Abstract
Progressive accumulation of Alzheimer’s disease-related pathology is associated with cognitive dysfunction. Differences in cognitive reserve may contribute to individual differences in cognitive function in the presence of comparable neuropathology. The protective effects of cognitive reserve could contribute differentially in early versus late stages of the disease. We investigated presynaptic proteins as measures of brain reserve (a subset of total cognitive reserve), and used Braak staging to estimate the progression of Alzheimer’s disease. Antemortem evaluations of cognitive function, postmortem assessments of pathologic indices, and presynaptic protein analyses, including the complexins I and II as respective measures of inhibitory and excitatory terminal function, were assayed in multiple key brain regions in 418 deceased participants from a community study. After covarying for demographic variables, pathologic indices, and overall synapse density, lower brain complexin-I and -II levels contributed to cognitive dysfunction (P < 0.01). Each complexin appeared to be dysregulated at a different Braak stage. Inhibitory complexin-I explained 14.4% of the variance in global cognition in Braak 0-II, while excitatory complexin-II explained 7.3% of the variance in Braak V-VI. Unlike other presynaptic proteins, complexins did not colocalize with pathologic tau within neuritic plaques, suggesting that these functional components of the synaptic machinery are cleared early from dystrophic neurites. Moreover, complexin levels showed distinct patterns of change related to memory challenges in a rat model, supporting the functional specificity of these proteins. The present results suggest that disruption of inhibitory synaptic terminals may trigger early cognitive impairment, while excitatory terminal disruption may contribute relatively more to later cognitive impairment.
Keywords: synaptic pathology, inhibitory terminals, cognitive decline, dementia, Braak staging, postmortem human brain, aging study
Introduction
Accumulation of multiple, age-related pathologies (such as amyloid plaques, phosphotau deposition and tangle formation, strokes, Lewy bodies, and hippocampal sclerosis) contributes to cognitive impairment [4, 9, 34, 38]. Similar to other diseases, staging approaches are proposed for several of these pathologies. Braak staging, based on using the anatomical distribution of tangle-containing neurons as an index of the stage of illness, demonstrates strong associations with cognitive impairment [10, 44]. However, age-related pathologies explain only part of cognitive impairment, and the wide variation in impairment related to pathologies may be explained by individual differences in cognitive reserve. In the face of comparable levels of pathology, individuals with greater reserve demonstrate better cognitive function. Positive psychological contributors to cognitive reserve include participation in cognitive and physical activities: risk factors include depressive symptoms and social isolation [58]. The neurochemical substrates for these experiential effects on cognitive reserve may include the number of functional synapses, or brain reserve [48]. Presynaptic terminals are enriched for specific protein families, and may be a critical structural component of brain (and thereby cognitive) reserve [8, 20]. Investigation of presynaptic proteins indicates these elements of brain reserve demonstrate protein-specific associations with cognition. A previous longitudinal, community-based study associated cognitive reserve with overall brain amounts of the presynaptic proteins complexin-I, complexin-II, and vesicle-associated membrane protein (VAMP), as well as with the ability of the three SNARE proteins (i.e. synaptosome-associated protein of 25 kDa [SNAP-25], syntaxin-1 and VAMP) to form functional complexes [20].
The Braak staging model implicates progressive changes and the possibility of spread of pathology between cortical regions [11, 24]. Pathological studies report relationships between specific types of plaque and neuritic or tangle pathologies, Braak stage, and cognitive function [44]. Cognitive reserve studies have not yet adopted similar strategies, and the possibility of differences in relationships between presynaptic proteins and cognitive function at different pathological stages remains unexplored.
Focusing on the presynaptic protein complexin-I allows investigation of inhibitory terminals, and complexin-II indexes excitatory terminals [41, 50, 56]. Evidence from clinical correlative studies as well as animal models suggests the balance of inhibitory and excitatory function demonstrates a relative loss of inhibition in the early phases of Alzheimer’s disease [36], while in the later phases excitatory functions are also impaired as global loss of synaptic function occurs [7, 46, 47]. These observations in illness, and in animal models of illness, are consistent with findings indicating presynaptic protein-specific patterns of change during learning and memory in normal animals [17, 41].
Synaptic function is intimately related to plasticity. As well as losses of synaptic terminals resulting in reduced plasticity, synaptic terminals become incorporated in the neuritic pathology, a form of dysplasticity [12, 54]. Again, specific presynaptic proteins appear to become involved early in dysplastic neurites, while other proteins show minimal involvement [29, 53].
Using a large, community-based sample of prospectively ascertained participants with no dementia at study entry [5, 9, 20], we sought to answer the question of whether or not inclusion of Braak staging would influence assessment of the contributions of the two complexin proteins to brain reserve. Secondary analyses were carried out to evaluate specific domains of cognitive function, and to assess the effects of complexins in specific anatomical regions. In exploratory studies investigating the potential mechanisms linking synaptic damage and AD pathology, we assessed the relationships between the neuritic plaque pathology and various components of the exocytotic machinery, including complexin and SNARE proteins. Preliminary animal studies were also performed to (1) confirm and quantify segregation of complexins into GABAergic and glutamatergic presynaptic terminals, and (2) to assess the associations between the complexins and cognitive performance in two different animal paradigms of learning and memory.
Materials and methods
Participants and cognitive evaluation
The Memory and Aging Project (MAP) is a large, community-based study of elderly participants with no dementia at study entry [5, 9, 20]. Comprehensive annual evaluations are carried out until death, when a brain autopsy is performed. Demographic, cognitive and pathological characteristics of the first available 418 deceased participants in MAP are summarized in Table 1. The Institutional Review Board of Rush University Medical Center approved the MAP protocols, including the informed consent and an Anatomic Gift Act for organ donation signed by participants at enrollment [5]. Nineteen cognitive tests were z-scored and averaged to make a global measure of cognition, and also to summarize five domains of cognition: episodic memory, semantic memory, working memory, perceptual speed or visuospatial skills [6]. Cognitive evaluations were performed annually from enrollment to death, with a median of 5 evaluations per participant. Scores obtained in the last assessment before death were used in the present analyses, and standardization used the mean and standard deviation of the overall baseline sample (see Table 1).
Table 1.
Demographic, cognitive and pathological characteristicsa of MAP participants, combined or by Braak stage
| Variable | All n = 418 |
Braak 0–II n = 76 |
Braak III–IV n = 246 |
Braak V–VI n = 96 |
|---|---|---|---|---|
| Demographic | ||||
| Female, no. (%) | 263 (63%) | 39 (51%) | 159 (64%) | 65 (68%) |
| Age at death, years | 88.9 ± 5.9 | 85.5 ± 7.3 | 89.5 ± 5.5 | 90.1 ± 4.6 |
| Education, years | 14.6 ± 2.9 | 15.0 ± 3.0 | 14.5 ± 2.9 | 14.4 ± 2.9 |
| APOE ε4 carriers, no. (%) | 98 (23%) | 12 (16%) | 47 (19%) | 39 (41%) |
| PMI, hours | 7.4 ± 4.5 | 8.0 ± 4.8 | 7.4 ± 4.1 | 7.1 ± 5.1 |
| Cognitive function proximate to death | ||||
| Global cognitive score | −0.78 ± 1.04 | −0.19 ± 0.81 | −0.61 ± 0.85 | −1.69 ± 1.10 |
| Episodic memory | −0.74 ± 1.18 | 0.01 ± 0.97 | −0.55 ± 0.87 | −1.81 ± 1.08 |
| Semantic memory | −0.59 ± 1.13 | −0.17 ± 0.87 | −0.39 ± 0.87 | −1.45 ± 1.45 |
| Working memory | −0.62 ± 1.08 | −0.24 ± 0.99 | −0.47 ± 0.97 | −1.30 ± 1.13 |
| Perceptual speed | −0.98 ± 0.98 | −0.52 ± 0.92 | −0.94 ± 0.94 | −1.43 ± 0.95 |
| Visuospatial ability | −0.51 ± 1.09 | −0.11 ± 1.08 | −0.47 ± 1.03 | −0.95 ± 1.13 |
| MMSE | 22.0 ± 8.4 | 26.2 ± 5.4 | 23.4 ± 6.9 | 15.1 ± 9.9 |
| Clinical diagnoses, no. in NCI:MCI:DEM | 141:120:157 | 41:27:8 | 92:73:81 | 8:20:68 |
| Pathological | ||||
| β-amyloid plaques, % area stained | 5.0 ± 4.8 | 2.3 ± 3.1 | 4.1 ± 4.4 | 9.2 ± 4.5 |
| Phosphotau tangles, % area stained | 6.1 ± 7.7 | 0.8 ± 0.7 | 4.1 ± 3.4 | 15.5 ± 10.4 |
| Braak stage, no. in 0:I:II / III:IV / V:VI | 5:29:42 | 121:125 | 90:6 | |
| Chronic macroinfarcts, no. (%) | 151 (36%) | 25 (31%) | 91 (37%) | 35 (36%) |
| Chronic microinfarcts, no. (%) | 108 (26%) | 19 (25%) | 64 (26%) | 25 (26%) |
| Lewy body disease, no. (%) | 81 (19%) | 11 (14%) | 49 (20%) | 21 (22%) |
| Hippocampal sclerosis, no. (%) | 35 (8%) | 7 (9%) | 22 (9%) | 6 (6%) |
Abbreviations: APOE ε4, Apolipoprotein E ε4 allele; DEM, dementia; MAP, Memory and Aging Project; MCI, mild cognitive impairment; MMSE, mini mental state examination; NCI, no cognitive impairment; NIA, National Institute on Aging; no., number of subjects; PMI, postmortem interval.
Values are mean ± standard deviation unless noted otherwise.
Postmortem tissue selection and pathologic examination
Brain autopsies and postmortem tissue preparation used standard uniform procedures [5, 6]. A board-certified neuropathologist performed evaluations blind to clinical and demographic data, using accepted criteria [43]. Immunohistochemical analyses quantified Aβ plaque load (10D5 or 4G8 antibodies) and tau-tangle density (AT8) [43]. Severity of AD pathology was evaluated blinded to clinical diagnosis using three complementary approaches: 1) a modified Braak stage with categories: no or transentorhinal tauopathy (i.e. Braak 0-II), limbic spread (Braak III-IV), or neocortical spread (Braak V-VI) [10], 2) CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) neuritic plaque score with 4 levels of severity (no, sparse, moderate or frequent plaques) [32], and 3) modified NIA/Reagan diagnosis, also including 4 levels of severity (no, low, intermediate, or high likelihood of AD), accounting for amyloid and tau pathologies [33]. The presence of cerebrovascular diseases (macroscopic and microscopic infarcts, arteriolosclerosis and atherosclerosis), Lewy bodies, and hippocampal sclerosis was also examined. For neurochemical assays, frozen grey matter samples from the entorhinal cortex proper (EC; Brodmann’s area [BA] 28; n = 283), mid hippocampus (HIP; including CA1/subiculum; n = 402), inferior temporal gyrus (IT; BA20; n = 417), middle-frontal gyrus of the dorsolateral prefrontal cortex (MF; BA46/9, n = 418), and calcarine (primary visual) cortex (CALC; BA17, n = 360) were carefully dissected on dry ice to avoid tissue thawing. Samples were immediately stored at −80° C until immunoassay.
Animals and memory task paradigms
Adult male Long-Evans rats (300–350 g, Charles-River, Montreal, QC, Canada) performed memory task paradigms. Detailed descriptions including standard manipulations, dietary restriction, elevated eight-arm radial maze characteristics, and memory task training and testing were reported previously [41]. Briefly, in the reference memory paradigm, rats needed to use spatial cues to recall which arms of the maze were baited with food pellets throughout the trials. In contrast, rats selected for the working memory task were trained to find the pellets without re-entering in the same maze arm. Performance to the specified criteria resulted in presynaptic hippocampal changes related to brain plasticity [17, 41]. At the end of the experiments, animals were anaesthetized with pentobarbital and killed by decapitation. Brain hemispheres were separated and prepared for quantitative immunohistochemical assays. The UBC Animal Care Committee approved all procedures.
Antibodies and immunoassays
Primary antibodies are listed in Supplementary Table S1. Production and characterization of mouse monoclonal antibodies against complexin-I, complexin-II, syntaxin-1, SNAP-25, VAMP and synaptophysin was described elsewhere [21, 50]. Peroxidase- and Alexa-Fluor 488/555/647-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) or Molecular Probes (Eugene, OR, USA), respectively.
Complexin-I and II isoforms, and SNARE proteins (syntaxin-1, SNAP-25, VAMP) were quantified in human brain samples by enzyme-linked immunosorbent assays (ELISA) on 384-well plates, using semi-automated standard protocols [2]. Further ELISA characterization studies were described elsewhere [37]. An overview of data acquisition in 384-well ELISA plates and later data transformation and standardization is provided in the Supplementary Fig. S1. Importantly, for comparative analyses, all plates included the same reference sample. Each brain homogenate was loaded at 4 different protein concentrations (range 0.225–3.6 μg) in duplicate on at least 2 different plates, on 2 different days.
Co-immunoprecipitation (IP) and Western blotting (WB) were used in characterization experiments as described elsewhere [36]. Subcellular extractions yielding proteins enriched in (1) nuclear- (along with cell debris), (2) cysosolic-, (3) membrane-, (4) synaptosomal- and (5) myelin-associated compartments were performed as reported [18, 36].
Immunohistochemistry and confocal microscopy
Quantitative immunohistochemical (IHC) assays in rats subjected to memory tasks and controls were performed in formalin-fixed paraffin-embedded brain tissue slides essentially as previously described [42, 57]. Immunofluorescence (IF) assays on paraformaldehyde-fixed, free-floating brain tissue sections were performed as reported [37, 41]. Briefly, brain tissue slabs from the HIP of n = 7 MAP participants with mild-to-moderate AD (NIA/Reagan = 3–2; Braak stages II-V), and rat brain hemispheres (−3.30 to −4.20 relative to bregma), were sliced coronally to a thickness of 40 μm. Antigens were retrieved at 80° C for 15 min in 20 mM citrate buffer, pH 6.0. Brain sections were incubated with Alexa Fluor-488/555/647-conjugated primary antibodies overnight at 4° C. Sections were laid on gelatin-coated slides, and images were obtained using a LSM 5 Pascal Module with a 63×/1.2 N.A. water immersion objective (Zeiss, Jena, Germany). Appropriate positive and negative controls were included in all experiments.
Data analysis and statistics
Immunoassay datasets were first log transformed and standardized as graphically represented in Supplementary Fig. S1, and as previously reported [20]. In exploratory analyses, standardized data across HIP, IT and MF were averaged to obtain a global score for the forebrain levels of a given protein. The global score excluded EC, as only a subset of cases with this region was available. Further analyses investigated region-specific variations in presynaptic protein levels. SNARE protein data (i.e. syntaxin-1, SNAP-25 and VAMP) were averaged to obtain a stable variable controlling for overall protein synthesis in presynaptic terminals, and indexing synapse density. Preliminary analyses were performed to detect all potential confounding variables that may interfere with neurochemical, pathologic and cognitive associations, including (but not limited to) psychotropic medication, alcohol and tobacco consumption, or comorbidity with other health conditions that might affect cognitive performance. Since none of these variables altered the neurochemical-cognitive associations, they were excluded from the final statistical modeling approaches. Other variables, such as years of education, that were not associated with the neurochemical variables, but could influence cognitive performance, were kept as covariates in follow-up models. Multiple regression analyses were conducted with global or domain-specific cognitive function as dependent variables, demographics (sex, age, education) and postmortem interval as covariates, and neurochemical (complexins I and II, SNARE proteins) and pathological indices as independent variables. Follow-up models included a 3-level-collapsed Braak staging variable (see Table 1), grouping Braak 0–II, III–IV and V–VI as an ordinal variable [55], and included in the regression models alone or in a statistical interaction with complexins I and II. Linear regression models were constructed as above, in parallel, for each of the three Braak levels separately. Similar tests were performed using CERAD or NIA/Reagan scales instead of Braak staging. To examine the contribution of complexins I and II to cognition, the adjusted R2 values in the reference models containing the covariates and no target predictors were subtracted from the adjusted R2 values in models including the predictor(s). Colocalization analyses between presynaptic proteins, or Aβ were performed with ImageJ 2.0 (NIH, Bethesda, MA, USA), using built-in unbiased algorithms [14, 36, 37]. For within-animal comparisons of complexins I and II colocalization with VGAT versus VGLUT1, data were analyzed with paired t-tests. All tests were two-tailed, and the significance was set to P < 0.05, unless otherwise stated. Statistical analyses were done in JMP 12.0 (SAS Institute, Cary, NC, USA) and/or R package version 3.2.3 [22]. Prism 6.0 (GraphPad, La Jolla, CA, USA) was used to plot the resulting data. In memory task experiments in rats, differences between groups were detected with one-way ANOVA followed by Dunnet’s test.
Results
Quantitative determination of complexin segregation into inhibitory (GABAergic) and excitatory (glutamatergic) terminals
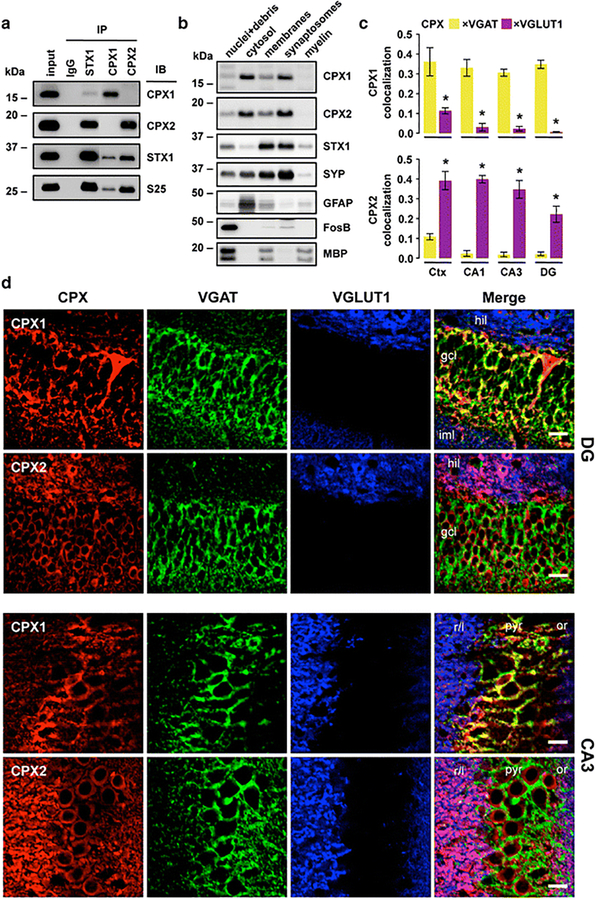
Although some of the biochemical and anatomical features of complexins I and II were reported previously [41, 42, 56], few studies compared and quantified both complexin isoforms using different and complementary brain tissue preparations. To further address the selectivity of antibodies recognizing complexin-I (SP33) and complexin-II (LP27) [2, 20, 37, 50], we immunoprecipitated syntaxin-1, and both complexins from human IT homogenates and further resolved the reactions by SDS-PAGE and immunobloting. No cross-reactivity was observed when complexin-II-IP products were probed with anti-complexin-I antibody, and vice versa (Fig. 1a). Both antibodies detected complexin proteins when anti-syntaxin-1 was used in IP experiments, indicating that complexin antibodies recognize peptides able to interact with SNARE proteins. However, syntaxin-1 was 4-fold enriched in complexin-II-IP products, compared to complexin-I co-IPs, either because syntaxin-1 has more affinity for complexin-II, or perhaps tissue homogenates contained greater amounts of complexin-II than complexin-I. At a subcellular level, distributions of complexins were very similar (Fig. 1b). Both isoforms were present in synaptosomal and cytosolic fractions, whereas syntaxin-1 was restricted to the synaptosome compartments.
Fig. 1.
Characterization and localization of complexins I (CPX1) and II (CPX2) in human and rat brain tissues. a Immunoprecipitation (IP) with specific antibodies against mouse IgG (as negative control), syntaxin-1 (STX1), complexin-I and complexin-II, using as input human crude cortical (inferior temporal) homogenates from subjects without cognitive impairment, psychiatric or neuropathological conditions. IP products, along with input samples, were resolved by immunoblotting (IB) with specific antibodies against complexins I and II, syntaxin-1, and SNAP-25 (S25). Note that complexin-I/II-IP products were loaded with some lanes of separation from the rest of the samples within the same SDS-gels. Resulting immunoblot captions were edited to cutoff non-informative lanes without altering image levels. b Sequential extraction of nuclear (with cell debris), soluble cytosolic, whole membranal, purified synaptosomal, and myelin-associated proteins from human cortical homogenates. Samples from each fraction were resolved by IB with antibodies against complexins I and II, syntaxin-1, and other markers for synaptic vesicles (synaptophysin [SYP]), cytosol (glial fibrillary acidic protein [GFAP]), nuclei (FosB), and myelin fragments (myelin basic protein [MBP]). Note that MBP strongly labels the nuclear fraction, as heavy myelin fragments precipitate along with cell debris. a–b Masses (in kDa) of prestained markers are indicated on the left side of immunoblots. c Quantitative colocalization analyses between complexin-I/II and each of the vesicular transporters for GABA (VGAT) and glutamate (VGLUT1) using laser-scan confocal microscopy in rat cortex (Ctx) and three hippocampal subfields. Bars represent mean±standard error of n = 4 rats, with at least 24 images analyzed per rat and brain area. *P < 0.001 (paired t-test). d Representative single- or merged-channel confocal images from triple co-immunolabeled rat brain sections described in (c) framing hippocampal dentate gyrus (DG; top panels) and CA3 subfield (bottom panels). Colors were arbitrarily assigned to maximize overlap visualization. Abbreviations: gcl, granule cell layer; hil, hilus; or, stratum oriens; iml, inner molecular layer; pyr, pyralmidal cell layer; r/l, stratum radiatum/lucidum. Scale bars: 10 μm
As expected [41], quantitative colocalization studies in rat brain slices showed that the wide majority of synaptic complexin-I expression occurs at GABAergic inhibitory (VGAT-positive) terminals, while complexin-II is predominantly contained in glutamatergic excitatory (VGLUT1-positive) presynaptic endings (Fig. 1c,d). This is best observed in the hippocampal subfields, where the segregation of inhibitory and excitatory terminals across the layers reduces potentially spurious overlapping between the fluorescent dyes (Fig. 1d). Although a residual expression of both isoforms in excitatory and inhibitory synapses cannot be eliminated, GABAergic complexin-I expression was 7.7–34.8-fold (P < 0.001) greater compared to that of glutamatergic, whereas complexin-II levels in glutamatergic terminals were 5.9–18.6-fold (P < 0.001) larger than in GABAergic endings, within the hippocampal subfields (Fig. 1c). Consistent with subcellular extractions, most complexin antibody signal arises from synapse-enriched areas, predominantly the neuropil. However, anti-complexin-I also immunolabeled a discrete number of cell bodies, possibly GABAergic interneurons, while anti-complexin-II slightly stained most hippocampal granule and pyramidal soma (Fig. 1d).
Hippocampal and cortical complexins are regulated during memory training tasks
Preliminary animal studies were performed to build on previous data showing the involvement of complexins I and II in cognitive processing - memory formation [49]. Rats were trained for two task learning paradigms respectively evaluating visuospatial skills (or reference memory), and working memory performance. These models were selected on the basis of their association with age-related cognitive impairment (related to cognitive domains for working memory, perceptual speed and visuospatial abilities), and also because both are known to be associated with behavioral and synaptic plasticity in rats [17, 41]. Interestingly, significantly elevated cortical levels of complexin-I, together with a non-significant reduction in the hippocampus, were found in animals reaching the criterion for the working memory task, but not in those trained for the reference memory test (Supplemetary Fig. S2a,c). In turn, upregulated complexin-II cortical (and borderline hippocampal) densities were associated with reaching criterion in reference memory acquisition, but not the working memory task (Supplementary Fig. S2b,d). These behavioral models suggest that the regulation of the balance between inhibitory (complexin-I) and excitatory (complexin-II) synapses occurring in the different memory consolidation processes may be region- and function-specific.
Association between complexin levels and cognitive function is AD stage-dependent
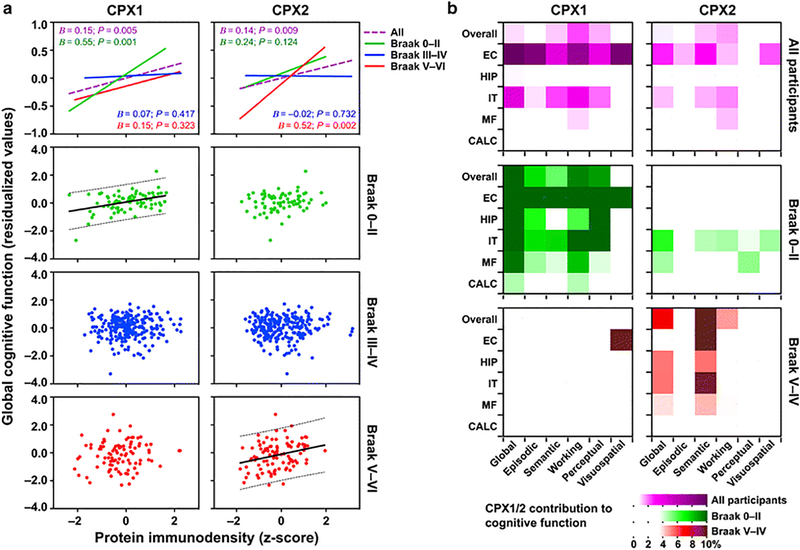
In preliminary analyses, without taking account of Braak stage but including demographic and pathological measures as well as the ubiquitous presynaptic SNARE proteins, analyses of complexin-I and II levels by ELISA in all available MAP samples revealed an association between each complexin and global cognition, accounting for 0.87–1.04% (P <0.01) of the total variance in cognition (Models 1–2 in Supplementary Table S2, and Fig. 2a). Brain regions contributing to the Braak staging model of AD progression showed different relationships between complexin-I and II levels and multiple domains of cognitive functioning (Fig. 2b and Supplementary Table S3). As well as previously used measures of tau and other pathologies, we added a term for Braak stage, and an interaction term with complexins I and II into the model of global cognitive function. Individuals were assigned to three Braak stage groupings: Braak 0-II (none or transentorhinal tau deposition), III-IV (limbic spread), or V-VI (neocortical spread). Remarkably, Braak stages themselves strongly contributed to the global cognition of MAP participants, despite controlling the analyses for overall brain deposition of pathological tau and amyloid (Supplementary Table S2, Model 4; and Supplementary Fig. S3a). In comparison, using similar statistical approaches, individuals separated by CERAD or NIA/Reagan assessments marginally or modestly differed in their cognitive performances, respectively (Supplementary Fig. S3b,c). These observations may indicate that the spread of the tauopathy (captured by Braak staging but not in the CERAD severity rating scale) is a qualitative hallmark of the progression of the disease, relatively independent from the pathologic load, which may be a better index for illness severity. As hypothesized, the interaction between Braak level and complexins I and II showed that each of the two proteins was most strongly associated with cognitive function at a different stage of AD (Model 4 in Supplementary Table S2). Variation in complexin-I contributed to brain reserve and cognitive function at early Braak stages, while variation in complexin-II had a similar effect in late Braak stages. The joint contribution of these interaction terms (complexins I and II-by-Braak) to the variance in cognitive function was 4.3% above the reference model. In similar models predicting cognitive function that included interaction terms between complexins I and II and overall tau density (instead of Braak staging), the interaction terms did not reach statistical significance (data not shown). Since the overall tau density is a fine indicator of the severity of AD, this later finding is consistent with the model that the Braak stage (i.e. the spread of disease) is the intrinsic component interacting with complexins and affecting cognition, regardless of illness severity. Accordingly, the robust Braak-stage-dependent associations between complexin protein levels and the cognitive function of MAP participants were rendered marginally significant when subjects were grouped by CERAD or NIA/Reagan scales (Supplementary Fig. S3d–f). Finally, inclusion in the models of a variable to control for possible effects of APOEΣ4 allele (alone or in interaction with complexins I or II) did not alter the above findings (data not shown). Of note, all variance in cognition explained by APOE genotype in our statistical models is fully mediated by AD-related pathologic indices.
Fig. 2.
Brain levels of complexins I (CPX1) and II (CPX2) are associated with cognitive function at early and late stages of Alzheimer’s disease (AD), respectively. a Scatterplots representing adjusted global cognitive function (residual values after controlling for demographics, age-related neuropathologies, and total synapse density) against overall brain levels of complexin-I (left panels) or complexin-II (right panels). In the two upper panels (scaled to represent the mean), fitted lines correspond to either all MAP participants (dashed purple), or grouped into Braak 0–II (n = 76, solid green), III–IV (n = 246, solid blue), or V–VI (n = 96, solid red). In bottom plots (scaled to show the range of individual values), points represent each of the participants’ values, segregated by Braak, along with fit (solid) and 95% prediction band (dotted) lines wherever significant associations were found. Unstandardized B-coefficients and P-values were calculated in the corresponding linear regression models summarized in Table 2 (by Braak group) and Supplementary Table S2 (for all participants). b Heatmaps representing percent contribution values of overall, entorhinal (EC), hippocampal (HIP), inferior temporal (IT), middle frontal (MF) and calcarine (CAL) immunodensities of complexin-I (left panels) or complexin-II (right panels) to the variance of each of the cognitive abilities of all MAP participants (upper purple panels), or those within Braak 0–II (middle green panels) or Braak V–VI (bottom red panels). Percent contribution values were estimated from the linear regression models summarized in Supplementary Tables S3 (all participants) and S4 (Braak-grouped). Note that color scales were chosen so that only significant contributions show high color intensity
To clarify the interpretation of the interactions, we performed analyses in each of the three Braak stage groupings separately (Table 2 and Fig. 2a). Consistent with the findings above, regression analyses indicated that in Braak 0-II, higher overall brain density of complexin-I contributed 14.4% of the variance in global cognitive function, while complexin-II did not contribute. In contrast, in Braak V-VI, higher overall density of complexin-II contributed 7.3% of the variance in global cognitive function, while complexin-I contribution was not significant. These relatively large contributions of inhibitory/excitatory complexins in early and late stages respectively contrast with the modest effects in the full cohort. This attenuation could be a consequence of the absence of significant associations between cognition and complexins in intermediate Braak stages (Table 2 and Fig. 2a), which comprise the majority of the MAP participants.
Table 2.
Linear regression models showing the associations of demographic, pathological and/or overall brain neurochemical variables, with global cognitive function in MAP participants by Braak stage
| Model and terms | Braak 0–II | Braak III–IV | Braak V–VI | |||
|---|---|---|---|---|---|---|
| Adj. R2 or Ba | P value | Adj. R2 or B | P value | Adj. R2 or B | P value | |
| Reference model | 0.3413 | 0.2259 | 0.2307 | |||
| Sex | 0.2204 | 0.0063* | −0.0541 | 0.3023 | 0.2829 | 0.0160* |
| Age at death | −0.0088 | 0.4265 | −0.0242 | 0.0062* | −0.0416 | 0.0827 |
| Education | 0.0636 | 0.0241* | 0.0240 | 0.1685 | 0.0109 | 0.7813 |
| PMI | −0.0056 | 0.7385 | 0.0036 | 0.7676 | 0.0237 | 0.2484 |
| Macroinfarcts | −0.2327 | 0.1924 | −0.5147 | <.0001* | 0.0103 | 0.9628 |
| Lewy bodies | −0.4268 | 0.0682 | −0.1392 | 0.2609 | 0.0418 | 0.8677 |
| Hippocampal sclerosis | −1.1800 | <.0001* | −0.5474 | 0.0017* | −0.8913 | 0.0405* |
| β-amyloid | −0.0936 | 0.2467 | −0.0244 | 0.5628 | −0.0561 | 0.6838 |
| Phosphotau | −0.0432 | 0.6785 | −0.0460 | 0.0019* | −0.0513 | <.0001* |
| Synapse densityb | 0.0111 | 0.9108 | −0.0559 | 0.2768 | −0.0848 | 0.5023 |
| Test model | 0.4852 | 0.2216 | 0.3036 | |||
| Sex | 0.2117 | 0.0037* | −0.0532 | 0.3137 | 0.3542 | 0.0022* |
| Age at death | −0.0132 | 0.1850 | −0.0234 | 0.0088* | −0.0326 | 0.1565 |
| Education | 0.0693 | 0.0087* | 0.0226 | 0.1990 | 0.0422 | 0.2742 |
| PMI | −0.0101 | 0.4999 | 0.0033 | 0.7891 | 0.0327 | 0.0987 |
| Macroinfarcts | −0.1726 | 0.2749 | −0.5094 | <.0001* | 0.0113 | 0.9568 |
| Lewy bodies | −0.6308 | 0.0039* | −0.1239 | 0.3248 | 0.1231 | 0.6088 |
| Hippocampal sclerosis | −0.7454 | 0.0069* | −0.5320 | 0.0028* | −0.8319 | 0.0478* |
| β-amyloid | −0.0374 | 0.6081 | −0.0241 | 0.5690 | −0.0494 | 0.7069 |
| Phosphotau | −0.0575 | 0.5454 | −0.0442 | 0.0033* | −0.0518 | <.0001* |
| Synapse densityb | −0.1581 | 0.1615 | −0.0682 | 0.2435 | −0.2149 | 0.0964 |
| CPX1 | 0.5466 | 0.0001* | 0.0748 | 0.4171 | −0.1476 | 0.3228 |
| CPX2 | 0.2355 | 0.1241 | −0.0284 | 0.7323 | 0.5189 | 0.0022* |
Unstandardized coefficient
Synapse density was estimated as the overall brain levels of the three SNARE proteins (syntaxin-1, SNAP-25 and VAMP) averaged
Statistically significant
Within Braak 0-II, complexin-I immunodensity appeared to be the greatest contributor to cognitive function (as reflected by standardized β-coefficients; data not shown), explaining more variance than Lewy body disease, hippocampal sclerosis, gender and years of education (see Test model in Table 2). At this illness stage, no associations were found between cognitive function and Aβ load or phosphotau deposition, indicating that AD-related pathology may exert its deleterious effects in intermediate and/or late stages, when the accumulation of neuritic plaques and NFTs reach higher densities (Table 1). In Braak III-IV, where synaptic protein levels had no significant effects, cognitive abilities seemed more influenced by a mixture of pathologic conditions (especially infarcts) and age (Table 2). Finally, phosphotau deposition was the strongest predictor of cognitive function at Braak V-VI stages, where complexin-II also showed a strong association with cognition.
The contributions of region-specific densities of complexins I and II to the different cognitive domains in MAP participants at early (0-II) or late (V-VI) stages of AD (intermediate stages were omitted as not significant) are schematized in the middle and bottom panels of Fig. 2b, and further detailed in Supplementary Table S4. Other than visuospatial skills, all cognitive domains were highly associated with overall brain complexin-I levels in early stages of the disease (5.63–9.45% contribution to variance, P < 0.02). In marked contrast, in Braak V-VI, greater overall levels of complexin-II, but not complexin-I, were associated specifically with better semantic memory (10.0% contribution to variance, P < 0.001) (Fig. 2b, and Supplementary Table S4).
Early loss of CPX1/2 from damaged terminals within neuritic plaques
To provide a better understanding of the relationship between presynaptic terminal degeneration and AD pathology, we co-stained available hippocampal sections from MAP participants at various stages of AD (n = 7, Braak II-V) using antibodies against complexins I and II, SNARE proteins, Aβ, and/or pathological tau (AT8 and Alz-50), with analysis using laser-scanning confocal microscopy. Neuritic plaques in early AD are interesting structures to study the evolution of synaptic pathology because they contain pre- and post-synaptic terminals, dystrophic neurites and the most common forms of neurotoxic soluble and insoluble tau and Aβ aggregates. Triple immunolabeling assays showed that complexins I and II, although not completely absent from the plaques, did not display overt colocalization with tau or Aβ within neuritic plaques (Fig. 3a–d). In contrast, focal colocalizations were noticed between VAMP (likely upregulated in these areas) or SNAP-25, and AD pathologic markers in these presumably unstructured and dystrophic neurites present within the plaque area (Fig. 3e–h). No gross differences were noticed between subjects at early and late stages of the disease, although the limited number of samples available did not allowed a reliable quantitative analysis of these observations. A relative abundance of SNARE proteins within dystrophic neurites, with an absence of complexins I and II, indicates that these molecules are differentially regulated under the influence of the pathologic stress of AD.
Fig. 3.
Differential localization patterns of complexins I (CPX1) and II (CPX2) versus SNARE proteins within β-amyloid (Aβ) plaques in the subiculum of participants with variable degrees of Alzheimer’s disease pathology. a, c, e, g Single- or merged-channel confocal images from triple co-immunolabeled sections with the antibodies against phosphotau (clone AT8), Aβ (clone 6F/3D) and CPX1 (a), CPX2 (c), vesicle-associated membrane protein (VAMP) (e), or SNAP-25 (S25) (g). In merged images, colors were arbitrarily assigned to maximize overlap visualization, and correspond to the colors of the labels in the single-channel images on the left. Areas occupied by neuritic plaques (as visualized by Aβ immunostaining) were grossly outlined in all channels. Scale bars: 20 μm. b, d, f, h Semi-quantitative colocalization data evaluating the number of overlapping pixels between phosphotau (yellow bars) or Aβ (magenta bars), and CPX1 (b), CPX2 (d), VAMP (f), or SNAP-25 (h), divided by the total number of pixels of the presynaptic proteins with an intensity over an unbiased threshold, and within the outlined areas. Bars represent mean ± standard deviation of all n = 7 MAP participants. Images next to the bar plots are ImageJ-generated bitmap outputs from the colocalization analyses of the image outlines on the left, representing the overlapping pixel intensities between phosphotau (yellow bitmaps) or Aβ (magenta bitmaps), and the corresponding presynaptic target.
4. Discussion
The present results support the hypothesis that the presynaptic complexin proteins are molecular components of brain reserve. Further, each complexin protein appears to contribute differently to brain reserve according to the pathological stage of Alzheimer’s disease. Additional support for different functional roles of complexins I and II was provided by an animal model of learning and memory.
Previous work using a smaller subset of the MAP cohort tissues used here (n = 253) showed that higher levels of both complexins I and II contributed to better cognitive function in old age and protected from cognitive decline [9, 20]. Here, we expanded the number of cases analyzed and pooled datasets together with those in the former studies to obtain a larger final cohort with n = 418 cases. As in the previous studies, the current complexin findings were additive with the effects of age-related pathologies. Expanding the sample allowed investigation of the possibility that stage of Alzheimer’s disease, and possibly extent of progression, could also contribute to cognitive outcome, and modify the role of brain reserve. Additionally, quantitative immunohistochemical studies solidify preliminary observations indicating that human GABAergic terminals are relatively enriched in complexin-I, and glutamatergic terminals in complexin-II [41]. The present findings in MAP participants provide empirical evidence suggesting that in the absence of widespread tauopathy and/or at the early stages of AD, cognitive performance largely relies on the ‘functionality’ of inhibitory synapses, as measured by complexin-I immunodensity. These findings are in agreement with other clinical and pathological observations that decreased inhibitory tone may be an early feature of Alzheimer’s disease [1, 23, 45], and that pan-synaptic terminal loss is a later feature of illness progression [25, 31, 35, 40]. The present animal study indicated a role for change in levels of cortical complexin-I in reference memory (largely perceptual and visuospatial) and for complexin-II in working memory. Changes in the ratio, but not the amounts of these proteins in hippocampus also help establish relatively specific roles for these proteins in learning and memory [41]. Complexin-II is reported to be involved in hippocampal long-term potentiation [49].
The results reported here provide some support for the relevance of treatment strategies in animal models of Alzheimer’s disease directed at increasing inhibitory tone [13, 26, 27, 51]. Limited evidence suggests pharmacotherapies directly targeting components of the presynaptic molecular machinery such as levetiracetam (which interacts with the vesicular protein SV2) may be of benefit in Alzheimer’s disease through modulating inhibitory tone [16, 28, 30, 39, 52]. Further research may also identify variation in the complexin genes as a factor contributing to variation in brain reserve in aging. Genetic variation in the CPLX2 gene appears to contribute to cognitive impairment in schizophrenia [3, 19].
The present study is not without limitations. The prominent involvement of complexin-I in the entorhinal cortex in the early Braak stages was consistent with the model of different components of brain reserve related to anatomical progression of Alzheimer’s disease, however a larger sample size would be needed to more fully explore this hypothesis. In addition, the association between cognitive function and inhibitory synapse preservation might be related to other features than just brain reserve, since at early stages the load of AD pathology might not be sufficient to impact on cognition. Thus, complexin-I loss could be an important feature of primary age-related tauopathy (PART), as many MAP participants in Braak I/II may satisfy PART criteria [15]. An unresolved question concerns the relationship between early life cognitive activity including years of education, and later life brain reserve. We did not detect statistically significant associations between years of education and complexins I or II, however the range of education among participants was limited. Likewise, a still larger sample size would allow exploration of the possibility that the slope of cognitive change, and the time between an inflection point of cognitive decline and death may relate to changes in brain reserve [58]. The statistical models were not controlled for stereological neuronal counts, which may affect overall synapse density and thereby complexin immunoreactivity. However, the models accounted for both SNARE protein levels (as an index of synapse density) and major neuropathologies causing neuronal cell death. While the immunocytochemical studies support the model that complexins are part of the neuropil component of brain reserve, other SNARE proteins may be part of reserve but also contribute to dysplasticity [29, 53]. Although the number of cases analyzed was limited, complexins appeared highly sensitive to the neurotoxicity elicited upon Aβ and/or phosphotau deposition in the surrounding neuropil, whereas SNARE proteins seemed undisturbed or, in the particular case of VAMP, overexpressed. More extensive immunocytochemical analyses of individual brain regions in a larger numbers of cases is needed to evaluate the relationships between presynaptic reserve and the diverse types of age-related pathologies. Likewise, although the animal study supported distinct roles for each complexin in memory functions, the translation of these findings to domains of cognitive function in humans requires extensive additional work.
The study also had strengths. Participants were free of dementia at baseline, and being a large, prospective community sample, were free of biases associated with clinic-based studies of help-seeking individuals. Detailed characterization of all forms of neuropathology was carried out, allowing a fairly comprehensive assessment of the relative contributions of pathology and presynaptic proteins (reserve) to cognitive function. Having antibodies detecting presynaptic proteins relatively enriched in specific (inhibitory and excitatory) terminal types allowed the associations with pathological staging to go beyond relatively non-specific effects to improve understanding of potential stage-related mechanisms. Notably, the relatively large postmortem cohort available in MAP study, together with the comprehensive cognitive evaluations and the extensive pathologic assessments, provided enough cases in all pathologically assessed groups to obtain reliable associations between cognitive and neurochemical datasets. The present findings are in agreement with prior data indicating that amyloid-based staging approaches do not efficiently separate cognitively healthy versus impaired individuals [44], whereas the tauopathy spread-based Braak staging does. Thus, the interaction between pathologic tau propagation and the inhibitory/excitatory imbalance could be focus of future research to understand brain mechanisms of cognitive reserve.
In summary, the evidence provided by the present study indicates differences in mechanisms of brain reserve related to phase of illness, as well as age-related pathology. Exploring interventions that could augment brain reserve may be a worthwhile strategy to complement attempts to delay or avert cognitive decline.
Supplementary Material
Acknowledgements
We thank Hong-Ying Li and Jenny Yang for their skillful technical assistance. The present work was financed with grants from the Canadian Institutes of Health Research (MT-14037, MOP-81112). The Memory and Aging Project represents a collaborative, multidisciplinary and prospective research supported by the National Institute on Aging (Grants R01AG17917, R01AG42210). Dr. W.G. Honer was supported by the Jack Bell Chair in Schizophrenia.
Conflict of interests Dr. W.G. Honer has received consulting fees or sat on paid advisory boards for: In Silico, Lundbeck/Otsuka, Eli Lilly, and Roche. Dr. A.M. Barr is on the advisory board or received consulting fees from Roche Canada, and received educational grant support from BMS Canada. The Organizations cited above had no role in (and therefore did not influence) the design of the present study, the interpretation of results, and/or preparation of the manuscript. All other authors have no financial interest on the reported data and declare that no competing interests exist.
References
- 1.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M (2012) Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74:467–474. doi: 10.1016/j.neuron.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barakauskas VE, Beasley CL, Barr AM, Ypsilanti AR, Li H-Y, Thornton AE, Wong H, Rosokilja G, Mann JJ, Mancevski B, Jakovski Z, Davceva N, Ilievski B, Dwork AJ, Falkai P, Honer WG (2010) A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacol 35:1226–1238. doi: 10.1038/npp.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begemann M, Grube S, Papiol S, Malzahn D, Krampe H, Ribbe K, Friedrichs H, Radyushkin KA, El-Kordi A, Benseler F, Hannke K, Sperling S, Schwerdtfeger D, Thanhäuser I, Gerchen MF, Ghorbani M, Gutwinski S, Hilmes C, Leppert R, Ronnenberg A, Sowislo J, Stawicki S, Stödtke M, Szuszies C, Reim K, Riggert J, Eckstein F, Falkai P, Bickeböller H, Nave K-A, Brose N, Ehrenreich H (2010) Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch Gen Psychiatry 67:879–888. doi: 10.1001/archgenpsychiatry.2010.107 [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurol 66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6 [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS (2005) The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiol 25:163–175. doi: 10.1159/000087446 [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA (2013) Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 33 Suppl 1:S397–403. doi: 10.3233/JAD-2012-129007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossers K, Wirz KTS, Meerhoff GF, Essing AHW, van Dongen JW, Houba P, Kruse CG, Verhaagen J, Swaab DF (2010) Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer’s disease. Brain 133:3699–3723. doi: 10.1093/brain/awq258 [DOI] [PubMed] [Google Scholar]
- 8.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA (2006) Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurol 67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20 [DOI] [PubMed] [Google Scholar]
- 9.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, Bennett DA (2013) Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. doi: 10.1002/ana.23964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K (2015) The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138:2814–2833. doi: 10.1093/brain/awv236 [DOI] [PubMed] [Google Scholar]
- 12.Brion JP, Couck AM, Bruce M, Anderton B, Flament-Durand J (1991) Synaptophysin and chromogranin A immunoreactivities in senile plaques of Alzheimer’s disease. Brain Res 539:143–150. [DOI] [PubMed] [Google Scholar]
- 13.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold K-H, Haass C, Staufenbiel M, Konnerth A, Garaschuk O (2008) Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321:1686–1689. doi: 10.1126/science.1162844 [DOI] [PubMed] [Google Scholar]
- 14.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S (2004) Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 86:3993–4003. doi: 10.1529/biophysj.103.038422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, Wisniewski T, Woltjer RL, Yamada M, Nelson PT (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. doi: 10.1007/s00401-014-1349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumbo E, Ligori LD (2010) Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav 17:461–466. doi: 10.1016/j.yebeh.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Rodger J, Hicks A, Mallet J, Laroche S (1996) Brain structure and task-specific increase in expression of the gene encoding syntaxin 1B during learning in the rat: a potential molecular marker for learning-induced synaptic plasticity in neural networks. Eur J Neurosci 8:2068–2074. [DOI] [PubMed] [Google Scholar]
- 18.Gray EG, Whittaker VP (1962) The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat 96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 19.Hass J, Walton E, Kirsten H, Turner J, Wolthusen R, Roessner V, Sponheim SR, Holt D, Gollub R, Calhoun VD, Ehrlich S (2014) Complexin2 modulates working memory-related neural activity in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci doi: 10.1007/s00406-014-0550-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, Schneider JA, Bennett DA (2012) Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry 2:e114. doi: 10.1038/tp.2012.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honer WG, Hu L, Davies P (1993) Human synaptic proteins with a heterogeneous distribution in cerebellum and visual cortex. Brain Res 609:9–20. [DOI] [PubMed] [Google Scholar]
- 22.Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comp Graph Stat 5:299–314. [Google Scholar]
- 23.Koliatsos VE, Kecojevic A, Troncoso JC, Gastard MC, Bennett DA, Schneider JA (2006) Early involvement of small inhibitory cortical interneurons in Alzheimer’s disease. Acta Neuropathol 112:147–162. doi: 10.1007/s00401-006-0068-6 [DOI] [PubMed] [Google Scholar]
- 24.Lewis J, Dickson DW (2015) Propagation of tau pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131:27–48. doi: 10.1007/s00401-015-1507-z [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H (2016) Implications of GABAergic neurotransmission in Alzheimer’s disease. Front Aging Neurosci 8:31. doi: 10.3389/fnagi.2016.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Lee SH, VandeVrede L, Qin Z, Ben Aissa M, Larson J, Teich AF, Arancio O, D’Souza Y, Elharram A, Koster K, Tai LM, LaDu MJ, Bennett BM, Thatcher GRJ (2016) A multifunctional therapeutic approach to disease modification in multiple familial mouse models and a novel sporadic model of Alzheimer’s disease. Mol Neurodegen 11:35. doi: 10.1186/s13024-016-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Lee SH, VandeVrede L, Qin Z, Piyankarage S, Tavassoli E, Asghodom RT, Ben Aissa M, Fà M, Arancio O, Yue L, Pepperberg DR, Thatcher GRJ (2015) Re-engineering a neuroprotective, clinical drug as a procognitive agent with high in vivo potency and with GABAA potentiating activity for use in dementia. BMC Neurosci 16:67. doi: 10.1186/s12868-015-0208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B (2004) The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA 101:9861–9866. doi: 10.1073/pnas.0308208101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masliah E, Honer WG, Mallory M, Voigt M, Kushner P, Hansen L, Terry R (1994) Topographical distribution of synaptic-associated proteins in the neuritic plaques of Alzheimer’s disease hippocampus. Acta Neuropathol 87:135–142. [DOI] [PubMed] [Google Scholar]
- 30.Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT (2008) Levetiracetam prevents kindling-induced asymmetric accumulation of hippocampal 7S SNARE complexes. Epilepsia 49:1749–1758. doi: 10.1111/j.1528-1167.2008.01687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minger SL, Honer WG, Esiri MM, McDonald B, Keene J, Nicoll JA, Carter J, Hope T, Francis PT (2001) Synaptic pathology in prefrontal cortex is present only with severe dementia in Alzheimer disease. J Neuropathol Exp Neurol 60:929–936. [DOI] [PubMed] [Google Scholar]
- 32.Mirra SS, Hart MN, Terry RD (1993) Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med 117:132–144. [PubMed] [Google Scholar]
- 33.National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer”s Disease (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer“s disease. Neurobiol Aging 18(4S):S1–2. [PubMed] [Google Scholar]
- 34.Neuropathology Group, Medical Research Council Cognitive Function and Aging Study (2001) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 357:169–175. [DOI] [PubMed] [Google Scholar]
- 35.Palop JJ, Mucke L (2009) Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol 66:435–440. doi: 10.1001/archneurol.2009.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Miguel A, Hercher C, Beasley CL, Barr AM, Bayer TA, Falkai P, Leurgans SE, Schneider JA, Bennett DA, Honer WG (2015) Loss of Munc18–1 long splice variant in GABAergic terminals is associated with cognitive decline and increased risk of dementia in a community sample. Mol Neurodegen 10:65. doi: 10.1186/s13024-015-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos-Miguel A, Honer WG, Boyda HN, Sawada K, Beasley CL, Procyshyn RM, Barr AM (2015) Exercise prevents downregulation of hippocampal presynaptic proteins following olanzapine-elicited metabolic dysregulation in rats: Distinct roles of inhibitory and excitatory terminals. Neurosci 301:298–311. doi: 10.1016/j.neuroscience.2015.06.022 [DOI] [PubMed] [Google Scholar]
- 38.Riley KP, Snowdon DA, Markesbery WR (2002) Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol 51:567–577. doi: 10.1002/ana.10161 [DOI] [PubMed] [Google Scholar]
- 39.Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu G-Q, Palop JJ, Mucke L (2012) Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci USA 109:E2895–903. doi: 10.1073/pnas.1121081109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saura CA, Parra-Damas A, Enriquez-Barreto L (2015) Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Front Cell Neurosci 9:1567. doi: 10.1038/npp.2011.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ, Falkai P, Phillips AG, Honer WG (2005) Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry 62:263–272. doi: 10.1001/archpsyc.62.3.263 [DOI] [PubMed] [Google Scholar]
- 42.Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V, Mann JJ, Dwork AJ, Falkai P, Phillips AG, Honer WG (2002) Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry 7:484–492. doi: 10.1038/sj.mp.4000978 [DOI] [PubMed] [Google Scholar]
- 43.Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 44.Serrano-Pozo A, Qian J, Muzikansky A, Monsell SE, Montine TJ, Frosch MP, Betensky RA, Hyman BT (2016) Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the Alzheimer disease continuum. J Neuropathol Exp Neurol 75:516–526 doi: 10.1093/jnen/nlw026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solodkin A, Veldhuizen SD, Van Hoesen GW (1996) Contingent vulnerability of entorhinal parvalbumin-containing neurons in Alzheimer’s disease. J Neurosci 16:3311–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spires-Jones TL, Hyman BT (2014) The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82:756–771. doi: 10.1016/j.neuron.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stargardt A, Swaab DF, Bossers K (2015) The storm before the quiet: neuronal hyperactivity and Abeta in the presymptomatic stages of Alzheimer’s disease Neurobiol Aging 36:1–11. doi: 10.1016/j.neurobiolaging.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 48.Stern Y (2009) Cognitive reserve. Neuropsychologia 47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi S, Ujihara H, Huang GZ, Yagyu KI, Sanbo M, Kaba H, Yagi T (1999) Reduced hippocampal LTP in mice lacking a presynaptic protein: complexin II. Eur J Neurosci 11:2359–2366. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T, Miyata T, Kaba H, Higuchi T, Okutani F (1995) Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett 368:455–460. [DOI] [PubMed] [Google Scholar]
- 51.Tong LM, Yoon SY, Andrews-Zwilling Y, Yang A, Lin V, Lei H, Huang Y (2016) Enhancing GABA Signaling during Middle Adulthood Prevents Age-Dependent GABAergic Interneuron Decline and Learning and Memory Deficits in ApoE4 Mice. J Neurosci 36:2316–2322. doi: 10.1523/JNEUROSCI.3815-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L (2013) Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70:1158–1166. doi: 10.1001/jamaneurol.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakabayashi K, Honer WG, Masliah E (1994) Synapse alterations in the hippocampal-entorhinal formation in Alzheimer’s disease with and without Lewy body disease. Brain Res 667:24–32. [DOI] [PubMed] [Google Scholar]
- 54.Wang D, Munoz DG (1995) Qualitative and quantitative differences in senile plaque dystrophic neurites of Alzheimer’s disease and normal aged brain. J Neuropathol Exp Neurol 54:548–556. [DOI] [PubMed] [Google Scholar]
- 55.Winship C, Mare RD (1984) Regression models with ordinal variables. Am Sociol Rev 49:512–525. [Google Scholar]
- 56.Yamada M, Saisu H, Ishizuka T, Takahashi H, Abe T (1999) Immunohistochemical distribution of the two isoforms of synaphin/complexin involved in neurotransmitter release: localization at the distinct central nervous system regions and synaptic types. Neurosci 93:7–18. [DOI] [PubMed] [Google Scholar]
- 57.Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG (1998) SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex 8:261–268. [DOI] [PubMed] [Google Scholar]
- 58.Yu L, Boyle PA, Segawa E, Leurgans S, Schneider JA, Wilson RS, Bennett DA (2015) Residual decline in cognition after adjustment for common neuropathologic conditions. Neuropsychol 29:335–343. doi: 10.1037/neu0000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.