Abstract
Importance
Differentiation between Ebola retinal lesions and other retinal pathologies in West Africa is important and the pathogenesis of Ebola retinal disease remains poorly understood.
Objective
To describe the appearance of Ebola virus disease (EVD) retinal lesions using multimodal imaging to enable inferences regarding potential pathogenesis.
Design
Consecutive, prospectively identified, cohort study
Setting
34 Military Hospital, Freetown, Sierra Leone
Participants
Fourteen EVD survivors of Sierra Leonean origin with identified Ebola virus retinal lesions. Mean age 37years (SD 8.8years) 43% Female.
Exposures
Ebola virus disease.
Main Outcomes and measures
Multimodal imaging findings including ultra-widefield (UWF) scanning laser ophthalmoscopy, fundus autofluorescence, swept source optical coherence tomography (OCT), Humphrey visual field analysis, and spatial analysis.
Results
141 Ebola virus retinal lesions were observed in 22 of 27 eyes of fourteen survivors on UWF imaging. 41 lesions were accessible to OCT imaging. Retinal lesions are predominantly non-pigmented with a pale grey appearance. Peripapillary lesions exhibit variable curvatures in keeping with the retinal nerve fiber layer projections. All lesions respect the horizontal raphe and spare the fovea. OCT demonstrates a ‘V’ shaped hyperreflectivity of the outer nuclear layer overlying discontinuities of the ellipsoid zone and interdigitation zone in the smaller lesions. Larger lesions cause a collapse of the retinal layers and loss of retinal thickness. Lesion shapes are variable but sharp angulations are characteristic. Perilesional areas of dark-without-pressure (thinned ellipsoid zone hyporeflectivity) of variable extent, accompany 89% of lesions.
Conclusions and relevance
We demonstrate OCT evidence of localized pathological changes seen at the level of the photoreceptors in small lesions. The relevance of associated areas of dark-without-pressure remains undetermined.
Introduction
We previously conducted a case-control study that identified retinal characteristics specific to survivors of Ebola virus disease (EVD) in Sierra Leone utilizing ultra-widefield (UWF) retinal imaging. Of all retinal lesions characterized in the previous study, only one lesion of characteristic morphological appearance was exclusively identified in Ebola survivors by two masked graders.1 On this basis, this lesion was deemed most likely to be secondary to Ebola virus infection. Identical lesions have been identified in other Ebola survivor cohort studies.2,3
This expanded analysis provides optical coherence tomography (OCT) interpretation and functional visual field (VF) analysis to provide further insights into the pathophysiology of Ebola retinal sequelae.
Methods
160 EVD survivors attended the ophthalmology clinic at 34 Military Hospital, Freetown between January 2016 and April 2017. Fourteen Ebola survivors met the eligibility criteria of at least one identified Ebola retinal lesions on UWF retinal imaging in keeping with the findings of our previous study.1 All fourteen were recalled and attended the clinic for examination including OCT of accessible Ebola retinal lesions. Thirteen of these survivors were recruited to this study, one was excluded due to increased lens opacity preventing fundus imaging. One further eligible patient was identified in March 2017 and directly enrolled into the study. OCT lesion appearance was categorized, and lesion grading concordance was compared by two masked ophthalmologists. Informed consent was obtained from all participants. The study was approved by the Office of Sierra Leone Ethics and Scientific Review Committee on 31st January 2017 and followed the tenets of the Declaration of Helsinki. Examination protocol is summarized in eMethods 1.
Results
Fourteen EVD survivors with 141 Ebola virus retinal lesions in twenty-two eyes (8 bilateral) were analyzed. OCT was obtained on 41 lesions from twenty eyes. Characteristics of all fourteen cases are summarized in eTable 1. Corresponding multimodal imaging and VFs are available in eFigures 2-23.
Retinal Lesions
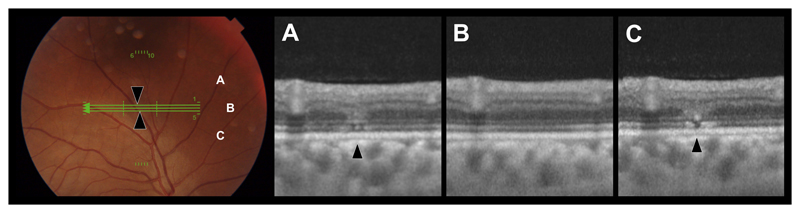
Ebola virus retinal lesions vary in size and shape although, distinctive linear borders with sharp angulations are characteristic (eFigure 11). Multimodal imaging features vary according to severity and extent of retinal structures involved. A lesion severity grading from 1 to 5 is outlined in eTable 2. Cohen’s kappa statistic of intergrader agreement was 0.77. OCT of the smallest lesions demonstrates multiple vertical discontinuities of the ellipsoid zone (EZ) and interdigitation zone (IZ) with overlying ‘V’ shaped increased reflectance of the outer nuclear layer (ONL) (Figure 1 & eFigure 21F). Lesions appear light grey in color on fundus photography and are predominantly non-pigmented.
Figure 1. Ebola Retinal Lesion.
Left - Color fundus image. Green lines indicate OCT scan locations for corresponding OCTs A-C. Black arrows indicate lesion sites. A-C) OCTs through Ebola retinal lesions demonstrating the close proximity of multifocal discontinuities of the ellipsoid zone with an overlying ‘V’ shaped increased reflectance of the outer nuclear layer (of equal reflectance of the adjacent outer plexiform layer).
Peripapillary lesions demonstrate variable curvatures depending on the optic disc perimeter location and resemble the arcuate anatomical pathways of the retinal nerve fiber layer (ganglion cell axons) (eFigures 4, 10 11 and 15). Visual acuity and color vision were preserved in all cases in the absence of other pathology. Corresponding absolute VF defects respecting the anatomical horizontal raphe were observed on 24-2 Humphrey visual field (HVF) analysis (eFigure 10G) and with a peripheral nasal 24-2 test protocol (eMethod 2, eFigure 10H).
Dark-without-pressure
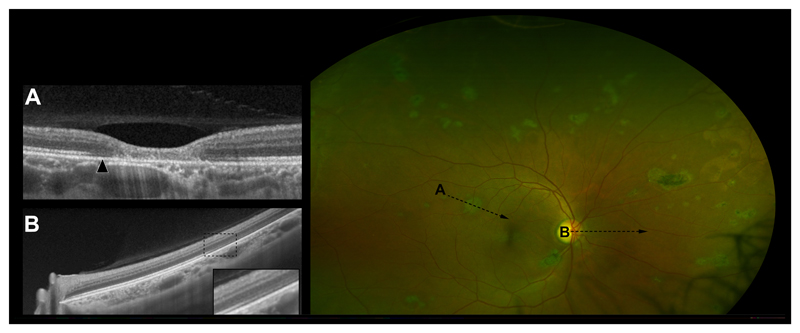
Well-defined areas of dark-without-pressure (DWP) which correspond to a thinned, hyporeflective EZ and an absent IZ on OCT (Figure 2) were seen adjacent to 88.7% of Ebola retinal lesions in this case series. The extent of DWP was variable, ranging from a confined circumferential marginal zone (eFigures 2, 6, 8, 14, 19, and 20), to larger defined areas (Figure 2 (190 disc areas), eFigures 5 (40 disc areas), 7 (38 disc areas), and 15 (89 disc areas) and in some cases 360-degree pan-retinal involvement (406 disc areas) (eFigure 16). The extent of DWP in some eyes appears associated with the density of Ebola retinal lesions (Figure 2, and eFigure 15). In all cases, DWP appears to spare the macula. No associated vitreous inflammation or vitreous traction was visible on OCT.
Figure 2. Extensive Dark-Without-Pressure.
A) OCT through an Ebola retinal lesion. Arrow indicates an area of perilesional dark without pressure (DWP) corresponding to a thinned hyporeflective EZ and absent IZ. B) OCT demonstrating the transition between the normal retina and the circumferential extension of an area of DWP nasally. The enlarged box highlights the transitional zone. C) Right eye UWF image. Multiple Ebola retinal lesions and associated areas of DWP.
120-point screen, 60-4 threshold tests or peripheral nasal 24-2 protocol (eFigure 10H) were unable to conclusively identify any definitive VF defect corresponding to areas of DWP. 24-2 HVF analysis of one survivor with right hemiparesis following acute infection demonstrated a right-sided homonymous hemianopia and left inferior quadrantanopia. (eFigure 19G and 20I).
Spatial Analysis
Aligned and amalgamated retinal images with corresponding Ebola retinal lesion loci and longitudinal axis are shown in eFigure 24. No overlapping axes or crossing of the horizontal raphe was observed.
Discussion
We present a multi-modal imaging analysis of a series of 14 EVD survivors with Ebola retinal lesions as characterized in our previous case-control study.1
While OCT analysis of larger lesions involving all retinal layers provides little insight into the pathogenesis, OCT observations of small lesions revealed multifocal fine discontinuities of the EZ and IZ with overlying increased reflectivity of the ONL (Figure 1). These findings mirror the histological appearance of early herpes simplex virus (HSV) retinopathy observed in the contralateral retina via a retrograde axonal transmission following unilateral anterior chamber viral inoculation.4,5 They have also been observed in the ipsilateral retina following unilateral anterior chamber viral inoculation5, although in all cases within this series, we did not identify signs of previous anterior chamber uveitis to suggest a direct anterior to posterior dissemination. The presence of peripapillary curvilinear lesions resembling the arcuate path of the ganglion cell axons, which respect the horizontal raphe (demonstrated both on imaging and VF analysis), provides further evidence that Ebola virus disease involves retinal ganglion cells and a lasting insult to their afferent photoreceptors.
Possible pathogenic mechanisms for the characteristic retinal lesions observed in ebola survivors could include retrograde neuronal transmission. Vascular ocular dissemination and involvement of the optic nerve leptomeninges has been demonstrated in a rhesus monkey model with acute fulminant Ebola infection.6
Dark-without-pressure
Although not specific to Ebola retinal lesions, the frequency of circumferential marginal zones of DWP around Ebola retinal lesions strongly suggests an association. This is supported by the correlation between Ebola retinal lesion density and the extent of DWP in some eyes (eFigure 15). Areas of DWP in this study correspond to a hyporeflective thinning of the second and loss of the third hyperreflective bands on OCT currently termed the ‘ellipsoid zone’ and ‘interdigitation zone’ respectively.7 Although controversy continues over the precise anatomical correlates of these bands8,9 recent cellular characterization using immunohistochemistry markers concurs that the second band is generated by the photoreceptor ellipsoids and probably the result of the high number of mitochondria that they contain, while the third band corresponds to the cone phagosomes located in the top of the RPE.10
Study Limitations
Due to the lack of histological evidence, pre-infection imaging or retinal imaging during acute infection, an absolute temporal association with EVD and the Ebola retinal lesions and associated DWP has yet to be established. We have not compared the OCT findings presented here to a control group of retinal lesions secondary to other pathologies to confirm characteristics are unique to Ebola retinal lesions.
Conclusion
In this study, we demonstrate pathological changes seen at the level of the photoreceptors on OCT in small lesions. We demonstrate associated areas of DWP which appear as a hyporeflectivity, thinned EZ in combination with an IZ absence on OCT. The importance of which remains to be determined. Follow up observations are ongoing. These findings suggest that ophthalmic evaluation of Ebola survivors would benefit from the inclusion of OCT analysis and visual field assessment in future outbreaks.
Supplementary Material
Key Points.
Question
Can multimodal imaging of Ebola retinal lesions inform our understanding of their pathogenesis?
Findings
In this prospective cohort of fourteen survivors, optical coherence tomography demonstrates a ‘V’ shaped increased reflectivity of the outer nuclear layer overlying discontinuities of the ellipsoid zone and interdigitation zone in the smallest lesions. A collapse of the overlying retinal structures is detected in larger lesions. Corresponding visual field defects respect the horizontal raphe. Perilesional areas of dark-without-pressure (ellipsoid zone hyporeflectivity) accompany 89% of lesions.
Meanings
In these survivors, findings are consistent with a neuronal rather than vascular pathogenesis. The significance of dark-without-pressure is undetermined.
Acknowledgments
We thank Optos PLC, for their generous donation of the Daytona Ophthalmoscope, which continues to improve patient care for the people of Sierra Leone; Topcon for the loan of their OCT device. Onlime SL Ltd for supplying the clinic with internet access. CBM Italia for their 2WIN autorefractor donation and the administration at 34 Military Hospital, Freetown, Sierra Leone, for facilitating the study and Mr. Ian Pearce for institutional peer review.
The funding bodies and organizations who provided equipment support for this research had had no role in the design and conduct of the study; data collection; management; analysis; interpretation of the data; preparation; review; approval of the manuscript or decision to submit the manuscript for publication.
P.J.S reports grants from Global Ophthalmology Awards Programme Grant supported by Bayer, The Dowager Countess Eleanor Peel Trust and Enhancing Research in Epidemic Situations (ERAES) funded by Wellcome Trust, non-financial support from Optos, CBM Italia, and Topcon during the conduct of the study; grants from National Institute for Health Research Health - Protection Research Unit in Emerging and Zoonotic Infections and BMA Humanitarian Fund outside the submitted work. F.S reports grants from Wellcome Trust Enhancing Research Activity in Epidemic Situations (ERAES) Programme award, Wellcome Trust, Bill & Melinda Gates Foundation, USA Department of Defense HIV/AIDS Prevention Program, Save the Children, UK, National Institute of Allergy and Infectious Diseases, USA, WHO & CDC, USA, and EU FP7 outside the submitted work. J.T.S reports grants from The Wellcome Trust Enhancing Research Activity in Epidemic Situations Programme, National Institute for Health Research Health - Protection Research Unit in Emerging and Zoonotic Infections during the conduct of the study. N.A.V.B reports personal fees from Abbvie, Wellcome Trust and Santen, outside the submitted work. M.G.S reports grants from National Institute for Health Research (NIHR) Health Protection Research Unit in Emerging and Zoonotic Infections at the University of Liverpool, and Wellcome Trust Enhancing Research Activity in Epidemic Situations (ERAES) Programme award during the conduct of the study; grants from Wellcome Trust and Bill & Melinda Gates Foundations, outside the submitted work. Other authors have nothing to declare.
Footnotes
Author Contributions: Conception and design: Steptoe, Scott and Semple. Data collection: Steptoe, Momorie, Fornah, Komba, and Emsley. Analysis and interpretation: Steptoe, Beare, Harding, Semple. Obtained funding: Steptoe, Semple and Scott. Steptoe wrote the first draft of the paper. Steptoe, Scott, Beare, Harding, and Semple revised the paper. All other authors reviewed and approved the final version. Steptoe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Overall responsibility (as Sponsor and Consortium Lead Investigator): Semple for University of Liverpool.
References
- 1.Steptoe PJ, Scott JT, Baxter JM, et al. Novel Retinal Lesion in Ebola Survivors, Sierra Leone, 2016. Emerg Infect Dis. 2017;23(7):1102–1109. doi: 10.3201/eid2307.161608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shantha JG, Crozier I, Varkey JB, et al. Long-term Management of Panuveitis and Iris Heterochromia in an Ebola Survivor. Ophthalmology. 2016;123(12):2626–2628.e2. doi: 10.1016/j.ophtha.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hereth-Hebert E, Bah MO, Etard JF, et al. Ocular Complications in Survivors of the Ebola Outbreak in Guinea. Am J Ophthalmol. 2017;175:114–121. doi: 10.1016/j.ajo.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN, Togni BI, Briones OC, Dawson CR. A microscopic study of herpes simplex virus retinopathy in mice. Invest Ophthalmol Vis Sci. 1987;28(7):1181–1190. [PubMed] [Google Scholar]
- 5.Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32(9):2462–2472. [PubMed] [Google Scholar]
- 6.Zeng X, Blancett CD, Koistinen KA, et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat Microbiol. 2017;2 doi: 10.1038/nmicrobiol.2017.113. 17113. [DOI] [PubMed] [Google Scholar]
- 7.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;121(8):1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF. Outer Retinal Bands. Investig Opthalmology Vis Sci. 2015;56(4):2505. doi: 10.1167/iovs.15-16456. [DOI] [PubMed] [Google Scholar]
- 9.Jonnal RS, Kocaoglu OP, Zawadzki RJ, Lee S-H, Werner JS, Miller DT. Author Response: Outer Retinal Bands. Investig Opthalmology Vis Sci. 2015;56(4):2507. doi: 10.1167/iovs.15-16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuenca N, Ortuño-Lizarán I, Pinilla I. Cellular Characterization of Optical Coherence Tomography and Outer Retinal Bands Using Specific Immunohistochemistry Markers and Clinical Implications. Ophthalmology. 2017 Oct; doi: 10.1016/j.ophtha.2017.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.