Abstract
Background/Aim. Accumulating evidence suggests that discoidin domain receptor tyrosine kinase 1 (DDR1) has an oncogenic role. Therefore, the aim of this study was to evaluate the potential utility of DDR1 and its post-transcriptional repressors, miR-199a-5p and miR-199b-5p, as prognostic factors in clear cell renal cell carcinoma (ccRCC). Patients and Methods. The expression of DDR1 in tumor and normal renal tissues of 56 patients with ccRCC was assessed by reverse transcription quantitative polymerase chain reaction, western blotting and immunohistochemistry. Renal cancer cells were transfected with specific RNA sequences to validate DDR1 as a putative miR-199a/b-5p target. Results. Decreased DDR1 mRNA and protein, as well as miR-199a/b-5p levels were found in ccRCC. Low DDR1 protein was associated with higher nuclear grade and shorter overall survival. DDR1 immunoreactivity was elevated in the nuclei and unchanged in the membrane/cytoplasmic compartment of tumor cells. DDR1 levels correlated with those of miR-199a/b-5p. In addition, we validated DDR1 as a target gene for miR-199a/b-5p in renal cancer cell lines. Conclusion. DDR1 expression is altered in ccRCC, but our findings do not support its oncogenic role. In-depth investigation will be necessary to elucidate the exact role and potential utility of miR-199a/b-5p in ccRCC.
Keywords: DDR1, miR-199a-5p, miR-199b-5p, clear cell renal cell carcinoma
Renal cell carcinoma (RCC) is considered one of the most lethal urological malignancies representing the sixth and tenth most frequently diagnosed cancers in men and women, respectively (1). The most prevalent histological subtype of RCC, clear cell renal cell carcinoma (ccRCC) comprises 70-80% of renal tumors (2). It is believed that clear cell tumors originate in the proximal convoluted tubule (PCT) (3,4). The majority of ccRCC sporadic cases are characteristically associated with the loss of function of von Hippel-Lindau (VHL) tumor-suppressor gene by somatic mutations, chromosomal loss or promoter hypermethylation (5). Loss or inactivation of VHL results in a sequence of events comprising accumulation of hypoxia inducible factors (HIFs), overexpression of HIF-driven genes such as vascular endothelial growth factor (VEGF), promotion of angiogenesis, cell proliferation and tumor growth (5). Surgical resection is the mainstay of treatment of localized renal tumors, although a significant number of these patients will eventually develop recurrent or metastatic disease (2). Better understanding of ccRCC molecular background allowed for introduction of new therapeutics that target VEGF and mammalian target of rapamycin (mTOR) pathways and can be applied in the adjuvant pharmacotherapy of advanced or metastatic tumors. However, the benefits of ccRCC adjuvant therapies are still far below expectations and complete and durable responses are rare (4). At present, RCC recurrence risk stratification models are primarily based on the American Joint Committee on Cancer (AJCC) pathological tumor-node-metastasis (TNM) classification and histopathological features (2,4,6). To date, despite many efforts, there is no consensus on the routine use of molecular biomarkers to improve the surveillance protocols for follow-up of ccRCC patients after nephrectomy and predict of pharmacotherapy responsiveness (2,4,6).
The discoidin domain receptor 1 (DDR1) is a unique collagen receptor that belongs to the tyrosine receptor family (7-9). DDR1 exists as homodimers at the plasma membrane which act as a collagen sensors that bind to native triple helix form of collagen types I to IV and VIII (10,11). DDR1 expression is predominant in epithelial cells and this receptor is a key molecule that maintains cell-to-extracellular matrix (ECM) crosstalk. DDR1 influences a number of physiological processes and also plays a significant role in a number of pathological processes including tissue fibrosis and neoplastic diseases (11). DDR1 is often deregulated in many human neoplasms including the cancers of lung, breast, liver, pancreas and ovary as well as leukemias (12,13). The involvement of DDR1 in the processes of tissue remodeling and its central role in the development of fibrotic lesions strongly suggest that abnormal expression of this receptor may affect, besides intracellular pathways, interactions of cancer cells with the ECM components (11). Therefore, in addition to the control of adhesion, proliferation and survival of cancer cells, DDR1 was proposed as a trigger of epithelial to mesenchymal transition (EMT) and its pro-invasive activity was proved in a variety of human cancer cell lines (12,13). However, the precise mechanisms by which DDR1 may contribute to oncogenesis have not been fully elucidated. The experimental data are often conflicting and the exact impact of DDR1 on carcinogenesis remains disputable (14,15).
So far, the prognostic significance of DDR1 in ccRCC was investigated in only one study performed on a Chinese population (16). It was reported that high levels of DDR1 immunoreactivity correlated with the progression of ccRCC and shorter overall survival (OS) of the patients (16). Interestingly, these findings (16) are discordant with the survival analysis available at the Human Protein Atlas website, demonstrating that high expression of DDR1 correlates with favorable prognosis in ccRCC (17). Therefore, the main purpose of the present study was to assess DDR1 expression in the paired, tumor and non-cancerous tissue samples of 56 patients with ccRCC by reverse transcription quantitative polymerase chain reaction (RT-qPCR), western blotting (WB) and immunohistochemistry (IHC), and evaluate the prognostic significance of DDR1. In addition, the mechanisms that could underlay altered DDR1 expression in ccRCC, comprising miR-199a-5p and miR-199b-5p, were investigated in ccRCC tumor tissue samples and renal cancer cell lines.
Patients and Methods
Ethical approval. This study was approved by the Bioethics Committee for Scientific Research at the University of Warmia and Mazury in Olsztyn, Poland (agreements no. 4/2010 and 44/2011). Written informed consent as specified in the Declaration of Helsinki (1964) was obtained from each patient.
Patients and the collection of samples. Specimens were obtained from postoperative material of 56 patients with histologically confirmed ccRCC (27 men and 29 women; mean age±SD, 63.9±11.0; range=27-83 years) who were operated on at the Department of Oncological Surgery, Warmia and Mazury Oncological Center in Olsztyn, Poland, between March 2010 and July 2014. None of patients included in the study had a second neoplastic disease or had previously undergone chemo- or radiotherapy. Clinical and demographic data were obtained at the time of enrollment. Data on the OS were collected and the median follow-up time was 40.6 months. During this time 22/56 (39.3%) patients died. Clinical staging was based on the AJCC criteria (18). The tumor and matched macroscopically unchanged renal tissue samples were obtained from surgically resected kidneys as previously described (19). Specimens for RT-qPCR or western blotting were immediately frozen in a liquid nitrogen and stored at –80˚C. Tissue fragments for routine histological evaluation and immunohistochemistry were fixed in 4% buffered formaldehyde, dehydrated in a series of ethanol solutions in ascending concentrations, cleared in xylene and processed into paraffin blocks. The tumor nuclear grading was characterized by pathologist according to the Fuhrman system (20).
DDR1 mRNA quantification. Total RNA was extracted and reverse transcribed as previously described (21,22). The levels of DDR1 transcripts in homogenates of paired tumor and renal tissue specimens were determined by RT-qPCR and normalized to peptidylprolylisomerase A (PPIA) and TATA box binding protein (TBP) mRNAs content. The reactions were carried out using TaqMan Fast Advanced Master Mix, and the respective TaqMan Gene Expression Assay (DDR1, #Hs01058430_m1; PPIA, #Hs99999904_m1; TBP, #Hs00427620_m1) in an ABI 7500/7500 Fast Real-Time PCR System (all: Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The thermocycling conditions were as follows: polymerase activation for 20 sec at 95˚C, followed by 40 cycles of denaturation for 3 sec at 95˚C and annealing/extension for 30 sec at 60˚C. All samples were amplified in duplicates. The ΔΔCq method (23) was used to determine the fold differences in DDR1 expression between the paired samples of ccRCC and unchanged renal tissue. Based on the median DDR1 transcript content in the tumor samples, patients were divided into two groups regarded as having ‘low’ or ‘high’ levels of DDR1 mRNA.
miR-199a-5p and miR-199b-5p quantitation. Total RNA was extracted and reverse transcribed as previously described (24). The levels of miR-199a-5p and miR-199b-5p in homogenates of paired tumor and normal renal tissue specimens were determined by RT-qPCR and normalized to small nucleolar RNA RNU48 content. The reactions were performed using TaqMan Universal PCR Master Mix and the respective TaqMan MicroRNA Assay (miR-199a-5p, #000498; miR-199b-5p, #000500; RNU48, #001006) in an ABI 7500/7500 Fast Real-Time PCR System (all: Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: polymerase activation for 10 min at 95˚C, followed by 40 cycles of denaturation for 15 sec at 95˚C and annealing/extension for 1 min at 60˚C. All samples were amplified in duplicates. The ΔΔCq method (23) was used to determine the fold differences in miRs expression between the paired samples of ccRCC and unchanged renal tissue. Based on the median miR-199a-5p or miR-199b-5p content in the tumor samples, the patients were divided into two groups regarded as having ‘low’ or ‘high’ levels of miR-199a-5p or miR-199b-5p.
DDR1 protein quantification. Protein extraction, SDS-PAGE and WB were carried out according to the modified method described previously (21). The levels of protein in homogenates of paired tumor and normal renal tissue specimens were determined using rabbit anti-human antibodies against DDR1 (diluted 1:500; #sc-532; Santa Cruz Biotechnology, Dallas, TX, USA) and actin beta (ACTB; diluted 1:100; #A2066; Sigma-Aldrich) as internal loading control. DDR1/ACTB optical density (OD) ratios were used to determine fold differences in expression between the paired samples of ccRCC and unchanged renal tissue. Based on the median DDR1 protein content in tumor specimens, patients were divided into two groups regarded as having ‘low’ or ‘high’ levels of DDR1 protein.
IHC and evaluation of immunoreactivity. DDR1 immunostaining of the tumor and non-cancerous kidney sections was carried out using the Autostainer Link48 (Dako Cytomation, Glostrup, Denmark) according to the previously described method (25). Rabbit antibody directed against human DDR1 was applied (1:300; #sc-532; Santa Cruz Biotechnology) whereas negative controls were obtained by omitting the primary antibody. The DDR1 immunostained sections were evaluated using Olympus BX53 light microscope (Olympus, Tokyo, Japan) by two independent pathologists in a blinded manner regarding the clinical data of the patients. In doubtful cases, re-evaluation was performed until a consensus was achieved. Immunoexpression of DDR1 was assessed in the cytoplasm of cancer cells and non-transformed, normal epithelial cells of the proximal convoluted tubules (PCTs). The membrane/cytoplasmic immunoreactivity of DDR1 was evaluated according to the immunoreactive score (IRS) of Remmele and Stegner (26). The IRS scale is based on the percentage of cells exhibiting positive reaction (0 points, absence of cells with positive reaction; 1 point, 1-10%; 2 points, 11-50%; 3 points, 51-80%; 4 points, >80% cells with positive reaction) and reaction intensity (0, no reaction; 1, low intensity reaction; 2, moderate intensity reaction; 3, intense reaction). The final score is the result of multiplication of both parameters and ranges from 0 to 12 points). The nuclear DDR1 immunoreactivity was evaluated semi-quantitatively based on the percentage of cells presenting positive expression of DDR1 and encoded as follows: 0 - absence of staining, 1 point when 1-10% cells stained, 2 points when 11-50%, 3 when 51-80%, and 4 points when 81-100%. Based on their DDR1 immunostaining in membrane/cytoplasm of ccRCC cells the patients were divided into two groups regarded as ‘negative’ (IRS=0) or ‘positive’ (IRS≥1). Similarly, depending on the absence or presence of DDR1 immunoexpression on the nuclei of ccRCC cells, patients were divided into two groups regarded as ‘negative’ (score=0) or ‘positive’ (score≥1), respectively.
Cell lines and cell culture. Human RCC cell lines Caki-1 (HTB-46), Caki-2 (HTB-47), ACHN (CRL-1611) and a control line, HK-2 (CRL-2190; immortalized cells established from normal epithelium of proximal tubule) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Caki-1 and Caki-2 cells were maintained in McCoy's 5A medium (Sigma-Aldrich; Merck, KGaA, Darmstadt, Germany), ACHN cells in Eagle's Minimum Essential Medium (ATCC) and HK-2 cells in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (Sigma-Aldrich; Merck, KGaA). All culture media were supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck, KGaA). Cell lines were incubated at 37˚C in 5% CO2.
DDR1 silencing by miR-199a-5p and miR-199b-5p. The transfections were carried out according to the manufacturer’s instructions using reverse transfection protocol adjusted for a 6-well plate culture. 2×105 cells were seeded into a well containing pre-activated mixture of 50 pmol mimic (miR-199a-5p, #MC10893; miR-199b-5p, #MC10553; Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA) or inhibitory (miR-199a-5p, #MH10893; miR-199b-5p, #MH10553; Ambion; Thermo Fisher Scientific, Inc) RNA diluted in Opti-MEM Reduced Serum Medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 7 μl of Lipofectamine RNAiMAX and incubated for 16 h prior to replacement with fresh medium. Nonsense mimic or inhibitor RNA sequences were used as negative controls (mirVana miRNa mimic Negative Control #1; mirVana miRNa inhibitor Negative Control #1; Ambion, Thermo Fisher Scientific, Inc.). Three independent transfections were carried out for every cell line. All transfections within a single experiment were performed in duplicates. The cells were harvested 72 h post-transfection and assayed for DDR1 expression levels using qPCR and WB as described above.
Statistical analyses.
The statistical analyses were carried out with Prism 6.04 (Graphpad, LA Jolla, CA, USA) and Statistica 13.1 (Statsoft, Tulsa, OK, USA). The DDR1 and miRs expression levels are expressed as mean±SEM. The differences in DDR1 or miR expression levels between the paired tumor and unchanged renal tissue specimens were examined by the Wilcoxon matched-pairs test. Fisher’s exact, Mann-Whitney U-test and Spearman’s rank correlation were used to assess correlations between the patients’ data and DDR1 or miR expression levels. Spearman’s correlation coefficient was used to determine the relationship between the DDR1 and miR-199a-5p or miR-199b-5p expression levels. Survival curves were plotted according to the Kaplan–Meier method while the significance of differences in OS between groups of patients was evaluated by the log-rank test. The uni- and multivariate survival associations were analyzed using the Cox proportional hazards regression model. The differences in DDR1 expression levels between the cells transfected with mimics or inhibitor RNA sequences and respective control incubations were examined by Analysis of Variance followed by the Least Significant Difference post hoc test. In all performed analyses results were considered statistically significant for p<0.05.
Results
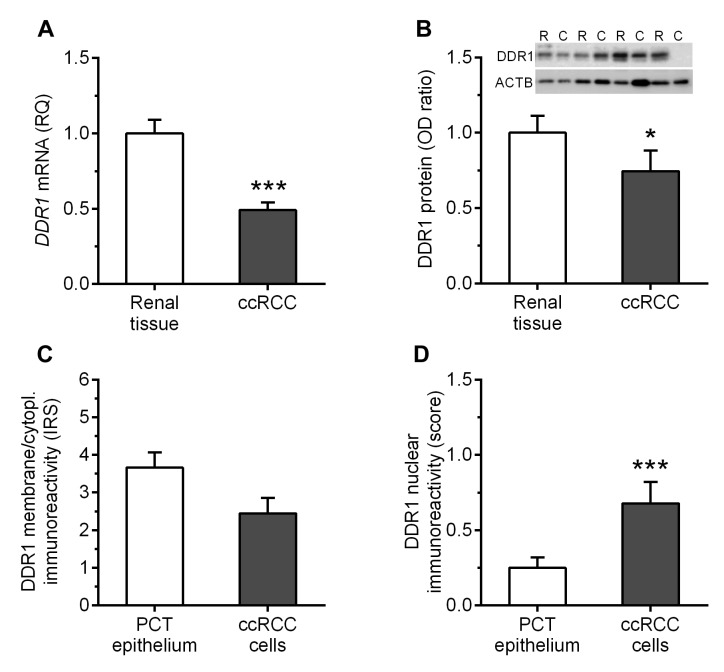
DDR1 expression is down-regulated in ccRCC homogenates. DDR1 transcripts were found in tumor and renal tissue samples of all patients included in the study. The tumor samples showed over 2-fold decrease in the expression levels of DDR1 mRNA as compared to the corresponding unchanged renal tissues (p<0.0001; Figure 1A). Western blotting analysis revealed that DDR1 protein was present in 84% (47/56) of ccRCC tumors and 95% (53/56) of normal kidney tissues samples. Consequently, the average DDR1 content in ccRCC specimens was reduced by nearly 1.4-fold as compared to the corresponding renal tissues (p=0.0113; Figure 1B).
Figure 1. Average levels of discoidin domain receptor tyrosine kinase 1 (DDR1) in the clear cell renal cell carcinoma (ccRCC) specimens and corresponding renal tissue samples were determined by (A) reverse transcription quantitative polymerase chain reaction, (B) western blot and (C, D) immunohistochemistry. *p<0.05; ***p<0.001; RQ: Relative quantification; OD: optical density; ACTB: actin beta; IRS: immunoreactivity score; PCT: proximal convolute tubule.
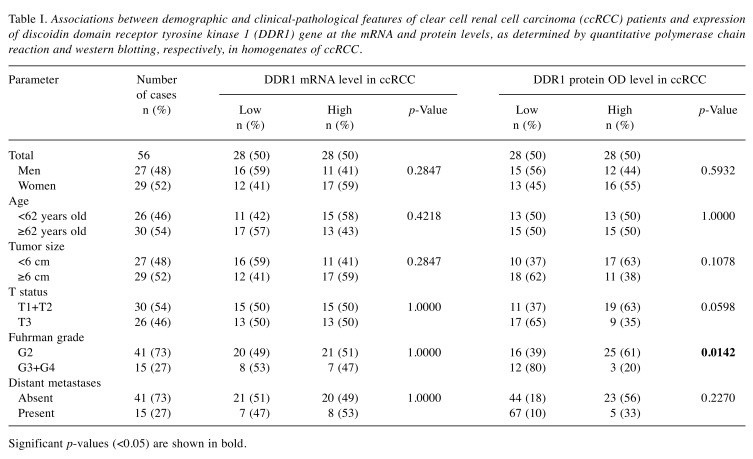
Decreased DDR1 protein levels in ccRCC are associated with higher nuclear grade. Low content of DDR1 protein in homogenates of ccRCC specimens correlated with higher Fuhrman nuclear grade (p=0.0142; Table I). Furthermore, the average DDR1 protein levels were over 2-fold lower in samples derived from G3/G4 tumors compared to G2 (p=0.0095).
Table I. Associations between demographic and clinical-pathological features of clear cell renal cell carcinoma (ccRCC) patients and expression of discoidin domain receptor tyrosine kinase 1 (DDR1) gene at the mRNA and protein levels, as determined by quantitative polymerase chain reaction and western blotting, respectively, in homogenates of ccRCC.
Significant p-values (<0.05) are shown in bold.
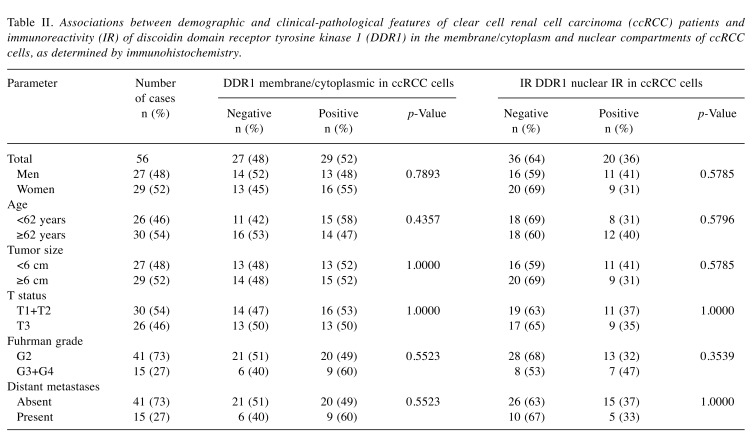
Altered membrane/cytoplasmic and nuclear DDR1 immunohistochemical reactions in ccRCC. DDR1 immuno-reactivity was observed in membrane/cytoplasmic and nuclear compartments of PCT epithelial cells (Figure 2A) and in the cancer cells of the analyzed specimens (Figure 2B). In addition to the PCT epithelial cells, IHC revealed the presence of DDR1 protein in cells of distal convoluted tubule and glomeruli in the sections of unchanged renal tissue. Among 56 tumor sections tested, the cancer cells of 29 (52%) cases exhibited membrane/cytoplasmic DDR1 immunoreactivity. The average levels of DDR1 immunoexpression in the membrane/ cytoplasm of cancer cells and PCT epithelial cells of corresponding renal tissues were similar and did not differ significantly (p=0.0712; Figure 1C). DDR1 immunoexpression in the nuclei of cancer cells was noted in 20/56 (36%) of ccRCC specimens. IHC revealed over 2.7-fold increase in nuclear immunoreactivity of DDR1 in cancer cells as compared to PCT epithelium of non-cancerous kidney sections (p=0.0044; Figure 1D). No correlations between DDR1 immunoreactivity and demographic or clinicopathological parameters of the patients were identified (Table II).
Figure 2. Evaluation of discoidin domain receptor tyrosine kinase 1 (DDR1) immunoexpression in the sections of (A) unchanged renal tissue and (B) tumor of representative patient with clear cell renal cell carcinoma was determined by immunohistochemistry. DDR1 protein was present in the membrane/cytoplasm of proximal convolute tubule epithelium and cancer cells. Most renal tissue specimens did not reveal nuclear immunoreactivity of DDR1 while approximately one third of tumor sections exhibited weak to moderate DDR1 immunoreactivity in the nuclei of cancer cells.
Table II. Associations between demographic and clinical-pathological features of clear cell renal cell carcinoma (ccRCC) patients and immunoreactivity (IR) of discoidin domain receptor tyrosine kinase 1 (DDR1) in the membrane/cytoplasm and nuclear compartments of ccRCC cells, as determined by immunohistochemistry.
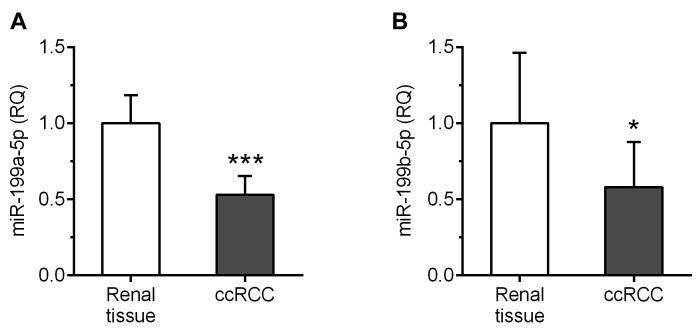
miR-199a-5p and miR-199b-5p are down-regulated in ccRCC homogenates. Both analyzed miRs were found in tumor and renal tissue samples of all patients included in the study. The average expression levels of miR-199a-5p and miR-199b-5p were nearly 2-fold reduced in ccRCC homogenates compared to corresponding renal tissues (p=0.0002 and p=0.0134; Figure 3A and B, respectively).
Figure 3. Average expression levels of miR-199a-5p and miR-199b-5p in the clear cell renal cell carcinoma (ccRCC) specimens and the corresponding renal tissue samples as determined by reverse transcription quantitative polymerase chain reaction. ***p<0.001; *p<0.05; RQ: Relative quantification.
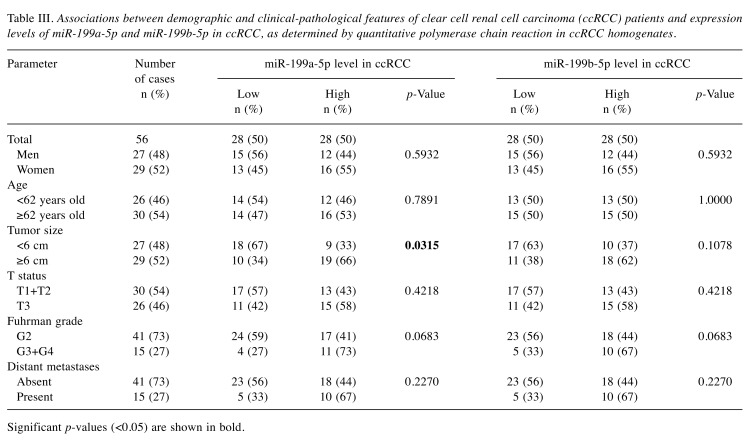
Expression levels of miR-199a-5p are associated with tumor growth. Higher content of miR-199a-5p correlated with bigger tumor size (p=0.0315; Table III). In addition, the average expression levels of miR-199a-5p were over 2-fold higher in tumors equal or bigger than 6 cm compared to those characterized by a smaller diameter (p=0.0249).
Table III. Associations between demographic and clinical-pathological features of clear cell renal cell carcinoma (ccRCC) patients and expression levels of miR-199a-5p and miR-199b-5p in ccRCC, as determined by quantitative polymerase chain reaction in ccRCC homogenates.
Significant p-values (<0.05) are shown in bold.
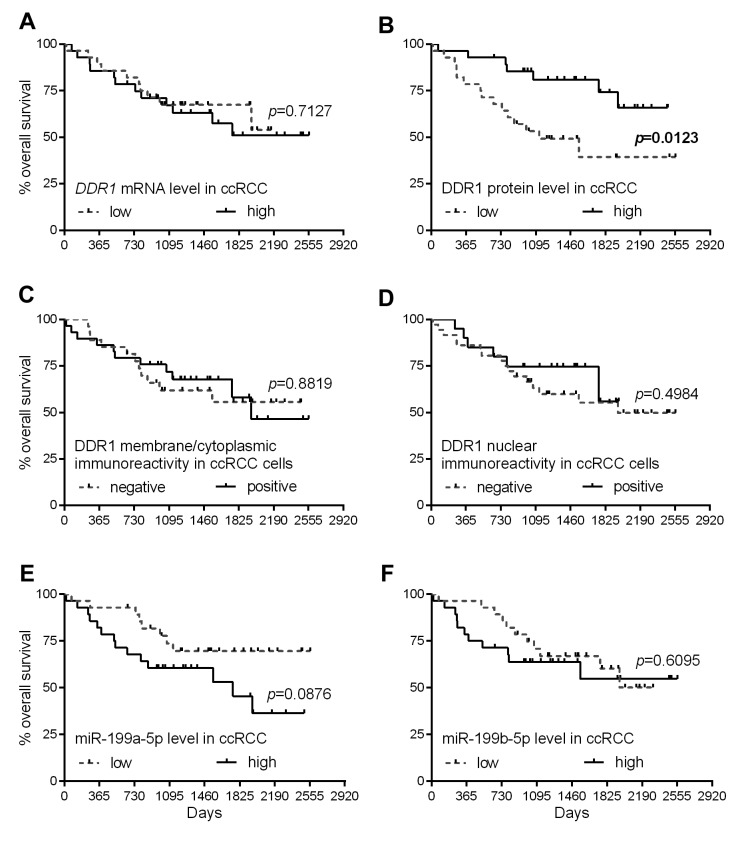
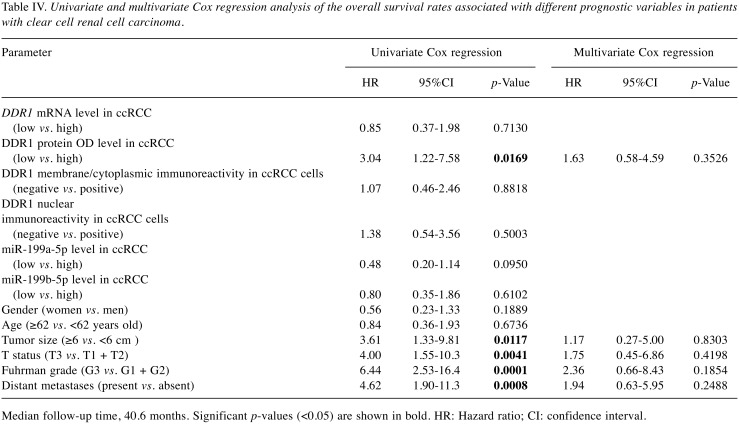
Low levels of DDR1 protein are associated with an unfavorable prognosis. Kaplan–Meier plots presenting the OS of patients with ccRCC classified in respect to the DDR1 expression levels are presented in Figure 4. Low levels of DDR1 protein expression were associated with shorter OS of the patients with ccRCC (median survival 37.7 months for low level of DDR1 protein group vs. ≥82.6 months for high levels of DDR1 protein group; Figure 4B). The expression of DDR1 transcript and DDR1 immunoreactivity did not correlate with OS of the patients (Figure 4A, C and D, respectively). No statistically significant relationships were disclosed between the expression of miR-199a-5p and miR-199b-5p and patients’ outcome (Figure 4E and F, respectively). Univariate Cox proportional hazards regression revealed that low levels of DDR1 protein, bigger tumor size, higher T-status of the primary tumor, higher Fuhrman nuclear grade and presence of distant metastases are significantly associated with OS of the patients (Table IV, left panel). However, the subsequent multivariate analysis revealed that none of the parameters achieved a status of an independent prognostic factor in the analyzed group of patients with ccRCC (Table IV, right panel).
Figure 4. Kaplan–Meier diagrams of overall survival of patients with clear cell renal cell carcinoma (ccRCC) regarding discoidin domain receptor tyrosine kinase 1 (DDR1) expression at the (A) mRNA and (B) protein OD levels, DDR1 immunoreactivity in (C) membrane/cytoplasm and (D) nuclear compartments, as well as (E) miR-199a-5p and (F) miR-199b-5p content in ccRCC tissue homogenates. p-Values for the corresponding log-rank test are shown.
Table IV. Univariate and multivariate Cox regression analysis of the overall survival rates associated with different prognostic variables in patients with clear cell renal cell carcinoma.
Median follow-up time, 40.6 months. Significant p-values (<0.05) are shown in bold. HR: Hazard ratio; CI: confidence interval.
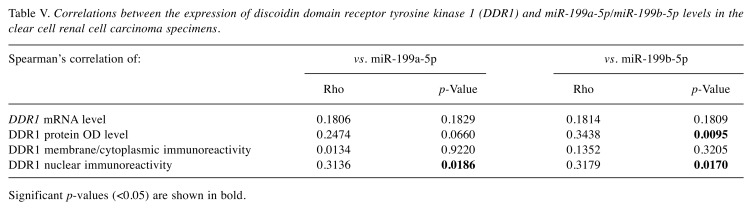
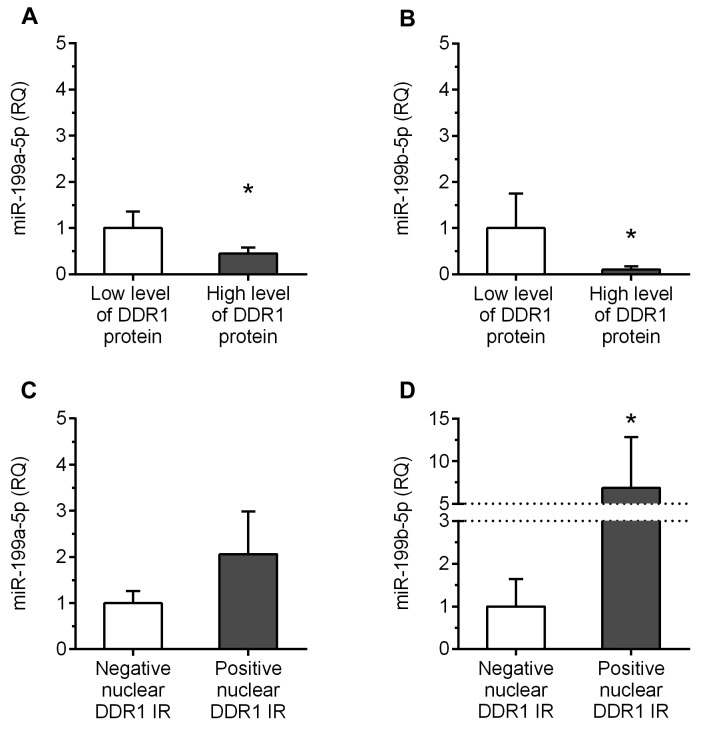
miR-199a-5p and miR-199b-5p correlate with expression of DDR1 at the protein level. Significant negative correlation was found between the levels of miR-199b-5p and DDR1 protein in ccRCC specimens (Rho=–0.3438, p=0.0095; Table V). The content of miR-199a-5p showed a tendency to be negatively associated with DDR protein levels (Rho=–0.2474, p=0.0660; Table V). In addition, the specimens with higher content of DDR1 protein (>median) were characterized by over 2 and 9-fold decrease in miR-199a-5p and miR-199b-5p expression levels, respectively, compared to tumors with lower DDR1 protein levels (p=0.0304 and p=0.0163; Figure 5A and B, respectively). In contrast, significant positive correlations were disclosed between the levels of miR-199a-5p and miR-199b-5p and DDR1 nuclear immunoreactivity score (Rho=0.3136, p=0.0186 and Rho=0.3179, p=0.0170, respectively; Table V). Furthermore, the average levels of miR-199b-5p in the specimens positive for nuclear DDR1 immunoexpression were approximately 7-fold increased compared to the tumors that did not reveal nuclear DDR1 immunoreactivity (p=0.0457; Figure 5D). Similarly, homogenates derived from nuclear-DDR1 positive tumors exhibited over 2-fold elevation in miR-199a-5p expression levels compared to tumors which lacked nuclear DDR1 immunoreactivity, however, this difference did not achieve statistical significance (p=0.0517; Figure 5C).
Table V. Correlations between the expression of discoidin domain receptor tyrosine kinase 1 (DDR1) and miR-199a-5p/miR-199b-5p levels in the clear cell renal cell carcinoma specimens.
Significant p-values (<0.05) are shown in bold.
Figure 5. Correlations between the expression levels of discoidin domain receptor tyrosine kinase 1 (DDR1) protein and miR-199a-5p/miR-199b- 5p in the clear cell renal cell carcinoma (ccRCC) specimens. Average expression levels of miR-199a-5p and miR-199b-5p in the tumors of patients with ccRCC in respect to (A and B) DDR1 protein OD and (C and D) nuclear DDR1 immunoreactivity. *p<0.05; RQ: Relative quantification; IR: immunoreactivity.
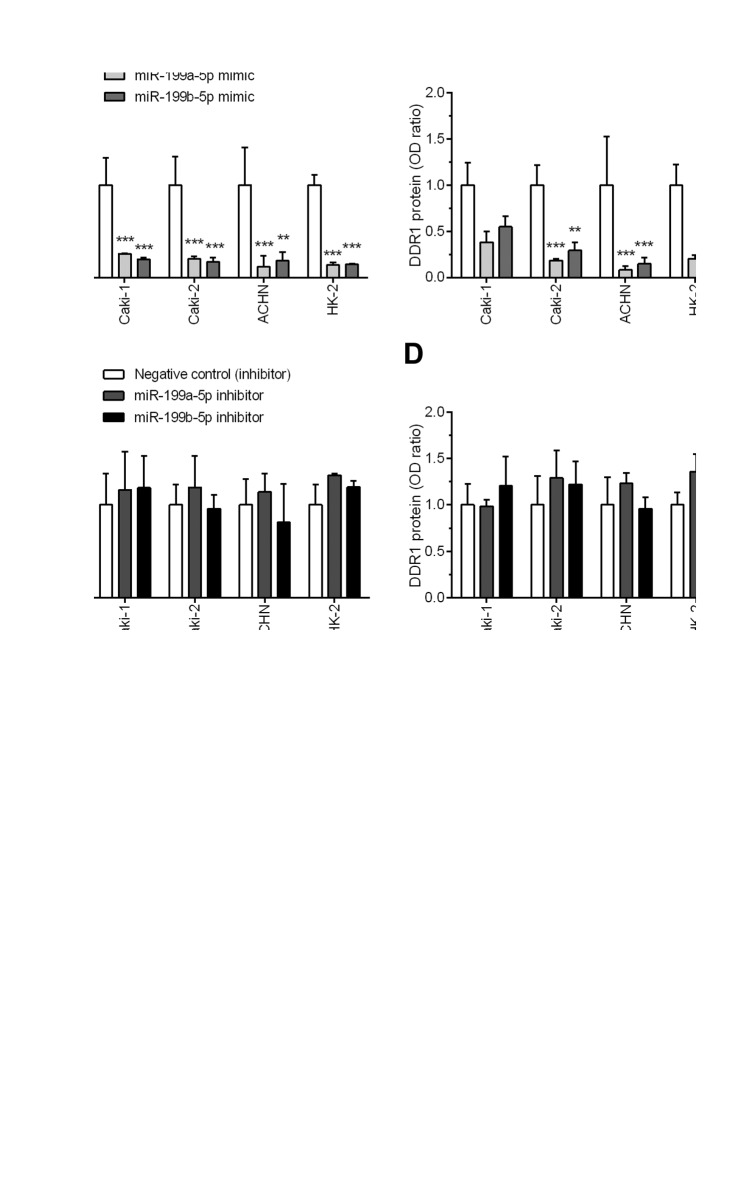
miR-199a-5p and miR-199b-5p regulate the expression of DDR1 in RCC cells. The expression of DDR1 transcript and protein was present in all analyzed cell cultures under control conditions, i.e. untreated or transfected by a nonsense RNA sequences (Figure 6E). Transfection with miR-199a-5p or miR-199b-5p mimics significantly reduced DDR1 mRNA in Caki-1 (p=0.0009 and p<0.0001, respectively), Caki-2 (p<0.0001), ACHN (p=0.0004 and p=0.0065, respectively) and HK-2 (p<0.0001) cell lines, ranging from 3.9 to 8.6-fold decrease compared to the cells transfected with nonsense mimic RNA sequences (Figure 6A). Furthermore, all the cell lines transfected with miR-199a-5p or miR-199b-5p exhibited reduced levels of DDR1 protein expression that ranged from 1.8 to 11.9-fold decrease as compared to negative controls, but this repression achieved a statistical significance in Caki-2 (p=0.0023 and p=0.0087, respectively) and ACHN (p=0.0022 and p=0.0039) cells only (Figure 6B and E). In contrast, transfection of the cell lines with miR-199a-5p or miR-199b-5p inhibitors had no significant influence on DDR1 expression levels (Figure 6C, D and E).
Figure 6. Effect of transfection with miR-199a-5p or miR-199b-5p mimics (A and B) or inhibitors (C and D) on discoidin domain receptor tyrosine kinase 1 (DDR1) expression in Caki-1, Caki-2, ACHN and HK-2 cells. DDR1 mRNA and protein levels were assessed by quantitative polymerase chain reaction and western blotting, respectively. (E) Representative western blots show DDR1 expression in cells transfected with miR-199a-5p or miR-199b-5p mimics, inhibitors and control RNA sequences as well as cells incubated in control conditions, in media with addition of Lipofectamine RNAiMAX only or without any additives (parental cell line). **p<0.01; ***p<0.001. ACTB: Actin beta.
Discussion
This study provides a novel insight into the significance of DDR1 as a putative prognostic factor in ccRCC. Reduced levels of DDR1 expression in the tumor tissues correlated with higher nuclear grade and shorter OS of the patients. Tumor cells exhibited altered DDR1 immunoreactivity reflected by the up-regulated nuclear expression of this protein. In addition, the putative post-transcriptional repressors of DDR1 expression, miR-199a-5p and miR-199b-5p, were down-regulated in the tumor specimens. To the best of our knowledge the present study is the first to demonstrate that miR-199a-5p and miR-199b-5p expression levels correlate with the content of DDR1 protein in tumor tissue samples collected from patients with ccRCC. Furthermore, these findings were validated in vitro by the transfection of three renal cancer cell lines and one PCT-derived epithelial cell line with respective mimic RNA sequences that resulted in significant DDR1 silencing.
In the present study, RT-qPCR, WB and IHC were used to investigate the expression of DDR1 gene in the tumor and normal renal tissue specimens collected from patients with ccRCC treated by nephrectomy. Tissue homogenates of ccRCC tumors exhibited consistent decrease in DDR1 expression at the mRNA and protein levels. Interestingly, IHC analysis revealed a differential pattern of DDR1 expression in cancer cells, disclosing the two distinct localizations of this protein within the membrane/cytoplasmatic and nuclear compartments. These findings are in accordance with the data available at The Human Protein Atlas website that indicate two major subcellular localizations for DDR1 protein expression in the nucleoplasm and cell membrane as it was shown in human MCF7 cells by immunofluorescence (27). The results of our study indicate that the levels of DDR1 protein could have a prognostic significance in ccRCC. Lower levels of DDR1 protein were associated with higher Fuhrman grade and worse patients’ outcome, although it did not achieve the status of independent prognostic factor in the analyzed cohort of patients. Our results are supported by the analysis of survival data of 528 patients with ccRCC provided by The Cancer Genome Atlas (TCGA) (28), which showed that low expression levels of DDR1 measured by RNA-seq is an unfavorable prognostic factor in this type of renal cancer (p<0.0001) (17). However, the latter and our observations are not in line with the results of an immunohistochemical study by Song et al. (16) who found that the immunoreactivity of DDR1 positively correlated with the progression of ccRCC and shorter OS of 119 patients with ccRCC. Although the same antibody directed against human DDR1 was used as in the study by Song and co-workers (16), similar correlations were not observed for membrane/cytoplasmic or nuclear DDR1 immunoreactivity. However, it has to be noted that Song et al. (16) neither distinguished the DDR1 immunoexpression in the cellular compartments nor quantified it, and, moreover, did not show representative microphotographs.
Conflicting results suggesting that DDR1 could either promote or suppress tumor progression, have been disclosed using other types of human cancers (15). An oncogenic role of DDR1 was proposed on the basis of a number of studies that investigated patients’ samples and/or cancer cell lines derived from the tumors that arose in the lung (29,30), breast (31,32), ovary (33), pancreas (34), liver (35), prostate (36), large intestine (37), stomach (38), liver (35), pancreas (34), and skin (39). Analogous observations were made in the case of glioma (40) and acute myeloid leukemia (41,42). In most of these studies DDR1 overexpressed and its levels correlated with the progression of disease and worse patients’ outcome. Nevertheless, several studies demonstrated that DDR1 can have also a putative tumor suppressing role. Low levels of DDR1 immunoreactivity were associated with worse patients’ outcome in non-small cell lung cancer (43) and triple-negative breast cancer (44). Furthermore, it has been shown that loss of DDR1 protein is associated with aggressive and metastatic potential of breast cancer cells (45). Various mechanisms of tumor suppressing activity of DDR1 were proposed, including the involvement of DDR1 in the up-regulation of antiapoptotic factors such as Bcl-2-interacting killer (46) and block of EMT by promotion of E-cadherin stability and E-cadherin-mediated adhesion (47) or decrease of actomyosin contractility at cell-to-cell junctions (48). Interestingly, while the epithelial ovarian cancer generally exhibited DDR1 overexpression, its silencing by CpG methylation was concomitant with the progression of EMT as observed in the tumor samples and cell lines (49). Altogether, the results of our and other studies emphasize the controversial nature of DDR1 in human malignancies and denote that its exact role may depend on many factors including stage of the disease and overall pathological or physiological context. Thus, the role of DDR1 in a given cancer type is of particular interest because this receptor is considered as a putative target of novel adjuvant therapies that are in preclinical development (14), however, lack of consensus regarding the role of DDR1 in many cancers does not support anticancer use of DDR1 inhibitors. Indeed, the analysis of DDR1 phosphorylation in two types of RCC showed that it may be phosphorylated in papillary, rather than in clear cell renal tumors (50). Therefore, DDR1 inhibitors could be less, if any, beneficial in the treatment of ccRCC than it was expected on the basis of recent in vitro studies (16).
In the present study, the expression of miR-199a-5p and miR-199b-5p was found, in line with the previous studies, to be reduced in ccRCC (51,52). Despite the overall down-regulation of miR-199a-5p in ccRCC, in our study, its higher (as compared to median value) expression levels correlated with bigger tumor size and tended to correlate with higher Fuhrman grade. Nonetheless, none of the analyzed miRs revealed to have a prognostic significance in our cohort of patients. In contrast, in the studies of Si et al. (51) and Tsukigi et al. (52) down-regulation of miR-199a-5p was associated with higher T status and unfavorable prognosis. However, these discrepancies may be the result of heterogeneity of RCC subtypes included in the cited studies and/or usage of RNU6 for the quantification of miR expression (51,52), since this small nuclear RNA is not recommended as miRs’ normalizer in RCCs (53).
Previous studies documented that DDR1 expression can be regulated at the transcription level by factors such as tumor protein p53 (TP53) and zinc finger E-box binding homeobox 1 (13) or promoter CpG methylation (49). DDR1 expression may be also controlled post-transcriptionally by the two highly similar miRs, miR-199a-5p and miR-199b-5p as found in hepatocellular carcinoma (35), acute myeloid leukemia (42), colorectal cancer (37), cutaneous squamous cell carcinoma (39) or triple-negative breast cancer (54). The expression levels of miR-199a-5p or miR-199b-5p were negatively correlated with DDR1 protein content in the ccRCC tissues, suggesting that those two miRs may repress DDR1 in ccRCC. To validate this hypothesis, RCC cell lines Caki-1, Caki-2, ACHN and PCT epithelium HK-2 cells were transfected with RNA sequences that mimicked or inhibited miR-199a-5p or miR-199b-5p. Transfection with 199a-5p and miR-199b-5p mimics resulted in a noticeable reduction in DDR1 expression at the transcript and protein levels. However, no significant effect of miRs inhibitors was observed. The latter could be attributed to the low levels of endogenous miR-199a-5p and miR-199b-5p expression in the renal cancer cell lines as it was reported previously by Tsukigi et al. (52).
The results of the present study disclose that DDR1 immunoreactivity can be found in two distinct subcellular localizations within the ccRCC cells. Nuclear immunoexpression of DDR1 was up-regulated in cancer cells and was positively correlated with the contents of miR-199a-5p and miR-199b-5p. These observations suggest that those miRs, besides their ability to repress overall DDR1 expression, could also have an impact on the trafficking of DDR1 protein and may promote DDR1 nuclear localization in ccRCC cells. However, the exact, direct or indirect, mechanisms underlaying this phenomenon remain unknown. To date, five DDR1 isoforms resulting from alternative splicing, deletions and frame-shift mutations have been described (55) and it has been proposed that the role of DDR1 in human cancer can be isoform-specific (13). Herein, we showed that miR-199a-5p and miR-199b-5p target DDR1 expression in renal cancer cells and the isoform-dependent character of this process cannot be excluded. A study carried out on mouse embryonic stem cells demonstrated that alternative transcript variants of oncologically relevant genes can be targeted by distinct miRs (56). In another study the tumor-associated miRs were shown to act by targeting the splicing machinery and as a result they potentiated the expression of cancer-specific isoforms of pyruvate kinase M1/2 glycolytic enzyme during the tumor development (57). Further studies are necessary to identify the mechanisms and relationships within the miR-199a/b-5p/DDR1 network and evaluate their prognostic and predictive relevance in ccRCC.
In summary, the first comprehensive investigation analyzing the expression of receptor tyrosine kinase DDR1 in a cohort of patients with ccRCC is presented. Evidence is provided indicating that DDR1 expression is altered in ccRCC and DDR1 protein levels correlate with clinicopathological parameters and OS of the patients. However, DDR1 could not serve as an independent prognostic factor in ccRCC. Our findings do not support an oncogenic role of DDR1 in this cancer type. In addition, we demonstrated that the expression levels of miR-199a-5p and miR-199b-5p are down-regulated in ccRCC. Since the altered DDR1 expression is correlated with the levels of miR-199a-5p and miR-199b-5p, the exact role of these miRs in ccRCC should be in-depth investigated.
Conflicts of Interest
The Authors declare that they have no competing interests regarding this study.
Authors’ Contributions
BEK, JK and ZK initiated and designed the study. JGo and PK collected clinical samples; JGo collected clinicopathological and survival data. JGr and PD performed immunohistochemistry and evaluated immunoreactivity scores. BEK, JK, ASJ and AEK performed cell cultures, qPCR and western blot assays. BEK performed the statistical analysis. The manuscript was drafted by BEK and JK and proof-read by ZK. JK and BEK were managing the project.
Acknowledgements
This study was supported by the National Science Centre (Poland) grant no. 2012/05/B/NZ4/01832. The Authors wish to thank Dr. Aleksandra Piotrowska from the Department of Human Morphology and Embryology, Division of Histology and Embryology, Wroclaw Medical University for technical support.
References
- 1.Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, Gore JL, Sun M, Wood C, Russo P. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. doi: 10.1016/j.eururo.2018.08.036. PMID: 30243799. DOI: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greef B, Eisen T. Medical treatment of renal cancer: New horizons. Br J Cancer. 2016;115(5):505–516. doi: 10.1038/bjc.2016.230. PMID: 27490806. DOI: 10.1038/bjc.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haake SM, Rathmell WK. Renal cancer subtypes: Should we be lumping or splitting for therapeutic decision making. Cancer. 2017;123(2):200–209. doi: 10.1002/cncr.30314. PMID: 27861752. DOI: 10.1002/cncr.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11(9):517–525. doi: 10.1038/nrurol.2014.194. PMID: 25112856. DOI: 10.1038/nrurol.2014.194. [DOI] [PubMed] [Google Scholar]
- 5.Keefe SM, Nathanson KL, Kimryn Rathmell W. The molecular biology of renal cell carcinoma. Semin Oncol. 2013;40(4):421–428. doi: 10.1053/j.seminoncol.2013.05.006. PMID: 23972705. DOI: 10.1053/j.seminoncol.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Shoji S, Nakano M, Sato H, Tang XY, Osamura YR, Terachi T, Uchida T, Takeya K. The current status of tailor-made medicine with molecular biomarkers for patients with clear cell renal cell carcinoma. Clin Exp Metastasis. 2014;31(1):111–134. doi: 10.1007/s10585-013-9612-7. PMID: 23959576. DOI: 10.1007/s10585-013-9612-7. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci USA. 1993;90(12):5677–5681. doi: 10.1073/pnas.90.12.5677. PMID: 8390675. DOI: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1(1):25–34. doi: 10.1016/s1097-2765(00)80004-0. PMID: 9659900. DOI: 10.1016/S1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 9.Vogel WW. Discoidin domain receptors: Structural relations and functional implications. Faseb J. 1999;13(Suppl):S77–82. doi: 10.1096/fasebj.13.9001.s77. PMID: 10352148. DOI: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- 10.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23. doi: 10.1016/s1097-2765(00)80003-9. PMID: 9659899. DOI: 10.1016/S1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 11.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5. PMID: 24725424. DOI: 10.1016/B978-0-12-800180-6.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borza CM, Pozzi A. Discoidin domain receptors in disease. Matrix Biol. 2014;34:185–192. doi: 10.1016/j.matbio.2013.12.002. PMID: 24361528. DOI: 10.1016/j.matbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: New players in cancer progression. Cancer Metastasis Rev. 2012;31(1-2):295–321. doi: 10.1007/s10555-012-9346-z. PMID: 22366781. DOI: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrogio C, Darbo E, Lee SW, Santamaría D. A putative role for discoidin domain receptor 1 in cancer chemoresistance. Cell Adh Migr. 2018;12(4):394–397. doi: 10.1080/19336918.2018.1445954. PMID: 29505315. DOI: 10.1080/19336918.2018.1445954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriet E, Sala M, Abou Hammoud A, Tuariihionoa A, Di Martino J, Ros M, Saltel F. Multitasking discoidin domain receptors are involved in several and specific hallmarks of cancer. Cell Adh Migr. 2018;12(4):363–377. doi: 10.1080/19336918.2018.1465156. PMID: 29701112. DOI: 10.1080/19336918.2018.1465156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, Chen X, Bai J, Liu Q, Li H, Xie J, Jing H, Zheng J. Discoidin domain receptor 1 (DDR1), a promising biomarker, induces epithelial to mesenchymal transition in renal cancer cells. Tumor Biol. 2016;37(8):11509–11521. doi: 10.1007/s13277-016-5021-2. PMID: 27020590. DOI: 10.1007/s13277-016-5021-2. [DOI] [PubMed] [Google Scholar]
- 17.Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnström H, Glimelius B, Sjöblom T, Edqvist PH, Djureinovic D, Micke P, Lindskog C, Mardinoglu A, Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352):eaan2507. doi: 10.1126/science.aan2507. PMID: 28818916. DOI: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 18.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. Kidney. In: American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th edition. Springer, New York, NY. 2002:323–328. [Google Scholar]
- 19.Godlewski J, Krazinski BE, Kowalczyk AE, Kiewisz J, Kiezun J, Kwiatkowski P, Sliwinska-Jewsiewicka A, Maslowski Z, Kmiec Z. PLAGL1 (ZAC1/LOT1) expression in clear cell renal cell carcinoma: Correlations with disease progression and unfavorable prognosis. Anticancer Res. 2016;36(2):617–624. PMID: 26851016. [PubMed] [Google Scholar]
- 20.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–663. doi: 10.1097/00000478-198210000-00007. PMID: 7180965. [DOI] [PubMed] [Google Scholar]
- 21.Kowalczyk AE, Krazinski BE, Godlewski J, Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A, Kiezun J, Wierzbicki PM, Bodek G, Sulik M, Kmiec Z. Altered expression of the PLAGL1 (ZAC1/LOT1) gene in colorectal cancer: Correlations to the clinicopathological parameters. Int J Oncol. 2015;47(3):951–962. doi: 10.3892/ijo.2015.3067. PMID: 26134521. DOI: 10.3892/ijo.2015.3067. [DOI] [PubMed] [Google Scholar]
- 22.Godlewski J, Krazinski BE, Kowalczyk AE, Kiewisz J, Kiezun J, Kwiatkowski P, Sliwińska-Jewsiewicka A, Wierzbicki PW, Kmieć Z. Expression and prognostic significance of EP300, TP53 and BAX in clear cell renal cell carcinoma. Anticancer Res. 2017;37(6):2927–2937. doi: 10.21873/anticanres.11646. PMID: 28551630. DOI: 10.21873/ anticanres.11646. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. PMID: 11846609. DOI: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Kowalczyk AE, Krazinski BE, Godlewski J, Grzegrzolka J, Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A, Dziegiel P, Kmiec Z. SATB1 is down-regulated in clear cell renal cell carcinoma and correlates with MIR-21-5p overexpression and poor prognosis. Cancer Genomics Proteomics. 2016;13(3):209–218. PMID: 27107063. [PubMed] [Google Scholar]
- 25.Kowalczyk AE, Godlewski J, Krazinski BE, Kiewisz J, Sliwinska-Jewsiewicka A, Kwiatkowski P, Pula B, Dziegiel P, Janiszewski J, Wierzbicki PM, Kmiec Z. Divergent expression patterns of SATB1 mRNA and SATB1 protein in colorectal cancer and normal tissues. Tumor Biol. 2015;36(6):4441–4452. doi: 10.1007/s13277-015-3084-0. PMID: 25874491. DOI: 10.1007/s13277-015-3084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immuno-histochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8(3):138–140. PMID: 3303008. [PubMed] [Google Scholar]
- 27.Thul PJ, Åkesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM, Bäckström A, Danielsson F, Fagerberg L, Fall J, Gatto L, Gnann C, Hober S, Hjelmare M, Johansson F, Lee S, Lindskog C, Mulder J, Mulvey CM, Nilsson P, Oksvold P, Rockberg J, Schutten R, Schwenk JM, Sivertsson Å, Sjöstedt E, Skogs M, Stadler C, Sullivan DP, Tegel H, Winsnes C, Zhang C, Zwahlen M, Mardinoglu A, Pontén F, von Feilitzen K, Lilley KS, Uhlén M, Lundberg E. A subcellular map of the human proteome. Science. 2017;356(6340):eaal3321. doi: 10.1126/science.aal3321. PMID: 28495876. DOI: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–49. doi: 10.1038/nature12222. PMID: 23792563. DOI: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valencia K, Ormazábal C, Zandueta C, Luis-Ravelo D, Antón I, Pajares MJ, Agorreta J, Montuenga LM, Martínez-Canarias S, Leitinger B, Lecanda F. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. 2012;18(4):969–980. doi: 10.1158/1078-0432.CCR-11-1686. PMID: 22223527. DOI: 10.1158/1078-0432.CCR-11-1686. [DOI] [PubMed] [Google Scholar]
- 30.Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, Lee DG, Lee YC, Moon WS, Chung MJ. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24(2):311–319. doi: 10.3892/or_00000861. PMID: 20596615. DOI: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
- 31.Alves F, Vogel WF, Aszo A. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. PMID: 11283268. DOI: 10.1128/MCB.21.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belfiore A, Malaguarnera R, Nicolosi ML, Lappano R, Ragusa M, Morrione A, Vella V. A novel functional crosstalk between DDR1 and the IGF axis and its relevance for breast cancer. Cell Adh Migr. 2018;12(4):305–314. doi: 10.1080/19336918.2018.1445953. PMID: 29486622. DOI: 10.1080/19336918.2018.1445953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan J, Yahata T, Adachi S, Yoshihara K, Tanaka K. Identification of receptor tyrosine kinase, discoidin domain receptor 1 (DDR1), as a potential biomarker for serous ovarian cancer. Int J Mol Sci. 2011;12(2):971–982. doi: 10.3390/ijms12020971. PMID: 21541037. DOI: 10.3390/ijms12020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo Y, Yang M, Liu W, Yang J, Fu X, Liu D, Li J, Zhang J, Hua R, Sun Y. High expression of DDR1 is associated with the poor prognosis in Chinese patients with pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2015;34(1):88. doi: 10.1186/s13046-015-0202-1. PMID: 26297342. DOI: 10.1186/s13046-015-0202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, Beckebaum S. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. PMID: 20799954. DOI: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimada K, Nakamura M, Ishida E, Higuchi T, Yamamoto H, Tsujikawa K, Konishi N. Prostate cancer antigen-1 contributes to cell survival and invasion though discoidin receptor 1 in human prostate cancer. Cancer Sci. 2008;99(1):39–45. doi: 10.1111/j.1349-7006.2007.00655.x. PMID: 17970783. DOI: 10.1111/j.1349-7006.2007.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Liu J, Jiang B, Chen J, Fu Z, Bai F, Jiang J, Tang Z. MiR-199a-5p loss up-regulated DDR1 aggravated colorectal cancer by activating epithelial-to-mesenchymal transition related signaling. Dig Dis Sci. 2014;59(9):2163–2172. doi: 10.1007/s10620-014-3136-0. PMID: 24711074. DOI: 10.1007/s10620-014-3136-0. [DOI] [PubMed] [Google Scholar]
- 38.Hur H, Ham I-H, Lee D, Jin H, Aguilera KY, Oh HJ, Han S-U, Kwon JE, Kim Y-B, Ding K, Brekken RA. Discoidin domain receptor 1 activity drives an aggressive phenotype in gastric carcinoma. BMC Cancer. 2017;17(1):87. doi: 10.1186/s12885-017-3051-9. PMID: 28143619. DOI: 10.1186/s12885-017-3051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BK, Kim I, Yoon SK. Identification of miR-199a-5p target genes in the skin keratinocyte and their expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2015;79(2):137–147. doi: 10.1016/j.jdermsci.2015.05.005. PMID: 26026896. DOI: 10.1016/j.jdermsci.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239–248. doi: 10.1007/s11060-005-6874-1. PMID: 16234985. DOI: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- 41.Chiaretti S, Li X, Gentleman R, Vitale A, Wang KS, Mandelli F, Foà R, Ritz J. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin Cancer Res. 2005;11(20):7209–7219. doi: 10.1158/1078-0432.CCR-04-2165. PMID: 16243790. DOI: 10.1158/1078-0432.CCR-04-2165. [DOI] [PubMed] [Google Scholar]
- 42.Favreau AJ, Cross EL, Sathyanarayana P. miR-199b-5p directly targets PODXL and DDR1 and decreased levels of miR-199b-5p correlate with elevated expressions of PODXL and DDR1 in acute myeloid leukemia. Am J Hematol. 2012;87(4):442–446. doi: 10.1002/ajh.23129. PMID: 22374871. DOI: 10.1002/ajh.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96(5):808–814. doi: 10.1038/sj.bjc.6603614. PMID: 17299390. DOI: 10.1038/sj.bjc.6603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toy KA, Valiathan RR, Núñez F, Kidwell KM, Gonzalez ME, Fridman R, Kleer CG. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):9–18. doi: 10.1007/s10549-015-3285-7. PMID: 25667101. DOI: 10.1007/s10549-015-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takai K, Drain AP, Lawson DA, Littlepage LE, Karpuj M, Kessenbrock K, Le A, Inoue K, Weaver VM, Werb Z. Discoidin domain receptor 1 (DDR1) ablation promotes tissue fibrosis and hypoxia to induce aggressive basal-like breast cancers. Genes Dev. 2018;32(3-4):244–257. doi: 10.1101/gad.301366.117. PMID: 29483153. DOI: 10.1101/gad.301366.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assent D, Bourgot I, Hennuy B, Geurts P, Noël A, Foidart J-M, Maquoi E. A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells. PLoS One. 2015;10(3):e0116006. doi: 10.1371/journal.pone.0116006. PMID: 25774665. DOI: 10.1371 /journal.pone.0116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh Y-C, Wu C-C, Wang Y-K, Tang M-J. DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol Biol Cell. 2011;22(7):940–53. doi: 10.1091/mbc.E10-08-0678. PMID: 21289093. DOI: 10.1091/mbc.E10-08-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13(1):49–58. doi: 10.1038/ncb2133. PMID: 21170030. DOI: 10.1038/ ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung VY, Tan TZ, Huang RL, Lai HC, Huang RYJ. Loss of discoidin domain receptor 1 (DDR1) via CpG methylation during EMT in epithelial ovarian cancer. Gene. 2017;635(3-4):9–15. doi: 10.1016/j.gene.2017.09.001. PMID: 28887161. DOI: 10.1016/j.gene.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Haake SM, Li J, Bai Y, Kinose F, Fang B, Welsh EA, Zent R, Dhillon J, Pow-Sang JM, Chen YA, Koomen JM, Rathmell WK, Fishman M, Haura EB. Tyrosine kinase signaling in clear cell and papillary renal cell carcinoma revealed by mass spectrometry-based phosphotyrosine proteomics. Clin Cancer Res. 2016;22(22):5605–5616. doi: 10.1158/1078-0432.CCR-15-1673. PMID: 27220961. DOI: 10.1158/ 1078-0432.CCR-15-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Si T, Liu C, Xu K, Gui Y. Association of miR-199a expression with clinicopathologic characteristics and prognosis of renal cell carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32(11):1568–1571. PMID: 23174576. DOI: 10.3969/ j.issn.1673-4254.2012.11.008. [PubMed] [Google Scholar]
- 52.Tsukigi M, Bilim V, Yuuki K, Ugolkov A, Naito S, Nagaoka A, Kato T, Motoyama T, Tomita Y. Re-expression of miR-199a suppresses renal cancer cell proliferation and survival by targeting GSK-3β. Cancer Lett. 2012;315(2):189–197. doi: 10.1016/j.canlet.2011.10.008. PMID: 22093618. DOI: 10.1016/j.canlet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Wotschofsky Z, Meyer HA, Jung M, Fendler A, Wagner I, Stephan C, Busch J, Erbersdobler A, Disch AC, Mollenkopf HJ, Jung K. Reference genes for the relative quantification of microRNAs in renal cell carcinomas and their metastases. Anal Biochem. 2011;417(2):233–241. doi: 10.1016/j.ab.2011.06.009. PMID: 21741950. DOI: 10.1016/j.ab.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Wu A, Chen Y, Liu Y, Lai Y, Liu D. miR-199b-5p inhibits triple negative breast cancer cell proliferation, migration and invasion by targeting DDR1. Oncol Lett. 2018;16(4):4889–4896. doi: 10.3892/ol.2018.9255. PMID: 30250555. DOI: 10.3892/ol.2018.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alves F, Saupe S, Ledwon M. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. FASEB J. 2001;2(1):1321–1323. doi: 10.1096/fj.00-0626fje. PMID: 11344127. DOI: 10.1096/fj.00. [DOI] [PubMed] [Google Scholar]
- 56.Salomonis N, Schlieve CR, Pereira L, Wahlquist C, Colas A, Zambon AC, Vranizan K, Spindler MJ, Pico AR, Cline MS, Clark TA, Williams A, Blume JE, Samal E, Mercola M, Merrill BJ, Conklin BR. Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proc Natl Acad Sci USA. 2010;107(23):10514–10519. doi: 10.1073/pnas.0912260107. PMID: 20498046. DOI: 10.1073/pnas.0912260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taniguchi K, Ito Y, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Sugiyama T, Futamura M, Otsuki Y, Yoshida K, Uchiyama K, Akao Y. Organ-specific PTB1-associated microRNAs determine expression of pyruvate kinase isoforms. Sci Rep. 2015;5:8647. doi: 10.1038/srep08647. PMID: 25721733. DOI: 10.1038/srep08647. [DOI] [PMC free article] [PubMed] [Google Scholar]