Abstract
Exosomes play essential roles in intercellular communications. The exosome was discovered in 1983, when it was found that reticulocytes release 50-nm small vesicles carrying transferrin receptors into the extracellular space. Since then, our understanding of the mechanism and function of the exosome has expanded exponentially that has transformed our perspective of inter-cellular exchanges and the molecular mechanisms that underlie disease progression. Cancer cells generally produce more exosomes than normal cells, and exosomes derived from cancer cells have a strong capacity to modify both local and distant microenvironments. In this review, we summarize the functions of exosomes in cancer development, metastasis, and anti-tumor or pro-tumor immunity, plus their application in cancer treatment and diagnosis/prognosis. Although the exosome field has rapidly advanced, we still do not fully understand the regulation and function of exosomes in detail and still face many challenges in their clinical application. Continued discoveries in this field will bring novel insights on intercellular communications involved in various biological functions and disease progression, thus empowering us to effectively tackle accompanying clinical challenges.
Keywords: exosome, extracellular vesicles, intercellular communication, cancer, metastasis, immunity
1. Exosomes and other major types of extracellular vesicles
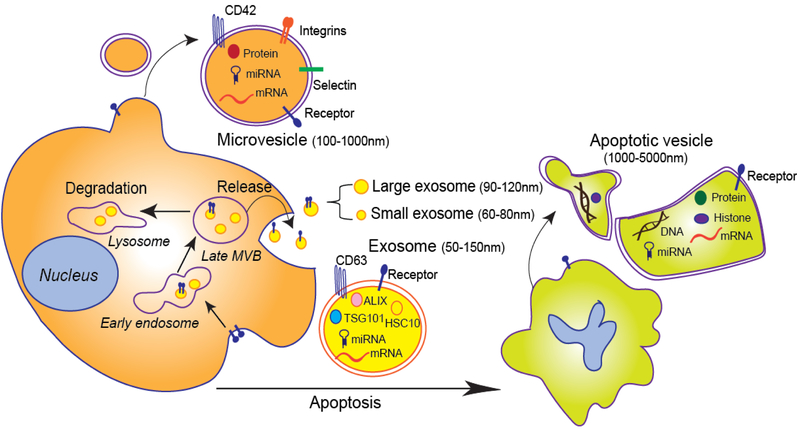
Exosomes are the most broadly investigated group among the three main subgroups (exosomes, microvesicles, and apoptotic vesicles, i.e., ApoEVs) of extracellular vesicles (EVs) released from mammalian cells. Exosomes arise from the membranes of multivesicular bodies (MVB) [1, 2] and are cup-shaped in morphology under electron microscopy, with diameters ranging from 50 nm to 150 nm [3]. After ultracentrifugation (100,000g), relatively pure exosomes can be isolated by an additional sucrose gradient step, with exosomes at a sucrose density from 1.13 to 1.19 g/mL. Membrane protein CD63, ALG2-interacting protein X (ALIX), tumor susceptibility gene 101 protein (TSG101), and proteasome component HSC10 are highly enriched on exosomes [4]. Recently, Lyden and colleagues identified two exosome subpopulations: large exosome vesicles (Exo-L), sized 90–120 nm, and small exosome vesicles (Exo-S), sized 60–80 nm [5].
Microvesicles, sometimes called microparticles, are generated from the plasma membrane and have diameters ranging from 100 nm to 1000 nm [6]. Membrane proteins such as the integrin GPIB (CD42) and P-selectin are enriched on microvesicles [7]. While both exosomes and microvesicles are released from healthy cells, the ApoEVs are released from apoptotic cells or dying cells. The ApoEVs range in diameter from 1000 nm to 5000 nm, and they contain the nuclear protein histone and DNAs [8]. Besides the three main subtypes, other EVs include membrane particles, exosome-like vesicles, neutrophil-originating EVs (ectosomes) [9], prostate-originating EVs (prostasomes) [10, 11], migrasomes [12], oncosomes[13], large oncosomes [14] and others [15].
While exosomes and other EV subtypes have different origins and take different cargos (Figure 1), they all have the capacity to communicate with other cells and modulate local or distant microenvironments. Distinguishing the three main subtypes of EVs unambiguously on the basis of size, density, or morphology is difficult, because these properties overlap somewhat between the subtypes. In many published papers, therefore, all membrane-released EVs are referred to simply as EVs without attempts to distinguish them as exosomes, microvesicles, or other subtypes. However, strict criteria for distinguishing exosomes and other EV subtypes can help us understand their functions more thoroughly and are beneficial for reliably comparing and better understanding the findings from different study groups. In this review, we focus on exosomes’ function in tumor development, metastasis, and immunity. Although other types of EVs, such as migrasomes, oncosomes and large oncosomes have also been associated with cancer development [12, 13, 16], their contributions will not be extensively discussed in this review, in the interest of focus and space.
Figure 1. Exosomes and other extracellular vesicles: biogenesis and secretion in eukaryotic cells.
First, exosomes fuse into early endosomes and multivesicular bodies (MVBs). Late MVBs fuse with plasma membrane to release exosomes or with lysosomes for degradation. Exosomes can be further categorized as large exosomes and small exosomes. CD63, ALIX, TSG101, and HSC10 are enriched in exosomes; many mRNAs, microRNAs (miRNAs), proteins, and receptors are also carried by exosomes. Microvesicles bud directly from the plasma membrane, not from MVBs. CD42, integrins, and selectin are enriched in microvesicles; microvesicles also carry multiple receptors, proteins, miRNAs, and mRNAs. Apoptotic vesicles are derived from apoptotic cells. They contain DNAs and histone besides proteins, receptors, mRNAs, and miRNAs.
2. Exosome biogenesis, composition and regulation
The process of exosome biogenesis involves several steps: 1) the inward budding of the plasma membrane forms early endosomes; 2) the early endosomes generate late endosomes and MVBs containing intraluminal vesicles, which are also called exosomes; and 3) upon fusion of late MVBs with the plasma membrane, exosomes are released; if these MVBs fuse with lysosomes, the MVBs are degraded [17] (Figure 1). Generally, the endosomal sorting complex required for transport (ESCRT) machinery, such as ESCRT-0, -I, -II, and -III and accessory proteins, control exosome biogenesis and formation and vesicle scission [18–20]. Sphingolipid ceramide [21], the small GTPase ADP ribosylation factor 6 (ARF6), and its effector phospholipase D2 (PLD2) [22] also control exosome biogenesis, independent of the ESCRT machinery. In melanocytes, melanosomal protein PMEL [23] and tetraspanin CD63 [24] directly participate in ESCRT-independent endosomal sorting.
The RAB family, especially RAB27A, RAB27B, RAB11, RAB35, and RAB7, regulate exosome secretion [25–27]. The controlling of exosome secretion by various RAB proteins is dependent on the cellular context. For example, RAB27A and RAB27B control exosome release in HeLa cells [25], while RAB7 controls exosome release in MCF-7 breast cancer cells [27]. Vacuolar protein sorting protein 33b controls the maturation and secretion of exosomes in hematopoietic stem cells and leukemia-initiating cells [28]. Soluble N-ethyl- maleimide-sensitive factor (NSF)-attachment protein receptor complex (SNARE) [29, 30] and the microenvironment pH [31] are also involved in the fusion between MVBs with the plasma membrane and thereby the regulation of exosome release. Unlike exosomes, microvesicles and ApoEVs bud directly from the plasma membrane, not from MVBs. The mechanism of microvesicle and ApoEV release are not well known, but ARF6 [32] and the small GTPase RHOA [33] were reported to control microvesicle shedding; caspase-3 was required for detachment of ApoEVs from the membrane [34].
Nearly all mammalian cells secrete and take up exosomes [35, 36]. Besides cancer cells, cells such as adipocytes [37], immune cells [38, 39], and brain resident cells [40, 41] have been reported to release or take up exosomes in physiologic and pathologic conditions. Exosomes carry many cargos: proteins, lipids, nucleic acids (mRNA, microRNAs [miRNAs], and DNA), and metabolites [42–45]. The ExoCarta database (http://exocarta.org/) lists thousands of proteins, mRNAs, and miRNAs that are candidate exosomes cargos [46], but the cargos vary in different cell lines. The mechanism by which exosomes selectively package their cargos remains unclear. It has been reported that it required special sequences to sort mRNA or miRNAs in exosomes [47, 48], but reliable criteria for the selection of proteins, lipids, and even RNAs into exosomes are unknown. Patton and colleagues found that colon cancer cells with mutant KRAS have different miRNA content in their exosomes than cells with wild-type KRAS: miR-10b was enriched in the exosomes from wild-type KRAS cancer cells, whereas miR-100 was enriched in the exosomes from mutant KRAS cancer cells [48]. This finding indicates that KRAS might control exosomal miRNA packaging. Lyden and colleagues showed that Exo-S specifically carry the proteins associated with endosomes and MVBs, while Exo-L carry proteins associated with plasma membrane, cell-cell contact and junctions, late endosomes, and Golgi network proteins. Their findings further demonstrate that cargos are selectively packaged in exosomes [5].
Exosome uptake by recipient cells is processed by endocytosis, receptor-ligand interaction, or fusion with the cell membrane. The uptake of exosomes is not random, but depends on interactions between proteins on the surface of the exosomes and recipient cells. Several reports have suggested that adhesion-associated molecules on the surface of exosomes, such as tetraspanins, glycoproteins, and integrins, determine which cells accept exosomes [49, 50]. For example, exosomes containing tetraspanin 8 (TSPAN8) and integrin α4 were easily taken up by CD54+ pancreas cells [49]. TSPAN8-α4 integrin (CD49d) in exosomes contributed to exosome binding to and uptake by endothelial cells, thereby promoting angiogenesis [50]. Integrin CD47 expression in engineered exosomes facilitated uptake by tumor cells through micropinocytosis [51]. To demonstrate that recipient cells can take up mRNAs loaded in EVs in vivo, Rheenen and colleagues transfected Cre recombinase into malignant tumor cells such that the EVs released from these cells contained Cre mRNA. After the reporter-expressing recipient benign tumor cells took up these EVs, their color switched from pre-labeled red to green due to Cre-LoxP recombination [52]. These data showed that mRNA in exosomes can be transferred to and function in recipient cells. Although the authors did not distinguish EVs from exosomes in this paper, it is very likely that exosomes also can transfer mRNAs between cells.
3. Exosome function in cancer development
Exosomes have been widely studied for their roles in intracellular communication, especially during tumor development. Exosome-associated RNAs, miRNAs, proteins, DNAs, and even metabolites can change the fate of recipient cells by autocrine and paracrine signaling. First, exosomal proteins can change the fate of exosome-releasing cells themselves via an autocrine pathway. For example, the exosomes derived from chronic myeloid leukemia cells contained a cytokine, TGFβ1, which binds to the TGFβ1 receptor on the leukemia cells, thereby promoting tumor growth through activation of ERK, AKT, and anti-apoptotic pathways in the producer cells [53]. Second, exosomal DNA changes cell survival itself. It has been debated whether exosomes carry DNA, because exosomes come from MVB in cytoplasm but do not connect to the nucleus. However, several groups have detected double-stranded DNA fragments and DNA mutation in exosomes [54]. Immuno-gold labeling of double-stranded DNA detected by transmission electron microscopy revealed the presence of DNA in some exosomes that was still in cell plasma before their secretion [55]. If exosome secretion was inhibited, the accumulation of nuclear DNA in the cytoplasm resulted in cell cycle arrest or apoptosis through activation of reactive oxygen species-dependent DNA damage response. Thus, exosome secretion of harmful cytoplasmic DNA from cells supported cell survival and maintained cellular homeostasis [55].
Compared to these autocrine effects, paracrine mechanisms through which exosomes mediate intercellular interactions and modulate the microenvironment have been well studied. Cargos in exosomes or EVs serve as external stimuli for recipient cells, thereby modifying the signaling pathways in recipient cells. Because of the heterogeneity of cancer cells, exosomes or EVs from host cancer cells can activate the receptors or change miRNA or RNA expression in the neighboring cancer cells to alter their biological phenotypes. For example, glioma cells transferred EVs with the oncogenic receptor EGFRvIII to neighboring glioma cells lacking this receptor, thereby activating the AKT pathway in neighboring glioma cells and conferring on these cells the capacity for anchorage-independent growth [13]. Similarly, mutant KRAS, along with other oncogenes such as EGFR and SRC, can be transferred by exosomes to recipient colon cancer cells of wild-type KRAS, promoting tumor invasion [56]. Breast cancer cells released exosomes harboring PD-L1, allowing its transfer to other cancer cells expressing low- or no- PDL1, promoting tumor evasion of immune surveillance [57]. Apoptotic vesicles also contribute to the survival of tumor cells. Apoptotic glioblastoma cells released ApoEVs to transfer spliceosomal proteins such as RBM11 and small nuclear RNAs (snRNAs) to change the mRNA splicing (MDM4, CCND1) in recipient cells, resulting in tumor aggressiveness and drug resistance [58].
Exosome not only transfer between cancer cells, they also transfer between cancer cells and stromal cells: stromal cells accept exosomes derived from cancer cells to generate a pro-tumor microenvironment; reciprocally, cancer cells take the exosomes released from stromal cells to facilitate cancer cell proliferation or invasion. For example, tumor-derived exosomes promoted endothelial cell proliferation and angiogenesis [50, 59]. The exosomes from MDAMB-231 breast cancer cells and U87 glioma cells endowed normal fibroblasts and epithelial cells with transformed characteristics of cancer cells, such as enhanced anchorage-independent growth and survival through the enzyme transglutaminase and its substrate fibronectin [60]. Breast cancer cell–derived exosomes triggered adipose-derived mesenchymal stem cells to transform to tumor-associated myofibroblasts via the TGFβ-SMAD–mediated signaling pathway [61]. Some cancer cells released TGFβhigh cancer exosomes, which trigged transition of fibroblasts into α-smooth muscle actin positive myofibroblasts [62].
Tumor cell–derived exosomes can also regulate endothelial cell characteristics to promote angiogenesis, especially in hypoxic conditions. Exosomes presenting tetraspanins can promote tumor growth by increasing angiogenesis [63]. For example, cancer cell–derived exosomes enriched with TSPAN8 and integrin subunit α4 enhanced endothelial cell proliferation and angiogenesis through upregulation of angiogenesis-related genes [50]. NOTCH ligand Delta-like 4 (DLL4) presented by cancer cell–derived exosomes increased vessel density and branching in vivo [64]. Soluble E-cadherin, a potent inducer of angiogenesis, was expressed at greater levels in the exosomes of ovarian cancer cells. Soluble E-cadherin carried by exosome was heterodimerized with vascular-endothelial cadherin on endothelial cells to active β-catenin and NF-κB signaling for angiogenesis [65]. Hypoxic conditions stimulated tumor cells, such as glioblastoma, to release exosomes, which enhanced angiogenesis by upregulating protease-activated receptor 2 (PAR2) in epithelial cells [66]. Under hypoxic conditions, lung cancer cells produced more exosomes enriched with miR-23a, which suppressed its target prolyl hydroxylases 1 and 2 (PHD1 and PHD2), resulting in the accumulation of hypoxia-inducible factor-1-alpha (HIF1A) in endothelial cells. Exosomal miR-23a also targeted to the tight junction protein ZO1 to increase vascular permeability and cancer migration [67]. In hypoxic bone marrow, multiple myeloma–derived exosomal miR-135b inhibited its target, factor-inhibiting hypoxia-inducible factor 1 (FIH1AN), in endothelial cells, thereby enhancing endothelial tube formation under hypoxic conditions [68].
Stromal cells also change the fate of tumor cells via exosomes. Activated stromal cells around breast cancer cells were found to release exosomes containing cytoplasmic unshielded RNA RN7SL1, which activated the viral RNA pattern recognition receptor RIG-1 signaling, resulting in an inflammatory response and tumor progression [69]. Cancer-associated fibroblast-derived exosomes (CAF-DEs) containing abundant ADAM10 enhanced cancer cell motility through the GTPase RHOA and maintained stem cell status through Notch signaling in cancer cells [70]. In addition, CAF-DEs carried metabolic cargos, including amino acids, lipids, and TCA-cycle intermediates. After prostate and pancreatic cancers took in CAF-DEs, glycolysis and glutamine-dependent reductive carboxylation were increased in cancer cells, thereby promoting tumor growth under nutrient deprivation or nutrient-stressed conditions [45, 71].
4. Exosomes induce drug resistance in cancers
Exosomes and EVs have robust impacts on drug resistance and induce drug resistance through multiple mechanisms. First, exosomes released from tumor cells can help the cells expel cytotoxic drugs, as has been observed in melanoma and ovarian cancer [72–75]. Second, drug-sensitive cells become drug resistant by taking up exosomes derived from drug-resistant cells. For example, a multidrug resistant leukemia subline transferred exosomes containing P-glycoprotein to drug-sensitive cells [76]. MiRNAs such as miR-30a, miR-222, or miR-100–5p carried by exosomes induced drug-sensitive cells to become resistant possibly through regulating MAPK or mTOR pathway [77, 78]. Expression of glutathione S-transferase P1 (GSTP1), an enzyme that has been reported to detoxify several anticancer drugs by conjugating them with glutathione [79], was much higher in exosomes derived from doxorubicin-resistant cells. When exosomal GSTP1 was transferred to sensitive cells, it conferred drug resistance to sensitive cells, and numbers of circulating GSTP1-containing exosomes were negatively correlated with clinical outcome of chemotherapy in breast cancer patients [79]. Exosomal long-non-coding RNA (lncRNA) mediated sunitinib drug resistance in renal cell carcinoma, since lncRNA competed for binding of miR-34 and miR-449 to their target RNAs, thereby increasing the expression of AXL and MET in sensitive cells to spread sunitinib resistance [80]. EVs released by HER2+ cells that are resistant to HER2-targeted drugs contained immune-regulated proteins TGFβ1 and PDL1, which made cells that had been sensitive to HER2-targeted drugs resistant. In fact, TGFβ1 expression was higher in EVs isolated from the serum of patients with HER2+ breast cancer that did not respond to HER2-targeted drugs trastuzumab or lapatinib [81]. Third, stromal exosomes can also induce drug resistance in cancer cells. For example, exosomes were transferred from the TME stroma to breast cancer cells to expand therapy-resistant tumor-initiating cells by exosome-RNA mediated activation of the STAT1-NOTCH3 pathway in the cancer cells [82]. Macrophage-derived exosomes decreased the sensitivity of pancreatic cancer cells to gemcitabine, an effect mediated by transfer of miR-365, which activated the enzyme cytidine deaminase to make pancreatic cancer cells resistant to this chemotherapy agent [83]. The other mechanisms of EV-based drug resistance have been comprehensively reviewed by McNamee and O’Driscoll [84].
5. Exosome function in cancer metastasis
The cancer metastatic process comprises several steps. It begins with local invasion by cancer cells, then cancer cells enter the circulation (intravasation) via the lymphatic system or the blood vessel. In the circulation, the cancer cells need to survive and disseminate to different organs, exiting the circulation to enter the parenchyma of distant organs (extravasation). Finally, extravasated cancer cells can die, remain dormant or outgrown to succeed in colonization in distant organs. Exosomes have extensive impact on each step of metastasis.
5.1. Exosomes in cancer cell migration and invasion
First, exosomes control cell polarity and directional cell movement. Fibrosarcoma cells secreted exosomes contain fibronectin that bound with cellular integrin receptors to facilitate integrin clustering and strong adhesion formation at the leading edge to promote cell migration [85]. Cancer-associated fibroblast–secreted CD81-positive exosomes were loaded with WNT ligand WNT11 and were taken up by breast cancer cells. The CD81/WNT11-positive exosomes promoted breast cancer cell protrusion, invasion, and metastasis via activating autocrine WNT-planar cell polarity signaling [86]. Second, exosomes derived from cancer cells could modulate the extracellular matrix (ECM) to promote cell invasion and metastasis. For example, CD151/TSPAN8-positive exosomes derived from the rat pancreatic adenocarcinoma cell line (ASML) recruited integrins and proteases, which degraded collagen and fibronectin to modify ECM [87]. Exosomes from RAB27B high-expressing metastatic breast cancer cells contained activated matrix metallopeptidase 2 (MMP2), a protease that degrades the ECM [88]. Third, exosomes could unlock the tight junctions to enhance tumor cells intravasation. Exosomes containing miR-105 from breast cancer reduced the expression of ZO1 in endothelial cells, thus destroyed the tight junctions of endothelial cells [89]. Fourth, exosomes derived from cancer cells could promote recipient cells’ epithelial–mesenchymal transition (EMT), which is an essential process for cancer invasion and metastasis. Many EMT factors delivered by exosomes, such as HIF1α, matrix metalloproteinase 13 (MMP13), casein kinase II α (CKIIA), annexin A2, and latent membrane protein 1 (LMP1), contributed to the metastatic features of recipient cells [90–93]. Exosomes also may contain anti-metastasis cargos. For example, bladder carcinoma cell exosomes contained miR-23b, which deterred cell invasion and metastasis. However, release of these miR-23b–containing exosomes reduced miR-23b level inside the parental cells, promoting their metastasis [94].
Other EVs have similar capacities to promote cancer cell invasion. For example, less malignant tumor cells that took up EVs from highly malignant tumor cells showed enhanced migratory behavior and metastatic capacity by in vivo imaging [52]. The secretion of EVs by breast cancer cells can help metastatic cancer cell extravasation across the blood-brain- barrier (BBB). These EVs from brain metastatic cancer cells transferred miR-181c into endothelial cells of the BBB, resulting in destruction of cell-cell contacts and therefore breakdown of the BBB to allow brain metastasis [95]. Large oncosomes, which are a subgroup of gigantic EV (size >1,000 nm to >10,000 nm), were reported to promote amoeboid migration of metastatic prostate cancer cells [16]. Migrasomes are migration-dependent EVs that are assembled on retraction fibers, present at the trailing edge of migrating cells. As cells migrate, the migrasomes are released into the extracellular environment and are taken up by surrounding cells, but the exact function of the migrasomes in the recipient cells is unclear [12].
5.2. Exosomes in pre-metastatic niches
Primary tumors release systemic signals, such as cytokines or exosomes, to prepare metastatic sites. The importance of exosomes in building these pre-metastatic niches for disseminated cancer cells was revealed by two reports that tumor cell–derived exosomes conditioned lymph nodes or lung tissue to become favorable niches for the metastatic colonization and outgrowth of melanoma cells [96, 97]. Hood et al. reported that melanoma-derived exosomes can travel to sentinel lymph nodes through lymphatic trafficking. These exosomes prepared lymph nodes for melanoma metastasis via the activation of molecular signals that modulated ECM deposition and vascular proliferation [96]. The exosomes from pancreatic cancer possessed increased levels of miRNAs, such as miR-494 and miR-542–3p, which downregulate cadherin-17, thereby increasing proteases, adhesion molecules, and other proteins to prepare pre-metastatic niches in lungs or lymph nodes for tumor cell hosting [97]. In prostate cancer, exosomes released by the cancer cells impaired osteoclast differentiation but promoted osteoblast activity to regulate the microenvironment of bone metastases [98, 99]. However, some exosomes cannot reach the pre-metastatic organ without the help of the soluble fraction or the adhesive matrix. Specifically, exosomes from rat pancreatic cancer had to combine with the cell-released soluble fraction, such as CD44v, c-Met, UPAR, and C3, to establish pre-metastatic niches within the lung or lymph node [100]. In addition, microvesicles released by CD105+ renal cell carcinoma stem cells triggered angiogenesis and promoted the formation of a pre-metastatic niche in the lung [101]. The CD105+ microvesicles contained a selected pattern of miRNAs that induce endothelial cell growth, invasion of matrix, and resistance to apoptosis [101].
Lyden and colleagues have advanced our understanding of the novel function of exosomes in pre-metastatic niches. They found that exosomes from melanoma cells delivered MET, a receptor tyrosine kinase, to bone marrow progenitor cells. After taking up the MET+ exosomes, bone marrow progenitor cells activated hepatocyte growth factor (HGF)-MET signaling to support tumor metastasis to bones [102]. The exosomes from pancreatic cancer cells were taken up by Kupffer cells, contributing to establishment of pre-metastatic niches in the liver. Pancreatic cancer cell-derived exosomes deliver macrophage-inhibitory factor (MIF) that made Kupffer cells release more TGFβ, which in turn increased fibronectin production and recruited bone marrow–derived macrophages for liver pre-metastatic niche formation [103]. Strikingly, exosomes derived from breast cancer contained different integrin patterns, which predetermined the future metastatic organ. Specifically, high expression of integrins α6β4 and α6β1 in breast cancer derived exosomes primed for lung metastasis, while high expression of integrin αvβ5 in exosomes primed for liver metastasis [104].
Additionally, it was reported that snRNAs in exosomes derived from lung cancer or melanoma promoted lung pre-metastatic niches by activating TLR3 and releasing cytokines which recruited neutrophils into the lung [105]. Another striking new mechanism for facilitation of metastasis is increase of nutrient availability to cancer cells in the pre-metastatic niche by exosomal miR-122. Glucose uptake by niche cells such as lung fibroblasts and brain astrocytes were suppressed by miR-122 through the downregulation of the glycolytic enzyme pyruvate kinase, thereby increasing nutrient availability for metastatic cancer cell growth [106].
In contrast, exosomes from poorly metastatic melanoma cells lack the capacity to generate pre-metastatic niches in the bone but inhibit cancer cell in the secondary organ sites. These “non-metastatic” exosomes stimulate an innate immune response in the bone marrow through pigment epithelium-derived factor (PEDF) on the outer surface of the exosome. This exosome-induced immune response caused cancer cell clearance at the pre-metastatic niche via recruitment of monocytes, natural killer (NK) cells, and macrophages [107]. In another study, exosomes derived from parental lung cancer cells contained miR-192, which inhibited interleukin 8 (IL8), intercellular adhesion molecules, and CXCL1 in the endothelial precursor cells of the bone microenvironment, thus preventing effective metastatic angiogenesis and impairing colonization. However, the exosomes from highly metastatic subpopulations harbored less miR-192, promoting bone-metastasis niches [108].
5.3. Stromal exosomes in metastatic dormancy and outgrowth
In metastatic organ sites, stroma-derived exosomes can regulate survival, dormancy or outgrowth of disseminated cancer cells. Bone marrow mesenchymal stem cell-released exosomes transferred miR-23b to metastatic cancer cells, resulting in cancer cell dormancy through the suppression of MARCKS, which promotes cell cycling and motility [109]. In contrast, astrocytes in the brain microenvironment can release exosomes containing miR-19, which downregulated PTEN expression in metastatic tumor cells, thereby promoting the outgrowth of brain metastasis [41].
The non-random pattern of metastasis can be explained by the “seed and soil” hypothesis, which indicates successful metastasis depends not only on the intrinsic factors of cancer cells (the “seed”) but also on the preferential microenvironment of select organs (the “soil”) that allow cancer cells to survive and metastases can only prosper when the appropriate seed was implanted in its suitable soil [110]. The effect of exosomes on educating the stromal cells in the distant organs for building pre-metastatic niches complements the “seed and soil” hypothesis, revealing that the cancer cells release exosomes to modify the selected “soils” before they arrive. Meanwhile, the exosome interactions between cancer cells and stromal cells in the distant organs also highlight the bi-directional (seed-soil) co-evolution during the metastasis process. Figure 2 and 3 briefly summarizes various functions of exosomes in cancer development and metastasis.
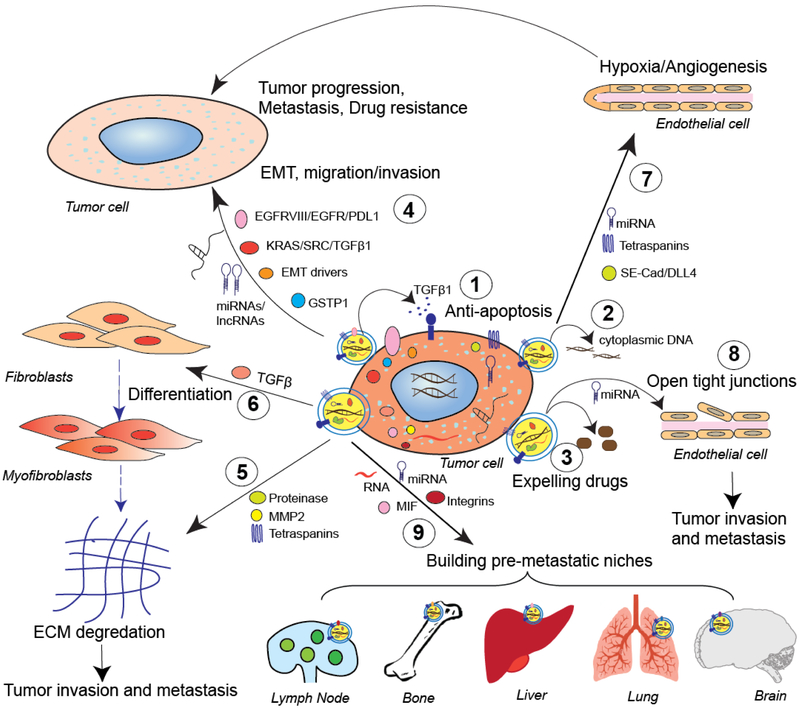
Figure 2. Functions of cancer cell-derived exosome in tumor progression and metastasis.
Tumor-derived exosomes (1) inhibit apoptosis of tumor cells through secretion of TGFβ1 or other ligands and (2) pump cytoplasmic DNA out for cellular homeostasis. (3) Exosomes expel cytotoxic drugs, resulting in tumor cell drug resistance. (4) Tumor-derived exosomes transfer their cargos (such as EGFRVIII, KRAS, SRC, TGFβ1, EMT drivers, PDL1, GSTP1, lncRNAs, or miRNAs) to other tumor cells to induce EMT, migration and invasion, or drug resistance in recipient cells, thereby promoting tumor progression and metastasis. Tumor exosomes can (5) reprogram the ECM through proteinase, MMP2, or tetraspanins, or (6) induce fibroblast differentiation to myofibroblasts through TGFβ1, which further induces ECM degradation; they also can (7) enhance endothelial cell proliferation and angiogenesis by transferring soluble E-cadherin (SE-Cad), DLL4, tetraspanins, or miRNAs, and (8) open tight junctions in endothelial cells by miRNAs, resulting in tumor progression and metastasis. (9) Tumor exosomes carry specific integrins, macrophage-inhibitory factor, mRNAs, or miRNAs, which allow them to establish pre-metastatic niches in lymph nodes, bone, liver, lung, and brain.
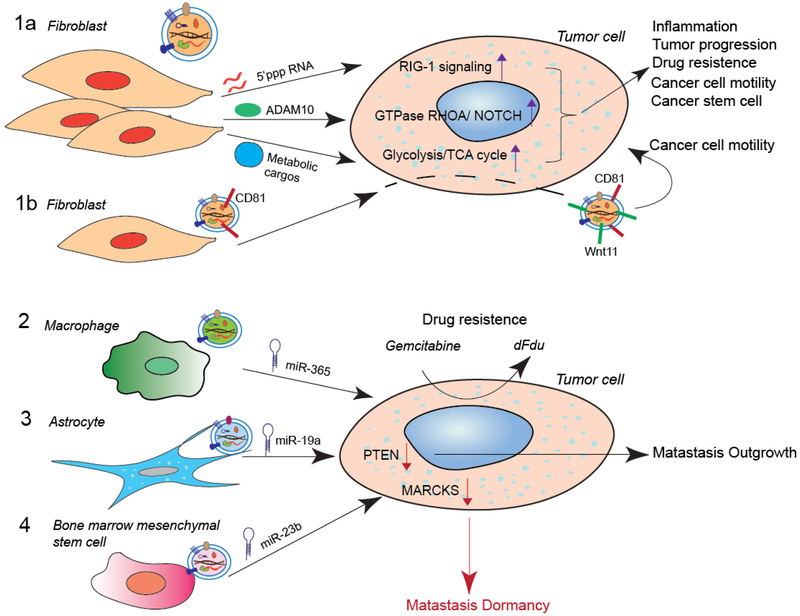
Figure 3. Examples of stromal cell-derived exosomes in tumor progression and metastasis.
1a) Activated fibroblasts secrete exosomes that are taken up by cancer cells and that transfer unshielded RNAs, protein ADAM10, metabolic cargos and other molecules, to induce inflammation, tumor growth, drug resistance, cancer cell motility and/or cancer stem cell phenotypic traits. 1b) Fibroblast-secreted CD81-positive exosomes are loaded with WNT11 by cancer cells, and these exosomes promote cancer cell motility and metastasis through an autocrine mechanism. 2) Macrophage-derived exosomes promote pancreatic cancer cell resistance to gemcitabine through miR-365. 3) Astrocytes promote the outgrowth of brain metastasis through exosomal miR-19a. 4) Bone marrow mesenchymal stem cells induce bone metastasis dormancy through exosomal miR-23b.
6. Exosome function in cancer immunity
Besides the strong capacity to regulate tumor metastasis, another important function of exosomes in cancer is modulating tumor immune response. The evidence for exosome-mediated intercellular antigen transfer was first reported by Thery and colleagues 20 years ago [38, 39]. They showed that Epstein-Barr virus–transformed B cells released exosomes containing major histocompatibility complex class (MHC)-II, which activated CD4+ T cells, whereas dendritic cell (DC)–secreted exosomes expressed MHC-I, which activated CD8+ T cells in vitro. Recently, the success of immune checkpoint therapy in several cancer types has boosted the interest in further exploration of immune dysregulation in tumors, including modification of tumor immunity by exosomes.
6.1. Exosomes in anti-tumor immune response
Tumor-derived exosomes or EVs have been reported to activate immune responses. Exosomes derived from tumor cells present neo-antigens and/or MHC-peptide complexes to prime and activate T cells by direct presentation and cross-presentation through DCs, or directly activate NK cells or macrophages [111–115]. For example, tumor-derived exosomes transferred tumor antigens heat-shock proteins (HSP70–80) and MHC-I molecules to DCs, which induced potent CD8+ T cell–dependent antitumor effects on mouse tumors [111, 116]. HSP70 on the exosome surface also stimulated NK cell migration and cytolytic activity [117], macrophage activity [118] and induced stronger anticancer immune responses of helper T cells [119]. Exosomes derived from RAB27A-overexpressing tumor cells were capable of upregulating MHC-II, CD80, and CD86 on DCs, in turn promoting CD4+ T cell proliferation [120]. Exosomes from patients’ melanoma delivered MART1 tumor antigens to DCs for cross-presentation to cytotoxic T lymphocytes specific to MART1 [121].
Exosomal DNA regulates tumor-associated inflammation and immunity. Guan and colleagues reported nucleic acid–rich EVs released from Hippo pathway kinase LATS1/2– depleted tumor cells induced antitumor immunity by stimulating the TLR-IFN pathway, whereas cancer cells with high LATS1/2 expression suppressed cancer immunity [122]. Treatment of breast cancer cells with the antitumor agent topotecan that triggered DNA double-strand breaks can induce exosome release. Consequently, exosomal DNA derived from topotecan-treated cancer cells triggered DC activation and subsequent CD8+ T cell activation via CGAS-STING signaling [123].
The capacity of exosomes to express antigens and MHC complexes and induce helper T cell immune responses raises the possibility that exosomes could be used as anticancer vaccines. Remarkably, ApoEVs from melanoma B16-ovalbumin provided the highest antitumor protection compared to micro-vesicles and exosomes. Mice immunized with ovalbumin-pulsed ApoEVs vaccine had significantly longer tumor-free survival than mice immunized with ovalbumin-pulsed exosome vaccine or ovalbumin-pulsed microvesicle vaccine. Protection by ApoEVs against melanoma challenge might be related to their expression of proteins associated with “immunogenic cell death,” such as high-mobility group box protein B1 (HMGB1) and calreticulin, on their surface [124].
6.2. Pro-tumor immune reaction
Tumor exosomes also have exhibited strong pro-tumor immune reactions. Tumor cell-derived exosomes could inhibit T cell and NK cell activation and promote regulatory T cell function [115, 125]. For example, cancer cell-derived exosomes expressed TGFβ and ligands for NKG2D to downregulate the surface NKG2D expression on NK cells and CD8+ T cells, thus blocked their activity [126, 127]. Exosomes that present FAS ligand, TNF-related apoptosis-inducing ligand, or galectin-9 induced the apoptosis of activated T cells [128–130]. Exosomes released by melanoma cells stimulated high levels of reactive oxygen species (ROS) in neighboring T cells to inhibit their function [131]. TGFβ in cancer exosomes upregulated the generation of Foxp3+ T regulatory cells [132].
Three recent studies simultaneously showed cancer cell–derived exosomes or EVs harboring PD-L1 which inhibited T cell functions, thereby promoting tumor growth. One reported that breast cancer cell-derived exosomes carried PD-L1, which was transferred to cancer cells that lacked or expressed only low levels of PD-L1 and also blocked T cell activity through interaction with programmed death protein, PD1 [57]. Another showed that PD-L1 was expressed on the surface of some glioblastoma-derived EVs. PDL1-containing EVs in the serum or plasma of glioblastoma patients were positively correlated with tumor burden [133]. The third reported that exosomes from human melanoma, breast cancer, or lung cancer carried PD-L1 on their surface, and that increased circulating exosomal PD-L1 can be used to predict patient response to anti-PD1 therapy [134].
Besides regulating T cells, cancer–derived exosomes inhibit the activity of DCs and increase expansion of myeloid-derived suppressor cells (MDSCs). Pancreatic cancer– derived exosomes were found to inhibit DC cytokines via downregulation of TLR4 expression by miR-203 [135]. Lung cancer–derived exosomes blocked DC differentiation via downregulation of surface markers, including CD80, MHC-II, and CD86, but upregulated CD11B and PD-L1 expression [136]. Tumor exosomes converted myeloid cells to MDSCs by secreting PGE2 and TGFβ [137], or IL6, TNFα, and CCL2 [138]. They also activated MDSCs and triggered their suppressive function in an HSP72/TLR2-STAT3–dependent manner [139, 140]. In glioma and lung carcinoma mouse models, exosome-recipient cells were mainly CD45+ leukocytes; among them, CD11b+Gr1+ MDSCs is the major subpopulation. After taking up the cancer–derived exosomes, the immunosuppressive function of the MDSCs was enhanced [141]. The hypoxia-inducible expression of miR-10a and miR-21 in glioblastoma exosomes mediated MDSC expansion and activation through downregulation of RAR-related orphan receptor alpha (RORA) and PTEN in MDSCs [142].
Tumor cell–derived exosomes also stimulate the polarization of macrophages towards the cancer-promoting M2 phase. For example, breast cancer–derived exosomes altered macrophage polarization to M2 phase via glycoprotein 130/STAT3 signaling. Specifically, glycoprotein 130 was enriched in the cancer-derived exosomes, which activated the STAT3 pathway in bone marrow–derived macrophages, resulting in IL6 secretion and polarization to M2 phase [143]. SNAIL-expressing human head and neck cancer cells produced miR-21–enriched exosomes, which were engulfed by CD14+ human monocytes, in turn suppressed the expression of M1 markers and increased that of M2 markers [144]. In hypoxic conditions, pancreatic cancer cell-derived exosomes induced macrophages to the M2 phenotype in a HIF1α or HIF2α-dependent manner, thereby facilitating the migration, invasion, and EMT of cancer cells [145]. Colon cancer cells harboring mutant p53 selectively shed miR-1246–enriched exosomes, which triggered neighboring macrophages into the cancer-promoting M2 phase with increased activity of TGFβ [146]. Breast cancer cell–derived exosomes transferred activated EGFR to host macrophages, which inhibited their production of type I interferons and antiviral immunity, resulting in compromise of innate immunity [147]. Additionally, tumor cell–derived exosomes also induced PD-L1 expression in macrophages. Gastric cancer–derived exosomes effectively induced generation of PD-L1+ tumor-associated macrophages and impaired CD8+ T cell function via IL10 [148], while glioblastoma-derived exosomes traversed to the monocytes, the precursor to macrophages, and skewed them toward the immune-suppressive M2 phenotype, inducing PD-L1 expression via activation of STAT3 or phosphorylated p70S6 kinase and ERK1/2 [149]. A complex report indicated that oral squamous cell carcinomas-derived exosomes transferred TSP1 to polarize macrophages to the M1-like phenotype, which were activated through P38 MAPK, AKT, and SAPK/JNK signaling at the early phase. However, it was found that exosome-activated M1 macrophages still had a pro-tumor effect because they significantly facilitated the migration of the cancer cells [150]. Similarly, melanoma- or breast cancer–derived exosomes activated M1 macrophages to secrete pro-inflammatory cytokines through stimulation of the NF-κB pathway, but these macrophages still facilitated cancer metastasis and immune escape [151, 152].
Therefore, exosomes exert both immune-activating and immune-suppressive functions in cancer. The effect of activating immunity mainly depends on antigen presentation by exosomes, while the effect of exosomes’ immune inhibition mainly depends on their carried ligands, proteins, and miRNAs, which inhibit the activity of cytotoxic T cells or increase immune-suppressive cells (Figure 4). Understanding the underlying mechanisms of both functions should benefit efforts to target or utilize exosomes in cancer treatment.
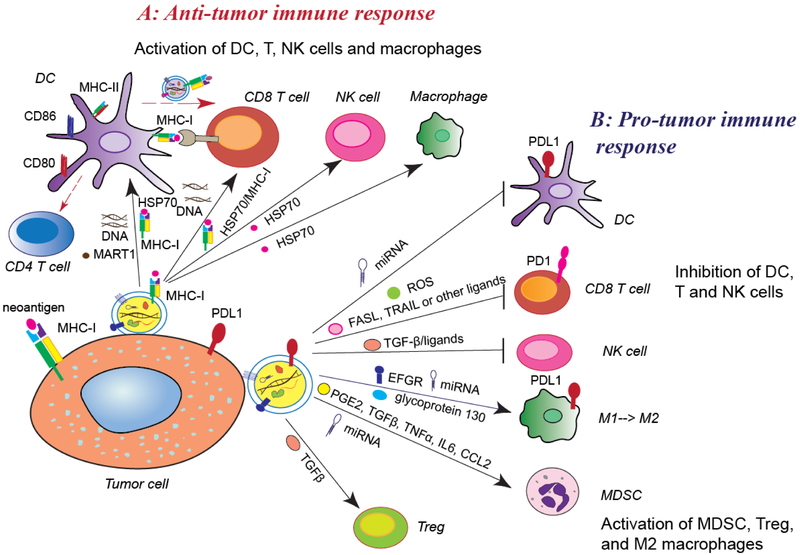
Figure 4. Exosomes’ functions in tumor immunity.
A. Anti-tumor immune response: Tumor exosomes present neo-antigens (such as HSP70, MART1) with MHC-I complex to dendritic cells (DCs) or directly to activate T cells. Tumor exosomes increase CD80, CD86, and MHC-II expression in DCs, which further activates CD4+ T cells. Exosomal DNAs trigger activation of DCs and CD8+ T cells. Tumor exosomes induce the activation of natural killer (NK) cells and macrophages by transferring HSP70. DCs release exosomes containing the antigens and MHC-I complex to activate cytotoxic T cells to inhibit tumor growth. B. Pro-tumor immune response: tumor exosomes also repress the function of DCs, T cells, and NK cells, enhancing the populations of myeloid derived suppressive cells (MDSCs) and regulatory T cells (Treg) and skewing macrophage function toward the M2 phenotype through various signaling pathways. Tumor exosomes carry PD-L1 from tumor cells and transfer it to DCs or macrophages, and then block T cell function.
7. Exosomes as therapeutic tools
Exosomes are a type of natural nanoparticle bio-vehicle, which are stable, membrane-permeable, and can even pass through the BBB. Because exosomes “recognize” specific cells, delivery of therapeutic cargos by exosomes can have better efficacy and fewer off-target effects than other bio-vehicles, such as liposomes. Therefore, exosomes have become a promising tool for delivery and transfer of drugs, miRNAs, small-interfering RNAs (siRNAs), short hairpin RNAs (shRNAs), and other compounds that remain stable in exosomes for the treatment of cancer and other diseases [153]. Several strategies for improving exosomes’ tumor cell-targeting specificity and uptake by tumors have been reported; for example, exosomes were engineered to express target ligands such as lysosome-associated membrane protein 2b (LAMP2B) and a tumor-targeting integrin [154]. An impressive example of exosomes delivering siRNAs or shRNAs that depleted oncogenes to inhibit tumor growth was reported by Kalluri and colleagues. They purified CD47+ exosomes from the supernatants of human fibroblast cultures and then introduced siRNA or short hairpin RNA (shRNA) that targeted oncogene KRASG12D [51]. This kind of engineered exosome (iExosomes) have a reduced clearance from monocytes and macrophages while increasing the uptake by the tumor cells. In a study, treatment of pancreatic tumor–bearing mice with iExosomes that specifically target KRAS-mutant tumor cells suppressed metastasis and dramatically prolonged survival [51], demonstrating the potential of exosomal miRNA-based approaches for effective targeting of KRAS or other mutations in cancer patients.
Strikingly, exosome-mediated delivery of drugs or siRNAs can pass the BBB. DC-derived exosomes delivered siRNAs to the brain in mice. Alvarez-Erviti et al. transduced the DCs to express exosomal membrane protein LAMP2B, which was fused to the neuron-specific RVG peptide. Purified DC exosomes were loaded with exogenous siRNA targeting BACE1, which is important in pathogenesis of Alzheimer disease; these iExosomes were then intravenously injected into mice. The exosomes specifically entered neurons, microglia, and oligodendrocytes in the brain, resulting in knockdown of the BACE1 gene in the mouse model. This study demonstrated the feasibility of exosome-specific systemic delivery and, more importantly, shows that exosome can pass biological barriers, shedding light on the possibility of new RNAi-based therapy for brain tumors, brain metastases, and neurodegenerative diseases [155].
Plant-derived EVs are also used to efficiently deliver drugs, siRNAs, or proteins to specific cells or tissue such as intestine, colon or liver [156]. Plant derived EVs have several benefits: they are edible, low toxicity and easily scaled up for mass production. It was reported that grapefruit-derived EVs delivered STAT3 inhibitor JSI-124 to prevent mouse glioblastoma tumor growth, when grapefruit-derived EVs were intranasally administrated. Gapefruit-derived EVs also can co-deliver paclitaxel with folic acid to increase the targeting efficiency to CT26 or SW620 colon cancer cells which express folate receptors. These EVs inhibited the colon cancer growth in mouse model [157].
Another promising area for exosomes is in anticancer vaccination, because exosomes can deliver or present tumor-derived antigens that activate cytotoxic T cells. One good example is the DC exosome vaccine. DC-derived exosomes express MHC-I and MHC-II molecules and induced antitumor immunity. Compared to DC vaccine, which is rapidly eliminated by antigen-specific cytotoxic T lymphocytes, the DC-derived exosome vaccine is relative long-lived, and thus is considered an alternative to and replacement for DC vaccine. DC-derived exosome vaccines have been tested in several phase I clinical trials [158–161]. In these clinical trials, no grade 2 or greater toxicity was observed, indicating the safety of exosome administration. In a follow-up phase II clinical trial testing the clinical benefit of DC exosomes for patients with non-small cell lung cancer after chemotherapy cessation, patients were administered granulocyte macrophage–colony-stimulating factor (GM-CSF) and IL4 to stimulate production of DCs from monocytes; the DCs were then induced to mature with IFNγ and loaded with MHC-I and MHCII restricted cancer antigens. Exosomes were isolated from these modified DCs and injected into the patients. No objective tumor response nor detectable T cell response was identified in this clinical trial, but NK cells were activated [162]. Although the phase II clinical result is not as favorable as expected, hope remains that DC-derived exosomes, after further optimization, represent a viable new vaccine strategy for cancer immunotherapy. In one of the phase I clinical trials, exosomes isolated from ascites of patients with colon cancer were injected into the patients as a vaccine. Administered with GM-CSF, the ascites-derived exosomes were safe and well tolerated and yielded a tumor-specific antitumor cytotoxic T cell response [160]. However, as already described, tumor-derived exosomes carry numerous oncogenes, mRNAs, and miRNAs, which induce tumor progression and metastasis, besides tumor antigens, the safety of tumor-derived exosome vaccine is still uncertain.
Given that cancer cell-derived exosomes can induce angiogenesis, promote metastasis, and modify pre-metastatic niches [104], a third potential clinical application of exosomes in cancer is depleting tumor exosomes from the circulatory system in order to block cancer metastasis. Inhibiting exosome assembly and release from tumor cells via inhibitors [139] or shRNAs [25], or eliminating exosomes from cancer patients’ circulation by extracorporeal purification, reduces numbers of exosomes in the circulation. For example, the ADAPT system is designed to selectively capture and remove circulating HER2+ exosomes [163]. Recently Datta et al. screened 4580 compounds to identify those that modulate exosome biogenesis and/or release by aggressive prostate cancer cells. Twenty-two of these compounds were found to be either potent activators or inhibitors of exosomes. The potent inhibitors, including tipifarnib, neticonazole, climbazole, ketoconazole, and triadimenol, might be utilized to deplete exosomes in cancer patients [164].
In summary, substantial evidence supports the application of exosomes for cancer treatment based on experiments in cell cultures or experimental models. Currently, however, only a few clinical trials of exosome for cancer therapy are underway. The main challenges of exosome application in the treatment of cancer or other diseases include, but are not limited to, 1) how to efficiently load exogenous miRNAs, siRNAs, shRNAs, or drugs into exosomes and further increase cell-specific delivery; 2) how to prevent immune reactions when utilizing nonautologous exosomes, which carry MHC-I or II; 3) how to increase cytotoxic T cell activation when using DC exosomes as a vaccine; 4) how to prolong the half-life of exosome vaccines or bioengineered exosomes in the body and avoid rapid clearance by immune cells, liver, or kidney; 5) how to prevent, when depleting tumor-derived exosomes from the blood of patients, the loss of non–tumor-promoting exosomes and their physiological function in the whole body; and 6) how to control the quality of exosomes to be administered to patients and the technological challenges related to clinical grade production [165]. Because of the complexity of exosome biology and these clinical challenges, carefully developing standard criteria for exosome quality and improving their efficacy in vivo are crucial before widely employing exosomes in clinical trials.
8. Exosomes as biomarkers
Besides the treatment of disease, other significant applications of exosomes include their use as biomarkers for disease diagnosis and prognosis. Because exosomes can be detected in bodily fluids such as blood, urine, saliva, and cerebrospinal fluid, they represent ideal noninvasive or less-invasive biomarkers for cancer diagnosis. For example, double-stranded DNAs in exosomes can be used as clinical biomarkers for cancer diagnosis because they reflect mutations in primary and/or metastatic cancer cells [44]. Circulating exosomal DNA enabled the detection of KRASG12D and TP53R273H mutations, which are potential biomarkers of pancreatic cancer [166]. Additionally, PD-L1 level on exosomes from plasma was associated with disease progression in head and neck cancers [167, 168] and predicted melanoma patient response to treatment with PD1 antibody [134]. Furthermore, certain proteins or miRNAs in exosomes isolated from cancer patient plasma are associated with tumor metastasis or relapse [169, 170]. Elevated expression of miRNA-191, −21, and −451a in serum exosomes seems to be a biomarker of pancreatic cancer [171]. miRNA analysis of EVs or exosomes from cerebrospinal fluid showed that miR-21 can serve as a biomarker for glioblastoma development and prediction of tumor recurrence or metastasis [172, 173]. Circulating exosomal miR-17–5p and miR-92a-3p were associated with pathologic stage and grade of colon cancers [174].
Although numerous publications present various exosomal miRNAs as potential biomarkers of breast cancer, prostate cancer, pancreatic cancer, melanoma, and other cancers, their utilization as clinical biomarkers still faces challenges. Most of these exosomal miRNA studies were conducted in a small patient cohort or only in a mouse model. In these studies, miRNA levels in plasma exosomes varied widely in the single cohort, and results from different groups were heterogeneous even when studying the same cancer type. The methods of exosome isolation from plasma and miRNA extraction from exosomes were not identical in the various study groups, and the studies lacked a common endogenous miRNA control for quantification of exosomal miRNAs. These problems affect the reliability of circulating exosomal miRNAs as cancer biomarkers in clinical diagnosis or prognosis.
Kalluri and colleagues discovered a serum exosomal biomarker for pancreatic cancer [175]. The exosomes from pancreatic cancers expressed at high levels a cell surface proteoglycan, glypican-1 (GPC1), which can be detected in serum as a highly sensitive and specific biomarker of pancreatic cancer, better than classic biomarker CA19–9 [175]. GPC1+ exosomes in serum can distinguish patients with pancreatic cancer from normal individuals and from patients with a benign pancreatic disease. GPC1+ exosome level was found to be associated with tumor burden and patient survival [175]. Following this finding, however, Lai et al. compared circulating exosomal GPC1 and miRNA levels in healthy subjects and in patients with pancreatic carcinoma or chronic pancreatitis and found that circulating exosomal GPC1 was not a good diagnostic marker for carcinoma. They found, in contrast, that high levels of miR-10b, miR-21, miR-30c, and miR-181a and a low level of miR-let7a in exosomes more reliably and promptly differentiated pancreatic carcinoma from normal controls and patients with benign pancreatic disease in their cohort relative small cohort [176]. The discrepancies between these two reports might be due to different methods used to detect circulating exosomal GPC1. The first group quantified exosomal GPC1 by an antibody-based assay, whereas the second group used liquid chromatography-tandem mass spectrometry. While these findings exemplify the power of utilizing exosomes for cancer diagnosis, the inconsistency indicates that GPC1 as a biomarker of pancreatic cancer requires further validation in larger patient cohorts and also highlights the importance of standardized methodologies for identifying exosomal biomarkers.
Another good example of exosomal protein as a biomarker is the urine exosome gene expression assay for prostate cancer. This assay detects RNA expression of ERG, PCA3, and SPDEF, which have known functions in prostate cancer initiation and progression. As a screening test, the urine exosome gene expression assay result combined with standard clinical data, comprising of prostate-specific antigen (PSA) level, age, race, and family history, can improve identification of patients with higher-grade prostate cancer over elevated prostate-specific antigen level alone, thereby reducing the number of unnecessary biopsies [177]. A urine-based liquid biopsy platform for the exosome gene expression assay, ExoDx Prostate (IntelliScore), has been developed for non-invasive detection of prostate cancer and there is an is ongoing clinical trial investigating the reliability of this urine test in predicting high-grade prostate cancer at the time of initial prostate biopsy.
In summary, applying exosome biomarkers in cancer diagnosis requires high sensitivity and specificity. Many miRNAs or proteins carried by exosomes have been considered as potential biomarkers, but the sensitivity and specificity of these candidates were not as good as the classic serum biomarkers of cancer, and most have not shown a prognostic or diagnostic significance in large patient cohorts. The technology of isolating exosomes from the serum, urine, or other bodily fluids and the methods of quantifying miRNAs or proteins also need to be further standardized.
Since research on these applications of exosomes is on the fast track, diagnostic applications of exosomes in cancer has an optimistic future.
9. Conclusion
Looking back over the more than 35-year history of exosome investigations, it is remarkable how rapidly our knowledge of exosomes has expanded. A multitude of studies have explored the functions of exosomes under different physiologic and pathologic conditions. Several hallmarks of exosomes have emerged, especially in cancer biology. First, extensive evidence shows that exosomes are not merely waste particles but rather critical mediators of intercellular communications. The cells control the cargos inside exosomes, with the effect of changing their own fate or that of other cells. As satellites of host cells, exosomes, contain substantial bio-information and function beyond initial expectations. Second, exosomes have a robust impact on tumor progression and metastasis. Strikingly, exosomes can predict sites of metastasis and build pre-metastatic niches, depending on their interactions with stromal cells. Third, exosomes can induce both effective pro-tumor and antitumor immune responses, but cancer cell–derived exosomes show much stronger immune suppression than immune activation in the advanced stage. Exosome vaccines, based on exosomes carrying antigens, are being tested in clinical trials. Given the specific characters of exosomes, their use as a new platform for cancer therapy and as cancer biomarkers is promising despite the technical challenges. Deep understanding of the mechanisms underlying exosome function should advance their application in the clinic. It should be noted that in many preclinical studies, the function of exosomes on tumor progression or immunity were mainly determined by “gain-of-function” experiments, such as the adoptive transfer of exosomes, and this may not accurately represent the actual physiological function of exosomes. Although blocking exosome secretion by knocking down RAB27A or RAB27B was used for “loss-of-function” exosome phenotypes in some studies, more rigorous “loss-of-function” techniques may be required to thoroughly uncover the essential function of exosomes and move the field further ahead. We anticipate that advances made in the exosome field will lead to breakthroughs in clinical applications to benefit patients.
Acknowledgments
We thank members of the Yu laboratory for helpful discussions. We thank Kathryn L. Hale in the Department of Scientific Publications of MD Anderson Cancer Center for editing the manuscript. This work was supported by National Institutes of Health grants R01-CA112567–06 (D.Y.), R01CA184836 (D.Y.), R01 CA208213 (D.Y.), R21CA223102 (D.Y.), and the China Medical University Research Fund (D.Y.). D. Yu is the Hubert L. & Olive Stringer Distinguished Chair in Basic Science of MD Anderson Cancer Center.
Abbreviations
- EV
extracellular vesicle
- MVB
multivesicular body
- Exo-L
large exosome
- Exo-S
small exosome
- ApoEV
apoptotic extracellular vesicle
- ESCRT
endosomal sorting complex required for transport
- SNARE
soluble NSF attachment protein receptor complex
- miRNA
microRNA
- snRNA
small nuclear RNA
- CAF-DE
cancer-associated fibroblast–derived exosome
- lncRNA
long non-coding RNA
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- BBB
blood-brain barrier
- NK
natural killer
- IL
interleukin
- MHC
major histocompatibility complex
- DC
dendritic cell
- MDSC
myeloid-derived suppressor cell
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
- GM-CSF
granulocyte macrophage–colony-stimulating factor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We apologize for not being able to cite all the relevant original research and review articles due to space limitation.
References
- [1].Pan BT, Teng K, Wu C, Adam M, Johnstone RM, Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes, The Journal of cell biology, 101 (1985) 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Harding C, Heuser J, Stahl P, Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes, J Cell Biol, 97 (1983) 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thery C, Ostrowski M, Segura E, Membrane vesicles as conveyors of immune responses, Nat Rev Immunol, 9 (2009) 581–593, DOI: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- [4].Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C, Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation, J Extracell Vesicles, 1 (2012), DOI: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhaes A, Ferreira JA, Osorio H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D, Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat Cell Biol, 20 (2018) 332–343, DOI: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colombo M, Raposo G, Thery C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles, Annu Rev Cell Dev Biol, 30 (2014) 255–289, DOI: 10.1146/annurevcellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- [7].Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ, Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules, Blood, 94 (1999) 3791–3799. [PubMed] [Google Scholar]
- [8].van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R, Classification, functions, and clinical relevance of extracellular vesicles, Pharmacol Rev, 64 (2012) 676–705, DOI: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- [9].Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA, Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils, Exp Cell Res, 285 (2003) 243–257. [DOI] [PubMed] [Google Scholar]
- [10].Ronquist G, Hedstrom M, Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A, Biochim Biophys Acta, 483 (1977) 483–486. [DOI] [PubMed] [Google Scholar]
- [11].Ronquist G, Brody I, Gottfries A, Stegmayr B, An Mg2+ and Ca2+-stimulated adenosine triphosphatase in human prostatic fluid--part II, Andrologia, 10 (1978) 427–433. [DOI] [PubMed] [Google Scholar]
- [12].Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, Chen L, Yan X, Du Y, Yu L, Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration, Cell Res, 25 (2015) 24–38, DOI: 10.1038/cr.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J, Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells, Nat Cell Biol, 10 (2008) 619–624, DOI: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- [14].Meehan B, Rak J, Di Vizio D, Oncosomes - large and small: what are they, where they came from?, J Extracell Vesicles, 5 (2016) 33109, DOI: 10.3402/jev.v5.33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP, Isolation of Extracellular Vesicles: General Methodologies and Latest Trends, Biomed Res Int, 2018 (2018) 8545347, DOI: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, Demichelis F, Freeman MR, Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease, Cancer Res, 69 (2009) 5601–5609, DOI: 10.1158/0008-5472.CAN-08-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ, Extracellular vesicles in cancer - implications for future improvements in cancer care, Nat Rev Clin Oncol, 15 (2018) 617–638, DOI: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- [18].Henne WM, Stenmark H, Emr SD, Molecular mechanisms of the membrane sculpting ESCRT pathway, Cold Spring Harb Perspect Biol, 5 (2013), DOI: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Katzmann DJ, Babst M, Emr SD, Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I, Cell, 106 (2001) 145–155. [DOI] [PubMed] [Google Scholar]
- [20].Hanson PI, Cashikar A, Multivesicular body morphogenesis, Annual review of cell and developmental biology, 28 (2012) 337–362, DOI: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- [21].Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M, Ceramide triggers budding of exosome vesicles into multivesicular endosomes, Science, 319 (2008) 1244–1247, DOI: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- [22].Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavik J, Machala M, Zimmermann P, Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2, Nat Commun, 5 (2014) 3477, DOI: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- [23].Babst M, MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between, Curr Opin Cell Biol, 23 (2011) 452–457, DOI: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G, The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis, Dev Cell, 21 (2011) 708–721, DOI: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C, Rab27a and Rab27b control different steps of the exosome secretion pathway, Nat Cell Biol, 12 (2010) 19–30; sup pp 11–13, DOI: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- [26].Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M, Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C, The Journal of cell biology, 189 (2010) 223–232, DOI: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G, Syndecan-syntenin-ALIX regulates the biogenesis of exosomes, Nature cell biology, 14 (2012) 677–685, DOI: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- [28].Gu H, Chen C, Hao X, Wang C, Zhang X, Li Z, Shao H, Zeng H, Yu Z, Xie L, Xia F, Zhang F, Liu X, Zhang Y, Jiang H, Zhu J, Wan J, Wang C, Weng W, Xie J, Tao M, Zhang CC, Liu J, Chen GQ, Zheng J, Sorting protein VPS33B regulates exosomal autocrine signaling to mediate hematopoiesis and leukemogenesis, J Clin Invest, 126 (2016) 4537–4553, DOI: 10.1172/JCI87105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [29].Zylbersztejn K, Galli T, Vesicular traffic in cell navigation, The FEBS journal, 278 (2011) 4497–4505, DOI: 10.1111/j.1742-4658.2011.08168.x. [DOI] [PubMed] [Google Scholar]
- [30].Fader CM, Sanchez DG, Mestre MB, Colombo MI, TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways, Biochimica et biophysica acta, 1793 (2009) 1901–1916, DOI: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [31].Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S, Microenvironmental pH is a key factor for exosome traffic in tumor cells, J Biol Chem, 284 (2009) 34211–34222, DOI: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C, ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles, Curr Biol, 19 (2009) 1875–1885, DOI: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li B, Antonyak MA, Zhang J, Cerione RA, RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells, Oncogene, 31 (2012) 4740–4749, DOI: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Muhsin-Sharafaldine MR, McLellan AD, Tumor-Derived Apoptotic Vesicles: With Death They Do Part, Front Immunol, 9 (2018) 957, DOI: 10.3389/fimmu.2018.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kowal J, Tkach M, Thery C, Biogenesis and secretion of exosomes, Curr Opin Cell Biol, 29 (2014) 116–125, DOI: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- [36].Munson P, Shukla A, Exosomes: Potential in Cancer Diagnosis and Therapy, Medicines (Basel), 2 (2015) 310–327, DOI: 10.3390/medicines2040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR, Adipose-derived circulating miRNAs regulate gene expression in other tissues, Nature, 542 (2017) 450–455, DOI: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ, B lymphocytes secrete antigen-presenting vesicles, J Exp Med, 183 (1996) 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S, Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes, Nat Med, 4 (1998) 594–600. [DOI] [PubMed] [Google Scholar]
- [40].Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D, Hypothalamic stem cells control ageing speed partly through exosomal miRNAs, Nature, 548 (2017) 52–57, DOI: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D, Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth, Nature, 527 (2015) 100–104, DOI: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nature cell biology, 9 (2007) 654–659, DOI: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- [43].Raposo G, Stoorvogel W, Extracellular vesicles: exosomes, microvesicles, and friends, The Journal of cell biology, 200 (2013) 373–383, DOI: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D, Double-stranded DNA in exosomes: a novel biomarker in cancer detection, Cell Res, 24 (2014) 766–769, DOI: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D, Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism, Elife, 5 (2016) e10250, DOI: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mathivanan S, Fahner CJ, Reid GE, Simpson RJ, ExoCarta 2012: database of exosomal proteins, RNA and lipids, Nucleic Acids Res, 40 (2012) D1241–1244, DOI: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Batagov AO, Kuznetsov VA, Kurochkin IV, Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles, BMC genomics, 12 Suppl 3 (2011) S18, DOI: 10.1186/1471-2164-12-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N, Levy S, Zhang B, Coffey RJ, Patton JG, KRAS-dependent sorting of miRNA to exosomes, Elife, 4 (2015) e07197, DOI: 10.7554/eLife.07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rana S, Yue S, Stadel D, Zoller M, Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection, The international journal of biochemistry & cell biology, 44 (2012) 1574–1584, DOI: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- [50].Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M, Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation, Cancer Res, 70 (2010) 1668–1678, DOI: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- [51].Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R, Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature, 546 (2017) 498–503, DOI: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SI, Wurdinger T, Pegtel DM, van Rheenen J, In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior, Cell, 161 (2015) 1046–1057, DOI: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Raimondo S, Saieva L, Corrado C, Fontana S, Flugy A, Rizzo A, De Leo G, Alessandro R, Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism, Cell communication and signaling : CCS, 13 (2015) 8, DOI: 10.1186/s12964-015-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kalluri R, LeBleu VS, Discovery of Double-Stranded Genomic DNA in Circulating Exosomes, Cold Spring Harb Symp Quant Biol, 81 (2016) 275–280, DOI: 10.1101/sqb.2016.81.030932. [DOI] [PubMed] [Google Scholar]
- [55].Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, Hara E, Exosomes maintain cellular homeostasis by excreting harmful DNA from cells, Nat Commun, 8 (2017) 15287, DOI: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ, Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS, Mol Cell Proteomics, 12 (2013) 343–355, DOI: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, Cha JH, Hou J, Hsu JL, Sun L, Hung MC, Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth, Cell Res, 28 (2018) 862–864, DOI: 10.1038/s41422-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, Zhang S, Wang J, Komarova S, Wang J, Yamaguchi S, Alsheikh HA, Shi J, Chen D, Mohyeldin A, Kim SH, Shin YJ, Anufrieva K, Evtushenko EG, Antipova NV, Arapidi GP, Govorun V, Pestov NB, Shakhparonov MI, Lee LJ, Nam DH, Nakano I, Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma Via Intercellular Transfer of Splicing Factors, Cancer Cell, 34 (2018) 119–135 e110, DOI: 10.1016/j.ccell.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr., Carter BS, Krichevsky AM, Breakefield XO, Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers, Nat Cell Biol, 10 (2008) 1470–1476, DOI: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA, Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells, Proc Natl Acad Sci U S A, 108 (2011) 4852–4857, DOI: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cho JA, Park H, Lim EH, Lee KW, Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells, Int J Oncol, 40 (2012) 130–138, DOI: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- [62].Webber J, Steadman R, Mason MD, Tabi Z, Clayton A, Cancer exosomes trigger fibroblast to myofibroblast differentiation, Cancer Res, 70 (2010) 9621–9630, DOI: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- [63].Gesierich S, Berezovskiy I, Ryschich E, Zoller M, Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029, Cancer research, 66 (2006) 7083–7094, DOI: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- [64].Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, Sargent I, Li JL, Harris AL, New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes, Blood, 116 (2010) 2385–2394, DOI: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- [65].Tang MKS, Yue PYK, Ip PP, Huang RL, Lai HC, Cheung ANY, Tse KY, Ngan HYS, Wong AST, Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface, Nat Commun, 9 (2018) 2270, DOI: 10.1038/s41467-018-04695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M, Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells, Proc Natl Acad Sci U S A, 108 (2011) 13147–13152, DOI: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL, Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1, Oncogene, 36 (2017) 4929–4942, DOI: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- [68].Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH, Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1, Blood, 124 (2014) 3748–3757, DOI: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, Minn AJ, Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer, Cell, 170 (2017) 352–366 e313, DOI: 10.1016/j.cell.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shimoda M, Principe S, Jackson HW, Luga V, Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas C, Hess FM, Ohtsuka T, Okada Y, Ailles L, Ludwig A, Wrana JL, Kislinger T, Khokha R, Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state, Nat Cell Biol, 16 (2014) 889–901, DOI: 10.1038/ncb3021. [DOI] [PubMed] [Google Scholar]
- [71].Achreja A, Zhao H, Yang L, Yun TH, Marini J, Nagrath D, Exo-MFA - A 13C metabolic flux analysis framework to dissect tumor microenvironment-secreted exosome contributions towards cancer cell metabolism, Metab Eng, 43 (2017) 156–172, DOI: 10.1016/j.ymben.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F, Federici C, Iessi E, Parmiani G, Arancia G, Belardelli F, Fais S, Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs, Journal of the National Cancer Institute, 96 (2004) 1702–1713, DOI: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- [73].Chen KG, Valencia JC, Lai B, Zhang G, Paterson JK, Rouzaud F, Berens W, Wincovitch SM, Garfield SH, Leapman RD, Hearing VJ, Gottesman MM, Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas, Proceedings of the National Academy of Sciences of the United States of America, 103 (2006) 9903–9907, DOI: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB, Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells, Molecular cancer therapeutics, 4 (2005) 1595–1604, DOI: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- [75].Yin J, Yan X, Yao X, Zhang Y, Shan Y, Mao N, Yang Y, Pan L, Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients, Journal of cellular and molecular medicine, 16 (2012) 337–348, DOI: 10.1111/j.1582-4934.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE, Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells, Leukemia, 23 (2009) 1643–1649, DOI: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- [77].Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, Tang JH, Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs, PLoS One, 9 (2014) e95240, DOI: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X, Shen B, Liu S, Yan D, Feng J, Cisplatin-resistant lung cancer cell-derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100–5p-dependent manner, Int J Nanomedicine, 12 (2017) 3721–3733, DOI: 10.2147/IJN.S131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yang SJ, Wang DD, Li J, Xu HZ, Shen HY, Chen X, Zhou SY, Zhong SL, Zhao JH, Tang JH, Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer, Gene, 623 (2017) 5–14, DOI: 10.1016/j.gene.2017.04.031. [DOI] [PubMed] [Google Scholar]
- [80].Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, Wang X, Wang Y, Xu ZY, Gao L, Yang Q, Xu B, Li YM, Fang ZY, Xu ZP, Bao Y, Wu DS, Miao X, Sun HY, Sun YH, Wang HY, Wang LH, Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA, Cancer Cell, 29 (2016) 653–668, DOI: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- [81].Martinez VG, O’Neill S, Salimu J, Breslin S, Clayton A, Crown J, O’Driscoll L, Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles, Oncoimmunology, 6 (2017) e1362530, DOI: 10.1080/2162402X.2017.1362530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ, Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways, Cell, 159 (2014) 499–513, DOI: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G, Shlomi T, Gil Z, Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma, Cancer Res, 78 (2018) 5287–5299, DOI: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- [84].Namee NM, O’Driscoll L, Extracellular vesicles and anti-cancer drug resistance, Biochim Biophys Acta Rev Cancer, 1870 (2018) 123–136, DOI: 10.1016/j.bbcan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- [85].Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM, Directional cell movement through tissues is controlled by exosome secretion, Nature communications, 6 (2015) 7164, DOI: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL, Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration, Cell, 151 (2012) 1542–1556, DOI: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- [87].Yue S, Mu W, Erb U, Zoller M, The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding, Oncotarget, 6 (2015) 2366–2384, DOI: 10.18632/oncotarget.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, Bracke M, Seabra MC, Gahl WA, De Wever O, Westbroek W, Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis, J Natl Cancer Inst, 102 (2010) 866–880, DOI: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE, Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis, Cancer Cell, 25 (2014) 501–515, DOI: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J, Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes, Oncogene, 33 (2014) 4613–4622, DOI: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].You Y, Shan Y, Chen J, Yue H, You B, Shi S, Li X, Cao X, Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis, Cancer science, 106 (2015) 1669–1677, DOI: 10.1111/cas.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KA, Dyrskjot L, Orntoft TF, Larsen MR, Ostenfeld MS, Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors, Proteomics, 14 (2014) 699–712, DOI: 10.1002/pmic.201300452. [DOI] [PubMed] [Google Scholar]
- [93].Yoshizaki T, Kondo S, Wakisaka N, Murono S, Endo K, Sugimoto H, Nakanishi S, Tsuji A, Ito M, Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma, Cancer letters, 337 (2013) 1–7, DOI: 10.1016/j.canlet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- [94].Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, Bui KH, Fristrup N, Christensen EI, Nordentoft I, Morth JP, Jensen JB, Pedersen JS, Beck M, Theodorescu D, Borre M, Howard KA, Dyrskjot L, Orntoft TF, Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties, Cancer Res, 74 (2014) 5758–5771, DOI: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- [95].Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lotvall J, Nakagama H, Ochiya T, Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier, Nat Commun, 6 (2015) 6716, DOI: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hood JL, San RS, Wickline SA, Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis, Cancer Res, 71 (2011) 3792–3801, DOI: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- [97].Rana S, Malinowska K, Zoller M, Exosomal tumor microRNA modulates premetastatic organ cells, Neoplasia, 15 (2013) 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Karlsson T, Lundholm M, Widmark A, Persson E, Tumor Cell-Derived Exosomes from the Prostate Cancer Cell Line TRAMP-C1 Impair Osteoclast Formation and Differentiation, PLoS One, 11 (2016) e0166284, DOI: 10.1371/journal.pone.0166284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ye Y, Li SL, Ma YY, Diao YJ, Yang L, Su MQ, Li Z, Ji Y, Wang J, Lei L, Fan WX, Li LX, Xu Y, Hao XK, Exosomal miR-141–3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer, Oncotarget, 8 (2017) 94834–94849, DOI: 10.18632/oncotarget.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zoller M, CD44v6 dependence of premetastatic niche preparation by exosomes, Neoplasia, 11 (2009) 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G, Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche, Cancer Res, 71 (2011) 5346–5356, DOI: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- [102].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D, Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET, Nature medicine, 18 (2012) 883–891, DOI: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D, Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver, Nature cell biology, 17 (2015) 816–826, DOI: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature, 527 (2015) 329–335, DOI: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X, Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils, Cancer Cell, 30 (2016) 243–256, DOI: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- [106].Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O’Connor ST, Li S, Chin AR, Somlo G, Palomares M, Li Z, Tremblay JR, Tsuyada A, Sun G, Reid MA, Wu X, Swiderski P, Ren X, Shi Y, Kong M, Zhong W, Chen Y, Wang SE, Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis, Nat Cell Biol, 17 (2015) 183–194, DOI: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, Filleur S, Bhowmick R, Henkin J, Miller SD, Ifergan I, Lee Y, Osman I, Thaxton CS, Volpert OV, Pre -metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche, Nat Commun, 8 (2017) 1319, DOI: 10.1038/s41467-017-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Valencia K, Luis-Ravelo D, Bovy N, Anton I, Martinez-Canarias S, Zandueta C, Ormazabal C, Struman I, Tabruyn S, Rebmann V, De Las Rivas J, Guruceaga E, Bandres E, Lecanda F, miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization, Mol Oncol, 8 (2014) 689–703, DOI: 10.1016/j.molonc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T, Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells, Sci Signal, 7 (2014) ra63, DOI: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- [110].Fidler IJ, The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited, Nat Rev Cancer, 3 (2003) 453–458, DOI: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- [111].Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L, Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming, Nat Med, 7 (2001) 297–303, DOI: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- [112].Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X, More efficient induction of HLAA*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells, Clinical cancer research : an official journal of the American Association for Cancer Research, 11 (2005) 7554–7563, DOI: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- [113].Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, Wan T, Yu Y, Cao X, Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells, Journal of molecular medicine, 84 (2006) 1067–1076, DOI: 10.1007/s00109-006-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE, Exosomes and immune surveillance of neoplastic lesions: a review, Biotechnic & histochemistry : official publication of the Biological Stain Commission, 87 (2012) 161–168, DOI: 10.3109/10520291003659042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V, Recent advances on the role of tumor exosomes in immunosuppression and disease progression, Seminars in cancer biology, 22 (2012) 342–349, DOI: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- [116].Altieri SL, Khan AN, Tomasi TB, Exosomes from plasmacytoma cells as a tumor vaccine, J Immunother, 27 (2004) 282–288. [DOI] [PubMed] [Google Scholar]
- [117].Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G, Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells, Cancer Res, 65 (2005) 5238–5247, DOI: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A, Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages, J Immunol, 180 (2008) 4299–4307. [DOI] [PubMed] [Google Scholar]
- [119].Cho JA, Lee YS, Kim SH, Ko JK, Kim CW, MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models, Cancer Lett, 275 (2009) 256–265, DOI: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- [120].Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X, Exosomes derived from Rab27aoverexpressing tumor cells elicit efficient induction of antitumor immunity, Mol Med Rep, 8 (2013) 1876–1882, DOI: 10.3892/mmr.2013.1738. [DOI] [PubMed] [Google Scholar]
- [121].Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L, Malignant effusions and immunogenic tumour-derived exosomes, Lancet, 360 (2002) 295–305, DOI: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- [122].Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, Guan KL, The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity, Cell, 167 (2016) 1525–1539 e1517, DOI: 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, Akira S, Matsuda T, Kawai T, DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity, J Immunol, 198 (2017) 1649–1659, DOI: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- [124].Muhsin-Sharafaldine MR, Saunderson SC, Dunn AC, Faed JM, Kleffmann T, McLellan AD, Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles, Oncotarget, 7 (2016) 56279–56294, DOI: 10.18632/oncotarget.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bobrie A, Colombo M, Raposo G, Thery C, Exosome secretion: molecular mechanisms and roles in immune responses, Traffic, 12 (2011) 1659–1668, DOI: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- [126].Lundholm M, Schroder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, Wikstrom P, Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion, PLoS One, 9 (2014) e108925, DOI: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z, Human tumor-derived exosomes down-modulate NKG2D expression, J Immunol, 180 (2008) 7249–7258. [DOI] [PubMed] [Google Scholar]
- [128].Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L, Tumour-released exosomes and their implications in cancer immunity, Cell death and differentiation, 15 (2008) 80–88, DOI: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- [129].Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP, Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis, Blood cells, molecules & diseases, 35 (2005) 169–173, DOI: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [130].Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F, Adhikary D, Mautner J, Busson P, Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells, Blood, 113 (2009) 1957–1966, DOI: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- [131].Soderberg A, Barral AM, Soderstrom M, Sander B, Rosen A, Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes, Free Radic Biol Med, 43 (2007) 90–99, DOI: 10.1016/j.freeradbiomed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- [132].Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z, Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2, Cancer Res, 67 (2007) 7458–7466, DOI: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- [133].Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, Hayes JL, Lee K, Balaj L, Passaro C, Rooj AK, Krasemann S, Carter BS, Chen CC, Steed T, Treiber J, Rodig S, Yang K, Nakano I, Lee H, Weissleder R, Breakefield XO, Godlewski J, Westphal M, Lamszus K, Freeman GJ, Bronisz A, Lawler SE, Chiocca EA, Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles, Sci Adv, 4 (2018) eaar2766, DOI: 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W, Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response, Nature, 560 (2018) 382–386, DOI: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L, Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203, Cell Immunol, 292 (2014) 65–69, DOI: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- [136].Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, Pan J, Qi C, Tumor exosomes block dendritic cells maturation to decrease the T cell immune response, Immunol Lett, 199 (2018) 36–43, DOI: 10.1016/j.imlet.2018.05.002. [DOI] [PubMed] [Google Scholar]
- [137].Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG, Induction of myeloid-derived suppressor cells by tumor exosomes, Int J Cancer, 124 (2009) 2621–2633, DOI: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, Michalek S, Grizzle W, Zhang HG, Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells, Am J Pathol, 176 (2010) 2490–2499, DOI: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F, Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells, J Clin Invest, 120 (2010) 457–471, DOI: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Diao J, Yang X, Song X, Chen S, He Y, Wang Q, Chen G, Luo C, Wu X, Zhang Y, Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3, Med Oncol, 32 (2015) 453, DOI: 10.1007/s12032-014-0453-2. [DOI] [PubMed] [Google Scholar]
- [141].Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sultmann H, Altevogt P, Umansky V, Momma S, Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment, Oncoimmunology, 4 (2015) e1008371, DOI: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Xue H, Li G, Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways, Oncogene, 37 (2018) 4239–4259, DOI: 10.1038/s41388-018-0261-9. [DOI] [PubMed] [Google Scholar]
- [143].Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, Wen SW, Wiegmans AP, Moller A, Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling, Front Immunol, 9 (2018) 871, DOI: 10.3389/fimmu.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Hsieh CH, Tai SK, Yang MH, Snail-overexpressing Cancer Cells Promote M2-Like Polarization of Tumor-Associated Macrophages by Delivering MiR-21-Abundant Exosomes, Neoplasia, 20 (2018) 775–788, DOI: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z, Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kgamma to Promote Pancreatic Cancer Metastasis, Cancer Res, 78 (2018) 4586–4598, DOI: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- [146].Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC, Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246, Nat Commun, 9 (2018) 771, DOI: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Gao L, Wang L, Dai T, Jin K, Zhang Z, Wang S, Xie F, Fang P, Yang B, Huang H, van Dam H, Zhou F, Zhang L, Tumor-derived exosomes antagonize innate antiviral immunity, Nat Immunol, 19 (2018) 233–245, DOI: 10.1038/s41590-017-0043-5. [DOI] [PubMed] [Google Scholar]
- [148].Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y, Tumor-derived exosomes induce PD1(+) macrophage population in human gastric cancer that promotes disease progression, Oncogenesis, 7 (2018) 41, DOI: 10.1038/s41389-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Gabrusiewicz K, Li X, Wei J, Hashimoto Y, Marisetty AL, Ott M, Wang F, Hawke D, Yu J, Healy LM, Hossain A, Akers JC, Maiti SN, Yamashita S, Shimizu Y, Dunner K, Zal MA, Burks JK, Gumin J, Nwajei F, Rezavanian A, Zhou S, Rao G, Sawaya R, Fuller GN, Huse JT, Antel JP, Li S, Cooper L, Sulman EP, Chen C, Geula C, Kalluri R, Zal T, Heimberger AB, Glioblastoma stem cell -derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes, Oncoimmunology, 7 (2018) e1412909, DOI: 10.1080/2162402X.2017.1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Xiao M, Zhang J, Chen W, Chen W, M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma, J Exp Clin Cancer Res, 37 (2018) 143, DOI: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy K, Szegletes Z, Varo G, Siklos L, Katona RL, Tubak V, Howard OM, Duda E, Minarovits J, Nagy K, Buzas K, Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro, Immunol Lett, 148 (2012) 34–38, DOI: 10.1016/j.imlet.2012.07.006. [DOI] [PubMed] [Google Scholar]
- [152].Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M, Wang SE, Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB, Sci Rep, 4 (2014) 5750, DOI: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M, A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy, Biochimica et biophysica acta, 1846 (2014) 75–87, DOI: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- [154].Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G, A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy, Biomaterials, 35 (2014) 2383–2390, DOI: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- [155].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nat Biotechnol, 29 (2011) 341–345, DOI: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- [156].Zhang M, Viennois E, Xu C, Merlin D, Plant derived edible nanoparticles as a new therapeutic approach against diseases, Tissue Barriers, 4 (2016) e1134415, DOI: 10.1080/21688370.2015.1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Zhang L, Xiang X, Wang B, Yan J, Miller D, Zhang HG, Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids, Nat Commun, 4 (2013) 1867, DOI: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bourderioux M, Nguyen-Khoa T, Chhuon C, Jeanson L, Tondelier D, Walczak M, Ollero M, Bekri S, Knebelmann B, Escudier E, Escudier B, Edelman A, Guerrera IC, A new workflow for proteomic analysis of urinary exosomes and assessment in cystinuria patients, Journal of proteome research, 14 (2015) 567–577, DOI: 10.1021/pr501003q. [DOI] [PubMed] [Google Scholar]
- [159].Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK, A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, Journal of translational medicine, 3 (2005) 9, DOI: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G, Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer, Mol Ther, 16 (2008) 782–790, DOI: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L, Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial, J Transl Med, 3 (2005) 10, DOI: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N, Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC, Oncoimmunology, 5 (2016) e1071008, DOI: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Marleau AM, Chen CS, Joyce JA, Tullis RH, Exosome removal as a therapeutic adjuvant in cancer, J Transl Med, 10 (2012) 134, DOI: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, Southall N, Hu X, Lal M, Mondal D, Ferrer M, Abdel-Mageed AB, High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer, Sci Rep, 8 (2018) 8161, DOI: 10.1038/s41598-018-26411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B, Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper, J Extracell Vesicles, 4 (2015) 30087, DOI: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P, Tachezy M, Bockhorn M, Gebauer F, Haltom AR, Melo SA, LeBleu VS, Kalluri R, Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer, Cancer Biol Ther, 18 (2017) 158–165, DOI: 10.1080/15384047.2017.1281499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL, Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients, Clin Cancer Res, 24 (2018) 896–905, DOI: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL, Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer, Clin Cancer Res, 23 (2017) 4843–4854, DOI: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].An M, Lohse I, Tan Z, Zhu J, Wu J, Kurapati H, Morgan MA, Lawrence TS, Cuneo KC, Lubman DM, Quantitative Proteomic Analysis of Serum Exosomes from Patients with Locally Advanced Pancreatic Cancer Undergoing Chemoradiotherapy, J Proteome Res, 16 (2017) 1763–1772, DOI: 10.1021/acs.jproteome.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D, Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis, Cancer Cell, 30 (2016) 836–848, DOI: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A, Utsumi T, Sato H, Iwama T, Ijiri M, Sakatani A, Tanaka K, Nomura Y, Ueno N, Kashima S, Moriichi K, Mizukami Y, Kohgo Y, Okumura T, An elevated expression of serum exosomal microRNA-191, - 21, −451a of pancreatic neoplasm is considered to be efficient diagnostic marker, BMC Cancer, 18 (2018) 116, DOI: 10.1186/s12885-018-4006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S, Kalinina J, Hua W, Kesari S, Mao Y, Breakefield XO, Hochberg FH, Van Meir EG, Carter BS, Chen CC, MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development, PLoS One, 8 (2013) e78115, DOI: 10.1371/journal.pone.0078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Shi R, Wang PY, Li XY, Chen JX, Li Y, Zhang XZ, Zhang CG, Jiang T, Li WB, Ding W, Cheng SJ, Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients, Oncotarget, 6 (2015) 26971–26981, DOI: 10.18632/oncotarget.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Fu F, Jiang W, Zhou L, Chen Z, Circulating Exosomal miR-17–5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer, Transl Oncol, 11 (2018) 221–232, DOI: 10.1016/j.tranon.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R, Glypican-1 identifies cancer exosomes and detects early pancreatic cancer, Nature, 523 (2015) 177–182, DOI: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M, A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer, Cancer Lett, 393 (2017) 86–93, DOI: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G, Brown G, Wei JT, Thompson IM Jr., Carroll P, A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy, JAMA Oncol, 2 (2016) 882–889, DOI: 10.1001/jamaoncol.2016.0097. [DOI] [PubMed] [Google Scholar]