Abstract
Background
Bariatric embolization is a new endovascular procedure to treat patients with obesity. However, the safety and efficacy of bariatric embolization are unknown.
Purpose
To evaluate the safety and efficacy of bariatric embolization in severely obese adults at up to 12 months after the procedure.
Materials and Methods
For this prospective study (NCT0216512 on ClinicalTrials.gov), 20 participants (16 women) aged 27–68 years (mean ± standard deviation, 44 years ± 11) with mean body mass index of 45 ± 4.1 were enrolled at two institutions from June 2014 to February 2018. Transarterial embolization of the gastric fundus was performed using 300- to 500-µm embolic microspheres. Primary end points were 30-day adverse events and weight loss at up to 12 months. Secondary end points at up to 12 months included technical feasibility, health-related quality of life (Short Form-36 Health Survey ([SF-36]), impact of weight on quality of life (IWQOL-Lite), and hunger or appetite using a visual assessment scale. Analysis of outcomes was performed by using one-sample t tests and other exploratory statistics.
Results
Bariatric embolization was performed successfully for all participants with no major adverse events. Eight participants had a total of 11 minor adverse events. Mean excess weight loss was 8.2% (95% confidence interval [CI]: 6.3%, 10%; P < .001) at 1 month, 11.5% (95% CI: 8.7%, 14%; P < .001) at 3 months, 12.8% (95% CI: 8.3%, 17%; P < .001) at 6 months, and 11.5% (95% CI: 6.8%, 16%; P < .001) at 12 months. From baseline to 12 months, mean SF-36 scores increased (mental component summary, from 46 ± 11 to 50 ± 10, P = .44; physical component summary, from 46 ± 8.0 to 50 ± 9.3, P = .15) and mean IWQOL-Lite scores increased from 57 ± 18 to 77 ± 18 (P < .001). Hunger or appetite decreased for 4 weeks after embolization and increased thereafter, without reaching pre-embolization levels.
Conclusion
Bariatric embolization is well tolerated in severely obese adults, inducing appetite suppression and weight loss for up to 12 months.
Published under a CC BY-NC-ND 4.0 license.
Summary
Bariatric embolization is a feasible and well-tolerated procedure that produces weight loss and reduces appetite for up to 1 year.
Key Points
■ Bariatric embolization is a feasible procedure that was performed with 100% technical success in 20 adults with severe obesity.
■ Bariatric embolization is well tolerated, with a major complication rate of 0%.
■ Bariatric embolization can produce substantial weight loss (ie, mean excess weight loss of 11% ± 10% at 12 months).
■ Participants who underwent bariatric embolization showed evidence of metabolic change, with decreases in hemoglobin A1c and total cholesterol (independent of weight loss) and increases in high-density lipoprotein levels.
Introduction
Obesity is a major cause of morbidity and mortality (1). Traditional treatments (eg, diet modification, exercise, behavioral therapy, pharmacotherapy) have limited effectiveness, which has driven the development of surgical interventions (2). Roux-en-Y gastric bypass, sleeve gastrectomy, and gastric banding are effective at initiating and maintaining weight loss but pose risks of morbidity and mortality (3). These surgical procedures were initially thought to induce weight loss by restricting stomach volume and/or by causing macronutrient malabsorption. However, surgically induced metabolic changes may play a more important role than mechanical restriction, as exemplified by changes in hormonal profiles after bariatric surgery (4–6). The lack of hormonal changes after noninvasive therapies may explain why these interventions fail to induce large and sustained weight loss (5,7–9). Thus, there is a need for minimally invasive techniques that influence appetite-mediating hormones in a safer, less invasive, and more cost-effective manner than surgical options.
Transarterial embolization of the gastric fundus (ie, bariatric embolization) is an image-guided procedure to treat obesity, which has yielded promising initial results (10–12). It aims to induce metabolic changes similar to those induced by bariatric surgery by targeting the endocrine functions of the gastric fundus, which play a role in stimulating appetite. Bariatric embolization delivers embolic microspheres into the gastric arteries to induce localized ischemia (13–16) and has modified appetite hormones in several animal models, leading to reductions in weight (13–16). Early clinical trials have produced promising short-term results (11,17–19). We hypothesized that bariatric embolization would be well tolerated and would lead to sustained weight loss; the Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) Trial was designed to assess this, as well as whether weight loss for up to 12 months could be maintained.
Materials and Methods
Our study was performed under a physician-initiated investigational device exemption from the U.S. Food and Drug Administration and was approved by the institutional review boards at The Johns Hopkins Hospital (Baltimore, Md) and Mount Sinai Hospital (New York, NY). All participants provided written informed consent. Data were protected in a manner compliant with the Health Insurance Portability and Accountability Act of 1996. We received financial support from Merit Medical and Siemens Healthcare and material support from Merit Medical and SureFire Medical. The funding organizations were not involved with the study design or in the collection, analysis, or interpretation of data. The authors had full control of the data, its analysis, and all information submitted for publication.
Study Design and Setting
The BEAT Obesity Trial (ClinicalTrials.gov identifier NCT 0216512) was a prospective, open-label, single-arm, two-center study to evaluate the safety and efficacy of bariatric embolization to treat severe obesity. Participants were recruited from June 2, 2014, to February 16, 2018. Study protocol details, including the study calendar and eligibility criteria, are provided in Appendix E1 (online) and a previous publication (19). Critical components and variations from the previous methods are described here.
Participants were evaluated at baseline (before undergoing embolization) and at 1 week, 2 weeks, 1 month, 3 months, 6 months, and 12 months after embolization (Table E1 [online]). Nuclear medicine gastric motility testing was performed at the 1- and 6-month visits for the first five participants. For the remaining 15 participants, a gastric motility test was performed at the 1-month visit. A subsequent gastric motility test was performed at the 6-month follow-up visit only if results of the 1-month test were abnormal.
Participants
Inclusion and exclusion criteria are listed in Table 1. Each participant was evaluated by a multidisciplinary team (C.R.W., L.J.C., A.M.F., and O.A., with 11, 30, 8, and 2 years of experience, respectively) who conducted a comprehensive history and physical examination and screening tests as previously described (19). Participants at Johns Hopkins had intake assessments at the institution’s weight management center, consisting of history and physical examination focusing on factors and health conditions associated with obesity, psychologic and dietary assessments, and physiologic evaluation, including measurement of resting metabolic rate. Participants at Mount Sinai were assessed by a registered dietician with 6 years of experience.
Table 1:
Characteristics of 20 Participants in the Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) Trial
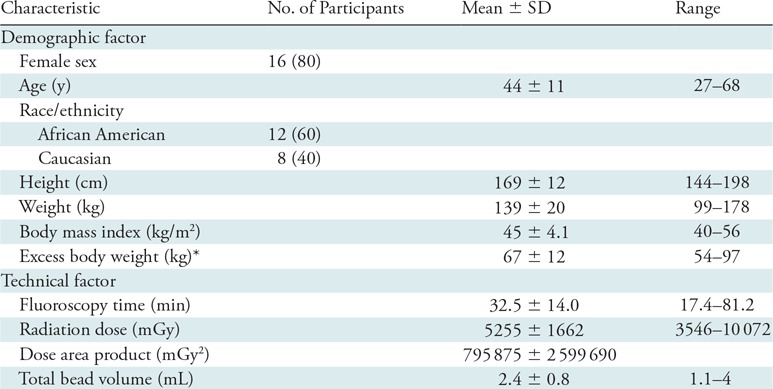
Note.—Data in parentheses are percentages. SD = standard deviation.
*Calculated as total weight–ideal body weight per Devine formula (20).
Twenty participants were included in this pilot trial. Five of these participants’ safety and efficacy data were reported previously (19). Of the 20 participants, 15 were treated at Johns Hopkins and five at Mount Sinai. The first five participants were encouraged, but not required, to attend pre-embolization weight management counseling. The subsequent 15 participants were required to attend four weight management sessions (Johns Hopkins) or dietician visits (Mount Sinai) during the 5-week period before embolization. All participants were encouraged to attend further visits with their weight management teams during the study. Participants at Johns Hopkins were compensated financially for their time, in adherence with institutional review board regulations.
Study End Points
Study end points were those reported previously (19). The primary safety end point was 30-day adverse events as defined by the American Society for Metabolic and Bariatric Surgery. The primary efficacy end point was weight loss (percentage change from baseline) during the study period. Secondary end points were technical feasibility (the ability to embolize the gastric fundus), mucosal changes seen during 1- and 3-month endoscopy, gastric-emptying studies, 3-day hunger assessments, quality-of-life scores, and metabolic panel laboratory changes (19,20). We measured health-related quality of life by using the Short Form-36 Health Survey (SF-36) and the impact of weight on quality of life by using the IWQOL-Lite (Quality of Life Consulting, Durham, NC).
Embolization Procedure
Transarterial embolization of the gastric fundus was performed with fluoroscopic guidance using 300- to 500-µm calibrated embolic microspheres. The embolization procedures and follow-up evaluations were conducted in the same fashion as described previously (19). All procedures were performed by experienced interventional radiologists (C.R.W., 15 procedures; B.P.H., 14 procedures; A.M.F., five procedures; K.H., one procedure; with 10, 5, 8, and 15 years of experience, respectively). Access to the celiac artery was obtained through a femoral or radial artery approach, which was chosen according to physician preference. When femoral access was performed, an SOS Omni selective catheter (Angiodynamics, Latham, NY) or Simmons catheter (Maestro; Merit Medical, Jordan, Utah) was used. When radial access was performed, a 5-F Ultimate Radial catheter (Merit Medical) or 5-F Jacky (Terumo Medical, Tokyo, Japan) was used. Celiac angiography was performed at the beginning of the procedure to assess participants’ vascular anatomy. A 2.9-F high-flow coaxial microcatheter (Maestro; Merit Medical) was used to perform selective angiography to assess distal blood supply to the left gastric artery (LGA), hepatic artery, splenic artery, gastroduodenal artery, and gastroepiploic artery (GEA) (21). Embolization of one or more fundal arteries (LGA and GEA, if deemed appropriate by consensus of the operating physicians) was performed using 300- to 500-µm Embosphere microspheres (Merit Medical) (Fig 1). Embolization was taken to stasis, which was defined as the visual absence of the flow of contrast after five heartbeats. Arterial phase cone-beam CT was performed at the beginning of each procedure to determine fundal perfusion and to confirm fundal blood supply and at the end of the procedure to confirm appropriate distribution of embolization and microspheres.
Figure 1a:
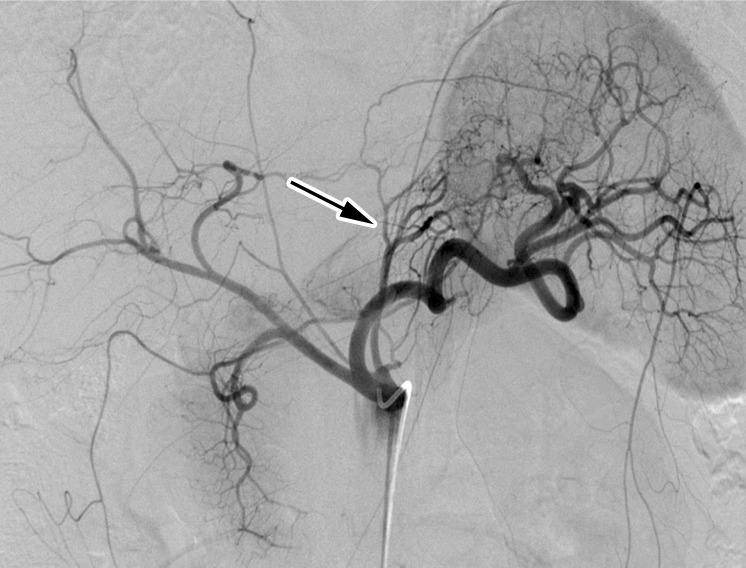
Bariatric embolization in a 41-year-old Caucasian woman with a baseline weight of 115 kg and a baseline body mass index (BMI) of 48. At 12 months after embolization, she had weight loss of 16 kg (30% excess weight loss) and a BMI of 34. (a) Pre-embolization celiac angiogram shows classic left gastric artery (LGA) (arrow) anatomy, with the LGA arising from the proximal celiac artery and left gastroepiploic artery (GEA) branching from the gastroduodenal artery. (b) Postembolization angiogram shows successful embolization of the gastric fundus via the LGA and left GEA.
Figure 1b:
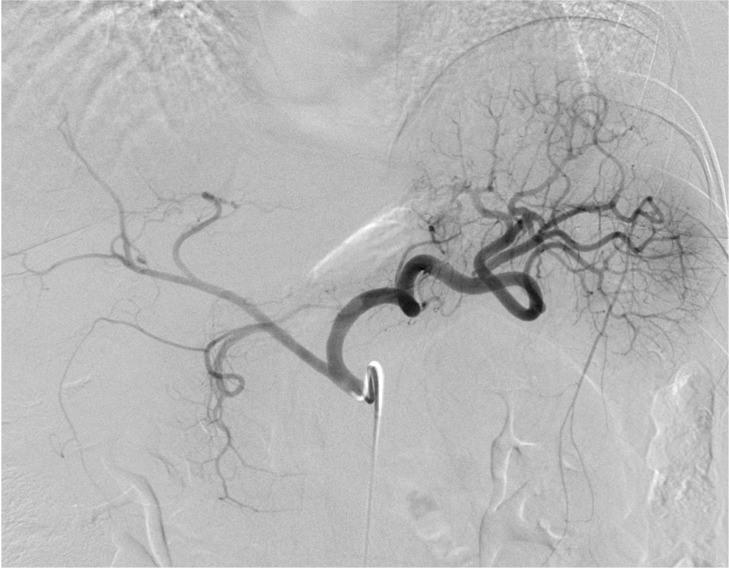
Bariatric embolization in a 41-year-old Caucasian woman with a baseline weight of 115 kg and a baseline body mass index (BMI) of 48. At 12 months after embolization, she had weight loss of 16 kg (30% excess weight loss) and a BMI of 34. (a) Pre-embolization celiac angiogram shows classic left gastric artery (LGA) (arrow) anatomy, with the LGA arising from the proximal celiac artery and left gastroepiploic artery (GEA) branching from the gastroduodenal artery. (b) Postembolization angiogram shows successful embolization of the gastric fundus via the LGA and left GEA.
Statistical Analysis
Data on weight, hematologic status, electrolyte levels, lipid levels, quality of life, and hunger were collected by trained clinical research staff and recorded on case report forms before being entered into an Excel spreadsheet (Microsoft Corp., Redmond, Wash; commercially available) for analysis. Demographic variables were described by using means and data ranges or standard deviations if the variable was normally distributed. Normality of the data was assessed by using histograms and tested with the Shapiro-Wilk test (22). Ninety-five percent confidence intervals (CIs) were calculated using the appropriate t distribution. Bootstrap methods were used to estimate 95% CIs for outcome measures that were non-normally distributed (23).
We used locally weighted scatterplot smoothing (LOWESS) curves to investigate the trajectory of excess weight loss over time. Although we collected multiple measurements over time per patient, forming a longitudinal data sample, our primary objective was to determine changes from pre- to post-intervention in our sample. Thus, a summary measure, weight change, was determined for each patient as weight at a given post-treatment time point minus weight at baseline, expressed as a percentage of excess weight lost (or gained). This reduction in repeated data is often used to simplify the interpretation of the treatment effect (24).
Because this was an exploratory study, we considered several summary measures of weight change from baseline at multiple post-treatment time points, rather than prespecifying a single post-treatment time point as the primary outcome. We considered the pretreatment-to-post-treatment change in excess weight loss and thus did not control for any baseline covariates. We used the one-sample t test with the null hypothesis that the pretreatment-to-post-treatment change would be zero. We confirmed the robustness of the t test with the nonparametric Wilcoxon signed-rank test for non-normal data. As a secondary analysis, we performed longitudinal analysis of the trajectory of weight loss over time by using linear and nonlinear (eg, cubic spline) terms. Similar pretreatment-to-post-treatment analyses were used for blood chemistry and quality-of-life measurements. Because this was an exploratory pilot study, we made no adjustments in P values for multiple comparisons. Plots of trends over time were created using Stata, version 14.2, software (StataCorp LP, College Station, Tex; commercially available). P values less than .05 were considered indicative of statistical significance.
Results
Participants
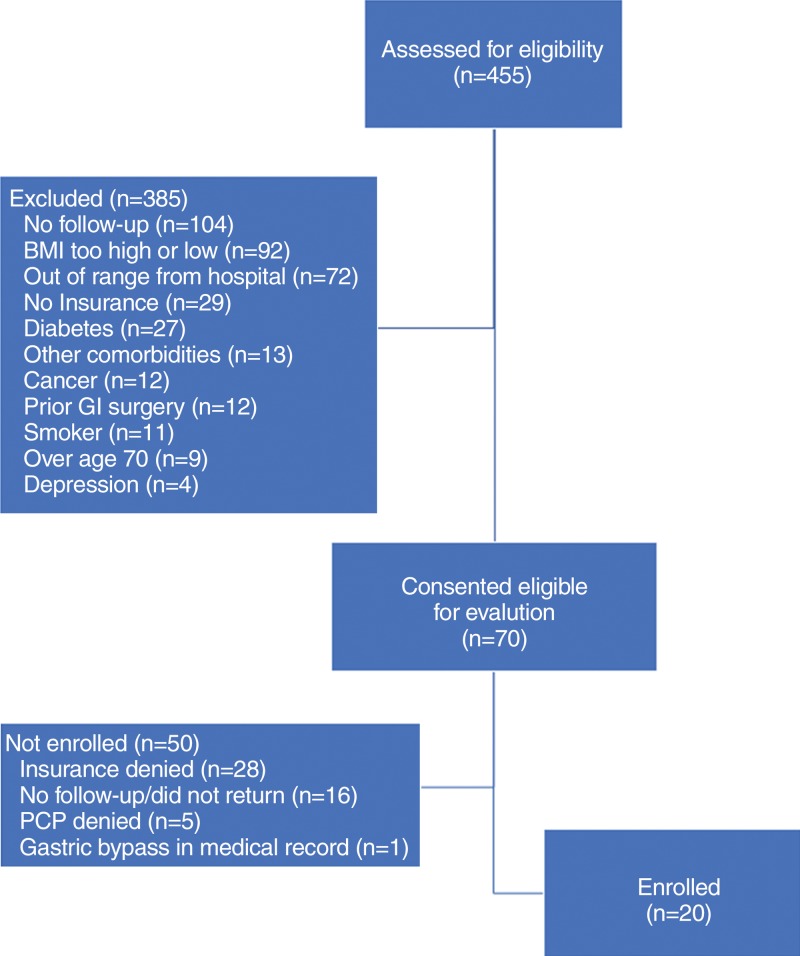
Twenty severely obese adults (16 women) were included (Fig 2). Mean (± standard deviation) participant age was 44 years ± 11, and mean body mass index value was 45 ± 4.1. Mean excess weight was 67 kg (range, 54–96 kg). Twelve participants identified as African American and eight identified as Caucasian (Table 1). As a result of loss to follow-up, 18 participants remained at 3 months, 16 at 6 months, and 15 at 12 months. Two additional participants were unable to attend the 6-month visit but attended the 12-month visit.
Figure 2:
Participant selection flowchart. BMI = body mass index, GI = gastrointestinal, PCP = primary care provider.
Adverse Events
Per study protocol, all participants were admitted to the hospital after embolization. Symptoms (eg, nausea, vomiting, epigastric pain) were treated supportively. All participants were discharged home 24–48 hours after admission, after resolution of any symptoms. There were no major adverse events. A total of 11 minor adverse events occurred in eight participants. One participant had subclinical pancreatitis, evident by transient elevation of lipase levels during the hospital stay. The participant was treated with supportive care and discharged within 48 hours in good condition. The participant was asymptomatic at 1-week follow-up and remained so.
Weight Loss
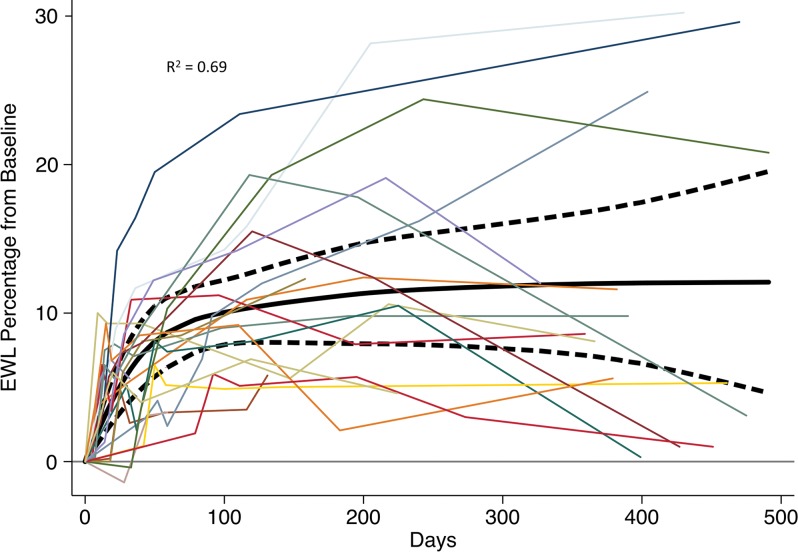
Participants experienced mean excess weight loss of 8.2% (95% CI: 6.3%, 10%) at 1 month (P < .001), 11.5% (95% CI: 8.7%, 14%) at 3 months (P < .001), 12.8% (95% CI: 8.3%, 17%) at 6 months (P < .001), and 11.5% (95% CI: 6.8%, 16%) at 12 months (P < .001) (Fig 3). Mean change in total weight was −7.6 kg (95% CI: −11 kg, −3.8 kg) at 12 months (P < .001). Analysis of the 10 participants at Johns Hopkins who underwent a more regimented weight management plan than the first five participants (19) had excess weight loss of 9.6% (95% CI: 6.3%, 13%) at 1 month (P < .001), 13.4% (95% CI: 8.8%, 18%) at 3 months (P < .001), 13.9% (95% CI: 5.6%, 22%) at 6 months (P = .006), and 12.1% (95% CI: 4.9%, 19%) at 12 months (P =. 008). The first five participants visited the weight management clinic a mean of 3.4 ± 2.7 times after embolization, despite encouragement to visit weekly. In contrast, each of the last 10 participants attended the weight management clinic at least 16 times.
Figure 3:
Excess weight loss (EWL) for the 20 participants who underwent bariatric embolization, plotted as percentage difference from baseline over time. Solid black line represents a fitted cubic spline model. Dotted lines represent 95% confidence interval.
Technical Feasibility
Bariatric embolization was performed successfully in all participants. The LGA and GEA were embolized in 16 participants; only the LGA was embolized in the other four participants (Table 1, Fig 1). The decision to embolize the GEA was made by the investigators based on its contribution to fundal blood supply. In one participant, an antireflux device (Surefire Medical, Westminster, Colo) was deployed to prevent nontarget embolization of the right gastric artery. Radial artery access was used in six participants, and femoral artery access in 14.
Gastric Changes
One participant demonstrated mild gastritis in the gastric body, or antrum, at the 3-month endoscopy. Another participant had delayed gastric emptying at 1 month; a repeat gastric motility test at 6-month follow-up was normal. Superficial, asymptomatic ulcers were found in eight participants at the 2-week endoscopy in locations consistent with fundal embolization (Fig 4). Although reported as a minor adverse event, some ulceration was expected on the basis of previous data (13,14,16,25). All ulcers healed by 3 months.
Figure 4a:
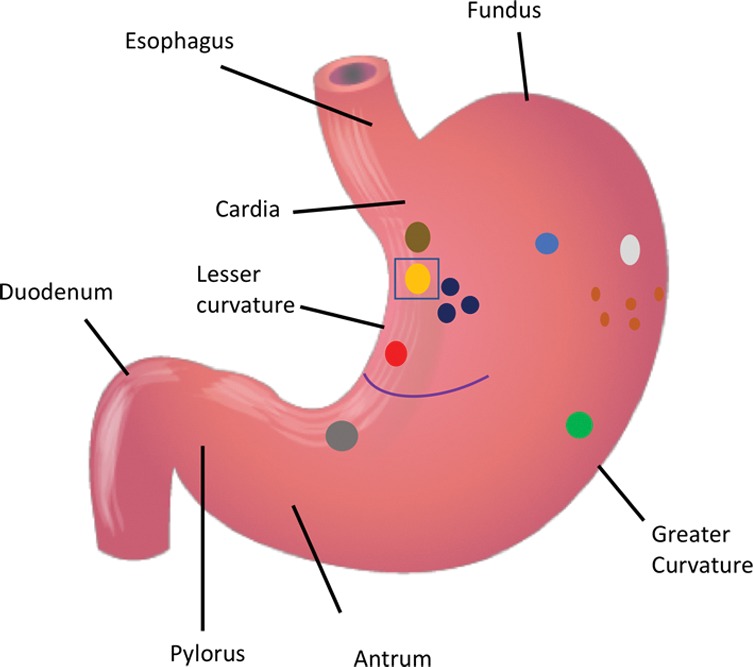
(a) Distribution of various gastric ulcerations observed during endoscopy 1 week after bariatric embolization. Relative sizes and shapes of ulcers are indicated by sizes and shapes of colored dots on diagram. Each color represents ulcers found on one participant (eg, the three black dots represent ulcers on one participant).The curved purple line represents a linear ulcer. The ulcer represented as a yellow oval and enclosed by a blue square corresponds to that shown in b–d. (b–d) Endoscopic images of the same location in one participant (48-year-old African American woman with a baseline weight of 127 kg): (b) at baseline, (c) at 2 weeks after embolization (arrow indicates a small, superficial gastric ulcer, measuring 1 cm on the longest axis), and (d) at 3 months after embolization (arrow indicates prior location of the ulcer).
Figure 4b:
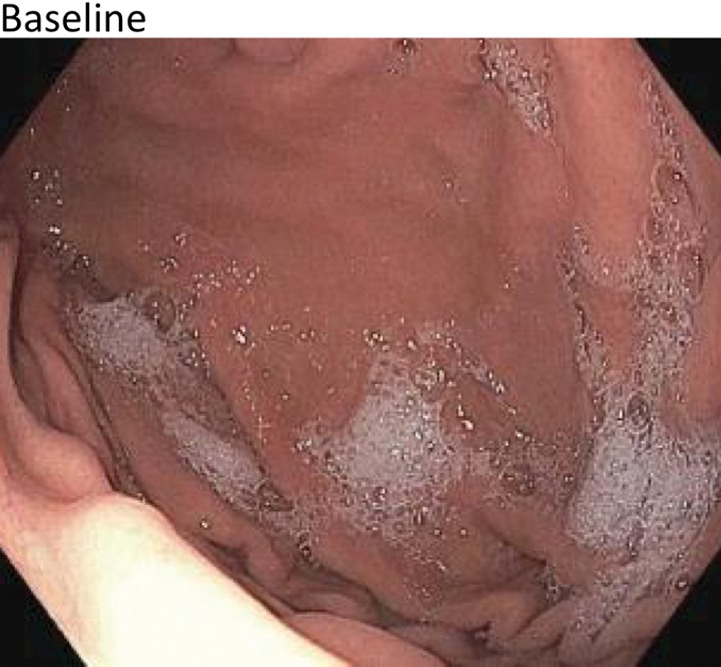
(a) Distribution of various gastric ulcerations observed during endoscopy 1 week after bariatric embolization. Relative sizes and shapes of ulcers are indicated by sizes and shapes of colored dots on diagram. Each color represents ulcers found on one participant (eg, the three black dots represent ulcers on one participant).The curved purple line represents a linear ulcer. The ulcer represented as a yellow oval and enclosed by a blue square corresponds to that shown in b–d. (b–d) Endoscopic images of the same location in one participant (48-year-old African American woman with a baseline weight of 127 kg): (b) at baseline, (c) at 2 weeks after embolization (arrow indicates a small, superficial gastric ulcer, measuring 1 cm on the longest axis), and (d) at 3 months after embolization (arrow indicates prior location of the ulcer).
Figure 4c:
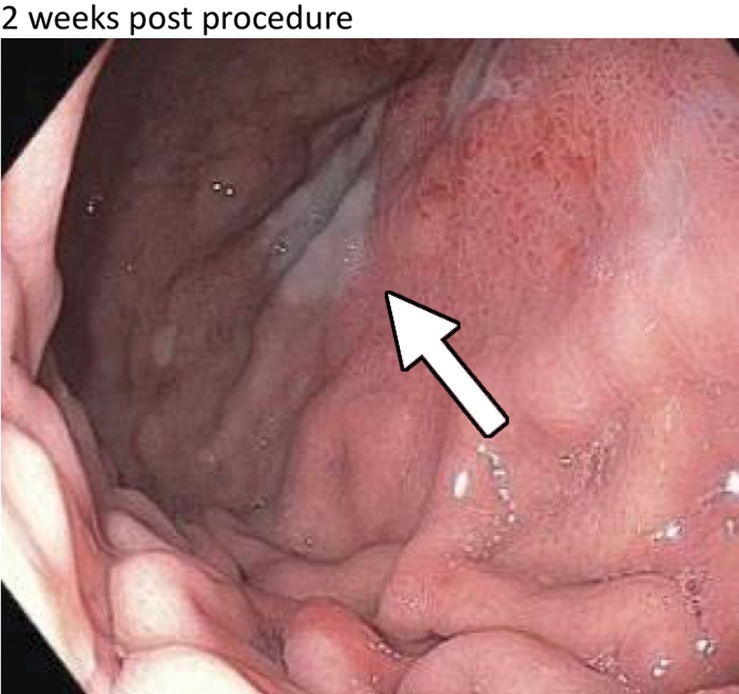
(a) Distribution of various gastric ulcerations observed during endoscopy 1 week after bariatric embolization. Relative sizes and shapes of ulcers are indicated by sizes and shapes of colored dots on diagram. Each color represents ulcers found on one participant (eg, the three black dots represent ulcers on one participant).The curved purple line represents a linear ulcer. The ulcer represented as a yellow oval and enclosed by a blue square corresponds to that shown in b–d. (b–d) Endoscopic images of the same location in one participant (48-year-old African American woman with a baseline weight of 127 kg): (b) at baseline, (c) at 2 weeks after embolization (arrow indicates a small, superficial gastric ulcer, measuring 1 cm on the longest axis), and (d) at 3 months after embolization (arrow indicates prior location of the ulcer).
Figure 4d:
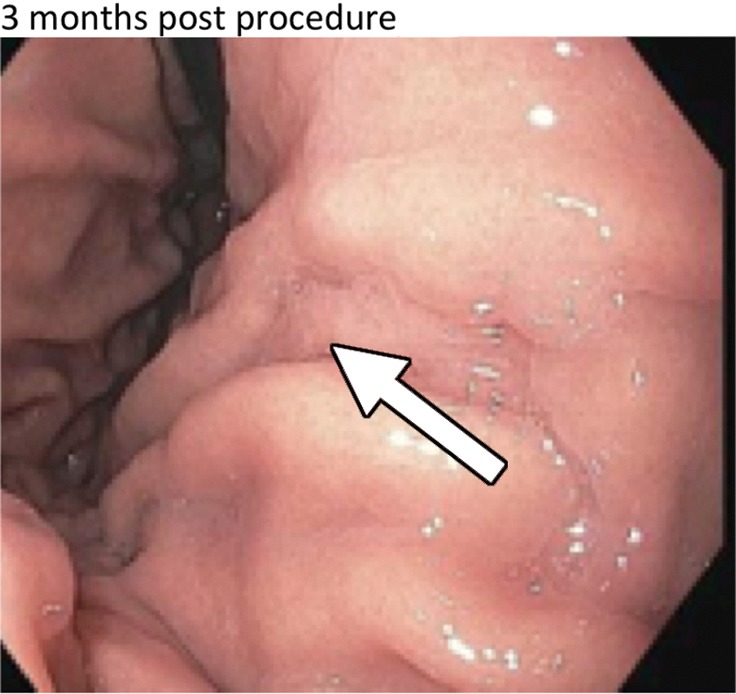
(a) Distribution of various gastric ulcerations observed during endoscopy 1 week after bariatric embolization. Relative sizes and shapes of ulcers are indicated by sizes and shapes of colored dots on diagram. Each color represents ulcers found on one participant (eg, the three black dots represent ulcers on one participant).The curved purple line represents a linear ulcer. The ulcer represented as a yellow oval and enclosed by a blue square corresponds to that shown in b–d. (b–d) Endoscopic images of the same location in one participant (48-year-old African American woman with a baseline weight of 127 kg): (b) at baseline, (c) at 2 weeks after embolization (arrow indicates a small, superficial gastric ulcer, measuring 1 cm on the longest axis), and (d) at 3 months after embolization (arrow indicates prior location of the ulcer).
Hunger Assessments
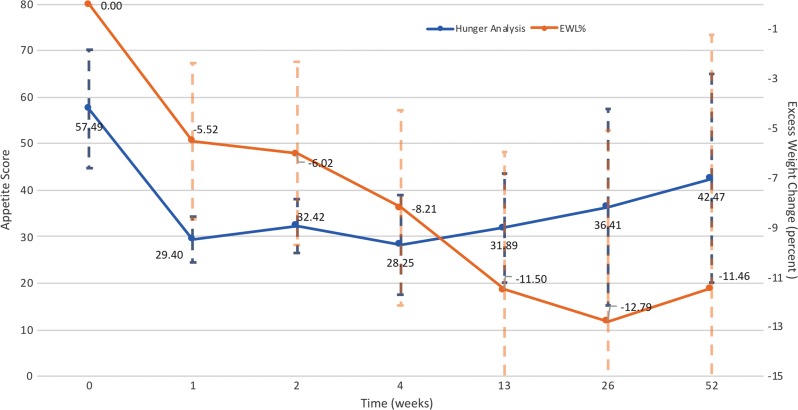
Mean hunger scores, representing hunger experienced during the 3 days after clinical assessment (Appendix E1 [online]) (19), decreased by a mean of 33 points (95% CI: −43, −23) 1 month after embolization from a baseline of 65 points (95% CI: 61, 68). Mean 3-day hunger scores continued to decrease from baseline at 3 months (mean change, −24; 95% CI: −32, −16 [bootstrap CI]) and 6 months (mean change, −40; 95% CI: −46, −35). A lower mean hunger score compared with baseline was observed at 12 months; however, only three participants had 3-day hunger scores at 12 months, so we were unable to determine significance. Hunger scores decreased as weight loss increased (Fig 5).
Figure 5:
Hunger and excess weight loss (EWL) over time. Hunger scores were generated on the basis of subjective questions regarding appetite during the previous 3 days—before eating breakfast, before eating lunch, during midafternoon, and after dinner. Hunger scores are compared with EWL at each time point. In general, decreases in hunger scores were correlated with an increase in EWL and vice versa.
Quality-of-Life Assessments
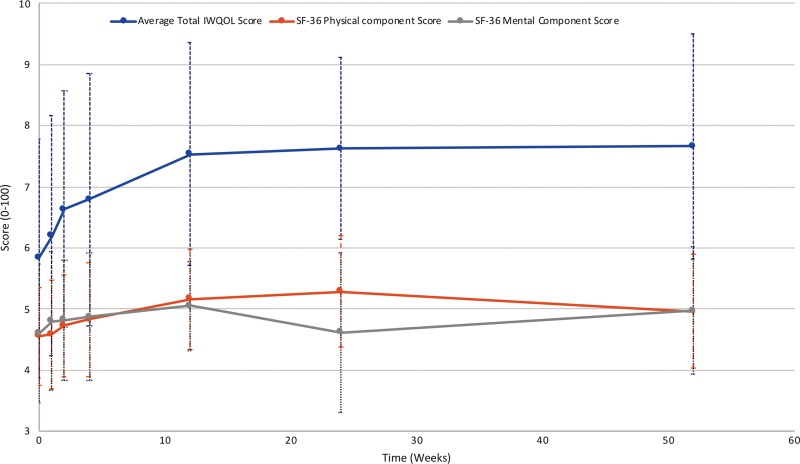
Participants had a baseline mean SF-36 physical component summary score of 46 ± 8.0 (n = 20) (national standardized value = 50) (Fig 6). This score increased to a peak of 53 ± 9.1 (n = 14) at 6 months (P = .01) and then decreased to 50 ± 9.3 (n = 16) at 12 months, which was not different than baseline (P = .15). The SF-36 mental component summary score followed a similar trajectory (Fig 6). The mean baseline score was 46 ± 11 (n = 20), which increased to a peak of 51 ± 7.3 (n = 15) at 3 months (P = .13). The score remained higher than baseline for the remaining follow-up period (46 ± 13 at 6 months and 50 ± 10 at 12 months; P = .81 and P = .44, respectively).
Figure 6:
Quality-of-life (QOL) measures. The Short Form-36 (SF-36) health questionnaire is scored from 1%–100%, with a national average of 50%. Impact of Weight on Quality of Life (IWQOL) is reported as points out of 100.
Mean IWQOL-Lite scores also increased gradually (Fig 6). The most rapid increase occurred early, with peak scores at 3 months for three domains (physical function, self-esteem, and public distress). In all domains, mean scores at 3 months had shown statistically significant increases from baseline. Scores remained stable after 3 months. At 12 months, mean physical function scores increased from 55 ± 18 to 70 ± 21 (P = .007); self-esteem scores increased from 50 ± 30 to 72 ± 25 (P = .011); sexual life scores increased from 61 ± 35 to 88 ± 25 (P = .003); public distress scores increased from 68 ± 19 to 79 ± 19 (P = .003); and work scores increased from 73 ± 17 to 88 ± 13 (P = .007). The mean total IWQOL-Lite score had increased by 19 ± 16 points at 12 months (P < .001).
Laboratory Values
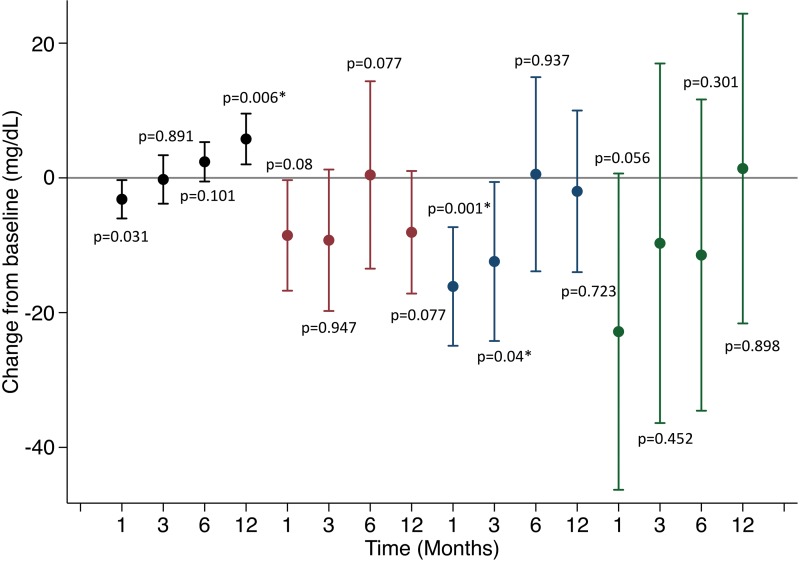
Total cholesterol decreased from baseline to 3 months (P = .04; Fig 7a). By 12 months, mean total cholesterol and low-density lipoprotein (LDL) levels were lower than their respective means at baseline (P = .08). Mean triglycerides initially decreased and then increased back to baseline levels (P = .06, 0.45, 0.30, and 0.90 at 1, 3, 6, and 12 months, respectively). Percentage changes in weight correlated negatively with changes in LDL at 12 months (r2 = 0.24). High-density lipoprotein (HDL) decreased at 1 month after embolization but increased at all subsequent time points (P = .03, .89, .10, and .006, respectively). When averaging the within-participant paired changes in HDL from baseline to 12-month follow-up, the mean change was 5.8 mg/dL (95% CI: 2.0 mg/dL, 9.5 mg/dL).
Figure 7a:
Changes in laboratory values over time. * indicates statistical significance. (a) Mean changes in lipids over time. High-density lipoprotein (black), low-density lipoprotein (red), total cholesterol (blue), triglycerides (green). (b) Changes in hemoglobin A1c (HbA1c) over time.
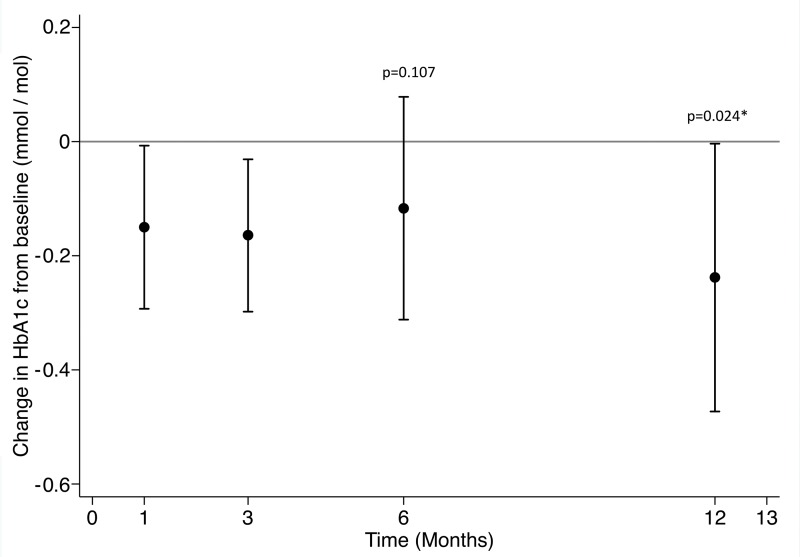
Mean blood glucose changes decreased at 12 months (change from baseline, −8.5 mg/dL; 95% CI: −19 mg/dL, 2.4 mg/dL; P = .11) (Fig 7b). Hemoglobin A1c decreased from a baseline of 5.9% ± 0.4% by 0.13 percentage points at 3 months and remained at similar levels at 6 months (5.8% ± 0.4%) and 12 months (5.7% ± 0.5%). Hemoglobin A1c at 12 months was lower than at baseline (P = .047). Change in hemoglobin A1c at 12 months did not correlate with weight change (r2 = 0.24).
Figure 7b:
Changes in laboratory values over time. * indicates statistical significance. (a) Mean changes in lipids over time. High-density lipoprotein (black), low-density lipoprotein (red), total cholesterol (blue), triglycerides (green). (b) Changes in hemoglobin A1c (HbA1c) over time.
Discussion
Transarterial embolization of the gastric fundus (ie, bariatric embolization) has yielded promising initial results for weight loss. The treatment goal is to target the endocrine functions of the gastric fundus to suppress appetite. In the current study, bariatric embolization using 300- to 500-µm microspheres was feasible, performed without major adverse events, and effective in reducing weight at 12 months in 20 adults with severe obesity, producing mean excess weight loss of 11% and mean total weight loss of 7.6 kg. Of the eight participants who experienced minor adverse events, most had superficial mucosal ulceration that was expected and resolved by 3 months.
In our study, peak weight loss occurred by 6 months. At 12 months, only three participants had returned to their baseline weight, and no participants were heavier than their baseline weight. Appetite was suppressed after embolization, with maximal suppression occurring at 1 month. Although appetite then increased steadily, it remained 26% lower at 12 months compared with baseline. Furthermore, participants reported improvements in quality of life after embolization. IWQOL scores for physical function, self-esteem, sexual life, and public distress improved at 3 months, and SF-36 scores peaked at 6 months. Clinical changes after bariatric embolization reflected improved cardiovascular health and prevention of diabetes. Total cholesterol, LDL, and triglycerides decreased over time, while mean HDL increased at 12 months. Hemoglobin A1c decreased significantly, although this decrease was not as clinically relevant as that in a patient with diabetes reported by Syed et al (18).
Of note, 5% weight loss is defined by the Food and Drug Administration as clinically relevant and is the benchmark by which low-risk drugs and devices are judged (26). Our weight loss results were consistent with those of previous studies. Kipshidze et al (11) reported five severely obese patients with mean weight reductions of 16% at 6 months and 17% at 24 months after embolization with 300- to 500-µm embolics (Biocompatibles UK, Surrey, England). Syed et al (18) reported four severely obese patients who had mean excess weight loss of 17% and total body weight loss of 8.5% at 6 months after embolization with 300- to 500-µm embolics. Bai et al (17) reported five obese patients who had mean weight loss of 8.3, 10, and 13 kg at 3, 6, and 9 months, respectively, after embolization with 500- to 700-µm polyvinyl alcohol particles. The decrease in the rate of weight loss in our study likely reflected a lessening of procedural efficacy at 6 months, consistent with previous findings (17), and was possibly caused by revascularization leading to reestablishment of normal hormone levels. It may be possible to repeat the embolization procedure to prolong the effect, but safety and efficacy of this are unknown.
Weight loss of 5%–10% has been found to reduce risks of cardiovascular disease and diabetes, including reduced hemoglobin A1c, increased HDL, reduced triglycerides, reduced blood pressure, and reduced need for diabetes and antihypertensive medications (27). The changes in hemoglobin A1c in our study, and the fact that they occurred independent of weight loss, may indicate that bariatric embolization alters the metabolic profile in ways similar to bariatric surgery, but to a lesser degree.
Although bariatric embolization is unlikely to promote weight loss as effectively as bariatric surgery, with losses up to 19% by gastric banding (28) and 36% by Roux-en-Y gastric bypass (29), it is at least as effective as some pharmacotherapies (ie, liraglutide, orlistat, lorcaserin), which induce mean weight loss of 2%–9% (28). Endoscopic bariatric procedures report similar results, with endoscopically placed gastric balloons producing 34% excess weight loss and gastric plication producing 23%–53% excess weight loss in 2 years, although with a high recidivism rate (30). However, bariatric surgery is reserved for the most obese patients because of its associated risks, and less than 1% of eligible patients choose to undergo such surgery (31–33). An advantage of bariatric embolization is that patients can achieve weight loss similar to that with pharmacotherapy after undergoing one procedure in combination with lifestyle changes, without requiring long-term adherence to scheduled medication doses, which can be difficult for some patients (34,35). It is important to reiterate that bariatric embolization is not proposed as a replacement for bariatric surgery, but as a supplemental method to facilitate weight loss with lifestyle modification.
The small sample size with an uneven distribution of participants between centers and the lack of a control group are limitations of the current study. In addition, several participants did not have continuous follow-up throughout the study or neglected certain questionnaires at several time points, resulting in insufficient data for analysis of some end points. Lifestyle and weight management counseling varied between study sites. Although participants were encouraged to adhere to a diet and exercise plan, compliance and regularity of visits varied, and weight management compliance before embolization was only required after the first five participants. This variability reflects real-world issues with weight loss programs, but it presents a challenge to establish the efficacy of bariatric embolization for weight management. Finally, African American women were over-represented in our study. Racial disparities in mean weight loss have been reported in clinical trials, with African American and Hispanic patients tending to lose less weight than Caucasian patients (20,36–38).
In conclusion, bariatric embolization is well tolerated and promotes clinically relevant weight loss in adults with severe obesity. It may provide needed assistance to patients who are struggling to succeed in lifestyle modification–based weight loss programs.
APPENDIX
SUPPLEMENTAL FIGURES
This was a physician-sponsored investigational device exemption study. The funding organizations were not involved with the study design or in the collection, analysis, and interpretation of data. Supported by National Institute of Biomedical Imaging and Bioengineering (R01EB017615 [C.R.W., D.L.K., A.A.] and T32EB006351 [J,V., O.A., F.N., K.P.]). C.R.W. and D.L.K. supported by Siemens Healthineers (Erlangen, Germany). C.R.W. and A.M.F. supported by Merit Medical (South Jordan, UT) (material and financial support). C.R.W. and A.M.F. supported by TriSalus Life Sciences (Westminster, CO) (material support).
Disclosures of Conflicts of Interest: C.R.W. Activities related to the present article: disclosed institutional grant from Merit Medical and Siemens Healthcare, and materials support from SureFire Medical. Activities not related to the present article: disclosed institutional grant and payment received for consultancy from BTG and Medtronic. Other relationships: disclosed no relevant relationships. G.O.A. disclosed no relevant relationships. A.M.F. Activities related to the present article: disclosed institutional grant from Merit Medical. Activities not related to the present article: disclosed receipt of payment from Embolx for board membership, from Terumo Medical for consultancy, and from Boston Scientific for lectures, including service on speakers’ bureaus; and royalties from Merit Medical. Other relationships: disclosed no relevant relationships. L.J.C. disclosed no relevant relationships. J.V. disclosed no relevant relationships. B.P.H. disclosed no relevant relationships. O.A. disclosed no relevant relationships. F.N. disclosed no relevant relationships. K.P. disclosed no relevant relationships. S.B. disclosed no relevant relationships. K.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed receipt of payment from Boston Scientific (as medical advisor) and BTG for consultancy, and grants received from BTG and Merit Medical. Other relationships: disclosed no relevant relationships. R.S.P. disclosed no relevant relationships. E.J.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed payment received from Boston Scientific, Medtronic, and C2 Therapeutics for consultancy. Other relationships: disclosed no relevant relationships. K.E.S. disclosed no relevant relationships. T.H.M. disclosed no relevant relationships. R.E.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed payment received from Neurotrope for consultancy. Other relationships: disclosed no relevant relationships. T.D. disclosed no relevant relationships. H.Z. disclosed no relevant relationships. D.L.K. Activities related to the present article: disclosed institutional grants from Siemens Healthcare and Merit Medical and payment from Merit Medical and SureFire for supplies. Activities not related to the present article: disclosed institutional grant from BTG. Other relationships: disclosed being a coinventor on a patent for an embolic held by The Johns Hopkins University. A.A. Activities related to the present article: disclosed consulting fee or honorarium from Trisalus Life Sciences and support received from Trisalus Medical for travel to meetings for the study or other purposes; founder of SureFire Medical (now TriSalus Life Sciences). Activities not related to the present article: disclosed payment received from Trisalus Life Sciences, BTG, and ABK Medical for consultancy. Other relationships: disclosed institutional grant from WL Gore, receipt of payment from Trisalus Life Sciences for patent and for travel/accommodations/meeting expenses, and stock options from Trisalus Life Sciences.
Abbreviations:
- BEAT Obesity
- Bariatric Embolization of Arteries for the Treatment of Obesity
- CI
- confidence interval
- GEA
- gastroepiploic artery
- HDL
- high-density lipoprotein
- IWQOL
- impact of weight on quality of life
- LDL
- low-density lipoprotein
- LGA
- left gastric artery
- SF-36
- Short Form-36 Health Survey
References
- 1.Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions—but do we have the will? Fertil Steril 2017;107(4):833–839. [DOI] [PubMed] [Google Scholar]
- 2.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology 2007;132(6):2253–2271. [DOI] [PubMed] [Google Scholar]
- 3.O’Rourke RW, Andrus J, Diggs BS, Scholz M, McConnell DB, Deveney CW. Perioperative morbidity associated with bariatric surgery: an academic center experience. Arch Surg 2006;141(3):262–268. [DOI] [PubMed] [Google Scholar]
- 4.Beckman LM, Beckman TR, Sibley SD, et al. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr 2011;35(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandarana K, Batterham RL. Shedding pounds after going under the knife: metabolic insights from cutting the gut. Nat Med 2012;18(5):668–669. [DOI] [PubMed] [Google Scholar]
- 6.Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab 2010;21(6):337–344. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002;346(21):1623–1630. [DOI] [PubMed] [Google Scholar]
- 8.Larder R, O’Rahilly S. Shedding pounds after going under the knife: guts over glory—why diets fail. Nat Med 2012;18(5):666–667. [DOI] [PubMed] [Google Scholar]
- 9.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365(17):1597–1604. [DOI] [PubMed] [Google Scholar]
- 10.Gunn AJ, Oklu R. A preliminary observation of weight loss following left gastric artery embolization in humans. J Obes 2014;2014:185349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kipshidze N, Archvadze A, Bertog S, Leon MB, Sievert H. Endovascular bariatrics: first in humans study of gastric artery embolization for weight loss. JACC Cardiovasc Interv 2015;8(12):1641–1644. [DOI] [PubMed] [Google Scholar]
- 12.Weiss CR, Gunn AJ, Kim CY, Paxton BE, Kraitchman DL, Arepally A. Bariatric embolization of the gastric arteries for the treatment of obesity. J Vasc Interv Radiol 2015;26(5):613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arepally A, Barnett BP, Montgomery E, Patel TH. Catheter-directed gastric artery chemical embolization for modulation of systemic ghrelin levels in a porcine model: initial experience. Radiology 2007;244(1):138–143. [DOI] [PubMed] [Google Scholar]
- 14.Arepally A, Barnett BP, Patel TH, et al. Catheter-directed gastric artery chemical embolization suppresses systemic ghrelin levels in porcine model. Radiology 2008;249(1):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bawudun D, Xing Y, Liu WY, et al. Ghrelin suppression and fat loss after left gastric artery embolization in canine model. Cardiovasc Intervent Radiol 2012;35(6):1460–1466. [DOI] [PubMed] [Google Scholar]
- 16.Paxton BE, Kim CY, Alley CL, et al. Bariatric embolization for suppression of the hunger hormone ghrelin in a porcine model. Radiology 2013;266(2):471–479. [DOI] [PubMed] [Google Scholar]
- 17.Bai ZB, Qin YL, Deng G, Zhao GF, Zhong BY, Teng GJ. Bariatric embolization of the left gastric arteries for the treatment of obesity: 9-month data in 5 patients. Obes Surg 2018;28(4):907–915. [DOI] [PubMed] [Google Scholar]
- 18.Syed MI, Morar K, Shaikh A, et al. Gastric artery embolization trial for the lessening of appetite nonsurgically (GET LEAN): six-month preliminary data. J Vasc Interv Radiol 2016;27(10):1502–1508. [DOI] [PubMed] [Google Scholar]
- 19.Weiss CR, Akinwande O, Paudel K, et al. Clinical safety of bariatric arterial embolization: preliminary results of the BEAT obesity trial. Radiology 2017;283(2):598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm 1974;8(11):650-655. [Google Scholar]
- 21.Biederman DM, Marinelli B, O’Connor PJ, et al. Transradial access for visceral endovascular interventions in morbidly obese patients: safety and feasibility. J Vasc Access 2016;17(3):256–260. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika 1965;52(3-4):591–611. [Google Scholar]
- 23.Efron B, Tibshirani R. Boostrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. 1986;1(1):54-75. [Google Scholar]
- 24.O’Connell NS, Dai L, Jiang Y, et al. Methods for analysis of pre-post data in clinical research: a comparison of five common methods. J Biom Biostat 2017;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxton BE, Alley CL, Crow JH, et al. Histopathologic and immunohistochemical sequelae of bariatric embolization in a porcine model. J Vasc Interv Radiol 2014;25(3):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration . Guidance for industry developing products for weight management. https://wayback.archive-it.org/7993/20170404224223/https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071612.pdf. Accessed September 7; 2018.
- 27.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(25 Suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu Dayyeh BK, Edmundowicz S, Thompson CC. Clinical practice update: expert review on endoscopic bariatric therapies. Gastroenterology 2017;152(4):716–729. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292(14):1724–1737. [DOI] [PubMed] [Google Scholar]
- 30.Verdam FJ, Schouten R, Greve JW, Koek GH, Bouvy ND. An update on less invasive and endoscopic techniques mimicking the effect of bariatric surgery. J Obes 2012;2012:597871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afonso BB, Rosenthal R, Li KM, Zapatier J, Szomstein S. Perceived barriers to bariatric surgery among morbidly obese patients. Surg Obes Relat Dis 2010;6(1):16–21. [DOI] [PubMed] [Google Scholar]
- 32.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg 2013;23(4):427–436. [DOI] [PubMed] [Google Scholar]
- 33.Funk LM, Jolles S, Fischer LE, Voils CI. Patient and referring practitioner characteristics associated with the likelihood of undergoing bariatric surgery: a systematic review. JAMA Surg 2015;150(10):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao Q, Ouwens MJ, Grandy S, Johnsson K, Kostev K. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes 2016;9:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemo B, Endevelt R, Porath A, Stampfer MJ, Shai I. Adherence to weight loss medications; post-marketing study from HMO pharmacy data of one million individuals. Diabetes Res Clin Pract 2011;94(2):269–275. [DOI] [PubMed] [Google Scholar]
- 36.DeLany JP, Jakicic JM, Lowery JB, Hames KC, Kelley DE, Goodpaster BH. African American women exhibit similar adherence to intervention but lose less weight due to lower energy requirements. Int J Obes 2014;38(9):1147–1152. [DOI] [PubMed] [Google Scholar]
- 37.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med 2008;35(2):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16(6):1413–1420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.