Abstract
Objective
To clarify the GRADE (grading of recommendations assessment, development and evaluation) definition of certainty of evidence and suggest possible approaches to rating certainty of the evidence for systematic reviews, health technology assessments and guidelines.
Study Design and Setting
This work was carried out by a project group within the GRADE Working Group, through brainstorming and iterative refinement of ideas, using input from workshops, presentations, and discussions at GRADE Working Group meetings to produce this document, which constitutes official GRADE guidance.
Results
Certainty of evidence is best considered as the certainty that a true effect lies on one side of a specified threshold, or within a chosen range. We define possible approaches for choosing threshold or range. For guidelines, what we call a fully contextualized approach requires simultaneously considering all critical outcomes and their relative value. Less contextualized approaches, more appropriate for systematic reviews and health technology assessments, include using specified ranges of magnitude of effect, e.g. ranges of what we might consider no effect, trivial, small, moderate, or large effects.
Conclusion
It is desirable for systematic review authors, guideline panelists, and health technology assessors to specify the threshold or ranges they are using when rating the certainty in evidence.
Keywords: GRADE, certainty of evidence, thresholds, guidelines, systematic reviews, health technology assessment
Introduction
The GRADE working group has designed a widely adopted structure for the development of clinical practice and public health guidelines (1). Formally assessing the trustworthiness of the available evidence represents a key component of the GRADE approach. GRADE offered a formal definition of certainty of evidence: “the extent of our confidence that the estimates of the effect are correct, or are adequate to support a particular decision or recommendation” (Box 1) (1). This definition suggests we are rating confidence or certainty in point estimates of effect. GRADE did not, however, present a coherent conceptual basis for rating certainty in such estimates.
Box 1. GRADE’s adopted definition of certainty of the evidence (1). Note that “quality of evidence” refers to the same concept as “certainty of the evidence” (see paragraph on definitions).
In the context of a systematic review, the ratings of the quality of evidence reflect the extent of our confidence that the estimates of the effect are correct. In the context of making recommendations, the quality ratings reflect the extent of our confidence that the estimates of an effect are adequate to support a particular decision or recommendation.
The aim of this paper is to present an alternative, and we believe more satisfactory, conceptualization. This alternative is grounded in the realization that, when deciding whether evidence regarding intervention effects is adequate to support a recommendation, we are not assessing our confidence in point estimates of effects, but rather our confidence in where effects lie relative to particular thresholds (2).
In the first part of our discussion we make evident that thresholds depend on the health care context, and therefore can vary. In the second part of our discussion we examine how authors can formally set thresholds, or ranges of values, in certainty of evidence assessments in systematic reviews. This fundamental point, and the associated approaches we suggest in this document, now constitutes official GRADE guidance.
With regard to the necessity for thresholds and the possibility they may vary, consider a societal choice to invest in the platelet inhibitor ticagrelor for patients following myocardial infarction. In a hundred typical patients, the drug may prevent one death over the course of a year, with limited adverse effects. In high income countries, the threshold for implementing ticagrelor may be less than a 1% mortality reduction, and policy makers may decide on widespread implementation. In this context we may have high certainty that the benefit exceeds our threshold for supporting the recommendation. In low income countries, the opportunity cost of offering ticagleror is likely to be prohibitive, and the threshold for implementation may be much higher (a 10% mortality reduction, or perhaps even larger). In this context we may have low certainty that the benefit exceeds our threshold for supporting the recommendation, and may even have high certainty that the benefit fails to exceed a threshold for supporting the recommendation and could warrant supporting a recommendation against.
Thus, given the same evidence regarding a particular outcome, in the context of a guideline, the certainty of the evidence can vary depending upon the context, and the health care question being asked. This is often surprising to those who first encounter the concept. Therefore, in the first part of the discussion below we will present another example illustrating this crucial concept.
Definitions
GRADE initially referred to “quality of evidence”; subsequently “confidence in the estimates” replaced “quality of evidence”; most recently “certainty of evidence” has often become the preferred term. These words all refer to the same concept, and we will use “certainty of evidence” throughout this paper.
As discussed above, certainty of evidence defined as adequacy to support a particular decision or recommendation varies with the health care context. We will refer to situations where the full health care question/context is made clear as “fully contextualized”. Such fully contextualized ratings are typically made in the setting of clinical practice guidelines. We will discuss the distinctions between assessments made that are fully contextualized, partly contextualized, and non-contextualized – the latter two typically made in context of systematic reviews and health technology assessments. Fully contextualized ratings – the focus of PART 1 of the discussion - include the possibility that some of the outcomes that are to be assessed may be societal or economic and may include issues of feasibility or equity (3).
Part 1
We are not rating certainty in point estimates, but rather certainty that the true effect lies in a particular range: an illustration from previous GRADE writings
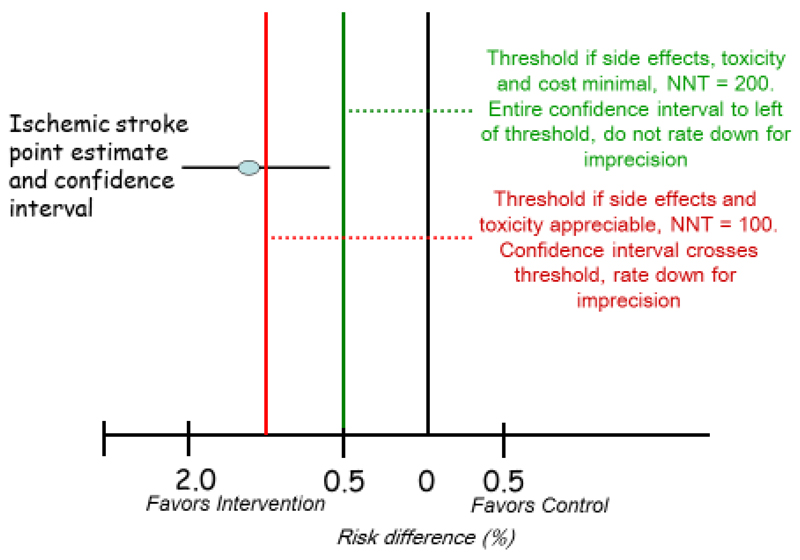
An illustration of rating our certainty that the effect lies above a particular threshold in GRADE is in the 6th article of the JCE series that deals with imprecision (2). In that article, we present a hypothetical systematic review of randomized control trials of an intervention to prevent major strokes that yields a pooled estimate of absolute reduction in strokes of 1.3%, with a 95% CI of 0.6% to 2.0% (Figure 1).
Figure 1. Rating certainty that the true effect lies in a particular range: an illustration from previous GRADE writings (2).
If there are no serious concerns about risk of bias, inconsistency, indirectness, or publication bias, the confidence interval will represent a reasonable estimate of a certainty range, the range of reasonably believable effects of the intervention; if there were such concerns, the certainty range (that is, the range in which we anticipate the true effect may lie, after considering not only precision but risk of bias, inconsistency, indirectness, and publication bias) would be wider and/or shifted compared to the 95% confidence interval, although its exact distribution would be difficult to ascertain (4).
This thought exercise occurs in the setting of a fully contextualized evidence certainty rating (typically, a guideline setting). We ask readers to first assume that the intervention is a drug with no serious adverse effects, minimal inconvenience, and modest cost that is equitable, feasible and acceptable to administer. Under these circumstances, even a small beneficial health effect would warrant a recommendation for the intervention because, overall, the desirable consequences would outweigh the undesirable consequences. For instance, given considerations about all the possible downsides or harms, we may recommend the intervention if it reduced the incidence of stroke by as little as 0.5% (vertical green line in Figure 1). Note: here, we set a threshold, 0.5%, that defines our willingness to recommend the intervention.
The entire CI (0.6% to 2.0%) around the effect on stroke reduction lies to the left of the clinical decision threshold of 0.5% and therefore excludes a benefit smaller than the threshold. We can – as we point out in the article - therefore conclude that the precision of the estimate is sufficient to support a recommendation: we are confident that the true effect lies above our threshold and there is no reason to rate down certainty as a result of imprecision. Assuming there are no serious concerns with risk of bias, inconsistency, indirectness, or publication bias, our certainty that the true effect lies above our threshold will be high, and any ensuing recommendation is likely to be strong.
Consider now a modification of this hypothetical scenario in which the same treatment is associated with more serious harm, such as a 0.5% absolute increase in myocardial infarction. Under these circumstances, we may be reluctant to recommend treatment unless the absolute stroke reduction was larger, for instance at least 1% (red line in Figure 1). Since the point estimate of 1.3% meets the threshold criterion, a recommendation in favor of treatment would still be appropriate, but the imprecision-generated uncertainty will result in only moderate certainty that the effect is above the threshold. Not only does it lead to lowering our certainty in the evidence, in this situation we are also likely to make a weak recommendation.
In this example, the certainty in effect size for the effect of stroke reduction does not change, but as our threshold for treatment changes (as the magnitude of undesirable health effects increases), the certainty that desirable consequences outweigh undesirable consequences decreases.
The logic of the previous example applies to all fully contextualized graded recommendations: given the undesirable consequences of an intervention, how certain are we that the health benefits lie above a threshold that makes it worthwhile to administer that intervention? Consider patients with atrial fibrillation who are deciding whether to use anticoagulant therapy to lower their risk for stroke. Such patients must weigh the anticipated stroke reduction against the undesirable health effects of anticoagulation, including the risk of bleeding. Thus, patients must ask themselves: how small a stroke reduction am I ready to accept given the bleeding risk associated with anticoagulation, and still use the drug? Depending on the bleeding risk, and patients’ values, the answer may be an absolute reduction in stroke risk of 1%, 2%, 3% or more. Furthermore, patients need to consider other undesirable health effects and consequences of anticoagulation: the burden of medication use, including lifestyle limitations and, if using warfarin, the need for monitoring.
Note, the question patients are asking relates not to the point estimate, but rather to the possible range in which the true effect lies. For instance, let us say a patient has chosen a threshold of 2%. For the decision to use or not use anticoagulation, it is immaterial whether the true effect is a 2.1% reduction in stroke or a 3% or larger reduction, as long as it is above the 2% threshold. Thus, we should ascertain our certainty that the true reduction is ≥2%, not our certainty that the point estimate represents the true effect. Although the point estimate might be above the relevant threshold, if the CI crosses the threshold, we would be less certain that the true effect is above the threshold (and thus rate down for imprecision).
We have framed the example as the magnitude of benefit required. It could as easily be framed as the acceptable magnitude of undesirable consequences (5). For instance, given a particular reduction in stroke, how great could the magnitude of increased bleeding be before patients would choose to forego anticoagulants.
We have discussed this logic in the context of individual patient decision-making. The process is, however, identical for a guideline panel. The panel members must ask themselves the extent to which they are certain the desirable and undesirable consequences lie in a range that would clearly mandate a recommendation for or against a particular management strategy. They are not therefore – as one might infer from the definition of certainty in Box 1 - rating their certainty in the point estimates of effect.
Considering uncertainty in both benefits and harms presents serious challenges to setting thresholds
To this point we have focused on uncertainty in the benefits, and not uncertainty in the harms. Simultaneously considering uncertainty in both benefits and harms raises additional challenges. Moreover, and perhaps even more challenging, we have focused on a single benefit outcome. What if, as is often the case, we have more than one benefit and more than one harm outcome associated with the intervention? The cognitive challenge of simultaneously considering quantitative thresholds when there are multiple desirable and undesirable outcomes is formidable – indeed, possibly beyond the capacities for all but a very few individuals. This is analogous to considering our certainty in total net benefit, as might be generated by a decision analysis model. The challenges do not, however, bear on the essential point that when we rate certainty in evidence, the process has to do with our certainty that true effects lie in a particular range or on one side of a particular threshold.
Part 2
Implementing the range/threshold approach to rating certainty of evidence – contextualization*
The atrial fibrillation example we have used represents a real (albeit simplified) clinical decision with the simultaneous consideration of all critical desirable and undesirable consequences of treatment. Setting the threshold for use of anticoagulants, and thus the rating of certainty, requires a judgment regarding the relative desirability of avoiding stroke, bleeding, and the burden associated with anticoagulation. We refer to patients’ judgments of relative desirability as value and preference judgments. A guideline panel will consider typical values and preferences for the patient group of interest.
In a clinical context, all outcomes associated with a given decision and the associated value and preference judgments are considered simultaneously, and so a decision about the tradeoff is “fully contextualized”. Clinical practice guideline panels should always be considering such fully contextualized settings, as may systematic reviews that are undertaken specifically to inform a guideline panel (Table 1).
Table 1. Possible ways of setting thresholds or ranges and what the certainty expressed will represent.
| Setting | Degree of contextualization | Threshold or range | How it is set | What the certainty rating represents |
|---|---|---|---|---|
| Primarily for systematic reviews and health technology assessment | Non-contextualized | See Appendix 1 for possible approaches | ||
| Primarily for systematic reviews and health technology assessment | Partially contextualized | Specified magnitude of effect | E.g. a small effect can be defined as an effect small enough that one might consider not using the intervention if adverse effects or costs are appreciable | Certainty in a specified magnitude of effect for one outcome (e.g. no or trivial, small, moderate or large effect) |
| Primarily for clinical practice guidelines | Fully contextualized | Threshold determined with considerations of all critical outcomes | Considering the range of possible effects on all critical outcomes, bearing in mind the decision(s) that need to be made, and the associated values and preferences | For each outcome, ratings represent our confidence that the direction of the net effect (positive or negative) will not differ from one end of the certainty range to the other. |
For both health technology assessments and systematic reviews, there is often a certain degree of contextualization of the results (e.g. by the choice of outcomes presented, the consideration of indirectness, and some notion of how the target audience values the outcomes). Thus, systematic review and health technology assessment authors should be explicit about the context they have in mind, and whether or not issues of feasibility and equity are influencing their judgments (usually, they will not). The specification of a complete set of values and preferences that allow the tradeoff between desirable and undesirable outcomes is not, however, in the authors’ purview. Their consideration of certainty in evidence is not, therefore, fully contextualized.
GRADE writings have recognized this key distinction between practice guidelines and systematic reviews, and therefore offered two definitions of certainty in evidence, one for the former setting and one for the latter (Box 1) (1). The process for setting thresholds for fully contextualized ratings of certainty may sometimes be challenging, but the need is clear. The process for setting thresholds for settings that are not fully contextualized is less clear. We will now address this issue.
Non-contextualized or partly contextualized ratings of certainty
Table 1 presents, in addition to the fully contextualized rating of certainty, reference to non-contextualized approaches that appear in an appendix, and description of a partly contextualized approach. The non-contextualized and partly contextualized approaches are relevant primarily for systematic reviews and health technology assessments.
The two non-contextualized approaches – of potential use primarily in systematic reviews and health technology assessments - do not represent guidance for applying GRADE but are part of a complete conceptualization of the certainty of evidence. One of these non-contextualized approaches presents certainty that the true effect lies within the 95% confidence interval. A second approach focuses on our certainty that a non-null effect is present. We include further details of these two approaches only in an appendix (appendix 1).
We will illustrate the application of the approaches using the example of the decision regarding whether to use shorter or longer duration of dual antiplatelet therapy (DAPT), i.e. aspirin and clopidogrel, or a related drug, in patients with coronary artery disease who have undergone placement of drug eluting stents in their coronary arteries (Table 3) (6). Critical outcomes in this case include death, myocardial infarction, serious bleeding, and stroke. Table 2 presents an evidence summary of the impact of longer-duration versus shorter-duration DAPT on these four outcomes.
Table 3. Possible certainty ratings for Myocardial infarction (MI) for longer-duration versus shorter-duration DAPT.
| Approaches | Examples of set thresholds or ranges | Certainty |
|---|---|---|
| Specified magnitude: small effect | The effect is small over a range of 4-11 fewer MIs per 1000 | We have low certainty that longer-duration DAPT gives a small decrease in the incidence of MI compared to shorter-duration DAPT (rating down for inconsistency and imprecision). |
| Threshold determined with considerations of all critical outcomes | Threshold based on the value we place on MI, bleeding, stroke and death | Overall, considering typical values and preferences (equal weight to MI and serious bleeding, high importance to mortality, aversion to taking medication with minimal net benefit), we have low certainty that longer-duration DAPT does not decrease MI sufficiently to outweigh the effects on survival, bleeding, and the burden associated with long-term use of additional medication. We have low certainty in the MI outcome because the overall balance between net benefit and net harm differs across the certainty range for MI (rating down for inconsistency and imprecision). |
Table 2. Longer-duration versus shorter-duration DAPT after drug eluting stents: Partial evidence profile†.
| Outcome | No. of participants (No. of studies) | Risk of bias | Inconsistency | Indirectness | Publication Bias | Relative Risk (95% CI) | Absolute Effect per 1000 treated (95% CI) per year |
|---|---|---|---|---|---|---|---|
| Total mortality | 28088 (9) | No serious limitations | No serious inconsistency | No serious indirectness | Undetected | 1.19 (1.04-1.36) | 2 more (0 more to 4 more) |
| Myocardial Infarction | 28088 (9) | No serious limitations | Serious inconsistency | No serious indirectness | Undetected | 0.73 (0.58-0.92) | 8 fewer (12 to 2 fewer) |
| Serious Bleeding | 26475 (8) | No serious limitations | No serious inconsistency | No serious indirectness | Undetected | 1.63 (1.34-1.99) | 6 more (3 more to 10 more) |
| Stroke | 28088 (9) | No serious limitations | No serious inconsistency | No serious indirectness | Undetected | 0.99 (0.71-1.37) | 0 more (2 fewer to 2 more) |
Partly contextualized ratings of certainty (typically used in systematic reviews and health technology assessments): Ranges of magnitude of effect
A partly contextualized option is to rate our certainty in a specific magnitude of effect (Table 1). For instance, we could consider whether the point estimate for a single outcome, were it accurate, represents a trivial, small, moderate, or large effect. We could then rate our certainty that the true effect for this outcome, expressed in absolute terms, lies within the boundaries of whatever we consider the range of a trivial, small, medium or large effect. This is completely analogous with our prior discussion of thresholds, but now we have two: one that represents the upper, and one the lower, limit of the designations small, medium, and large. This approach is likely to be particularly relevant for health technology assessments, or for systematic reviewers who believe the usefulness of their review will be enhanced by providing “plain language” to specify the magnitude of effect.
The challenge here is the specification of what we will consider trivial, small, moderate and large effects. This is likely to differ across outcomes. People may, for example, consider a reduction in deaths of 6 in 100 patients (e.g. from 20% to 14%) per year – or even less - a large effect. People are less likely to consider the same magnitude of effect as large if the outcome is much less serious (for instance, recurrent migraine headache). Ideally, in the future, consensus approaches could achieve a consensus regarding thresholds for trivial, small, moderate or large effects for a wide variety of outcomes.
To be optimally clear, the characterization of the size of effect would also require specification of the consequences against which the effect on the chosen outcome is being traded off. For example, one could specify that there are no harms, and a small effect would then be one large enough that, given the burden of administration, one would consider it worthwhile to use the intervention. For most interventions (e.g. taking one pill a day) this would be very small, and would represent the minimum of the range of a small effect. One would also require a way of specifying the upper range of what one considers a small effect, a challenge that remains in applying the approach.
Such thresholds would only apply to absolute effects (a relative risk reduction of 50% could mean a reduction from 2% to 1%, likely a small effect, or 40% to 20%, likely a large effect). If one used this approach in our example, one might specify that the effect of longer-duration DAPT on mortality was a small effect (2 in 1,000) and that we have high certainty it is small (the upper boundary of the confidence interval is 4 in 1,000, and there is no other reason to rate down certainty).
Considering myocardial infarction, one might specify that the point estimate represents an effect that is small (a reduction of 8 in 1,000) but we have only low certainty that the effect is indeed small. The rationale for this judgment would be as follows. The confidence interval includes 2 in 1,000 fewer, an effect that many might consider trivial, and 12 in 1,000 fewer, an effect that (arguably) many might consider moderate. In addition, however, we found serious inconsistency in results (point estimates of relative effect ranged from 0.49 to 1.08, I2 36%)‡, and this raises the possibility that the effect might either be trivial or no effect at all, or might be moderate. One could apply similar logic to bleeding and stroke outcomes.
When the CI of effect estimate overlaps the null effect, as in this case for the outcome of stroke (i.e., relative association measure of 1 or absolute association measure of 0), two conclusions are possible: 1) the evidence is imprecise (e.g., small number of events) and we are unable to reliably answer the question of effectiveness; or 2), the evidence is precise and the intervention is in fact not effective, or the effect is trivial. To make the latter inference (no or trivial effect), the CI needs to be sufficiently narrow to exclude the threshold of whatever one considers the lower boundary of a small effect. If the CI is tight and does not cross this threshold, one can infer that the effect is null or trivial (and presumably, the intervention, with respect to that effect, is not worth considering). If the CI is wider and is not contained within this threshold, the conclusion would be that the evidence is imprecise and cannot reliably exclude a small (or if very wide moderate or even large) effect.
In this context, the question is: how certain are we that the effect lies in a particular range, with boundaries on either side of a RR of 1.0? For stroke in our example, if one set boundaries at 0.70 and 1.38, certainty would be high. If one set narrower boundaries, one would rate down for imprecision. In terms of absolute effects, with a confidence interval of 2 fewer to 2 more strokes, one would likely conclude there is a precise estimate of a trivial or null absolute effect. Making a judgment that an intervention effect is no greater than trivial will, just as for the other ranges, require some degree of contextualization – for instance, the more important the outcome, the narrower the range in which one will be willing to conclude that there is no important effect.
Applying fully contextualized ratings (typically used in clinical practice guidelines) to individual outcomes
When, typically in the setting of a guideline or recommendation, we make fully contextualized ratings, we are simultaneously weighing the benefits and harms of every important outcome. Such contextualized ratings may best be addressed at the stage of the evidence to decision, rather than at an earlier point in the decision-making process (i.e. the evidence profile or summary of findings stage). At whatever stage a guideline panel decides to make the assessment, having separate certainty ratings for each outcome in the fully contextualized setting can inform patients, clinicians, and researchers as to where there are important gaps in medical knowledge.
The fully contextualized ratings of individual outcomes do not altogether resolve the issue of how best to rate the certainty in the net benefit. Indeed, alternative approaches to addressing certainty of evidence in the fully contextualized setting, and in particular an overall rating of certainty in net benefit, are issues of ongoing GRADE working group activity.
When simultaneously considering all outcomes, however, the lowest rating of certainty among the critical outcomes will generally provide an upper limit for the overall certainty in the balance between desirable and undesirable health outcomes (i.e., the net benefit). This is what GRADE currently refers to as the overall certainty of evidence (7) and thus – pending further conceptual development – it provides an interim approximation of certainty in the net benefit. Once an approach is developed for assessing certainty in the net benefit, fully contextualized ratings for individual outcomes may no longer be needed.
One approach to a fully contextualized rating of net benefit would be using a decision model, in which sensitivity analyses would highlight which outcomes are capable of tipping the model (altering the overall result from benefit to harm or vice versa) over a range of plausible values for those outcomes. The approach to fully contextualized ratings we are suggesting in this article is analogous to the decision model approach. The approach involves differential weighting of the importance of outcomes, and allows a guideline panel to, without creating a decision model, address net benefit.
Making fully contextualized ratings of certainty – and indeed, deciding if one recommends for or against an intervention - requires first specifying values. The values should be those of the patients, and GRADE (8) and others provide guidance regarding how to obtain estimates of those values. The process includes a systematic review of the relevant literature (9), the experience of the topic experts in conducting shared decision-making, consultation with patients and patient groups, and conduct of targeted surveys (10–12).
In the DAPT example, a guideline panel might note that they believe that typical patients would value a myocardial infarction and serious bleed similarly, place an appreciably greater value on stroke (say, 3 times the value of a bleed or myocardial infarction) and an even greater value on death (say, 5 times the value of a bleed or myocardial infarction).
Prior to considering uncertainty, the initial judgment regarding a recommendation would be its direction. That judgment can initially be based on the point estimates of effects and the associated values and preferences. On occasion, the point estimates may suggest a direction of recommendation but after full consideration of the certainty range for all outcomes the final recommendation may be in the opposite direction. Nevertheless, focusing on the point estimates provides a useful starting point. In this case, using the weights we have specified above, the reduction in MI of 8 in 1,000 over one year suggests a recommendation in favor of longer-duration DAPT, but the increase in bleeding of 6 and in death of 2 more than balances the benefit. Thus, the recommendation would be against the use of longer-duration DAPT.
Having decided on the direction, let us consider the certainty rating, beginning with the outcome of death. Since, for mortality, there are no serious limitations in risk of bias, consistency, directness, or publication bias, the certainty (high or moderate) depends on the judgment of precision. One might start the process of rating precision by looking at the point estimates of outcomes other than the one under consideration (in this case, outcomes other than death). Considering the values and preferences mentioned above (equal value on MI and bleeding) and ignoring mortality, if longer-duration DAPT reduced myocardial infarctions by 8 in 1,000, increased bleeds by 6 in 1,000, and did not change the incidence of stroke, we would recommend in favor of longer-duration DAPT (though barely, it would be a close call).
Now, what if longer-duration DAPT actually had no effect on mortality (a risk difference of 0, at one boundary of the confidence interval)? We would continue to recommend longer-duration DAPT. What about the other end of the confidence interval, an increase in 4 deaths. Were this the case, we would surely recommend against longer-duration DAPT. Thus, because the decision differs at the opposite ends of the confidence interval, we rate down our certainty for imprecision.
Note, making this judgment did not require the likely painful obligation of specifying the exact mortality threshold between recommending for or against. All we needed to know is that our decision differed at either end of the confidence interval and, therefore, the threshold must lie somewhere within the confidence interval, in this case between 0 and 4 deaths.
Let us now consider MI. Because we have already identified problems in inconsistency, the rating of certainty may begin as moderate, and we will examine whether to rate down for imprecision. Putting MI, the outcome under consideration, aside, and looking at the point estimates of the other three outcomes, would we recommend longer-duration DAPT? Given the increase in death of 2, and an increase in bleeding of 6, surely not.
Would the possible effect on MI change this recommendation? The minimum reduction in MI (2 events) would clearly not change the recommendation, but what about the maximum (12)? Given the value of death (5 X that of MI, so 2 increased deaths would have a weight of 10) and the point estimate of bleeding increase (6), the net disutility of death and bleeding is greater than the utility advantage of reducing MI even by 12/1,000 per year. Therefore, considering only point estimates, even the largest plausible reduction in MI is less than would be required to compensate for death and bleeding, and considering point estimates alone, we do not need to rate down for imprecision.
One should, however, also consider the uncertainty in estimates of effects on the other three outcomes. Could one imagine a constellation of possible effects of longer-duration DAPT on outcomes other than MI that would lead one to recommend longer-duration DAPT if the true effect on MI were a reduction in 12? Certainly: if there were an increase in death of only 1/1,000 rather than 2 (very plausible as a potential truth), the utility of the reduction of 12 MIs would be greater than the disutility associated with death and serious bleeding. Thus, given the uncertainty across outcomes, it is possible that the reduction in MI warrants use of longer-duration DAPT and we should rate down for imprecision.
Note again that the process did not require an exact specification of a threshold. The requirement was only to decide whether the decision changes depending on the extremes of the confidence interval. If it does not, then the entire confidence interval is on one side of the threshold (as it was when we considered only point estimates) in which case we needn’t rate down our certainty. Alternatively, the threshold may be within the confidence interval boundaries (as it was when we considered uncertainty in non-MI outcomes) therefore requiring rating down for imprecision. Our experience suggests that these decisions will be easier than attempting to define an exact threshold.
The consideration of optimal information size (OIS)
Previous GRADE writings have suggested using the OIS as a possible primary item for rating imprecision (2) (i.e. considering whether the total number of participants in the included trials is more than the number of patients generated by a conventional sample size calculation for a single adequately powered trial). This way of rating imprecision is not compatible with the approaches described in the current paper. However, whichever of our suggested approaches reviewers are using, they will sometimes confront large effect sizes with apparently satisfactory confidence intervals despite modest sample size. Because such findings are untrustworthy – experience has shown that these large effects typically decrease or disappear as data accumulate – they require consideration of the event rate using OIS or closely related alternative approaches (4).
Presentation of certainty of evidence in the context of clinical practice guidelines
Both fully and less or non-contextualized ratings represent, in the guideline context, options for presenting certainty (in, for instance, evidence to decision tables). An advantage of choosing less contextualized ratings is that such a presentation may be more useful for another group that wishes to adapt the guideline to a different context (with, for instance, different resource constraints or different typical values). Doing so, however, admits that this preliminary certainty rating often will differ from the fully contextualized rating that the panel must use in deciding on the direction and strength of their recommendation.
Indeed, the advantage of choosing the fully contextualized approach is that the simultaneous consideration of all outcomes, and the implications for certainty of evidence, determines the ultimate direction and strength of recommendations. High or moderate certainty with a large gradient between desirable and undesirable outcomes will dictate a strong recommendation. Low certainty, or a small gradient between desirable and undesirable outcomes, will generally dictate a weak recommendation.
In making the decision regarding whether to present less or non-contextualized ratings of evidence along with certainty ratings that drive the direction and strength of their recommendations, guideline panels may want to consider both what is optimal for the process of coming to the recommendation, and what will be most helpful for the ultimate consumers of the guideline. They should specify, clearly and explicitly, which approach to setting thresholds for effect they used.
Some limitations in the discussion
Bayesian thinking, and formal Bayesian statistics, would provide an alternative approach to the questions we have addressed in this article. This would be interesting to pursue, but is beyond the scope of the current discussion because, currently, guideline developers seldom use the approach.
Formal decision analysis based upon expected utility theory provides a structure for the simultaneous consideration of multiple outcomes, including uncertainty in estimates. Methodologists have suggested alternative quantitative approaches to decision-making that rely on explicit specification of values and preferences (13, 14). Decision analysis, as well as alternative quantitative approaches, could therefore be a potential solution to the challenges we raise in our discussion of the “fully contextualized” rating. Clinical practice guidelines seldom, however, involve formal decision analysis. Decision analysis has had even less impact on individual patient decision-making, and newer quantitative methods have not yet stood the test of time. Ultimately, such approaches may provide an alternative framework for the simultaneous consideration of all outcomes.”
Conclusions
This article has addressed the following question: when we rate certainty of evidence, what exactly is it in which we are rating our certainty. The answer we have provided is that we are rating our certainty that the true effect lies on one side of a particular threshold, or in a particular range. What follows from this is the desirability for systematic review authors, guideline panelists, and health technology assessors to specify the threshold or ranges they are using. We have presented how this might be done in the fully contextualized setting and presented alternatives for less-contextualized settings. Future research can assess which approaches are most useful for different settings and target groups, as well as how best authors can communicate the threshold or ranges they are using. Finally, although all our examples relate to intervention effects, the guidance that ratings of certainty should specify the relevant thresholds underlying the judgments also applies to questions of diagnosis and prognosis.
Supplementary Material
Acknowledgments
The authors would like to thank colleagues at the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) who, through participating in a series of seminars, contributed with valuable input on the initial draft of the presented approaches. The authors also thank all GRADE Working Group members who have contributed to the paper during group discussions at Grade Working Group meetings.
The Health Services Research Unit, University of Aberdeen, receives core funding from the Chief Scientist Office of the Scottish Government Health Directorates. The Parker Institute, Bispebjerg and Frederiksberg Hospital is supported by a core grant from the Oak Foundation (OCAY-13-309). SVK is funded by a NRS Scottish Senior Clinical Fellowship (SCAF/15/02), the Medical Research Council (MC_UU_12017/13 & MC_UU_12017/15) and Chief Scientist’s Office (SPHSU13 & SPHSU15).
Footnotes
We recognize that in the context of policy making and guideline adaptation contextualization may refer to considering local circumstances and other criteria such as available resources, legal frameworks, cultural issues, feasibility, and equity. We use the term contextualization here differently, that is only referring to clinical thresholds. Additionally, a recommendation might be considered to only become fully contextualized when it is interpreted in the context of a specific patient who has individual values and preferences.
The fifth domain, imprecision, is not presented in this table because the assessment of imprecision is dependent on the chosen threshold or range (Table 1).
Inconsistency and imprecision are related to each other and rating the certainty in the evidence should consider this relation, in particular, when random effects models are used to pool effect estimates. In this case, we recognize that the use of a random effects model (which we used to calculate this confidence interval) accounts for some of the inconsistency by widening this confidence interval. However, we believe not all of the inconsistency is expressed in the confidence interval and the true certainty range is wider than expressed.
References
- 1.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol. 2011;64(12):1283–93. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Welch VA, Akl EA, Pottie K, Ansari MT, Briel M, Christensen R, et al. GRADE Equity Guidelines 3: Health equity considerations in rating the certainty of synthesized evidence. J Clin Epidemiol. 2017 doi: 10.1016/j.jclinepi.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schunemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? J Clin Epidemiol. 2016;75:6–15. doi: 10.1016/j.jclinepi.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Schunemann HJ. Guidelines 2.0: do no net harm-the future of practice guideline development in asthma and other diseases. Curr Allergy Asthma Rep. 2011;11(3):261–8. doi: 10.1007/s11882-011-0185-8. [DOI] [PubMed] [Google Scholar]
- 6.Spencer FA, Prasad M, Vandvik PO, Chetan D, Zhou Q, Guyatt G. Longer-Versus Shorter-Duration Dual-Antiplatelet Therapy After Drug-Eluting Stent Placement: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(2):118–26. doi: 10.7326/M15-0083. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66(2):151–7. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726–35. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e1S–23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darzi AJ, Officer A, Abualghaib O, Akl EA. Stakeholders' perceptions of rehabilitation services for individuals living with disability: a survey study. Health Qual Life Outcomes. 2016;14:2. doi: 10.1186/s12955-016-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santesso N, Schunemann H, Blumenthal P, De Vuyst H, Gage J, Garcia F, et al. World Health Organization Guidelines: Use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2012;118(2):97–102. doi: 10.1016/j.ijgo.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Schunemann HJ, Hill SR, Kakad M, Vist GE, Bellamy R, Stockman L, et al. Transparent development of the WHO rapid advice guidelines. PLoS Med. 2007;4(5):e119. doi: 10.1371/journal.pmed.0040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puhan MA, Singh S, Weiss CO, Varadhan R, Boyd CM. A framework for organizing and selecting quantitative approaches for benefit-harm assessment. BMC Med Res Methodol. 2012;12:173. doi: 10.1186/1471-2288-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu T, Fain K, Boyd CM, Singh S, Weiss CO, Li T, et al. Benefits and harms of roflumilast in moderate to severe COPD. Thorax. 2014;69(7):616–22. doi: 10.1136/thoraxjnl-2013-204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.