Abstract
The biology of bone healing is a rapidly developing science. Advances in transgenic and gene-targeted mice have enabled tissue and cell-specific investigations of skeletal regeneration. As an example, only recently has it been recognized that chondrocytes convert to osteoblasts during healing bone, and only several years prior, seminal publications reported definitively that the primary tissues contributing bone forming cells during regeneration were the periosteum and endosteum. While genetically modified animals offer incredible insights into the temporal and spatial importance of various gene products, the complexity and rapidity of healing— coupled with the heterogeneity of animal models—renders studies of regenerative biology challenging. Herein, cells that play a key role in bone healing will be reviewed and extracellular mediators regulating their behavior discussed. We will focus on recent studies that explore novel roles of inflammation in bone healing, and the origins and fates of various cells in the fracture environment.
Keywords: bone repair, fracture healing, bone regeneration
Injuries to the appendicular skeleton heal through two distinct processes: Direct (primary) or indirect (secondary) healing. Primary healing involves a direct transition of mesenchymal cells to bone-forming osteoblasts (intramembranous ossification). Secondary healing progresses through a cartilage intermediate before bone is formed by osteoblasts (endochondral ossification). The cellular and molecular factors that coordinate fracture callus formation and resolution are complex and highly orchestrated. This review will primarily discuss secondary healing, since the vast majority of fractures that occur clinically heal in this manner.
The process of bone healing has a variety of cellular components required for the progression of healing (Fig. 1). Inflammatory cells (i.e., T-cells, B-cells, mast cells, macrophages, eosinophils, and neutrophils) are the initial cellular component of the fracture environment, followed by mesenchymal progenitor cells, endothelial cells, chondrocytes, osteoblasts, and finally osteoclasts. The process of fracture healing can be easily considered in discrete temporal segments; however, it is important to recognize that there is significant overlap of the temporal segments of healing, and associated cell-types coexist. This is an important concept to consider, because cell-to-cell signaling, in a heterotypic manner (across cell-types) is undoubtedly important. For example, both chondrocytes and osteoblasts can promote blood vessel in-growth through their production of vascular endothelial growth factor (VEGF).1 Conversely, endothelial cells promote bone formation through production of bone morphogenetic protein (BMP) and new data suggest that vasculature guides the formation of a cartilaginous template and stimulates conversion of hypertrophic chondrocytes to osteoblasts.2
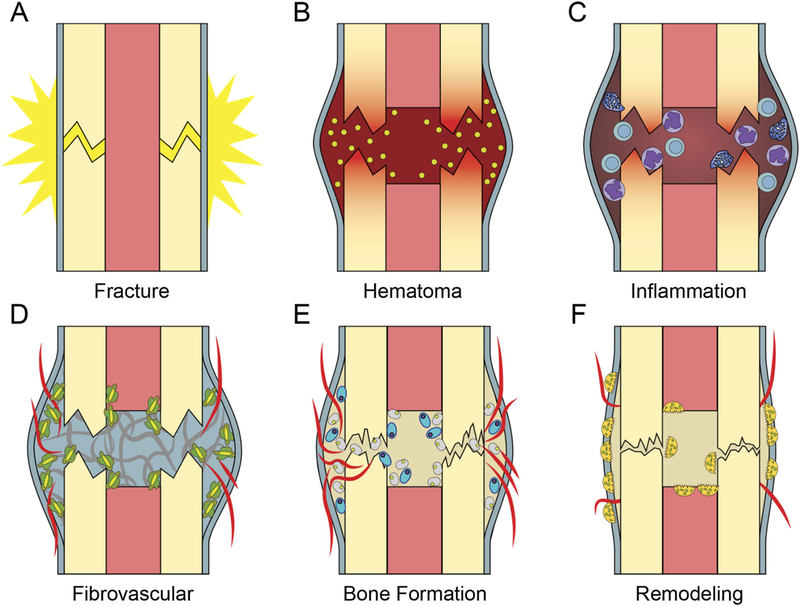
Figure 1.
Fracture healing is temporally-defined process. (A) At injury there is disruption of periosteum and bone (B) A clot forms immediately providing a provisional matrix. Platelet degranulation releases chemokines to recruit inflammation. (C) Inflammatory phase leads to a period of (D) Mesenchymal expansion and migration from the periosteum and endosteum and angiogenesis, (E) Bone is formed via both endochondral (blue large oval cells) and intramembranous ossification (smaller grey cells), (F) Osteoclasts (multinucleated cells) resorb primary bone and the process of remodeling restores bone shape and structure.
For this review we will consider the cells of fracture in a well-described and useful temporal sequence, familiar to many in the field. Where appropriate, we will discuss signaling factors regulating or produced by those cells, and in some cases consider signal transduction cascades and molecular programs that guide cellular physiology. While this review is not expected to be a comprehensive treatise on all known signaling factors, nor all known transcriptional regulators, we hope to present a detailed examination of the cells involved in bone regeneration.
INFLAMMATORY PHASE—INFLAMMATORY CELLS
Acute Inflammation
The acute pro-inflammatory response is essential for initiating fracture healing (Fig. 1B and C and 2). After fracture, bone architecture and vascular supply are disrupted (Fig. 1A). This results in a loss of mechanical stability, a decrease in tissue oxygenation and nutrient supply, and the release of bioactive factors at the site of injury.3,4 The inflammatory cells themselves, along with the cytokines and extracellular matrix they produce, appear essential in facilitating normal healing, as mice deficient in innate and adaptive immunity have significantly impaired endochondral bone repair.5
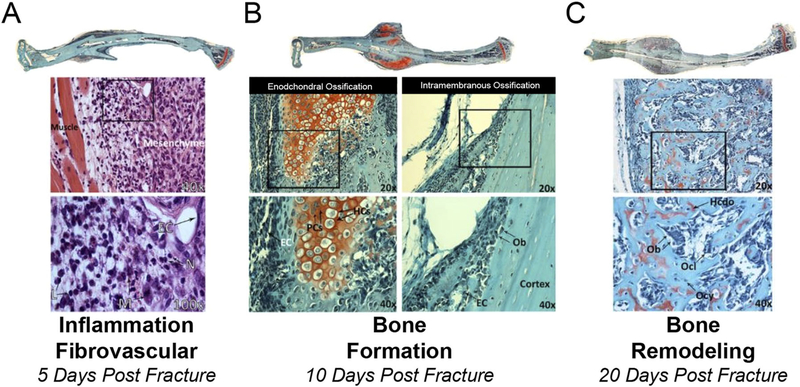
Figure 2.
Multiple cell-types present during the process of bone regeneration. Tibae were harvested 5 (A), 10 (B), and 20 (C) days post closed fracture and fixation with an intramedullary pin. Longitudinal histological sections were stained with H&E (A) or safranin-o (B and C) imaged at 2.5X and images stiched together and higher magnification images at 20X, 40X, and 100X obtained. (A) 5 day post-fracture undifferentiated mesenchymal cells are present in the callus and areas of inflammation remain (boxed area in 40X image is magnified in 100X) EC, endothelial cell; N, neutrophil; L, lymphocyte; M, macrophage. (B) 10 days post-fracture there is both endochondral ossification (red staining, safranin-o stains cartilage) and intramembranous bone formation occurring. Boxed areas in 20X images are magnified in 40X images. EC, endothelial cell; PC, proliferation chondrocytes; HC, hypertrophic chondrocytes; OB, osteoblast. (C) 20 days post-fracture. An extensive network of primary bone has formed and endochondral ossification is complete. Boxed areas in 20X images are magnified in 40X images. Ob, Osteoblast; Ocl, Osteoclast; Ocy, osteocyte; Hcdo, hypertrophic chondrocyte derived osteoblast.
Within the first minutes of fracture, a fibrin-rich blood clot forms to achieve hemostasis (Fig. 1B). The role of this fibrin-rich clot during fracture healing has been examined in mice lacking the key enzyme for fibrin degradation, plasminogen. While fibrin is not required for bone healing, repair does not properly progress without fibrinolysis. Specifically, the absence of plasminogen results in ectopic ossification and poor healing.6
Cytokines released by the clot (particularly during platelet degranulation) recruit inflammatory cells including lymphocytes, macrophages, eosinophils, and neutrophils.3,4,7,8 As one example, C-C Motif Chemokine Ligand 2 (also known as Monocyte Chemoattractant Protein-1) (CCL2 or MCP1) and its receptor Chemokine Receptor type 2 (CCR2) stimulate monocyte chemotaxis in the inflammatory response.9 CCL2 is expressed from days 1–3 in the fracture site.10 When subject to fracture, Ccl2-null and Ccr2-null mice both exhibit delayed fracture healing and decreased callus volume as a result of diminished mesenchymal cell infiltration and impaired vascularization.10,11
Inflammatory cells are deposited throughout the clot during hemorrhage and migrate to the injury site from local sources. While, the contribution of inflammatory cells derived from circulation versus those that are locally derived is not fully-understood, tissue resident macrophages, called ostealmacs, are necessary for fracture healing. One role of inflammatory cells, particularly neutrophils and macrophages, is debridement of injured and devitalized tissue. Inflammatory cells also produce cytokines that positively and negatively influence healing (Table S1).12–14 Some of these cytokines are detected at the fracture site within the first 24 h post-injury and are important for the expansion of the inflammatory response by acting on cells in the bone marrow, periosteum, and hematoma.15,16
Macrophages secrete the pro-inflammatory molecule Interleukin 1 (IL1). IL1 in-turn regulates expression of cyclooxygenases (Cox1 and Cox2), which are the enzymes that synthesize prostaglandins in injured tissues.17 Non-steroidal anti-inflammatory drugs, which inhibit cyclooxygenase activity, cause delays in fracture healing.14,18–22 These delays have been attributed to inhibition of Cox2 activity during fracture healing.14,15,23,24 Interestingly, marrow stromal cells derived from Cox2-null mice have diminished ability to form bone nodules in vitro, and this deficiency can be alleviated by the addition of prostaglandins to the culture media.15 However, eliminating signaling through IL1-beta does not appear to affect fracture repair.25
Other pro-inflammatory factors are also essential for fracture healing. For example, mice lacking the gene that encodes the Tumor Necrosis Factor alpha (TNF-alpha) receptor have a substantial delay in the onset of chondrocyte differentiation,26 and a delay in endochondral ossification.12 Interleukin 6 (IL6) has also been implicated in bone healing. Genetic ablation of IL6 in mice disrupts healing due to delayed callus mineralization, maturation, and conversion to bone.27 Early fracture healing in IL6-null mice is marked by decreases in osteocytes and callus strength.28 In addition to providing inflammatory cytokines, inflammatory cells also produce growth factors such as Fibroblast Growth Factors (FGF), Platelet-Derived Growth Factor (PDGF) and Transforming Growth Factor beta (TGF-beta), which initiate the repair process by facilitating proliferation and differentiation of the stem cells that give rise to the fracture callus.4,29,30
The multifactorial role of the acute pro-inflammatory response together contributes to its significance in healing and inhibition of inflammation is associated with delays in fracture repair.14,18–22,31 For example, depletion of macrophages during the early phases of fracture repair has been shown to reduce both callus size and chondrogenesis resulting in impaired fracture union.32,33 Defects in fracture healing can also be seen in mice lacking macrophage migration inhibitory factor (MIF).34 While endochondral bone regeneration is the primary mechanism of fracture repair, depletion of macrophages also impacts osteogenesis during intramembranous healing.35 Similarly, the absence of T and B lymphocytes and neutrophils also alters fracture healing.36–38
The role of complement factors in healing has been investigated by Ignatius and colleagues. Their work has demonstrated that mice deficient in C5, but not C3, show reduced bone healing. Interestingly, C5a activation is independent of C3.39 Furthermore, recent work demonstrates that both C5a receptors, C5aR1 and C5aR2, are required for bone healing, particularly in cartilageto-bone transition.40 Interestingly, this is in contrast to C5aR antagonists in polytrauma fracture healing, which has been shown to improve bone healing.41 As many cell types involved in bone healing express complement receptors, including, inflammatory cells, osteoclasts, and osteoblasts, fully understanding the role of complement in bone healing will require temporal and cell-type specific allelic disruption.
Resolving Inflammation
While the inflammatory phase of fracture healing begins during the earliest stages of repair, current evidence indicates that the inflammatory cells are also present throughout later phases and appear to undergo changes as healing proceeds (reviewed in ref.42). Analysis of fracture healing in mice lacking the TNF-alpha receptor reveals delays not only during acute inflammation, but in later stages of healing as well.12,26 Likewise, IL6 expression appears bimodal during fracture healing suggesting a temporally specific role for inflammatory cytokines during bone repair.43
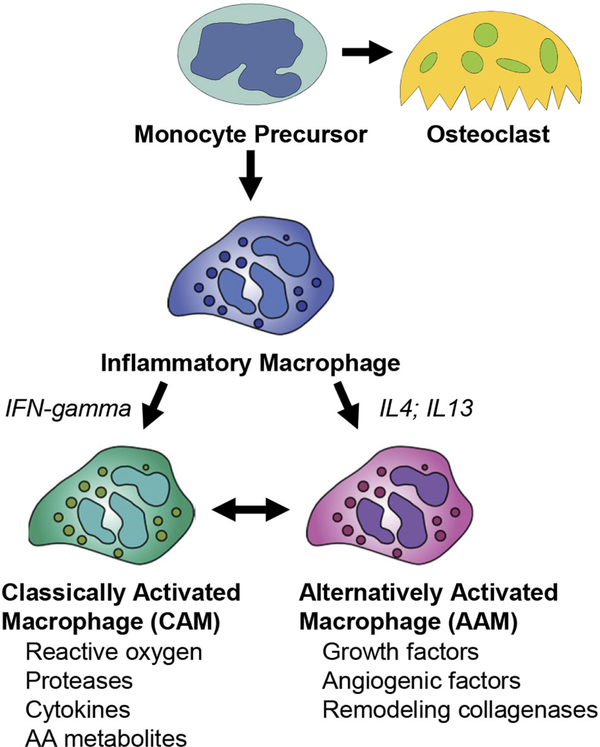
Changes in macrophage phenotype may explain this dual role in fracture healing. Macrophages can polarize along a continuum of pro- to anti-inflammatory states (Fig. 3). In the first few days post injury, pro-inflammatory macrophages are produced by “classical activation” that is typified by the innate immune response to bacterial pathogens and tissue injury through toll-like receptors (TLR). Classically activated macrophages (CAMs) are primed by exposure to interferon-gamma (IFN-gamma). Thereafter, pathogen-associated molecular pattern binding to TLR-family receptors on CAMs up-regulates the pro-inflammatory cytokines TNF-alpha, IL1 and IL6 through the NFkB pathway. Recent work from the Goodman laboratory has shown that CAMs indirectly promote osteogenesis by regulating MSC, albeit these studies have not been supported by in vivo studies.44
Figure 3.
Macrophage precursors develop into both classically activated and alternatively activated macrophages. Monocyte precursors give rise to both the osteoclast lineage and to inflammatory macrophages. Various factors, such as IFN-gamma, IL4, and IL13 control transitions between classically activated macrophages (CAM) and Alternatively activated macrophages (AAM).
Once macrophages have debrided the wound and are no longer classically activated, they can assume an anti-inflammatory state. Anti-inflammatory macrophages, also known as alternatively activated macrophages (AAMs), are generated through IL4 and IL13 signaling. In contrast to CAMs, alternative polarization of macrophages results in cellular activity that promotes collagen deposition and the return to tissue homeostasis. Production of TGF-beta, IL10 and arginase, as well as other secreted anti-inflammatory proteins, is associated with the repair of tissues following infectious and traumatic insults.45,46
Chronic Inflammation
Chronic, non-resolving inflammation is detrimental to fracture healing. Experimental evidence indicates fracture healing and osseointegration are disrupted in conditions where there is chronic, non-resolving inflammation, such as diabetes.47–49 Elevated TNF-alpha signaling may underlie some chronic inflammatory conditions,49 and evidence suggests blocking this pathway may have a therapeutic benefit in fracture healing.50
Aging is also associated with a non-resolving inflammatory state and impaired bone healing.16,51–54 In elderly animals, there are alterations to both progenitor and inflammatory cells.55 In particular, macrophages exhibit significant age-related alterations in function that change the inflammatory environment of aged animals and impact healing (reviewed in ref.56). Cutaneous wound healing is delayed in aged mice; however, these delays can be overcome by grafting macrophages derived from younger animals.57 Similarly, rejuvenation of the inflammatory system in aged animals significantly accelerates fracture repair.53,58,59 Thus, the functional capacity of juvenile macrophages appears to be more beneficial for healing than that of elderly macrophages. Compared to young mice, the innate and adaptive immunity cells of aged mice are more highly enriched during fracture healing.60 Bone regeneration is inhibited by increased CD8+ T cells which produce interferon-gamma and TNF-alpha and increased expression of CXCL8, CXCL9, and CXCL5 cytokines. The specific contribution of CD8+ T cells on fracture healing was demonstrated in a murine osteotomy model.61 Depletion of CD8+ T cells improved callus formation and bone mineral density. Conversely, increasing the CD8+ T cell population using adaptive transfer resulted in delayed callus formation and decreased bone mineral density. This work supports CD8+ T cell number as a potential prognostic marker for bone healing. Contrary to CD8+ T cells, IL-17A producing gamma delta T cells are essential for fracture repair. Loss of IL-17A disrupts proliferation and differentiation of MSCs resulting in delayed callus formation and lower bone mineral density.62
The cellular mechanisms causing dysregulation of immune cell function in aged animals is still being investigated. However, age-related changes to the macrophages appear to alter production of inflammatory cytokines. For example, decreases in Cox2 expression, an enzyme required for prostaglandin production, were observed during fracture healing in aged mice, and age-related delays were mitigated by activation of the prostaglandin receptor.54 Ultimately, understanding the altered functional characteristics of the macrophages may be essential for addressing the decreased healing in the elderly63; however, it is also important to consider that there are other non-inflammatory cell-autonomous explanations for altered healing with aging and metabolic conditions, such as reductions in progenitor cell number and function.64
FIBROVASCULAR PHASE—ENDOTHELIAL AND MESENCHYMAL PROGENITORS CELLS
Following inflammation, the angio-mesenchymal phase of repair begins (Fig. 1D and 2A). This phase has been termed the “fibrovascular phase” and is defined by vascular remodeling (angiogenesis and neovascularization) and recruitment of mesenchymal progenitor cells, sometimes referred to as mesenchymal stem cells (MSCs), that will ultimately differentiate into chondrocytes and osteoblasts to regenerate the fractured bone.
Revascularization
During the initial fracture trauma, the periosteal, cortical, and medullary vascular supply are disrupted leading to acute cellular necrosis and acidosis. The lack of vascularization causes local hypoxia, in which oxygen tension is lowered to 0.1–2%65–67 from 5%. Revascularization is required for perfusion of the callus with oxygen, nutrients, inflammatory and progenitor cells to facilitate repair, and the egress of waste products. In most cases, vascular supply is reestablished rapidly through the development of a new vascular network.68
Formation of the network occurs by two distinct processes: Angiogenesis and vasculogenesis. Angiogenesis is the process by which new blood vessels are formed by sprouting from existing vasculature. Vasculogenesis is de novo formation of blood vessels from in situ endothelial progenitor cells (EPCs) within the callus. Endothelial cells in forming callus vasculature can develop from a variety of sources, including, existing vessels of the periosteum and the intramedullary vasculature69; circulating EPCs70 that are increased during fracture repair71; or the bone marrow.72 Circulating EPCs are not only increased in rodent models, but are significantly increased in human patients at day three post-fracture.73
Vascular endothelial growth factor (VEGF) is a well-characterized driver of angiogenesis and vasculogenesis.74 VEGF is produced by a variety of cells in the fracture callus, including inflammatory cells and mesenchyme, but also osteoblasts and hypertrophic chondrocytes. VEGF binds the VEGF family of receptors VEGFR1 (FLT1) and VEGFR2 (FLK1) activating signaling cascades that lead to increased proliferation and sprouting of endothelial cells, and recruitment of EPCs to the fracture. In a model of distraction osteogenesis, blockade of VEGF activity via antibodies to VEGFR1 and VEGFR2 results in decreased vessel volume and reduction of callus formation.75 Neutralization of VEGF by the soluble VEGF receptor (IgGFlt) recapitulated this delay of callus vascularization.76
VEGF is a classical downstream target gene of hypoxia inducible factor 1-alpha (HIF1-alpha), which is stabilized in hypoxic77 and other conditions including when lacate levels are increased, as they are after fracture.67 Induction of HIF1-alpha and VEGF protein production peak at day 10 post-fracture in mice, during the period of endochondral ossification.2,78 Mice with increased expression of HIF1-alpha develop hyper-vascularized long bones with enhanced healing.79 On the other hand, HIF1-alpha-null mice,80 and mice with HIF1-alpha disrupted in osteoblasts,1 have delayed callus formation in fracture healing.
One interesting aspect of VEGF signaling is that during endochondral ossification, VEGF protein binds to the cartilage matrix until liberated by matrix metalloproteases (MMPs). MMPs are a family of extracellular proteases that degrade and remodel the extracellular matrix during development and repair. MMPs-2, −9, and −13 are robustly expressed during fracture repair and their absence results in impaired healing.13,81–84 While the Mmp2-null mutation delays only bone remodeling, the Mmp9- and Mmp13-null mutations affect bone formation by altering cartilage remodeling and vascularization. Importantly, administration of rVEGF to Mmp9-null mice during fracture healing rescues the null phenotype, indicating that VEGF release from the matrix by MMP9 is required for angiogenesis. MMP9 regulates VEGF availability.
The extracellular matrix (ECM) can also influence the angiogenic response to fracture healing. For example, thrombospondins (TSP) are a family of non-fibrillar matricellular proteins with a potent antiangiogenic function.85 Tsp2-null mice exhibit increased angiogenesis in the fracture callus,86,87 resulting in enhanced ischemic bone healing and alterations in callus composition in non-ischemic conditions. As such, targeting this pathway is an attractive therapeutic target for enhancing vascularity in bone regeneration. Osteopontin is also a modulator of fracture vascularization. Mice deficient in osteopontin show delayed angiogenesis and smaller calluses.88 Cell-type specific deletion of TSP2 and osteopontin using Cre-LoxP has not been described but would serve to better elucidate the mechanistic bases for these observations.
As the angiogenic response is a required event in fracture healing, deficiencies in angiogenesis result in delayed or insufficient fracture repair. Clinically, the non- or delayed-union rate increases from a basal level of 10–20% in the normal fracture population, to 46% when there is concomitant damage to the vasculature.89 Multiple preclinical models have been used to investigate the underlying mechanisms for this defect in healing. In experimental models of ischemia, fracture healing is significantly altered due to massive apoptosis of the periosteum.90 Similarly, de-vascularization of the periosteum proximal to the fracture site results in delayed healing and inhibits new bone formation.91 Some of the negative effects of ischemic bone fractures can be mitigated by environmental hyperoxia. In an experimental model of an ischemic tibia fracture, mice in hyperoxic conditions (50% environmental oxygen) demonstrated an increase in callus volume and cartilage content.67 The mice also were less likely to progress to non-union.
Co-morbidities such as aging, diabetes and smoking are also associated with delayed fracture healing, likely due to underlying vascular defects. Elderly and middle aged mice exhibit a decreased callus volume formation coupled with inhibited angiogenesis, and reduced expression of VEGF and MMP9 relative to juvenile fractures.92 In an obesity-induced model of type II diabetes mellitus, neovascularization of the fracture callus is inhibited resulting in decreased formation of woven bone.93 In distraction osteogenesis, cigarette smoking inhibits neovascularization and delayed tibial lengthening.94 Taken together, identifying clinically relevant conditions that affect angiogenesis are required to improve outcomes in fracture healing.
Mesenchymal Progenitor Cells
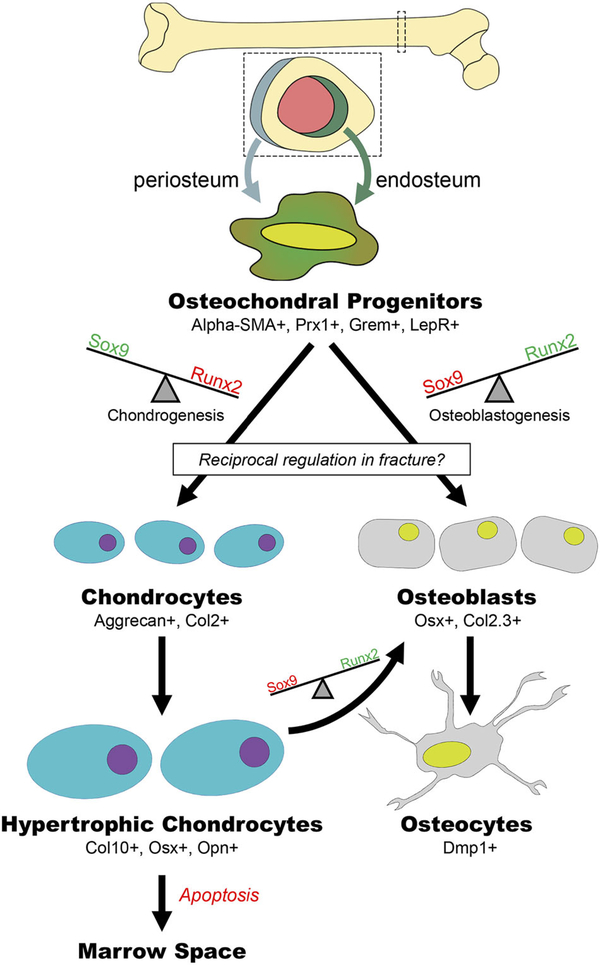
The other primary cellular component of the fibrovascular callus, is the mesenchymal progenitor cell (MSC). MSCs are multipotent cells that give rise to osteoblasts, chondrocytes, fibroblasts, myocytes, and adipocytes.95 While these cells are referred to as “stem cells,” it is notable that in most cases, criteria of stemness are not well-established for these cells. Even within the MSC population, sub-populations have been identified that differ in lineage potential and function. Nestin, an intermediate filament fiber, has been used to differentiate between populations of MSCs that are mesodermal- or neural crest-derived. Nestin-negative MSCs primarily contribute to skeletogenesis in the fetus whereas nestin-positive cells assume this role later in life.96 In bone fracture repair, quiescent MSCs reprise their developmental role as osteochondral progenitor cells (Figs. 1, 2, and 4).
Figure 4.
Mesenchymal precursors develop into both osteoblasts and chondrocytes. Osteochondral progenitors are activated at the time of bone injury and a balance in transcriptional activation results in the cells becoming either osteoblasts or chondrocytes. Hypertrophic chondrocytes can differentiate to become osteoblasts.
The majority of MSCs recruited to the fracture site are derived locally from the periosteum and bone marrow. The original experimental evidence for this came from Colnot who used lineage analysis to demonstrate that locally derived progenitors from the periosteum, endosteum, and bone marrow are the major cellular contributors to the fracture callus.97 Subsequently, using the mesenchymal marker α-Smooth Muscle Actin-9 (α-SMA9), Cre-recombination based fate mapping shows α-SMA9+ cells invade the fracture site from the periosteum 2-days post fracture, and by 6 days post fracture this α-SMA9+ population has robustly expanded to provide a large source of osteocyte progenitors.98,99 Gremlin-CreERT2 and LepR-Cre cells have also been shown to give rise to osteoblasts and chondrocytes in the fracture callus.100,101 Table S2 provides a summary of recent investigations in bone healing that employed Cre systems and includes additional Cre mice relevant to fracture biology that have yet to be studied. Together, these various Cre mice will be useful for better defining the spatial and temporal regulation of cells contributing to bone healing.
Recruitment of MSCs in the fracture repair program is under molecular regulation by cytokines released at the fracture site, particularly CXCL12, also known as stromal cell-derived factor 1 (SDF1). SDF1 is released by the injured periosteum and drives mobilization and homing of MSCs through CXCR4.102 Partial disruption of SDF1/CXCR4 in mouse allografts lead to decreased MSC chemotaxis and bone formation. In a live bone graft model of repair, both antibody sequestering of SDF1 and pharmacologic inhibition of its receptor CXCR4 resulted in inhibited MSC chemotaxis and decreased formation of bone in the callus.102 SDF1 is under transcriptional regulation by HIF1-alpha in response to ischemia,103 demonstrating a role for the hypoxic condition of the fracture environment in directing MSC recruitment, as well as vascularization. In a tibial fracture model in mice, SDF1 increased callus formation as well as induced expression of VEGF and Runx2 in the soft tissue callus, indicative of increased angiogenesis and osteogenesis.104 Recently, implantation of bone marrow derived MSCs (BM-MSCs) overexpressing SDF1 in a bone defect model resulted in improved new bone formation relative to BM-MSC implantation alone.105 It is notable that the SDF1/CXCR4 homing pathway is also required for EPC recruitment in tibial fracture healing. Cxcr4-null mice exhibit decreased callus formation as a result of inhibited EPC recruitment, decreased blood flow in the fracture site, and decreased VEGF and CD31 (an endothelial cell marker) expression in the callus one week post fracture.106 Exogenous SDF-1 was not sufficient to rescue this phenotype, indicating an exclusive requirement of CXCR4 in SDF-1 stimulated EPC recruitment.
Notch signaling is another potentially important factor in both regulating MSC number and activation. Mice with Notch signaling disrupted through Mx1-Cre mediated overexpression of the dominant negative mastermind (DnMAML) show alterations in callus size.38 Mice with complete disruption of canonical Notch signaling by Prx1-Cre mediated disruption of the Notch transcription factor CSL have non-unions. Notch signaling appears to be required for the proliferation and/or migration of mesenchymal progenitor cells.107
While the data using reporter mice support the concept that MSC in fracture callus are derived from periosteal and endosteal activation, there is still some potential that MSC could be derived from the circulation. Using CMV-Cre-R26R-LacZ-MSCs transplanted into a fractured mouse, it was shown that MSCs in circulation localize in the endosteum, but not periosteum, of the fracture site as early as 3 days post injury.108 To study if circulatory MSCs could contribute to fracture healing, BMP-2-Lac-Z-MSCs were transplanted which demonstrated BMP-2 expression in the endosteum. However, it is still unclear if MSCs in circulation contribute significantly to bone remodeling by differentiating into chondrocytes and osteoblasts or if they promote healing indirectly in a paracrine fashion through the release of growth factors and cytokines.
BONE FORMATION—OSTEOBLASTS AND CHONDROCYTES
Following the fibrovascular phase of healing, many of the MSC that formed the fibrovascular callus undergo differentiation to either osteoblasts or chondrocytes to initiate the bone formation phase of healing98 (Fig. 1E, 2, 4).
Differentiation of MSCs into bi-potential osteochondral progenitor cells is initially regulated by Sox9.109 Sox9 is required for chondrogenesis and genetic disruption studies demonstrate that absence of this transcription factor leads to the complete elimination of the cartilaginous anlagen in the developing skeleton.110 Conversely, in osteoblasts, downregulation of Sox9 in bi-potential cells releases repression of Runx2.111 Runx2 deletion results in a complete loss of osteoblasts in mouse embryos.112–114 However, disruption of Runx2 in chondrocytes is embryonic lethal and inhibits endochondral ossification.115 While Runx2 has been traditionally called a “master regulator” of osteoblastogenesis, it may play a larger upstream role as a regulator of bi-potential osteochondral progenitor cells. Runx2 transcriptionally regulates osteoblastogenesis in part through the transcription factor Sp7 (Osterix).114,116 Knock-out of Osterix is also associated with lack of osteoblasts, however, Osterix expression is absent following deletion of Runx2, suggesting Osterix is downstream.114,116 Sox9 also actively represses osteogenic potential by suppressing Runx2,111 thereby these opposing programs appear to act as a molecular switch between cartilage and bone fate in osteochondral progenitor cells.117
Factors regulating the decision of progenitor cells towards the chondrogenic or osteogenic fate are multifactorial, integrated and still being defined. Extrinsically, mechanical factors and oxygen tension are undoubtedly important variables regulating fate decision.118,119 These microenvironmental cell-extrinsic factors then lead to very specific cell-intrinsic regulation of chondrogenesis and osteoblastogenesis.
Increased motion has been shown to induce the formation of more chondrocytes and in-turn increases endochondral ossification,118,120,121 while stabilization results in the generation of more osteoblasts and direct bone repair via intramembranous formation.120 Specifically, strains smaller than 5% and hydrostatic pressures less than 0.15 MPa promote intramembranous formation.121 Morgan et al.122 have assessed strain distribution during healing in a loaded osteotomy model and then associated strain patterns with the type of bone formation determined histologically. Higher octahedral shear strain and maximum principal strain increased cartilage and decreased woven bone, while volumetric strain was less reliably associated with intramembranous bone versus a cartilage phenotype.
Another putative environmental signal that may regulate the fate decision of MSC is oxygen tension. The relationship between oxygen tension and MSC differentiation in vitro has been extensively investigated, and the preponderance of evidence suggests that hypoxia promotes a chondrogenic phenotype, whereas higher levels of oxygen promote osteoblast differentiation. In vivo, this relationship between oxygen and MSC fate decision has been computationally modeled and experimentally validated through directed callus oxygenation.123,124 However, other work has demonstrated that reducing inspired oxygen levels leads to problems with healing, but does not appear to alter the mode of fracture healing.67
Secreted growth factors also have a direct effect on MSC differentiation. BMPs are the classic osteogenic molecule associated with bone formation. In vitro BMPs directly stimulate MSC osteoblast differentiation and canonical bone programs characterized by the activity of the Runx2 and Sp7 (Osterix) transcription factors which are direct, downstream targets of BMP signaling.125 In vivo, after trauma, periosteal cells express BMP2 and BMP4 and over time they proliferate in response to BMP5 and BMP6.126,127 Notably, BMPs are also important in dictating chondrocyte differentiation, so that mice with conditional disruption of Bmp (in particular Bmp2) show non-unions.128–130 Signaling via BMP2 is also absent during intermediate stages of intramembranous repair, which is critical for preventing cartilage formation.131 At day 10 postinjury, BMPs (2, 4–8), extracellular BMP antagonists (BMP3 and noggin), BMP receptors (1A, 1B, and II), and effectors (p-Smads 1, 5, and 8) are not detected in osteoblasts, osteoclasts, or the periosteum within a fracture site’s new bone.132 Addition of recombinant human BMP2 (rhBMP2) to stabilized fractures results in formation of new cartilage primarily at the periosteal surface, which ultimately leads to a callus with increased cartilage and total volume, but no increase in intramembranous bone formation.131 The dual role of BMP signaling in regulating both osteoblast and chondrocyte differentiation of multipotent mesenchymal progenitors is not fully understood in the context of fracture repair. Presumably, co-acting factors, in association with as yet undefined epigenetic changes, influence the balance of key osteoblast transcription factors, such as Runx2 and Osterix, relative to key chondrogenic transcription factors, such as Sox9.
Another secreted growth factor family that could play a role in regulating MSC fate determination in bone healing is the Wnt family (reviewed in ref.133). In non-fracture environments, inhibiting beta-catenin activity in the osteoblast lineages leads to decreased bone mass and increased chondrogenesis,134–136 while ablation of Wnt inhibitors, DKK137 or sclerostin,138 increases bone formation and bone mass. While the developmental role of canonical Wnt has been demonstrated, less is known about its role during fracture healing.133 In areas of intramembranous ossification in murine femur fractures, Dishevelled and beta-catenin have been localized to osteoblasts lining regions of newly formed woven bone and in those destined to be trapped in new bone.139 Fracture studies in Wnt deficient mice suggest impaired healing compared to wild type littermates,140,141 likely as a result of disturbed osteoblast function since cartilage formation and osteoclasts numbers degrading the mineralized matrix are unaltered in its absence.142 Conversely, mice deficient in Sclerostin (an inhibitor of the canonical Wnt/beta-atenin pathway) heal single cortical, fully stabilized mid-diaphyseal femur fractures more robustly than wild type mice.143 This is in large part due to increased osteoblast numbers and bone surfaces 7–14 days post-injury. Importantly, a therapeutic benefit to fracture healing has been shown when canonical Wnt signaling was stimulated by adding a monoclonal antibody to the Wnt inhibitor DKK.144,145
Intramembranous Ossification—Osteoblasts
Direct differentiation of mesenchymal progenitors to osteoblasts is the exclusive mechanism of bone repair in fully stabilized defects (intramembranous ossification), but also occurs along the periosteal and endosteal surfaces of the bone in less stabilized fractures. (Fig. 1E, 2B and 4) Periosteal progenitor cells appear to have a bi-potent osteo-chondral potential, with differentiation linked to the mechanical microenvironment, as detailed previously. Osteogenic differentiation of the periosteal MSC gives rise to intramembranous bone locally along the bone surfaces adjacent to the fracture; while these same periosteal progenitor cells migrate into the fracture gap to undergo chondrogenesis. In contrast, endosteal stem cells exhibit uni-potent osteogenic potential. Intramembranous bone formation from these endosteal stem cells is thus responsible for rapidly bridging across the marrow cavity.97
Endochondral Bone Formation—Chondrocytes
Temporally, chondrogenic differentiation of fracture callus progenitor cells is closely aligned with resolution of the pro-inflammatory response and occurs on the fibrin scaffold that was generated as part of the hematoma. Spatially chondrogenesis occurs primarily in the fracture gap, with periosteal stem cells being the primary source of the chondrocytes146 (Fig. 1E, 2B and 4).
Following initial fate specification of the MSC to a chondrocyte, SOX9 expression plays an essential role in maintaining the cartilaginous phenotype and hypertrophic maturation.147–149 SOX9, along with transcriptional co-factors SOX5 and SOX6, regulate the expression of collagen II150–152 and aggrecan.153 These are the canonical extracellular matrix proteins of cartilage, and together make up ~90% of the dry weight of the tissue, imparting cartilage with its characteristic biophysical properties. This dense cartilage callus bridges the fracture gap and helps stabilize the defect. At this stage the cartilage tissue becomes avascular, repressing angiogenesis and vascular invasion.154
Conversion of the cartilage callus to bone occurs following a highly regulated maturation of chondrocytes from a proliferative through a hypertrophic state (Fig. 5).2 Hypertrophic maturation is distinguished morphologically by a dramatic increase in cell volume. Hypertrophic chondrocytes in the growth plate increase in size ~20-fold by taking on both volume and dry mass.155 At a molecular level, the hypertrophic chondrocyte is distinguished by the expression of collagen type X. While the exact function of collagen X is not clear, it is uniquely expressed by hypertrophic chondrocytes and matrix deposition is believed critical in priming the matrix for mineralization.
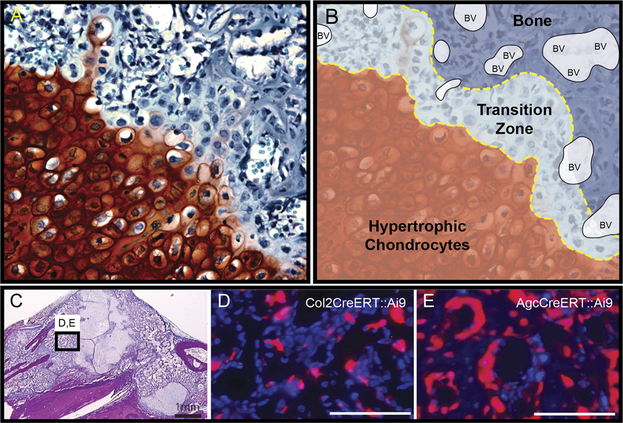
Figure 5.
Hypertrophic chondrocytes develop into osteoblasts and osteocytes. Tibiae were harvested post fracture and stained with (A) Safranin-O to define the chondrogenic front as outlined in panel (B). (B) shows zones of hypertrophic chondrocytes, transition zone, Bone, and blood vessels (BV). (C) is a low magnification H&E image showing the localization of panels D and E in areas of bone. Cells of bone can be traced to the chondrocyte lineage using the (D) Col2CreERT2:: Ai9 or the (E) AgcCreERT::Ai9 mouse with a tamoxifen pulse.
Chondrocyte hypertrophy represents a pivotal state during endochondral ossification. Hypertrophic chondrocytes are highly angiogenic and facilitate a second phase of vascular invasion into the cartilage callus by synthesizing VEGF,156–158 PDGF (platelet derived growth factor),159 and PlGF (placental growth factor).160 Adjacent to the invading vasculature, hypertrophic chondrocytes lose Sox9 expression, which subsequently relieves repression of osteogenic promoters Runx2 and beta-catenin.2,147 Subsequently, hypertrophic chondrocytes begin to express canonical markers of bone, including, alkaline phosphatase, osterix, osteopontin, and osteocalcin.161 Together, activation of osteogenic programs and angiogenesis result in calcification of the cartilage matrix.162 From a functional perspective this calcification provides additional rigidity to the fracture.
The molecular trigger for calcification is not completely clear, but BMPs likely play a key role in this process. BMP are expressed by both hypertrophic chondrocytes132 and vascular endothelial cells,163 suggesting that there are both cell-autonomous and paracrine effects of BMP signaling that may drive calcification. Invasion of the vasculature also provides hypertrophic chondrocytes with other systemic factors such as extracellular calcium, parathyroid hormone, vitamin D, and insulin-like growth factor that play a role in controlling mineral homeostasis during fracture repair. Whether it is BMP expression alone from the vascular endothelial cells that drives calcification of the cartilage, or whether additional secreted factors164 may also contribute to this process remains unclear.
Following calcification of the cartilage, bone formation occurs. In this vascularized transition zone between cartilage and bone, histological staining reveals hypertrophic chondrocytes entrapped in a bone matrix adjacent to the vasculature.2 As cartilaginous matrix is lost and bone matrix is laid down the large round hypertrophic morphology of chondrocytes is gradually converted into morphology characteristic of the osteocytes with cellular extensions existing in canaliculi. How this shape change is facilitated remains an outstanding question, but reductive cell division of the hypertrophic chondrocytes may be one mechanism enabling this morphogenesis.2 Similarly, the mechanism by which the cartilage matrix is degraded remains debated and will be discussed later.
The ultimate fate of the hypertrophic chondrocyte at the time of bone formation has recently been redefined both in the growth plate and fracture callus (Fig. 2 and 5). The traditional model held that hypertrophic chondrocytes were a terminally differentiated, post-mitotic cell, fated for apoptosis. According to this model, new bone was formed by osteoprogenitors or pre-osteoblasts that invade acellular cartilage matrix along with the vasculature.165 This dogmatic view of cell death in the hypertrophic chondrocyte overshadowed early work suggesting that chondrocytes could directly give rise to bone during endochondral ossification.166–169 However, more recently a number of genetic lineage tracing studies using chondrocyte-specific, temporally-regulatable promoters (Table S2) have clearly demonstrated that chondrocytes live and differentiate to become osteocytes both in the growth plate during development and during fracture repair164,170,171,2 (Fig. 5).
The mechanism by which chondrocytes transform into osteocytes remains poorly defined, but a few possibilities have been proposed. The osteocyte could just be the terminal fate of the chondrocyte, representing the natural phenotypic progression of these cells during maturation; or the chondrocyte could de-differentiate to a progenitor-like state prior to activating the osteoblast programs, and then becoming an osteoblast.2,164,172 Another proposed mechanism is that the hypertrophic chondrocytes undergo an asymmetric cell division, at which point one of the daughter cells becomes an osteoblast/osteocyte and the other undergoes apoptosis.173–175 These suggested pathways are not mutually exclusive. For example, activation of the stem cell genes may not truly impart multipotency, but rather reactivate the cell cycle or enable the chromatin remodeling required for osteoblast gene expression. Significantly more work is required to understand the molecular details that regulate conversion of hypertrophic chondrocytes to osteoblasts, and to understand how chondrogenic matrix is converted to an osteogenic matrix.
It should be noted, that some amount of apoptosis of hypertrophic chondrocytes and osteoblasts/cytes is required to create marrow space. Similarly, evidence suggests that in the growth plate at least some of the endochondral bone is formed by osteoblasts.165 Consequently, it is important to recognize that this new model does not exclude the possibility that chondrocyte apoptosis and invading osteoblasts contribute to the newly formed bone at the fracture site. Determining the contribution of the various cell sources will require more study utilizing cell-type-specific temporally-regulated Cre-based studies.
CALLUS REMODELING AND OSTEOCLASTS
Remodeling of the bony callus is traditionally considered the last stage of fracture repair. Remodeling must occur to degrade the provisional bone that is first produced, referred to as woven bone, and replace it with mature lamellar bone. A key component of callus remodeling is bone degradation by osteoclasts176 (Fig. 1F and2). Osteoclasts are myeloid lineage multinucleated cells that form tight attachments to the bone surface via a specialized membrane structure called the sealing zone.177 Vesicle trafficking delivers both soluble and membrane-bound lysosomal proteins to the sealing zone, and fusion of these transport vesicles with the intra-sealing zone plasma membrane creates the high-surface-area, manifold ruffled border that is the hallmark of a mature resorbing osteoclast.178–180 The acidic pH of Howship’s lacuna facilitates dissolution of hydroxyapatite crystals comprising the mineral component of bone while proteases digest the underlying collagenous matrix. Osteoclast mediated degradation of the bone liberates bonesequestered factors, such as TGF-beta as well as factors produced by the osteoclast itself, such as complement 3a, Wnt10b, BMP6, and SLIT3181,182 which are hypothesized to be critical in the subsequent stimulation of osteogenesis.183,184 Resorption is concluded with the apoptotic death of the osteoclast, an event that can be stimulated by the hormone calcitonin or 17-beta-estradiol-enhanced Fas ligand expression.185
Osteoclasts originate from hematopoietic monocyte/macrophage lineage precursors. Proliferation and survival of osteoclast precursors is stimulated by interaction between monocyte/macrophage colonystimulating factor (MCSF) and its receptor c-fms, which is present on both macrophages and osteoclasts. Bone marrow macrophages differentiate into osteoclasts upon stimulation with Receptor Activator of Nuclear Factor kappaB Ligand (RANKL) which binds to its receptor, RANK.186 Osteoclast differentiation occurs through multiple phases.176,187 Both MCSF and RANKL are required throughout the differentiation process and also contribute to the survival of mature osteoclasts. MCSF and RANKL are both necessary and sufficient for osteoclast formation and function, but multiple other cytokines and signaling pathways influence osteoclast differentiation, maturation, and survival.188–191
Both RANK and RANKL knockout mice have demonstrated the critical role of osteoclasts in physiological bone remodeling, but the role of osteoclasts in fracture repair has been investigated only recently.192,193 The medaka fin ray fracture model has allowed for longitudinal observation of the cellular contribution to fracture repair and has revealed a role for osteoclasts in two stages.194 Following the inflammatory phase of fracture, osteoclasts are recruited to smaller bone fragments which are partially resorbed. This partial resorption deburrs the edges of the fragments which are later incorporated into the callus, but whether osteoclast resorption of the fragments is necessary for their preservation in the growing fracture callus is unknown. Osteoclast activity is again induced near the conclusion of the healing process, wherein they remodel the hard callus and restore the bone to dimensions similar to those prior to injury. Inhibition of osteoclast protease activity using cathepsin K inhibitors during the bony callus remodeling phase results in calluses with greater mineral density, but also increases osteoclast surface and osteoblast numbers.195 Pharmacological disruption of osteoclastogenesis by inhibiting transient receptor potential cation channel subfamily V member 1 (TRPV1) has been used as a treatment strategy for post-menopausal bone loss. However, fracture studies using TRPV1 knockout mice demonstrated an essential role of osteoclasts in soft-callus formation and remodeling.196 The decreased osteoclast number in TRPV1 mice lead to enlarged malformed calluses and persistent fracture gaps. In addition, there was down regulation of RUNX2 and ALP in MSCs.
Remodeling during the process of endochondral bone formation is also necessary; however, the requirement for osteoclasts in this process are less-clear. Osteoclasts can be detected in histological sections of the endochondral callus and therefore are sometimes referred to as chondroclasts (though there is no evidence that these are a cell type distinct from osteoclasts). While osteoclasts are capable of resorbing cartilage, it is not clear that they have a functional role. Both human and animal studies have revealed that inhibiting osteoclast function does not significantly impact remodeling of the cartilaginous callus, but will delay bony callus remodeling.197–200
If not mediated through direct interaction with osteoclasts, degradation of the cartilage callus may be accomplished indirectly through other cells expressing MMPs. As discussed earlier, MMPs are a family of extracellular matrix proteins with a demonstrated functional role in fracture repair. MMPs are expressed by many of the cells involved in bone healing, including osteoblasts and chondrocytes, and have differential specificity towards the collagens and proteoglycans found in the cartilage matrix. MMP13 has high specificity towards both collagen II and aggrecan and is made by both hypertrophic chondrocytes and osteoblasts. Similarly MMP9, with specificity towards gelatin (or degraded collagen), is made by both vascular endothelial cells and macrophages.84,201 Interestingly, transplantation of wild type bone marrow into MMP9 mutants rescues the remodeling defect that is observed in the mutant animals,83 but this same experiment does not rescue the remodeling defect in the MMP13 mutant.81 These outcomes suggest that MMP9 expressed by cells derived from the hematopoietic system and MMP13 derived from the chondrocytes work in concert to remove cartilage during endochondral ossification.
CONCLUSIONS
While we have discretely discussed the various phases of healing, the reality is that there is overlap of all phases of healing. This spatiotemporal heterogeneity of fracture healing has made studies of fracture cell biology challenging. However, the utilization of genetically modified mice has permitted a more rapid advancement in our understanding of the cells involved in fracture repair (Table S2). Cell-specific reporter mice have permitted us to prospectively identify the cells of the callus to provide spatial resolution. The development of tissue-specific promoters driving inducible Creactivity enables additional temporal resolution for lineage tracing. Indeed, it was only relatively recently that the periosteal and endosteal origin of mesenchymal cells was definitively determined based on promoter reporter lineage tracing. Similarly, recent temporally defined lineage-specific data from Bahney et al. has been able to demonstrate the transdifferentiation of chondrocytes to osteoblasts.2 Traditional gene knockouts as well as tissue specific and temporally defined knockout models have permitted us to understand gene function in the context of defined cell types. This has been particularly true for probing the role of BMP. Over the next decade, this temporally-regulated, cell-type specific gene regulation will permit a more careful dissection of fracture cell biology. The next frontier will be to understand how multiple cell types and resultant signaling networks are integrated spatially over time to regulate healing. More advanced computational models of cellular behavior in complex environments will be useful for understanding these influences. From a translational perspective, advances in understanding the cell biology of fracture will then need to be extended to larger animal models, and to pathological fracture healing.
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Wang Y, Wan C, Deng L, et al. 2007. The hypoxiainducible factor {alpha} pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu DP, Ferro F, Yang F, et al. 2017. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 144:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Einhorn TA. 1995. Enhancement of fracture-healing. J Bone Joint Surg Am 77:940–956. [DOI] [PubMed] [Google Scholar]
- 4.Barnes GL, Kostenuik PJ, Gerstenfeld LC, et al. 1999. Growth factor regulation of fracture repair. J Bone Miner Res 14:1805–1815. [DOI] [PubMed] [Google Scholar]
- 5.Rapp AE, Bindl R, Recknagel S, et al. 2016. Fracture healing is delayed in immunodeficient NOD/scidIL2Rgammacnull mice. PLoS ONE 11:e0147465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuasa M, Mignemi NA, Nyman JS, et al. 2015. Fibrinolysisis essential for fracture repair and prevention of heterotopic ossification. J Clin Invest 125:3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claes L, Recknagel S, Ignatius A. 2012. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8:133–143. [DOI] [PubMed] [Google Scholar]
- 8.Miclau T. 2000. Current opinioin in orthopaedics. Current Issue 11:367–371. [Google Scholar]
- 9.Chu HX, Arumugam TV, Gelderblom M, et al. 2014. Role of CCR2 in inflammatory conditions of the central nervous system. J Cereb Blood Flow Metab 34:1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa M, Ito H, Kitaori T, et al. 2014. MCP/CCR2 signaling is essential for recruitment of mesenchymal progenitor cells during the early phase of fracture healing. PLoS ONE 9:e104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing Z, Lu C, Hu D, et al. 2010. Multiple roles for CCR2 during fracture healing. Dis Model Mech 3:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstenfeld LC, Cho TJ, Kon T, et al. 2001. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs 169:285–294. [DOI] [PubMed] [Google Scholar]
- 13.Colnot C, Thompson Z, Miclau T, et al. 2003. Altered fracture repair in the absence of MMP9. Development 130:4123–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstenfeld LC, Thiede M, Seibert K, et al. 2003. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res 21:670–675. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Schwarz EM, Young DA, et al. 2002. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest 109:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing Z, Lu C, Hu D, et al. 2010. Multiple roles for CCR2 during fracture healing. Dis Model Mech 3:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bankers-Fulbright JL, Kalli KR, McKean DJ. 1996. Interleukin-1 signal transduction. Life Sci 59:61–83. [DOI] [PubMed] [Google Scholar]
- 18.Sudmann E, Dregelid E, Bessesen A, et al. 1979. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest 9:333–339. [DOI] [PubMed] [Google Scholar]
- 19.Allen HL, Wase A, Bear WT. 1980. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand 51:595–600. [DOI] [PubMed] [Google Scholar]
- 20.Altman RD, Latta LL, Keer R, et al. 1995. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma 9:392–400. [DOI] [PubMed] [Google Scholar]
- 21.Engesaeter LB, Sudmann B, Sudmann E. 1992. Fracture healing in rats inhibited by locally administered indomethacin. Acta Orthop Scand 63:330–333. [DOI] [PubMed] [Google Scholar]
- 22.Giannoudis PV, MacDonald DA, Matthews SJ, et al. 2000. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br 82:655–658. [DOI] [PubMed] [Google Scholar]
- 23.Einhorn TA. 2003. Where are we in 2003? The role of cyclooxygenase-2 in bone repair. Arthritis Res Ther 5:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon AM, Manigrasso MB, O’Connor JP. 2002. Cyclooxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 17:963–976. [DOI] [PubMed] [Google Scholar]
- 25.Lange J, Sapozhnikova A, Lu C, et al. 2010. Action of IL-1beta during fracture healing. J Orthop Res 28:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerstenfeld LC, Cho TJ, Kon T, et al. 2003. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 18:1584–1592. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Ricciardi BF, Hernandez-Soria A, et al. 2007. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace A, Cooney TE, Englund R, et al. 2011. Effects of interleukin-6 ablation on fracture healing in mice. J Orthop Res 29:1437–1442. [DOI] [PubMed] [Google Scholar]
- 29.Hurley MM, Adams DJ, Wang L, et al. 2016. Accelerated fracture healing in transgenic mice overexpressing an anabolic isoform of fibroblast growth factor 2. J Cell Biochem 117:599–611. [DOI] [PubMed] [Google Scholar]
- 30.Wildemann B, Schmidmaier G, Ordel S, et al. 2003. Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-beta1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater 65:150–156. [DOI] [PubMed] [Google Scholar]
- 31.Mullis BH, Copland BA, Weinhold PS, et al. 2005. Effect of COX-2 inhibitors and non-Steroidal anti-inflammatory drugs on a mouse fracture model. Arch Orthop Trauma Surg 37:827–837. [DOI] [PubMed] [Google Scholar]
- 32.Raggatt LJ, Wullschleger ME, Alexander KA, et al. 2014. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol 184:3192–3204. [DOI] [PubMed] [Google Scholar]
- 33.Vi L, Baht GS, Whetstone H, et al. 2014. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 30:1090–1102. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Onodera S, Kondo E, et al. 2011. Impaired fracture healing in macrophage migration inhibitory factordeficient mice. Osteoporos Int 22:1955–1965. [DOI] [PubMed] [Google Scholar]
- 35.Alexander KA, Chang MK, Maylin ER, et al. 2011. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 26:1517–1532. [DOI] [PubMed] [Google Scholar]
- 36.Nam D, Mau E, Wang Y, et al. 2012. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS ONE 7:e40044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toben D, Schroeder I, El Khassawna T, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res 26:113–124. [DOI] [PubMed] [Google Scholar]
- 38.Dishowitz MI, Mutyaba PL, Takacs JD, et al. 2013Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PLoS ONE 8:e68726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehrnthaller C, Huber-Lang M, Nilsson P, et al. 2013. Complement C3 and C5 deficiency affects fracture healing. PLoS ONE 8:e81341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovtun A, Bergdolt S, Hagele Y, et al. 2017. Complement receptors C5aR1 and C5aR2 act differentially during the early immune response after bone fracture but are similarly involved in bone repair. Sci Rep 7:14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Recknagel S, Bindl R, Kurz J, et al. 2012. C5aR-antagonist significantly reduces the deleterious effect of a blunt chest trauma on fracture healing. J Orthop Res 30:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolar P, Schmidt-Bleek K, Schell H, et al. 2010. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 16:427–434. [DOI] [PubMed] [Google Scholar]
- 43.Blakytny R, Laumen S, Ignatius A, et al. 2009. Multiple roles for interleukin-6 (IL-6) in fracture healing. Orthop Proc 91-B:472. [Google Scholar]
- 44.Lu LY, Loi F, Nathan K, et al. 2017. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res 35:2378–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S, Martinez FO. 2010. Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604. [DOI] [PubMed] [Google Scholar]
- 46.Daley JM, Brancato SK, Thomay AA, et al. 2009. The phenotype of murine wound macrophages. J Leukoc Biol 87:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kayal RA, Tsatsas D, Bauer MA, et al. 2007. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 22:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colombo JS, Balani D, Sloan AJ, et al. 2011. Delayed osteoblast differentiation and altered inflammatory response around implants placed in incisor sockets of type 2 diabetic rats. Clin Oral Implants Res 22:578–586. [DOI] [PubMed] [Google Scholar]
- 49.Alblowi J, Kayal RA, Siqueira M, et al. 2009. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol 175:1574–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kayal RA, Siqueira M, Alblowi J, et al. 2010. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone Miner Res 25:1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mountziaris PM, Mikos AG. 2008. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev 14:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green E, Lubahn JD, Evans J. 2005. Risk factors, treatment, and outcomes associated with nonunion of the midshaft humerus fracture. J Surg Orthop Adv 14:64–72. [PubMed] [Google Scholar]
- 53.Xing Z, Lu C, Hu D, et al. 2010. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res 28:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naik AA, Xie C, Zuscik MJ, et al. 2008. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res 24:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geiger H, Van Zant G. 2002. The aging of lymphohematopoietic stem cells. Nat Immunol 3:329–333. [DOI] [PubMed] [Google Scholar]
- 56.Sebastian C, Espia M, Serra M, et al. 2005. MacrophAging: a cellular and molecular review. Immunobiology 210: 121–126. [DOI] [PubMed] [Google Scholar]
- 57.Danon D, Kowatch MA, Roth GS. 1989. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A 86:2018–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu C, Miclau T, Hu D, et al. 2005. Cellular basis for agerelated changes in fracture repair. J Orthop Res 23:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baht GS, Silkstone D, Vi L, et al. 2015. Exposure to a youthful circulaton rejuvenates bone repair through modulation of beta-catenin. Nat Commun 6:7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hebb JH, Ashley JW, McDaniel L, et al. 2018. Bone healing in an aged murine fracture model is characterized by sustained callus inflammation and decreased cell proliferation. J Orthop Res 36:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reinke S, Geissler S, Taylor WR, et al. 2013. Terminally differentiated CD8(þ) T cells negatively affect bone regeneration in humans. Science translational medicine 5:177ra36. [DOI] [PubMed] [Google Scholar]
- 62.Ono T, Okamoto K, Nakashima T, et al. 2016. IL-17 producing gammadelta T cells enhance bone regeneration. Nat Commun 7:10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lloberas J, Celada A. 2002. Effect of aging on macrophage function. Exp Gerontol 37:1325–1331. [DOI] [PubMed] [Google Scholar]
- 64.Mutyaba PL, Belkin NS, Lopas L, et al. 2014. Notch signaling in mesenchymal stem cells harvested from geriatric mice. J Orthop Trauma 28:S20–S23. [DOI] [PubMed] [Google Scholar]
- 65.Pennathur-Das R, Levitt L. 1987. Augmentation of in vitro human marrow erythropoiesis under physiological oxygen tensions is mediated by monocytes and T lymphocytes. Blood 69:899–907. [PubMed] [Google Scholar]
- 66.Heppenstall RB, Grislis G, Hunt TK. 1975. Tissue gas tensions and oxygen consumption in healing bone defects. Clin Orthop Relat Res 357–365. [DOI] [PubMed] [Google Scholar]
- 67.Lu C, Saless N, Wang X, et al. 2013. The role of oxygen during fracture healing. Bone 52:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu C, Marcucio R, Miclau T. 2006. Assessing angiogenesis during fracture healing. Iowa Orthop J 26:17–26. [PMC free article] [PubMed] [Google Scholar]
- 69.Yuasa M, Mignemi NA, Barnett JV, et al. 2014. The temporal and spatial development of vascularity in a healing displaced fracture. Bone 67:208–221. [DOI] [PubMed] [Google Scholar]
- 70.Tepper OM, Capla JM, Galiano RD, et al. 2005. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105:1068–1077. [DOI] [PubMed] [Google Scholar]
- 71.Lee DY, Cho TJ, Kim JA, et al. 2008. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 42:932–941. [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto T, Mifune Y, Kawamoto A, et al. 2008. Fracture induced mobilization and incorporation of bone marrowderived endothelial progenitor cells for bone healing. J Cell Physiol 215:234–242. [DOI] [PubMed] [Google Scholar]
- 73.Ma XL, Sun XL, Wan CY, et al. 2012. Significance of circulating endothelial progenitor cells in patients with fracture healing process. J Orthop Res 30:1860–1866. [DOI] [PubMed] [Google Scholar]
- 74.Otrock ZK, Mahfouz RA, Makarem JA, et al. 2007. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis 39:212–220. [DOI] [PubMed] [Google Scholar]
- 75.Jacobsen KA, Al-Aql ZS, Wan C, et al. 2008. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 23:596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Street J, Bao M, deGuzman L, et al. 2002. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 99:9656–9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams JM, Difazio LT, Rolandelli RH, et al. 2009. HIF-1: a key mediator in hypoxia. Acta Physiol Hung 96:19–28. [DOI] [PubMed] [Google Scholar]
- 78.Komatsu DE, Hadjiargyrou M. 2004. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 34:680–688. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Wan C, Deng L, et al. 2007. The hypoxiainducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Komatsu DE, Bosch-Marce M, Semenza GL, et al. 2007. Enhanced bone regeneration associated with decreased apoptosis in mice with partial HIF-1alpha deficiency. J Bone Miner Res 22:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Behonick DJ, Xing Z, Lieu S, et al. 2007. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS ONE 2:e1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colnot CI, Helms JA. 1902. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 100:245–250. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Yu YY, Lieu S, et al. 2013. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone 52:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colnot C, Thompson Z, Miclau T, et al. 2003. Altered fracture repair in the absence of MMP9. Development 130:4123–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adams JC, Lawler J. 2011. The thrombospondins. Cold Spring Harb Perspect Biol 3:a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taylor DK, Meganck JA, Terkhorn S, et al. 2009. Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing. J Bone Miner Res 24:1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miedel E, Dishowitz MI, Myers MH, et al. 2013. Disruption of thrombospondin-2 accelerates ischemic fracture healing. J Orthop Res 31:935–943. [DOI] [PubMed] [Google Scholar]
- 88.Duvall CL, Taylor WR, Weiss D, et al. 2007. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-Deficient mice. J Bone Miner Res 22:286–297. [DOI] [PubMed] [Google Scholar]
- 89.Dickson KFKS, Paiement G 1995. The importance of the blood supply in the healing of tibial fractures. Contemp Orthop 30:489–493. [PubMed] [Google Scholar]
- 90.Lu C, Miclau T, Hu D, et al. 2007. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res 25:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oetgen ME, Merrell GA, Troiano NW, et al. 2008. Development of a femoral non-union model in the mouse. Injury 39:1119–1126. [DOI] [PubMed] [Google Scholar]
- 92.Lu C, Hansen E, Sapozhnikova A, et al. 2008. Effect of age on vascularization during fracture repair. J Orthop Res 26:1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown ML, Yukata K, Farnsworth CW, et al. 2014. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 9:e99656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ueng SW, Lee SS, Lin SS, et al. 1999. Hyperbaric oxygen therapy mitigates the adverse effect of cigarette smoking on the bone healing of tibial lengthening: an experimental study on rabbits. J Trauma 47:752–759. [DOI] [PubMed] [Google Scholar]
- 95.Pittenger MF, Mackay AM, Beck SC, et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. [DOI] [PubMed] [Google Scholar]
- 96.Isern J, Garcia-Garcia A, Martin AM, et al. 2014. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3: e03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colnot C. 2009. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matthews BG, Grcevic D, Wang L, et al. 2014. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res 29:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grcevic D, Pejda S, Matthews BG, et al. 2012. In vivo fate mapping identifies mesenchymal progenitor cells. Stem cells 30:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou BO, Yue R, Murphy MM, et al. 2014. Leptin-receptorexpressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Worthley DL, Churchill M, Compton JT, et al. 2015. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160: 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitaori T, Ito H, Schwarz EM, et al. 2009. Stromal cellderived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60:813–823. [DOI] [PubMed] [Google Scholar]
- 103.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. 2004. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864. [DOI] [PubMed] [Google Scholar]
- 104.Li X, Gao Z, Wang J. 2011. Single percutaneous injection of stromal cell-derived factor-1 induces bone repair in mouse closed tibial fracture model. Orthopedics 34:450. [DOI] [PubMed] [Google Scholar]
- 105.Ho CY, Sanghani A, Hua J, et al. 2014. Mesenchymal stem cells with increased stromal cell-Derived factor 1 expression enhanced fracture healing. Tissue Eng Part A 21:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kawakami Y, Ii M, Matsumoto T, et al. 2015. SDF-1/ CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. J Bone Miner Res 30:95–105. [DOI] [PubMed] [Google Scholar]
- 107.Wang C, Inzana JA, Mirando AJ, et al. 2016. NOTCH signaling in skeletal progenitors is critical for fracture repair. J Clin Invest 126:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Granero-Molto F, Weis JA, Miga MI, et al. 2009. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem cells 27:1887–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akiyama H, Kim JE, Nakashima K, et al. 2005. Osteochondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A 102:14665–14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bi W, Deng JM, Zhang Z, et al. 1999. Sox9 is required for cartilage formation. Nat Genet 22:85–89. [DOI] [PubMed] [Google Scholar]
- 111.Zhou G, Zheng Q, Engin F, et al. 2006. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci U S A 103:19004–19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ducy P, Zhang R, Geoffroy V, et al. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754. [DOI] [PubMed] [Google Scholar]
- 113.Komori T, Yagi H, Nomura S, et al. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764. [DOI] [PubMed] [Google Scholar]
- 114.Otto F, Thornell AP, Crompton T, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771. [DOI] [PubMed] [Google Scholar]
- 115.Chen H, Ghori-Javed FY, Rashid H, et al. 2014. Runx2 regulates endochondral ossification through control of chondrocyte proliferation and differentiation. J Bone Miner Res 29:2653–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakashima K, Zhou X, Kunkel G, et al. 2002. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29. [DOI] [PubMed] [Google Scholar]
- 117.Eames BF, Sharpe PT, Helms JA. 2004. Hierarchy revealed in the specification of three skeletal fates by Sox9 and Runx2. Developmental biology 274:188–200. [DOI] [PubMed] [Google Scholar]
- 118.Carter DR, Beaupre GS, Giori NJ, et al. 1998. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res S41–S55. [DOI] [PubMed] [Google Scholar]
- 119.Thompson Z, Miclau T, Hu D, et al. 2002. A model for intramembranous ossification during fracture healing. J Orthop Res 20:1091–1098. [DOI] [PubMed] [Google Scholar]
- 120.Le AX, Miclau T, Hu D, et al. 2001. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res 19:78–84. [DOI] [PubMed] [Google Scholar]
- 121.Claes LE, Heigele CA, Neidlinger-Wilke C, et al. 1998. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res S132–S147. [DOI] [PubMed] [Google Scholar]
- 122.Morgan EF, Salisbury Palomares KT, Gleason RE, et al. 2010. Correlations between local strains and tissue phenotypes in an experimental model of skeletal healing. J Biomech 43:2418–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Burke D, Dishowitz M, Sweetwyne M, et al. 2013. The role of oxygen as a regulator of stem cell fate during fracture repair in TSP2-null mice. J Orthop Res 31:1585–1596. [DOI] [PubMed] [Google Scholar]
- 124.Burke DP, Kelly DJ. 2012. Substrate stiffness and oxygen as regulators of stem cell differentiation during skeletal tissue regeneration: a mechanobiological model. PLoS ONE 7:e40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luo W, Friedman M, Hankenson K, et al. 2011. Time series gene expression profiling and temporal regulatory pathway analysis of BMP6 induced osteoblast differentiation and mineralization. BMC Syst Biol 5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marsell R, Einhorn TA. 2009. The role of endogenous bonemorphogenetic proteins in normal skeletal repair. Injury 40:S4–S7. [DOI] [PubMed] [Google Scholar]
- 127.Cho TJ, Gerstenfeld LC, Einhorn TA. 2002. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone MinerRes 17:513–520. [DOI] [PubMed] [Google Scholar]
- 128.Tsuji K, Cox K, Gamer L, et al. 2010. Conditional deletion of BMP7 from the limb skeleton does not affect bone formation or fracture repair. J Orthop Res 28:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsuji K, Cox K, Bandyopadhyay A, et al. 2008. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am 90:14–18. [DOI] [PubMed] [Google Scholar]
- 130.Tsuji K, Bandyopadhyay A, Harfe BD, et al. 2006. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38:1424–1429. [DOI] [PubMed] [Google Scholar]
- 131.Yu YY, Lieu S, Lu C, et al. 2010. Bone morphogenetic protein two stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 47:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu YY, Lieu S, Lu C, et al. 2010. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone 46:841–851. [DOI] [PubMed] [Google Scholar]
- 133.Secreto FJ, Hoeppner LH, Westendorf JJ. 2009. Wnt signaling during fracture repair. Curr Osteoporos Rep 7:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Day TF, Guo X, Garrett-Beal L, et al. 2005. Wnt/betacatenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750. [DOI] [PubMed] [Google Scholar]
- 135.Hill TP, Spater D, Taketo MM, et al. 2005. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738. [DOI] [PubMed] [Google Scholar]
- 136.Glass DA 2nd, Bialek P, Ahn JD, et al. 2005. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764. [DOI] [PubMed] [Google Scholar]
- 137.MacDonald BT, Joiner DM, Oyserman SM, et al. 2007. Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balemans W, Ebeling M, Patel N, et al. 2001. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543. [DOI] [PubMed] [Google Scholar]
- 139.Zhong N, Gersch RP, Hadjiargyrou M. 2006. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone 39:5–16. [DOI] [PubMed] [Google Scholar]
- 140.McGee-Lawrence ME, Carpio LR, Bradley EW, et al. 2014. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone 66:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y, Whetstone HC, Lin AC, et al. 2007. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med 4:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heilmann A, Schinke T, Bindl R, et al. 2013. The Wnt serpentine receptor Frizzled-9 regulates new bone formation in fracture healing. PLoS ONE 8:e84232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McGee-Lawrence ME, Ryan ZC, Carpio LR, et al. 2013. Sclerostin deficient mice rapidly heal bone defects by activating beta-catenin and increasing intramembranous ossification. Biochem Biophys Res Commun 441:886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Komatsu DE, Mary MN, Schroeder RJ, et al. 2010. Modulation of Wnt signaling influences fracture repair. J Orthop Res 28:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jin H, Wang B, Li J, et al. 2015. Anti-DKK1 antibody promotes bone fracture healing through activation of betacatenin signaling. Bone 71:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Colnot C. 2008. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dy P, Wang W, Bhattaram P, et al. 2012. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell 22:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ikegami D, Akiyama H, Suzuki A, et al. 2011. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development 138:1507–1519. [DOI] [PubMed] [Google Scholar]
- 149.Leung VY, Gao B, Leung KK, et al. 2011. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet 7:e1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bell DM, Leung KK, Wheatley SC, et al. 1997. SOX9 directly regulates the type-II collagen gene. Nat Genet 16:174–178. [DOI] [PubMed] [Google Scholar]
- 151.Lefebvre V, Huang W, Harley VR, et al. 1997. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol 17:2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ng LJ, Wheatley S, Muscat GE, et al. 1997. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol 183:108–121. [DOI] [PubMed] [Google Scholar]
- 153.Han Y, Lefebvre V. 2008. L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Mol Cell Biol 28:4999–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hattori T, Muller C, Gebhard S, et al. 2010. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137:901–911. [DOI] [PubMed] [Google Scholar]
- 155.Cooper KL, Oh S, Sung Y, et al. 2013. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 495:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gerber HP, Vu TH, Ryan AM, et al. 1999. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5:623–628. [DOI] [PubMed] [Google Scholar]
- 157.Colnot CI, Helms JA. 2001. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 100:245–250. [DOI] [PubMed] [Google Scholar]
- 158.Zelzer E, McLean W, Ng YS, et al. 2002. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129:1893–1904. [DOI] [PubMed] [Google Scholar]
- 159.Andrew JG, Hoyland JA, Freemont AJ, et al. 1995. Platelet-derived growth factor expression in normally healing human fractures. Bone 16:455–460. [DOI] [PubMed] [Google Scholar]
- 160.Maes C, Coenegrachts L, Stockmans I, et al. 2006. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest 116:1230–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gerstenfeld LC, Shapiro FD. 1996. Expression of bonespecific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem 62:1–9. [DOI] [PubMed] [Google Scholar]
- 162.Gerstenfeld LC, Cruceta J, Shea CM, et al. 2002. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J Bone Miner Res 17:221–230. [DOI] [PubMed] [Google Scholar]
- 163.Matsubara H, Hogan DE, Morgan EF, et al. 2012. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 51:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bahney CS, Hu DP, Taylor AJ, et al. 2014. Stem cellderived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res 29:1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maes C, Kobayashi T, Selig MK, et al. 2010. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kahn AJ, Simmons DJ. 1977. Chondrocyte-to-osteocyte transformation in grafts of perichondrium-free epiphyseal cartilage. Clin Orthop Relat Res 299–304. [DOI] [PubMed] [Google Scholar]
- 167.Scammell BE, Roach HI. 1996. A new role for the chondrocyte in fracture repair: endochondral ossification includes direct bone formation by former chondrocytes. J Bone Miner Res 11:737–745. [DOI] [PubMed] [Google Scholar]
- 168.Roach HI. 1992. Trans-differentiation of hypertrophic chondrocytes into cells capable of producing a mineralized bone matrix. Bone Miner 19:1–20. [DOI] [PubMed] [Google Scholar]
- 169.Pritchard JJ, Ruzicka AJ. 1950. Comparison of fracture repair in the frog, lizard and rat. J Anat 84:236–261. [PMC free article] [PubMed] [Google Scholar]
- 170.Yang L, Tsang KY, Tang HC, et al. 2014. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A 111:12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou X, von der Mark K, Henry S, et al. 2014. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10:e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Song L, Tuan RS. 2004. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 18:980–982. [DOI] [PubMed] [Google Scholar]
- 173.Roach HI, Aigner T, Kouri JB. 2004. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis 9:265–277. [DOI] [PubMed] [Google Scholar]
- 174.Roach HI, Clarke NM. 2000. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J Bone Joint Surg Br 82:601–613. [DOI] [PubMed] [Google Scholar]
- 175.Roach HI, Erenpreisa J. 1996. The phenotypic switch from chondrocytes to bone-forming cells involves asymmetric cell division and apoptosis. Connect Tissue Res 35:85–91. [DOI] [PubMed] [Google Scholar]
- 176.Teitelbaum SL. 2007. Osteoclasts: what do they do and how do they do it? Am J Clin Pathol 170:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vaananen HK, Zhao H, Mulari M, et al. 2000. The cell biology of osteoclast function. J Cell Sci 113:377–381. [DOI] [PubMed] [Google Scholar]
- 178.Yang DQ, Feng S, Chen W, et al. 2012. V-ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast-mediated bone resorption. J Bone Miner Res 27:1695–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hirvonen MJ, Mulari MT, Buki KG, et al. 2012. Rab13 is upregulated during osteoclast differentiation and associates with small vesicles revealing polarized distribution in resorbing cells. J Histochem Cytochem 60:537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takahashi N, Ejiri S, Yanagisawa S, et al. 2007. Regulation of osteoclast polarization. Odontology 95:1–9. [DOI] [PubMed] [Google Scholar]
- 181.Pederson L, Ruan M, Westendorf JJ, et al. 2008. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A 105:20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim BJ, Lee YS, Lee SY, et al. 2018. Osteoclast-secretedSLIT3 coordinates bone resorption and formation. J Clin Invest 128:1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matsuoka K, Park KA, Ito M, et al. 2014. Osteoclastderived complement component 3a stimulates osteoblast differentiation. J Bone Miner Res 29:1522–1530. [DOI] [PubMed] [Google Scholar]
- 184.Tang Y, Wu X, Lei W, et al. 2009. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Krum SA, Miranda-Carboni GA, Hauschka PV, et al. 2008. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Feng X. 2005. RANKing intracellular signaling in osteoclasts. IUBMB Life 57:389–395. [DOI] [PubMed] [Google Scholar]
- 187.Yagi M, Miyamoto T, Sawatani Y, et al. 2005. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jules J, Zhang P, Ashley JW, et al. 2012. Molecular basis of requirement of receptor activator of nuclear factor kappaB signaling for interleukin 1-mediated osteoclastogenesis. J Biol Chem 287:15728–15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lam J, Takeshita S, Barker JE, et al. 2000. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fukushima H, Nakao A, Okamoto F, et al. 2008. The association of Notch2 and NF-kappaB accelerates RANKLinduced osteoclastogenesis. Mol Cell Biol 28:6402–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yasui T, Kadono Y, Nakamura M, et al. 2011. Regulation of RANKL-induced osteoclastogenesis by TGF-beta through molecular interaction between Smad3 and Traf6. J Bone Miner Res 26:1447–1456. [DOI] [PubMed] [Google Scholar]
- 192.Dougall WC, Glaccum M, Charrier K, et al. 1999. RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pettit AR, Ji H, von Stechow D, et al. 2001. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Clin Pathol 159:1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Takeyama K, Chatani M, Takano Y, et al. 2014. In-vivo imaging of the fracture healing in medaka revealed two types of osteoclasts before and after the callus formation by osteoblasts. Dev Biol 394:292–304. [DOI] [PubMed] [Google Scholar]
- 195.Gentile MA, Soung do Y, Horrell C, et al. 2014. Increased fracture callus mineralization and strength in cathepsin K knockout mice. Bone 66:72–81. [DOI] [PubMed] [Google Scholar]
- 196.He LH, Liu M, He Y, et al. 2017. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci Rep 7:42385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xue D, Li F, Chen G, et al. 2014. Do bisphosphonates affect bone healing? A meta-analysis of randomized controlled trials. J Orthop Surg Res 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ulrich-Vinther M, Andreassen TT. 2005. Osteoprotegerin treatment impairs remodeling and apparent material properties of callus tissue without influencing structural fracture strength. Calcif Tissue Int 76:280–286. [DOI] [PubMed] [Google Scholar]
- 199.Gerstenfeld LC, Sacks DJ, Pelis M, et al. 2009. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 24:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Einhorn TA. 2010. Can an anti-fracture agent heal fractures? Clin Cases Miner Bone Metab 7:11–14. [PMC free article] [PubMed] [Google Scholar]
- 201.Wu AC, Raggatt LJ, Alexander KA, et al. 2013. Unraveling macrophage contributions to bone repair. Bonekey Rep 2:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.