) of phase mapping and electrogram-based driver mapping strategy.
-
Analysis 3:
Complications in driver mapping strategy compared to those in conventional ablation therapy.
2.4 |. Assessment of study quality
Comparison of interventions usually does not allow a blinded study design; hence, we did not assess for blinded studies. Two independent cardiac electrophysiologists screened the eligible abstracts and full texts of all controlled trials, with disagreements solved by the opinions of a third cardiac electrophysiologist. The methodological quality of the studies was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines,18,19 Newcastle-Ottawa Scale,20 and Cochrane Collaboration’s tool.9
2.5 |. Statistical analyses
Data were pooled by the use of the Cochran-Mantel-Haenszel method, and the outcomes were compared with the results obtained from a random-effects model, which considered the heterogeneity among the trials. To avoid unnecessary heterogeneity, we formed a homogeneous group of studies according to the adjustment status of the estimated risk. The Cochran Q and I2 statistic were applied for the estimation of heterogeneity, and the funnel plots with Egger’s test for small-study effects were applied to evaluate the risks of bias. The pooled odds ratios (ORs) and the 95% confidence intervals (CIs) were determined for the outcomes. P value of <0.05 was considered statistically significant. The analyses were performed using the software RevMan 5.3 (Cochrane, London, UK) and Stata 11.0 (StataCorp LLC, College Station, TX, USA).
3 |. RESULTS
3.1 |. Study selection and characteristics
Of the 175 relevant full-text articles/clinical trials identified from database search or manual search, seven full manuscripts met the inclusion criteria for this present study (Figure 1). Two full RCTs,9,14 one quasiexperimental study,13 and four observational studies (three case-controlled studies12,15,16 and one cross-sectional study21) were found. The remaining reports were observational studies, predominantly descriptive studies, or small case series. Study characteristics and quality are summarized in Table 1 and in Supporting Information Tables S1–S3. Funnel plot with Egger’s test on the small-study effects of 1-year freedom from AF/AT recurrence with driver-guided versus conventional ablation for PAF plus PerAF patients revealed that there was no bias of heterogeneity in this study (Supporting Information Figure S1). Metaregression with the adjustment with heterogeneity of AF duration in the selected studies demonstrates no significant impact on the outcomes (adjusted R2 = −82.7%, P = 0.67, Supporting Information Figure S2).
FIGURE 1.
Flow diagram of literature search
TABLE 1.
Details of selected studies
| First author | Study type | Total number | PerAF | Control group | Treatment group | Risk of bias | Quality of methodologyb |
|---|---|---|---|---|---|---|---|
| Atienza14 | RCT | 232 | 117 | PVI | Driver (PAF) ± PVI (PerAF) | No serious limitation | High |
| Lin9 | RCT | 68 | 68 | PVI + CFAE | PVI then rotor ablation within CFAE regions | No serious limitation | High |
| Narayan13 | Quasiexperimental study | 107a | 76 | PVI ± LA roof line, AT/AFL ablation (PerAF) | Driver then PVI (PAF) ± LA roof line, AT/AFL ablation (PerAF) | Serious | Moderate |
| Haïssaguerre12 | Case-controlled study | 103 | 103 | PVI | Driver then LA roof + mitral isthmus line if AF persisted then PVI | Serious | Moderate |
| Jadidi15 | Case-controlled study | 85 | 85 | PVI | PVI then rotor in low-voltage areas (<0.5 mV) | Serious | Low |
| Seitz16 | Case-controlled study | 105 | 81 | PVI | Driver | Serious | Moderate |
| Sommer21 | Cross-sectional study | 20 | 20 | N/A | Driver then PVI (PAF) ± LA roof line, AT/AFL ablation (PerAF) | Serious | Low |
AF = atrial fibrillation; AT/AFL = atrial tachycardia/atrial flutter; CFAE = complex fractionated atrial electrogram; LA = left atrium; PAF = paroxysmal atrial fibrillation; PerAF = persistent atrial fibrillation; PVI = pulmonary vein isolation; RCT = randomized controlled trial.
Procedure numbers.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines.
3.2 |. Analysis 1: Efficacy (AF termination and 1-year freedom of AF/AT recurrence) of driver-guided ablation compared to conventional ablation therapy
Six studies with control groups reported relevant efficacy outcomes for both treatment and control groups in PAF and PerAF (Table 1). One study was excluded from this analysis because there was no control group.21 Five studies were extracted for evaluation of the driverguided ablation efficacy on PerAF.9,12–15 Overall, driver-guided ablation was associated with significantly improved 1-year freedom (OR: 0.44, 95% CI: 0.30–0.65, P < 0.001) from recurrent AF/AT and higher AF termination rate (OR: 4.62, 95% CI: 2.12–10.08, P < 0.001) than conventional AF ablation strategies (Figures 2A and 3A). In the subgroup analysis for PerAF, driver-guided ablation was associated with higher 1-year freedom from recurrent AF/AT (OR: 0.36, 95% CI: 0.22–0.60, P < 0.001) and higher AF termination rate (OR: 7.12, 95% CI: 1.24–41.04, P = 0.03) than conventional AF ablation strategies.
FIGURE 2.
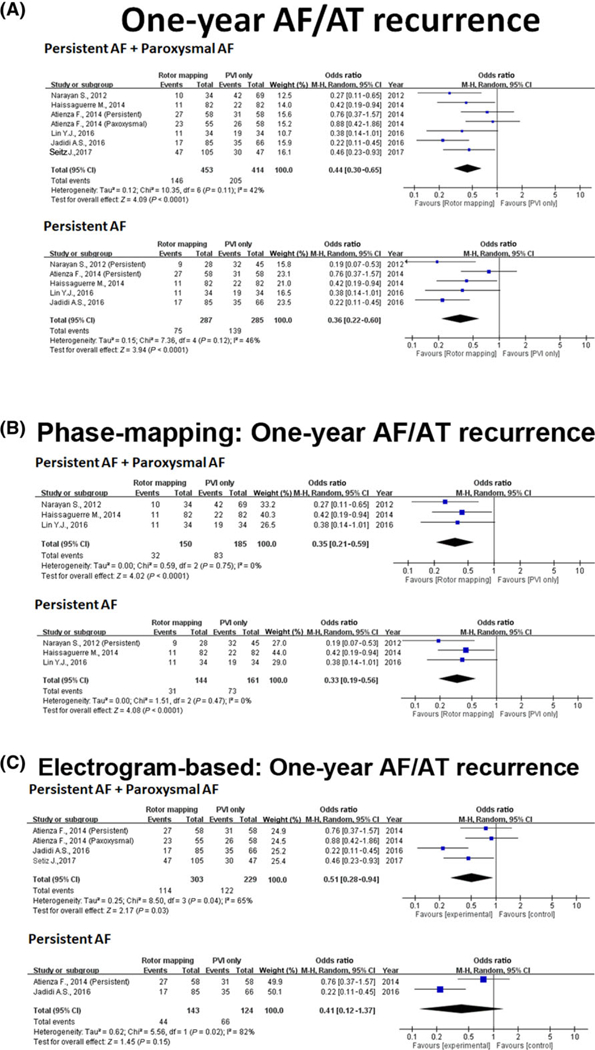
One-year freedom from AF/AT recurrence at 1 year. (A) Forest plot of 1-year freedom from AF/AT recurrence at 1 year with driver-guided versus conventional ablation. (B) Forest plot of 1-year freedom from AF/AT recurrence at 1 year with phase-mapping-based driver-guided versus conventional ablation. (C) Forest plot of 1-year freedom from AF/AT recurrence at 1 year with electrogram-based driver mapping versus conventional ablation. AF = atrial fibrillation; AT = atrial tachycardia [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
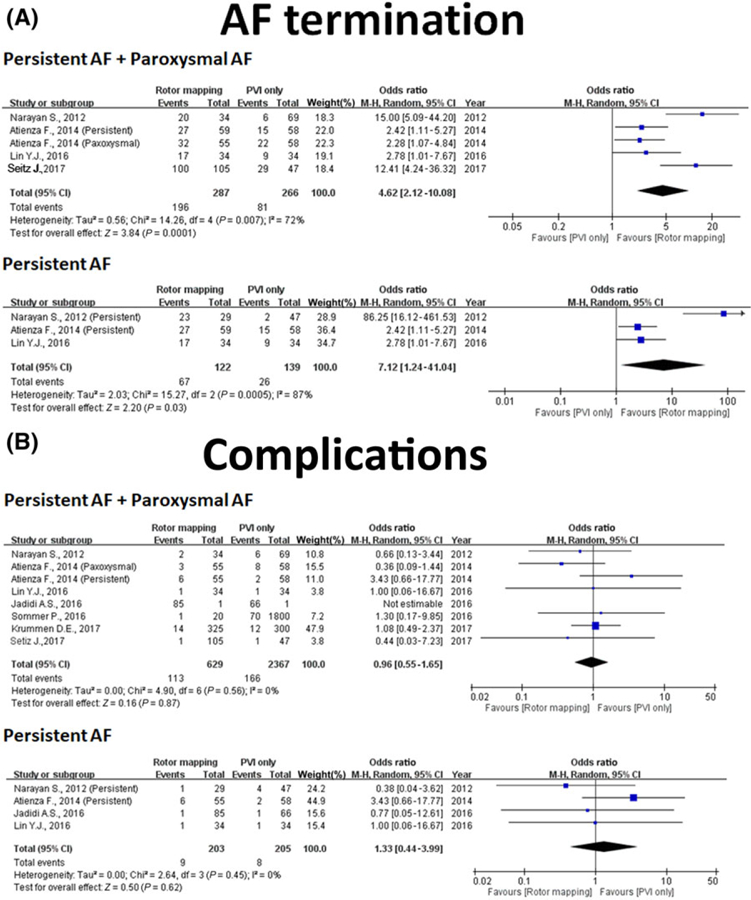
Analysis of AF termination and complication. (A) Forest plot of acute AF termination with driver-guided versus conventional ablation. (B) Forest plot of complication with driver-guided versus conventional ablation. AF = atrial fibrillation [Color figure can be viewed at wileyonlinelibrary.com]
3.3 |. Analysis 2: Subgroup analysis of efficacy (1-year freedom of AF/AT recurrence) by phase mapping and electrogram-based driver mapping strategy
Three studies used phase-mapping-based strategy9,12,13 and three used the electrogram-based driver mapping strategy to identify the drivers.14–16 One study that used phase mapping was excluded from this analysis because there was no control group.21 Five studies were extracted for evaluation of the driver-guided ablation efficacy on PerAF.9,12–15 In the pooled database, both phase mapping and electrogram-based driver mapping significantly improved the 1-year freedom from recurrent AF/AT (OR: 0.35, 95% CI: 0.21–0.59, P < 0.001 and OR: 0.51, 95% CI: 0.28–0.94, P < 0.001, respectively, Figures 2B and 2C). In the subgroup analysis of PerAF, phase mapping (OR: 0.33, 95% CI: 0.19–0.56, P < 0.001) significantly improved the 1-year freedom of recurrent AF/AT compared to electrogram-based driver mapping (OR: 0.41, 95% CI: 0.12–1.37, P = 0.15).
3.4 |. Analysis 3: Complications in driver mapping strategy compared to those in conventional ablation therapy
Seven studies with control groups reported relevant complications for both treatment and control groups in PAF or PerAF. One study was excluded from this analysis because there was no report regarding complications.12 Four studies were extracted for evaluation of the efficacy of driver-guided ablation on PerAF.9,13–15 Overall, driver-guided ablation-related complications did not differ from that in the control group in overall AF procedure and PerAF procedure (OR: 0.96, 95% CI: 0.55–1.65, P = 0.87 and OR: 1.33, 95% CI: 0.44–3.99, P = 0.62, respectively, Figure 3B).
4 |. DISCUSSION
4.1 |. Main findings
The current meta-analysis demonstrated that adjunctive driver-guided ablation in addition to conventional ablation could improve 1-year AF/AT freedom and increase AF termination rate during the procedure without risking additional potential complication. In patients with PerAF, phase mapping may be beneficial compared to electrogram-based driver mapping in the 1-year AF/AT freedom after ablation.
4.2 |. AF driver mapping technologies
A driver of a spiral wave is a rotation center with excitation rotating outward. Phase mapping has been the standard method to identify drivers in animal models of fibrillation.22 On the phase maps, a driver is defined as a phase singularity point around which the phase transitions through a complete cycle from −π to +π.23,24 There are mainly three phase mapping-guided settings used for driver detection: invasive focal impulse and driver modulation (Abbott, Abbott Park, IL, USA),13 noninvasive electrocardiographic imaging (ECGI),12 and electrogram similarity/phase mapping combined techniques.9 Several electrogram-based driver mapping techniques have also been used to demonstrate successful driver identification and ablation.
The heterogeneity of methodology used for AF driver mapping in published studies is a major limitation of the current data. Moreover, the available mapping systems capable of detecting AF drivers have major differences related to signal acquisition and processing.1 Focal Impulse and Rotor Mapping (FIRM) mapping13 used basket contact mapping and electrogram-based driver mapping technique,2 Haïssaguerre et al12 reported using noninvasive ECGI mapping technique with an array of body surface electrodes and phase-mapping-based technique,3 Lin et al9 reported using 20-poles double spiral catheter (1-mm electrodes with 4-mm spacing, St. Jude Medical, St. Paul, MN, USA) and nonlinear processing technique in signal processing with phase-mapping-based technique in high similarity index areas,4 Jadidi et al15 used doubleloop 20-pole catheter AFocus II HD (1-mm electrodes with 4-mm spacing, St. Jude Medical) or a 20-pole variable Lasso-Nav catheter (1 mm electrodes with 2–5-2 mm spacing, Biosense Webster, Diamond Bar, CA, USA) to identify repetitive rotational activity >70% of AF cycle length with electrogram-based driver mapping technique,5 Radiofrequency Ablation of Drivers of AF (RADAR-AF)14 used ablation catheter or circular mapping catheter with a dominant frequency/electrogram-based driver mapping technique, and6 Seitz et al16 used the 20-pole PentaRay catheter to identify the local regions displaying electrogram dispersion during AF. Because of the nonuniformed mapping technique and mapping materials, it is unknown if these mapping tools would detect the same drivers. The pooled efficacy effect estimates provided in this meta-analysis are based on the premise that these mapping tools are adequate for detecting AF drivers.
4.3 |. Comparison with previous meta-analyses
Previous meta-analyses of trials comparing additional driver-guided ablation with the traditional approach have supported the possible benefit of a combined approach of driver-guided ablation, which includes the phase mapping and electrogram-based technique, and PVI in improving single-procedure freedom from all arrhythmias in the population with mixed AF type.25,26 Another meta-analysis focused on studies using FIRM mapping (phase mapping based, RhythmView, Abbott Medical) to identify rotors in the mixed AF type.27 Although the pooled dataset favor the rotor ablation by using FIRM mapping, there was a marked heterogeneity between studies and wide variability in success rates between different centers performing rotor ablations.27 Mohanty et al28 conducted another meta-analysis comparing the PVI alone and PVI plus FIRM ablation. Unlike the previous meta-analysis, the PVI only group was extracted from other randomized trials, which were not related to FIRM ablation studies. Although the study design is debatable by comparing different strategies from different studies, the overall pooled estimate did not show any therapeutic benefit of PVI plus FIRM approach over PVI alone.28
These above-mentioned meta-analyses did not focus on ablation in PerAF patients alone, but on both PAF and PerAF patients. Additionally, the different driver mapping methodologies (phase mapping or electrogram-based mapping) have not been investigated. To the best of our knowledge, this is the first systematic review and meta-analysis reporting the comparison of the efficacy and safety of phase mapping and electrogram-based driver mapping for AF and PerAF patients. Our systematic review suggests that phase mapping may be superior to electrogram-based driver mapping technique for catheter ablation in PerAF patients.
4.4 |. Consideration of PerAF ablation
The optimal ablation strategy for persistent AF remains undetermined and an alternative approach must be explored. The results of the substrate and trigger ablation for reduction of AF—part II (STAR-AF II) trial have cast doubts on the efficacy of widely adopted strategies to modify the atrial substrate and have underscored an urgent need to identify the optimal ablation strategy for PerAF. Adjuvant ablation of the ganglion plexus failed to achieve significant improvement in PerAF ablation.29 Adjuvant elimination of drivers and non-PV triggers have been proposed as a potential strategy in PerAF patients.30
RADAR-AF and the study by Lin et al are the only full RCTs that test targeting the presumed AF drivers (defined as high-frequency sources using dominant frequency mapping in the former and as sites with high similarity indices using nonlinear phase-mapping in areas exhibiting complex fractionated atrial electrograms in the latter).9,14 The studies by Haïssaguerre et al, Narayan et al, and Jadidi et al were prospective studies with matched control patients to test the efficacy of driver elimination (defined as focal or reentrant activity by phase mapping using commercially available ECGI in the first study [against historical controls] and rotational activity with multiple electrodes in the latter two).12,15 The pooled data on the efficacy of PerAF driver-guided catheter ablation showed increased freedom from AF/AT relative to conventional strategies.
Additionally, phase mapping rather than electrogram-based driver mapping seemed to provide better freedom from AF/AT relative to conventional strategies. Although the data are promising, and the results favored phase-mapping driver identification, our meta-analysis included primarily nonrandomized studies. Overall, the evidence for the efficacy of AF driver ablation remains inconclusive. Further prospective randomized study with standardized driver identification and validation are warranted.
4.5 |. Limitations
Although the results are promising, existing studies are limited owing to the lack of consistent mapping tools. Therefore, the evidence to support ablative strategies targeting AF drivers remains inconclusive. Further, randomized and clinical trials with standardized mapping materials are needed. This study also included differences in clinical management between centers, reflecting differences in anticoagulation protocols, transseptal technique, and PVI ablation strategy between individual operators. Furthermore, the number of published RCT trials on driver-guided ablation is limited.
5 |. CONCLUSIONS
Pooled data on the efficacy of AF driver-guided catheter ablation suggested increased freedom from AF/AT relative to conventional strategies. Phase mapping appears to be superior to electrogram-based driver mapping technique to achieve better ablation outcomes in PerAF patients.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Center for Dynamical Biomarkers and Translational Medicine, Ministry of Science and Technology (Grant No. MOST 107–2314-B-010–061-MY2, MOST 106–2314-B-075–006-MY3, MOST 106–2314-B-010–046-MY3, MOST 106–2314-B-075–073-MY3), Szu-Yuan Research Foundation of Internal Medicine, and Taipei Veterans General Hospital (Grant No. V107B-014, V107C-060, and V107C-054).
Funding information
Szu-Yuan Research Foundation of Internal Medicine and Taipei Veterans General Hospital, Grant/Award Numbers: V107B-014, V107C-060, V107C-054; Ministry of Science and Technology, Taiwan, Grant/Award Numbers: 107–2314-B-010–061-MY2, MOST 106–2314-B-075–006-MY3, MOST 106–2314-B-010–046-MY3, MOST 106–2314-B-075–073-MY3
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Nattel S New ideas about atrial fibrillation 50 years on. Nature 2002;415:219–226. [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 3.Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879–1886. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo LW, Lin YJ, Chang SL, et al. Predictors and characteristics of multiple (more than 2) catheter ablation procedures for atrial fibrillation. J Cardiovasc Electrophysiol 2015;26:1048–1056. [DOI] [PubMed] [Google Scholar]
- 6.Lin YJ, Tsao HM, Chang SL, et al. Role of high dominant frequency sites in nonparoxysmal atrial fibrillation patients: Insights from high-density frequency and fractionation mapping. Heart Rhythm 2010;7:1255–1262. [DOI] [PubMed] [Google Scholar]
- 7.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005;112:789–797. [DOI] [PubMed] [Google Scholar]
- 8.Lin YJ, Tai CT, Kao T, et al. Frequency analysis in different types of paroxysmal atrial fibrillation. J Am Coll Cardiol 2006;47: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 9.Lin YJ, Lo MT, Chang SL, et al. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC Clin Electrophysiol 2016;2:667–678. [DOI] [PubMed] [Google Scholar]
- 10.Atienza F, Almendral J, Jalife J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009;6:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 12.Haïssaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–538. [DOI] [PubMed] [Google Scholar]
- 13.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol 2012;60:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: A noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol 2014;64: 2455–2467. [DOI] [PubMed] [Google Scholar]
- 15.Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 2016;9:e002962 10.1161/CIRCEP.115.002962 [DOI] [PubMed] [Google Scholar]
- 16.Seitz J, Bars C, Theodore G, et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: A wholly patient-tailored approach. J Am Coll Cardiol 2017;69:303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 19.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64: 401–406. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis 2011. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed December 21, 2018).
- 21.Sommer P, Kircher S, Rolf S, et al. Successful repeat catheter ablation of recurrent longstanding persistent atrial fibrillation with rotor elimination as the procedural endpoint: A case series. J Cardiovasc Electrophysiol 2016;27:274–280. [DOI] [PubMed] [Google Scholar]
- 22.Umapathy K, Nair K, Masse S, et al. Phase mapping of cardiac fibrillation. Circ Arrhythm Electrophysiol 2010;3:105–114. [DOI] [PubMed] [Google Scholar]
- 23.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature 1998;392:75–78. [DOI] [PubMed] [Google Scholar]
- 24.Bray MA, Wikswo JP. Considerations in phase plane analysis for non-stationary reentrant cardiac behavior. Phys Rev E Stat Nonlin Soft Matter Phys 2002;65:051902. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez FD, Birnie DH, Nair GM, et al. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J Cardiovasc Electrophysiol 2017;28:1371–1378. [DOI] [PubMed] [Google Scholar]
- 26.Baykaner T, Rogers AJ, Meckler GL, et al. Clinical implications of ablation of drivers for atrial fibrillation: A systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2018;11:e006119 10.1161/CIRCEP.117.006119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parameswaran R, Voskoboinik A, Gorelik A, et al. Clinical impact of rotor ablation in atrial fibrillation: A systematic review. Europace 2018;20:1099–1106. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty S, Mohanty P, Trivedi C, et al. Long-term outcome of pulmonary vein isolation with and without focal impulse and rotor modulation mapping: Insights from a meta-analysis. Circ Arrhythm Electrophysiol 2018;11:e005789 10.1161/CIRCEP.117.005789 [DOI] [PubMed] [Google Scholar]
- 29.Driessen AHG, Berger WR, Krul SPJ, et al. Ganglion plexus ablation in advanced atrial fibrillation: The AFACT study. J Am Coll Cardiol 2016;68:1155–1165. [DOI] [PubMed] [Google Scholar]
- 30.Hung Y, Lo LW, Lin YJ, et al. Characteristics and long-term catheter ablation outcome in long-standing persistent atrial fibrillation patients with non-pulmonary vein triggers. Int J Cardiol 2017; 241:205–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


