Abstract
Implantation of drug-eluting stents (DESs) via percutaneous coronary intervention is the most popular treatment option to restore blood flow to occluded vasculature. The many devices currently used in clinic and under examination in research laboratories are manufactured using a variety of coating techniques to create the incorporated drug release platforms. These coating techniques offer various benefits including ease of use, expense of equipment, and design variability. This review paper discusses recent novel DES designs utilizing individual or a combination of these coating techniques and their resulting drug release profiles.
Keywords: Coronary stents, Drug-eluting stents, Stent coatings, Drug release kinetics, Interventional cardiology
Background
Atherosclerosis is a disease characterized by clogged vasculature due to buildup of fatty substances along the vessel wall known as plaque or atheroma [1]. This condition is a subset of a cardiovascular disease (CVD), the leading annual cause of death, affecting millions of lives worldwide. In the U.S. alone, incidence of cardiovascular procedures has tripled in the last decade and this trend is expected to continue due to the aging population, increasing numbers of obese and diabetes mellitus patients [2]. Treatments for atherosclerosis are necessary to restore blood flow and can include atherectomy (direct removal of the atheroma), angiogenesis (formation of new blood vessels from existing vasculature), brachytherapy (localized radiotherapy), bypass grafting (circumventing blocked vessel with clean vessel from a donor), and angioplasty (reopening of vessel using balloon or stent) [3].
Balloon Angioplasty
Angioplasty, as a treatment for CVD, emerged in the 1980s in the form of balloon angioplasty, a minimally invasive procedure to open the blocked vessel by inflating a balloon, on the end of a catheter, at the site of the obstruction. In the late 1980s and early 1990s, after balloon angioplasty had become a popular treatment option, it was found that 4–8% of patients suffered abrupt closure of vessels and more than 20% required emergency coronary artery bypass surgery due to occurrences of endothelial dysfunction with rapid gathering of platelets and fibrin, disruption of atheromatous plaque, elastic recoil of the vessel, and postprocedural arterial shrinkage [4,5]. To prevent these complications, a more permanent solution was developed in the form of bare metal stent (BMS) implantation.
BMSs
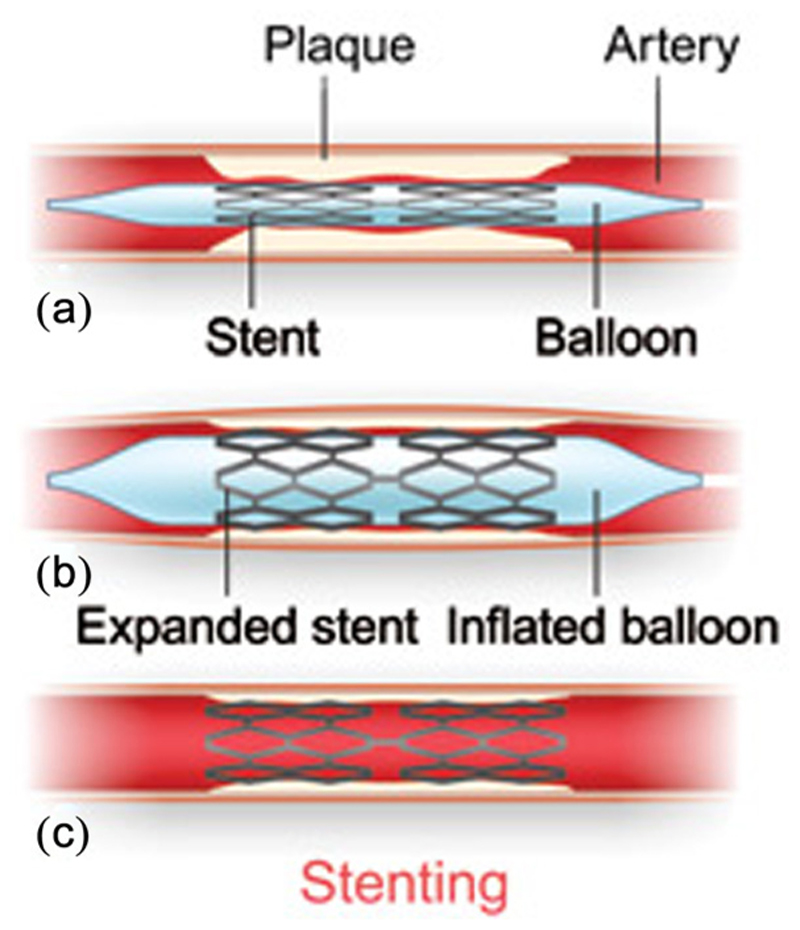
BMSs became the standard of care for angioplasty operations in the late 1980s after positive results from clinical trials in North America and Europe [3]. BMSs are implanted using minimally invasive techniques via a catheter to the occlusion site (Fig. 1). There, the wire mesh cage of the BMS acts as structural support to hold open the vessel and re-establish the blood flow. Unfortunately, implantation of the BMS causes an endothelial injury to the surrounding cells, eliciting an inflammatory response, activating white blood cells and platelets, and causing the release of vasoconstrictors, cytokines, and growth encourages smooth muscle cell and myofibroblast migration toward the lumen and overproliferation at the site of the stent [7].
Fig. 1. Diagram of stent implantation.
The metal stent is fed via a catheter to the occlusion site and locally expanded (Reproduced with permission from Texas Heart Institute [6]. Copyright 2014 by Texas Heart Institute).
These cascading events are known as neointimal hyperplasia, an example of restenosis, and can result in vessel re-occlusion at the implant site, necessitating re-intervention in 20–25% of patients within 6 months of stenting [3]. When defined as greater than 50% reduction in luminal diameter, restenosis was found by Birkenhauer et al. to occur in 30–50% of patients at a 6-month follow-up as determined by intravascular ultrasound (IVUS) [7]. Another complication after stenting with BMS is stent thrombosis (ST), but the use of IVUS during implantation and the establishment of dual antiplatelet therapy regimens for patients after the procedure reduced the rate of ST with BMS to its current 1.2% [3]. To combat the high incidence of restenosis, researchers developed BMS with the addition of a localized drug release platform in order to stop the overproliferation of vascular smooth muscle cells (VSMCs) at the implant site.
First Generation DESs
The first generation DESs were approved by the U.S. Food and Drug Administration (USFDA) in the two years following the introduction of the technology to Europe in 2002. Among them are Cypher™ (Cordis Corporation, Freemont, CA, approved April 2003 [8]) and Taxus™ (Boston Scientific, Marlborough, MA, approved March 2004 [9]). The Cypher™ stent elutes sirolimus from a nonbiodegradable porous polymer layer made of poly(ethylene-vinyl acetate) (PEVA) and poly(butyl methacrylate) (PBMA). The Taxus™ stent releases paclitaxel from a nonbiodegradable, nonporous, coating of poly(-styrene-b-isobutylene-b-styrene) (SIBS). These devices, as the latest evolution of cardiovascular technology, quickly became the popular choice of implant, with more than 90% of all DES implantations occurring in the U.S. and Europe by 2005 [3].
Soon, problems arose involving stent complications after insertion. Multiple meta-analyses of patients who received DES showed errors with various aspects of the devices. It was determined that the permanent polymer coating on the first generation DES was linked to chronic inflammation and delayed arterial healing [10]. Joner et al. found similar results in published animal studies showing comparable devices to the approved DES resulted in substantial decreases in arterial healing relative to BMS [11]. The same group reviewed autopsies of DES and BMS patients, finding that, of the DES cases, 61% had late ST (LST) (i.e., ST > 30 days after implantation), while only 8% of the BMS cases did [11]. LST-related events confirmed by angiography, autopsy-confirmed ST, target vessel-related death, or myocardial infarction (MI) were shown to be twice as frequent in patients with first generation DES compared to those with BMS (2.6% versus 1.3%) [12]. A meta-analysis by Farb et al. revealed that after 6 months, DES implant sites showed incomplete healing, fibrin deposition, and the presence of inflammatory cells, suggesting a hypersensitivity reaction to the DES polymers [13]. Another analysis reviewed autopsies to study the long-term effects of Cypher™ and Taxus™ finding constant fibrin deposition and less endothelialization compared with BMS. It has also been shown that across different time points, BMS showed greater vascular healing when compared to first generation DES [11]. With this wealth of information, in 2007, the USFDA acknowledged a small but significant increased risk of LST with DES [14].
Currently Available Stents
To address the shortcomings of the first generation DES, researchers have created dozens of novel stent designs by incorporating new drugs with numerous degradable and nondegradable polymers. Thrombosis, which took hold as a significant problem associated with first generation DES, still exists to varying degrees with currently available devices. The cause of thrombosis is not entirely agreed upon, but due to disparities about what constitutes thrombosis, the Academic Research Consortium outlined a uniform definition for ST, including the distinction between probable and definite ST [15,16]. It is generally accepted that early ST is defined as incidence occurring within 30 days of implantation and LST after 30 days.
Most incidents of ST occur within 30 days [17] but the overall risk of ST with DES is between 0.5% and 3.1% [18]. A collaboration between hospitals in Rotterdam, The Netherlands, and Bern, Germany, found that over a period of three years, patients implanted with sirolimus-eluting stents (SES) and paclitaxel-eluting stents had a 2.9% risk of ST three years after implantation. This report also found that the risk of ST increased 0.6% every year [19]. LST has been shown to have a mortality rate of 45–75% with 25–65% suffering nonfatal MI [3,20,21]. However, this data may underestimate the problem of ST because most events happen outside of hospitals so angiographic or autopsy evidence is not available [12].
While there is very little agreement as to the physiologic mechanism behind ST, multiple studies have been performed to find commonalities between DES with higher rates of ST. Kolandaivelu et al. found that thin-strutted stents were 1.5-fold less thrombogenic than thick-strutted DES with the same polymer and drug concentrations [22]. In addition, stent malapposition and underexpansion caused by inadequate stent deployment have been shown to significantly increase thrombogenicity of the stent [22,23]. Cook et al. found that 80% of patients who presented with ST showed stent-wall malapposition when imaged with IVUS [24]. It has also been suggested that incomplete endothelialization and the presence of fibrin long after 30 days of implantation establish the critical pathology underlying LST [11].
Drug Release Kinetics
It is believed that VSMCs start to hyperproliferate 24 hrs after stents implantation and continue for around 2 weeks [25,26]. Therefore, antirestenotic drugs need to be delivered for at least 3 weeks after stent implantation to prevent smooth muscle cell proliferation and migration into the stent [25,27,28]. Several methods exist to deliver drugs to vessels in order to stop restenosis including perivascular, intraluminal, and endoluminal drug delivery [29]. DES is an example of endoluminal drug delivery because the drug is release slowly, disrupting smooth muscle cell cycle and proliferation [30]. The physical mechanisms determining the rate at which a drug is released from the stent include drug molecule diffusion through a polymer layer, drug release via osmotic pressure, and ion exchange to release ionized drugs [25].
DES Coating Design
Multiple variables can be adjusted to optimize drug release for cardiovascular stent applications, including biocompatible polymers and antirestenotic drugs. While the presence of a polymer coating on a DES is not required, the process to choose one can be difficult because it has been previously shown that most polymers listed as biocompatible and used in medicine can cause vascular inflammation [31]. A more detailed study performed by van der Giessen et al. showed that poly(lactic-co-glycolic acid) (PLGA), poly(caprolactone) (PCL), poly(hydroxybutyrate valerate), polyorthoester, poly(ethylene oxide)/poly(butylene terephthalate), polyurethane, silicone, and poly(ethylene terephthalate) encompass both biodegradable and nonbiodegradable polymers, all cause inflammatory reactions [32].
A polymer showing superior stability in animal models does not ensure viability in vivo. The SIBS polymer coating on Taxus™ stents was shown to not have any polymer degradation after 2.5 yearr implantation in rabbit and porcine models [33], but has consistently shown higher incidence of ST even when compared to the Cypher™ stent [34]. Some have postulated that stents should be entirely degradable so as not to illicit an inflammatory reaction in the vessel [35].
The active ingredient in DES is the released drug. For that reason, careful consideration has been taken when choosing the pharmacologic agents incorporated in the second and third generation DES designs. Roiron et al. published a meta-analysis of DES showing that SES, and its derivatives, had less incidence of restenosis and target lesion revascularization than paclitaxel (and its derivatives) when compared to BMS [34]. Everolimus-eluting stents (EES), used in second generation DES, were shown in multiple clinical trials to have decreased rates of restenosis and ST compared to commercially available BMS [36]. In addition, EES were shown to have the lowest incidence of ST compared to BMS and other DES throughout early and late time periods [10,17].
Stent Coating Techniques
The physiologic function of the DES first comes to fruition with the technique used to coat the stent, creating the designed release platform. Many techniques exist, each offering its own benefits and drawbacks that must be considered when choosing between them.
Dip Coating
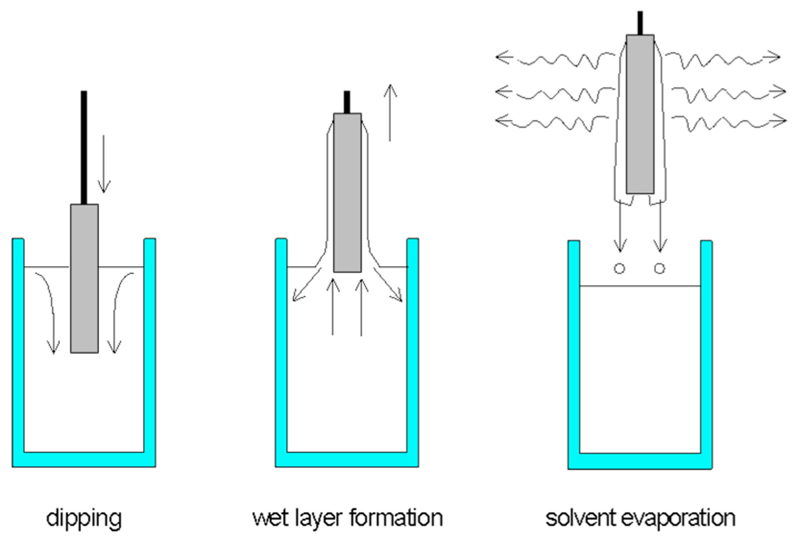
Dip coating is a basic technique that does not require extensive machinery or time. It involves submerging the stent in a solution of typically drug and/or polymer in a solvent. The stent is then left to dry, allowing for evaporation, in the air or an oven (as shown in Fig. 2 [37]). Using this method, the polymer, drug, and their respective concentrations can vary. For instance, Jang et al. coated stents with either a low dose or high dose of curcumin without the presence of any polymer. This group found that equal masses of drug were released from each design when immersed in a release medium, at 37°C, over the same length of time. As such, the low dose design (50 μg) released all of its drug within 3 days while the high dose (500 μg) only released 25% of its loaded drug in 21 days [38]. In 2012, a team led by Acharya dip coated a nitric oxide prodrug, S-nitrosoglutathione, loaded inside a degradable layer of PCL onto metal stents. The group measured the release profiles of this formulation in multiple layers by coating the stents up to three times. They found that an increase in the number of dip coats correlated to an increased burst release effect but the overall drug release evened out at around 70–80% by day 11 for all designs [39]. Utilizing multiple coating techniques in the same design, Song et al. first deposited titanium oxide (TiO2) thin films on BMS using plasma-enhanced chemical vapor deposition then grafted heparin, a-lipoic acid, and abciximab onto the hydroxylated stent surface using dip coating [40]. All release profiles were performed by incubating samples in PBS (pH 7.4) at physiologic temperature with 10% (v/v) ethanol with no agitation. Abciximab samples stopped releasing drug after 10 days while heparin-loaded samples continued to release some drug up for 4 weeks [40]. The group published their release pro-files as a function of drug concentration released at each day, rather than a cumulative (of the total drug) release percentage, and did not include any significance figures or error bars for their data, making additional comparisons with other designs more difficult.
Fig. 2. Dip-coating schematic.
With a controlled speed, stent is submersed in the coating solution then removed and allowed for solvent evaporation, leaving the drug/polymer solution on the stent surface (Reproduced with permission from Schmidt and Menning [37]. Copyright 2000 by Sol Gel Gateway).
Not all release platforms are studied with the three-dimensional model of a metal stent, some preliminary testing is performed on two-dimensional models, relieving the material burden of needing large quantities of BMS. Park et al. utilized the layer-by-layer (LbL) dip-coating technique to coat hyaluronic acid films with PEG-PLGA micelles layered between charged linear polyethylene imines [41]. The group compared the release of coumarin 30 and paclitaxel loaded inside the polymeric micelles. Coumarin 30 samples were kept at room temperature in physiologic buffer with 0.05% (w/v) polysorbate 20 and exhibited a burst release of 54% in the first 2 days followed by a prolonged total release of 100% by day 25. Samples containing paclitaxel-loaded micelles, with either 10 or 20 coated layers, were incubated at 37°C in physiologic buffer. Paclitaxel release profiles showed that samples coated with ten layers released 100% of the encapsulated drug within 3hrs and those coated with 20 layers release 60% total drug in 6 hrs, 80% in 3 days, and 100% by day 15 [41]. The problem with solvent-based coating techniques, like dip coating, is that they can result in complications such as bridging, pooling, and lack of uniformity, especially with coating thicknesses less than 0.5 mm [42,43]. These complications create difficulties when progressing stent designs toward commercialization. Dip coating can be used when performing introductory studies on a novel stent but more advanced techniques may be required to increase production of the device.
Electrotreated Coating
To expand available options for coating techniques, researchers have begun incorporating electrical stimulus into their stent coating techniques to assist in drug/polymer deposition onto the stent surface or to increase polymerization on an already deposited drug release layer. Okner et al. performed the latter to include the presence of an electrocoated tricopolymer loaded with paclitaxel on its stent. The release pro-files of the electropolymerized coatings were evaluated with and without the presence of a methacrylate-derivative polymer topcoat (to act as a drug diffusion barrier). The presence of a topcoat reduced the burst release of the design from 30% to 17% in 1 day but did not change the subsequent rate of release, leading to a total drug release of 80% without the diffusion barrier and 60% with the diffusion barrier [44]. In 2009, Levy et al. published the work with their DES design of electrocoating 4-(1-dodecyloxy)-phenyldiazonium tetrafluoroborate (C12-phenyldiazonium) onto metal stents in order to increase polymer adhesion, finding that electrocoated stent showed a larger burst release of 80% loaded paclitaxel after 1 day versus 55% release from purely spray-coated SS stents [43]. That same year, Shaulov et al. coated a SS stent by electrochemically reducing 4-(2-bromoethyl)benzenediazonium tetrafluoroborate (BrD) onto the surface to form carbon–metal covalent bonds [45]. This coating was used to initiate atom transfer radical polymerization of methyl methacrylate into PMMA brushes on the SS exterior. BrD, PMMA-coated BrD, and BMS samples were then spray coated with a paclitaxel-loaded PEVA/PBMA solution, with some coated with an additional PBMA topcoat to act as a drug diffusion barrier. BrD and BMS samples showed a one-day burst release of 52% and 64.5%, respectively, while the presence of a topcoat reduced the burst releases of each design by over 30% [45]. The presence of a PBMA topcoat in each of the designs changed the overall shape of the release curve but resulted in equivalent cumulative release percentages at day 21.
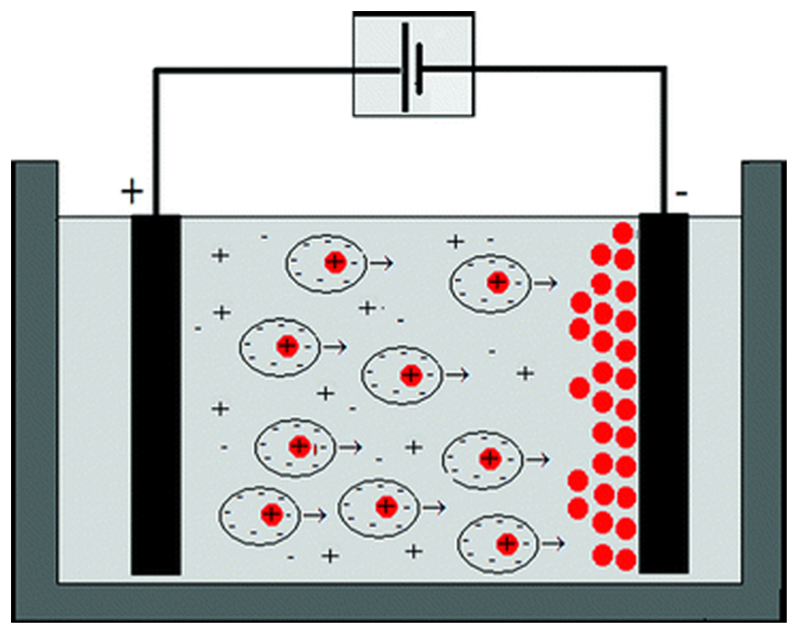
Electrophoretic deposition (EPD) is a technique that uses an electric field (either in a dry environment or in solution) to attract charged particles onto the stent surface, resulting in the formation of a drug release layer; a schematic of the apparatus is shown in Fig. 3 [46]. Wang et al. used EPD to coat 316L SS stents with a thin layer of carbon nanotubes (CNTs) topped with a composite layer of CNTs and rapamycin-loaded magnetic mesoporous silica nanoparticles (MMSNs). Their design resulted in a burst release of 50% loaded rapamycin in the first day followed by a slower rate maxing out at 98% loaded drug release by day 10 [47]. Their design was found to have faster endothelialization than the Firebird II stent (a previous but similarly designed stent); however, the nontoxicity of their stent design can be called into question based on the presence of CNTs.
Fig. 3. EPD in solution schematic.
Drug and polymer particles are deposited onto the stent surface via an electrostatic attraction within the coating apparatus (Reproduced with permission from Ammam [46]. Copyright 2012 by Royal Society of Chemistry).
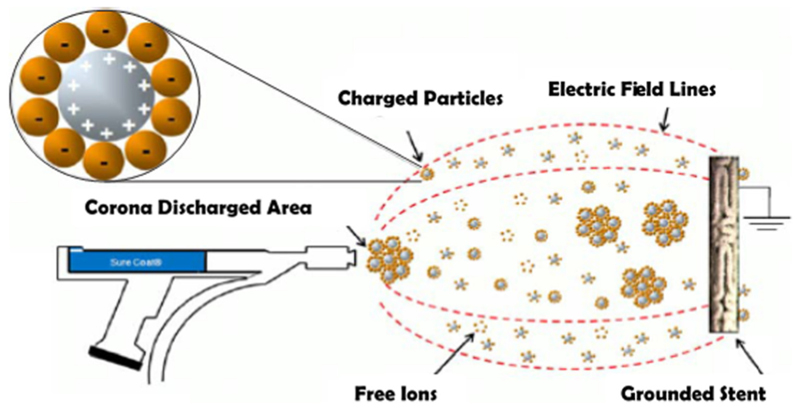
Utilizing electrostatic dry powder deposition (Fig. 4), Nukala et al. coated stents with sirolimus-loaded PEVA and PBMA microparticles, comparing the resulting release profiles to those of the Cypher stent [48]. Though this design used the same polymers as the Cypher stent, it exhibited a three-day burst release of 50% compared to 35% release by Cypher as well as a 100% total release after 25 days compared to 85% release by Cypher [48].
Fig. 4. Electrostatic dry powder deposition schematic.
Charged drug and polymer particles are deposited onto the stent surface via the apparatus shown above (Reproduced with permission from Nukala et al. [48]. Copyright 2009 by Springer Science+Business Media, LLC.).
Designing another DES coated using multiple techniques, Liu et al. deposited N-nitrosomelatonin (NOMela)-loaded PLGA nanoparticles using EPD onto SS 316L stents then used dip coating to create a diffusion barrier of collagen [49]. This work studied the release profiles of a model hydrophobic and hydrophilic drug after immersing the stent in PBS (pH 7.4) with and without 5% (v/v) Tween 80, respectively. Samples including a collagen top layer showed a burst release of 50–70% in 24 hrs with another 20% of the encapsulated drug released between day 2 and day 14 [49]. Overall, the integration of an electrical stimulus to aid in stent coating presents itself as an interesting development but the safety and efficacy of the electrotreated stents have not been evaluated in clinical models, only in noninferiority animal models.
Plasma-Treated Coating
Most recently, groups have started to include plasma treatments into their coating designs in order to strengthen the chemical bonds in the drug release layer via polymer crosslinking. This technique involves exposing the base metal or polymer coated stent surface to a gaseous plasma beam for varying lengths of time. A group led by Hagiwara evaluated the potential of this as a release platform for DES by plasma-treating silicon wafers coated with curcumin-loaded PEVA, studying specifically the effects of exposure to argon, oxygen, and nitrogen plasma over varying lengths of time between 0 s and 45 s. They found that untreated stents released up to 120 μg of drug in 14 days while highly treated samples only released between 5 and 50 μg (depending on the gas) in the same time frame [50]. The release curves of samples plasma-treated for longer time periods appeared to plateau even though they had not released nearly as much of their loaded drug as the untreated samples, suggesting that the plasma treatment caused the curcumin to get trapped in the crosslinked PEVA. While this technique presents a relatively simple way to increase the intermolecular strength of the stent coating, it is still in the fledgling stages of development, for this application, so most related studies have only been to establish its effect on drug release profiles.
Spray Coating
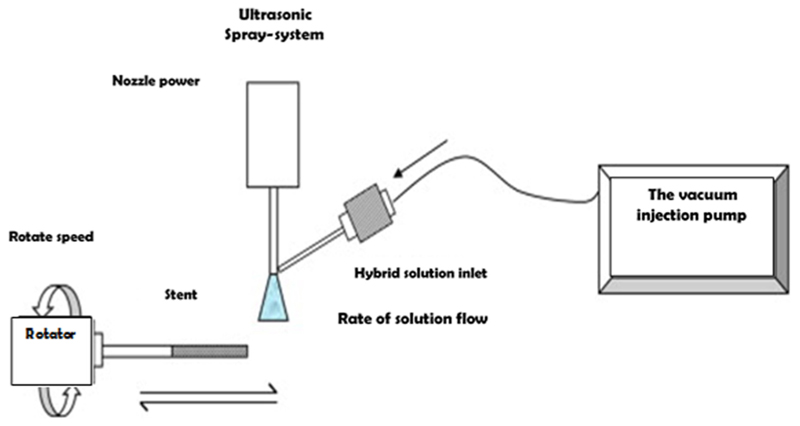
The most commonly utilized stent coating techniques include ultrasonic atomization, electrohydrodynamic jetting, and air-brush spray coating. These techniques use apparatuses that spray polymer and drug solutions (using various solvents) onto a stent, enabling consistent deposit of a uniform drug release layer(s) onto the stent surface. For the purposes of this discussion, an amalgamation of these techniques will be referred to as spray coating. Spray coating can be performed using several systems, one of which is described in Fig. 5. This technique allows for higher variability of coating designs, resulting in better optimization of the release profile. In general, this technique exhibits a logarithmic release curve characterized by a burst release, caused by the presence of the drug at the boundary layer between the stent and the surrounding vascular environment, followed by a slower release of drug to enable long-term therapeutic effects.
Fig. 5. Spray coating system schematic.
Drug/polymer and solvent solution(s) is sprayed onto rotating stent to provide consistent coating along the stent’s luminal surface (Reproduced with permission from Shanshan et al. [60]. Copyright 2012 by Elsevier B.V. Publishing).
The team of Gallo and Mani developed a dual DES (DDES) to release paclitaxel and nitric oxide (NO) from abluminal and luminal stent surfaces, respectively. They first dip coated the stent with phosphonoacetic acid, then spray coated with paclitaxel, and dip coated with NO (polymer-free design). They found that stents coated with both drugs released less of each drug than their respective individually coated controls, showing a 50% burst release of paclitaxel in the first hour with 75% total released over 28 days [51]. Another polymer-free stent was created by Su et al., who spray-coated abluminal surfaces of 316L SS stents with Duraflo heparin and sirolimus. The group varied the number of layers of each drug and measured their corresponding release profiles in PBS (7.4) with either 10% or 20% (v/v) of ethanol incubated at 37°C with rotation at 120 rpm. Increased ethanol concentrations in the release medium increased the cumulative release of heparin with a total sirolimus release of 42% after 10 days (30% burst release in first day) [52]. The total release of each drug plateaus after 5 days, suggesting that significant amounts of the drug were trapped on the stent surface.
The difficulty with polymer-free release designs is that there is no rate-controlling mechanism for drug release. For this reason, most designs incorporate a nonbiodegradable or biodegradable polymer to optimize the release rate of the drug. Huang et al. spray-coated cobalt–chromium (CoCr) stents with a sirolimus and triflusal-loaded biodegradable polymer to treat restenosis and thrombosis, respectively. This DDES showed a 30% burst release of sirolimus in the first day with a subsequent linear release up to 60% at day 14 and 70% at day 30. In the same design (i.e., second drug release on the same stent), triflusal exhibited a burst release of 60% in the first day with almost 100% release by day 5, due to the hydrophilicity of the drug [53]. Accordingly, the inclusion of triflusal in this design was to combat early thrombosis rather than LST. Stents coated with a bilayer composed of a sirolimus, PLLA, 50/50 PLGA, and polyvinyl pyrrolidone (PVP) base layer with a PVP top layer (diffusion barrier) were designed by Thakkar et al. This design had a total sirolimus release of 65–85% in 48 days in a PBS (pH 7.4) release medium in an orbital shaker (60rpm) at physiologic temperature, with a burst release of 50–60% in the first 2 days [54]. The addition of the PVP top layer actually yielded a greater initial burst release and was concluded to have enhanced the overall drug elution rate. The group went a step further and showed that the in vitro cumulative release percentage for one of their stent designs matched its in vivo release at the same time scale. Data showing in vivo release profiles for their other designs were not included, making similarity comparisons across research platforms difficult. Petersen et al. developed a DDES for the abluminal release of sirolimus and the luminal release of atorvastatin from a PLA-coated metallic stent. While the release studies were performed at room temperature in a supplemented 0.9 wt.% NaCl solution, stents tempered at 80 °C released 60% of encapsulated drug over 1000 hrs [55].
The group of Raval et al. have published release profiles for multiple stent designs over the past few years. One such design involved spray-coating biodegradable polymers, PLCL and PVP, loaded with dexamethasone or sirolimus onto CoCr L605 stents. In PBS (pH 7.4) at physiologic temperature with 60 rpm agitation, release profiles showed 30% burst release of dexamethasone in 1 day, 50% in 2 days, and 80% release after 14 days [56]. Their sirolimus-loaded stent had a burst release of 20% and 27% with the presence of a PVP or 75/25 PLCL topcoat, respectively [57]. Release kinetic evaluation determined that drug release was based both on Fickian diffusion and effect of surface erosion during the initial burst phase. Their team also examined the effects of varying lactic acid to glycolic acid ratios in PLGA and PVP spray-coated stents. They found that the burst release effect was inversely proportional to the amount of LA in PLGA, with the highest burst release effect occurring through the 50:50 (LA:GA) PLGA formulation (50% release in the first day) [58].
A group led by Liu created completely degradable PCL stents by micro-injection moulding, then coating a drug release layer containing paclitaxel and PLGA onto the surface. They specifically compared the release profile effects of dip coating and spray coating this layer onto the stent surface. Dip coating was shown to have 100% drug release in 7 days with a near linear release. On the other hand, spray coating with multiple layers showed an exponential release plateauing between day 7 and day 21 before exhibiting a secondary burst release [59]. Total release time points moved from day 24 to day 30 to day 36 as the number of spraycoated layers increased. The development of a sirolimus-loaded 75/25 PLGA spray-coated SS stents led to the secondary conclusion that ethyl acetate as solvent for spray coating led to a smoother coating with lower toxicity compared to acetone [60]. This research also showed that increasing the amount of PLGA in the drug release layer led to a decrease in the burst release effect and a reduction in the total amount of drug released over the same time scale.
An emulsion of PLGA, ReoPro (abciximab), and PVA was sprayed onto 316L SS disks to study its potential as a release platform for DES. This research also found that increasing the wt.% of PVA correlated to an increased release rate of ReoPro, resulting in a burst release of 56% over the first 4 days [61]. Another group measured the release profiles of stents coated with a covalently grafted phospholipid/PEG mixed monolayer loaded with echinomycin. They found that the presence of a top layer of the monolayer without drug decreased the total drug release from 50% in 45 days to less than 30%, and an increase in the drug mass sprayed onto the stent yielded a slower release rate for the system [62]. The team of Bian et al. developed a novel coating for 316L SS stents by spraying sirolimus-loaded and paclitaxel-loaded poly(ethylene carbonate) (PEC) onto their surfaces. This design showed a steady release of sirolimus up to 48% at day 7 followed by a slower release up to 90% at day 54 and 10% paclitaxel release at day 4 followed by a steady release rate up to 32% at day 60 [63]. The addition of a PEC topcoat reduced the relative rates of each drug culminating in 62% sirolimus release at day 54 and 28% paclitaxel release at day 60. Their team decided that there was a need for a topcoat in the release design even though the release profile of the system without it could be deemed sufficient.
In order to evaluate the release profiles of paclitaxel-loaded PEVA compared to a blend of PEVA and PLGA (85:10), BMSs were spray coated and immersed in PBS (pH 7.4) with 0.05 wt.% Tween 80 at physiologic temperature and 120 rpm rotation [64]. The research showed that the PEVA control had 35% paclitaxel release in 5 days but only 40% total release by day 30. Stents coated with the PEVA/PLGA blend released 40% paclitaxel in 5 days and 65% total release at day 30, suggesting that the addition of PLGA allows for a more readily degradable drug release structure. Another group used plasma polymerization to attach l-lactide to the surface of 3D springs (to mimic the structure of BMS) and then sprayed a solution of sirolimus and PLGA onto the surface [65]. Plasma-treated samples showed a more linear drug release with 100% released by day 56. Those without plasma treatment had a larger percentage drug release in the first week (55% versus 40%) followed by a slower, relatively linear, release up to complete release by day 56 [65]. Finally, a DES that released monoclonal antibody (mAb), SZ-21, rather than an antirestenotic drug was developed by Wang et al. The stent was spray coated with SZ-21-loaded PLLA and release profiles established in solutions with flow rates of either 10mL/min or 20mL/min. Stents immersed in both solutions showed nearly linear drug release with those in 10 mL/min solution releasing 80% mAb at 312 hrs and those in 20 mL/min solution releasing 90% mAb at 220 hrs [66]. This data does follow intuition by showing that a faster flow rate resulted in more rapid release of the encapsulated compound.
Applying spray-coating techniques to manufacture DES and study resulting drug release profiles is a popular option in this area of cardiac research. Its popularity may be due, in part, to the idea that this is the easiest technique for scaling up stent manufacturing by outputting high volumes of consistently coated devices. The difficulty with evaluating this technology is that the immense number of variables manipulated makes broad comparisons between the individual designs nearly impossible.
Future of DES
Most of the groups designing third generation DES aim to address the problems with current DES, as detailed previously, including restenosis, acute thrombosis, and LST.
Bioabsorbable Stents
One of the possible solutions to this problem, not already discussed in this paper, is thought to be the development of bioabsorbable or bioresorbable stents. These stents offer multiple potential benefits including (1) a reduction in ST because no foreign materials would remain at the occlusion site after degradation, (2) no metal cage, allowing the vessel to return to normal vasomotion, adaptive shear stress, late luminal enlargement, and late expansive remodeling, (3) the possible elimination of the need for long-term continuation of dual antiplatelet therapy (DAPT), (4) resorption of the scaffold allows for an easier second surgery if a new device is needed, (5) the absence of metal in the stent scaffold allows for use of imaging modalities to more accurately externally visualize the device, and (6) tuneable resorption duration with multiple drugs encapsulated inside the stent [67]. Work categorizing the clinical benefits of bioresorbable scaffolds was published by Serruys et al. who showed that these devices were easily detectable by ultrasound and optical coherence tomography at 12 months postimplantation and had a one-year major adverse cardiac event rate similar to comparable clinically approved metallic stents [68]. Their research found that full endothelialization was not yet achieved within 12 months for these devices, so some work may need to be done to improve on those figures.
Nonthrombogenic Polymers
Considering associated with and the occurrence of thrombosis in currently available devices, materials chosen for use in DES should ideally display nonthrombogenic characteristics. The nanocomposite polymer polyhedral oligomeric silsesquioxane poly(carbonate urea) (POSS-PCU) has been shown to support endothelial cell growth without significant cell toxicity and can therefore be used in CVDs [69]. Furthermore, POSS-PCU has shown safety and efficacy in large animal models [70] and has shown to be nonthrombogenic through minimal adherence of platelets compared to currently approved vascular device materials [71]. While the next generation DES needs to be nonthrombogenic, more tailored designs for drug release platforms must be developed to match the therapeutic profiles necessary to combat the hyperproliferation of VSMCs.
Stents Promoting Re-Endothelialization
Another popular idea on how to address the shortcomings of current DES in novel devices is to design stents that promote the growth of new endothelium in the blocked vessel in order to restore healthy function of local tissue. Some devices, like the COMBO stent developed by OrbusNeich Medical [72], do this by attaching antibodies to the stent surface to promote the adhesion of circulating endothelial progenitor cells. As a nonpharmacologic tool to promote re-endothelialization, work with a rat model showed that daily exercise training after percutaneous coronary intervention (PCI) resulted in complete re-endothelialization of injured vessels and abolished neointima formation after balloon angioplasty and stenting [73]. However, some researchers believe that the inclusion of local delivery of an antirestenotic drug is unavoidable in order to treat neointimal hyperplasia [67].
Limitations
These examples represent widely studied designs but do not include all novel designs to address issues with current DES. Researchers have taken many different approaches to develop the optimal stent, and a future review may be able to detail additional designs including those using electrospinning processes or drug reservoirs for long-term drug release. The majority of the researches discussed are benchtop studies of novel stents because the scope of this analysis does not include clinical studies. The vast number of publications reporting clinical results requires its own review paper due to the abundance of information available.
Conclusions
While there are dozens of recent articles detailing novel DES designs, determination of optimal individual characteristics between the designs is incredibly difficult due to their extensive variability. When developing a new DES, each group must choose between various approved drugs, biodegradable/bioabsorbable/bioresorbable polymers, and coating techniques. After creating the device, the group must choose between release mediums to perform studies in, instrumentation to quantify released drug, and length of time to perform release studies. Within each of these categories exist several options, making direct comparisons between devices virtually impossible. To optimize DES designs using currently available materials, the exact physiology timeline of restenosis must be determined in order to know what drugs need to be delivered when. The publication of intravascular healing responses will encourage exact engineering of drug release technologies to fit the application of DESs.
Acknowledgment
Funding for this review in the form of a fellowship was provided for Ms. Livingston by the Whitaker International Program, and in part by The Wellcome Trust (099722).
References
- [1].NHS. Atherosclerosis. National Health Service; Redditch, UK: 2014. http://www.nhs.uk/conditions/atherosclerosis/Pages/Introduction.aspx. [Google Scholar]
- [2].Rao SV, Califf RM, Kramer JM, Peterson ED, Gross TP, Pepine CJ, Williams DO, Donohoe D, Waksman R, Mehran R, Krucoff MW. Postmarket Evaluation of Breakthrough Technologies. Am Heart J. 2008;156(2):201–208. doi: 10.1016/j.ahj.2008.01.036. [DOI] [PubMed] [Google Scholar]
- [3].Newsome LT, Kutcher MA, Royster RL. Coronary Artery Stents: Part I. Evolution of Percutaneous Coronary Intervention. Anesth Analg. 2008;107(2):552–569. doi: 10.1213/ane.0b013e3181732049. [DOI] [PubMed] [Google Scholar]
- [4].Kuntz RE, Piana R, Pomerantz RM, Carrozza J, Fishman R, Mansour M, Safian RD, Baim DS. Changing Incidence and Management of Abrupt Closure Following Coronary Intervention in the New Device Era. Cathet Cardiovasc Diagn. 1992;27(3):183–190. doi: 10.1002/ccd.1810270306. [DOI] [PubMed] [Google Scholar]
- [5].Serruys PW, Kutryk MJB, Ong ATL. Coronary-Artery Stents. N Engl J Med. 2006;354(5):483–495. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]
- [6].Texas Heart Institute. Balloon Angioplasty and Stents. Texas Heart Institute; Housto, TX: 2015. http://www.texasheartinstitute.org/HIC/Topics/Proced/angioplasty.cfm. [Google Scholar]
- [7].Birkenhauer P, Yang Z, Gander B. Preventing Restenosis in Early Drug-Eluting Stent Era: Recent Developments and Future Perspectives. J Pharm Pharmacol. 2004;56(11):1339–1356. doi: 10.1211/0022357044797. [DOI] [PubMed] [Google Scholar]
- [8].Wood S. Sirolimus-Eluting Stent Gets U.S. Regulatory Approval at Last. Medscape. 2003 http://www.medscape.com/viewarticle/785641. [Google Scholar]
- [9].U.S. FDA. TAXUS Express2 Paclitaxel-Eluting Coronary Stent System–P030025. U.S. Food and Drug Administration; Silver Spring, MD: 2013. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/deviceapprovalsandclearances/recently-approveddevices/ucm081189.htm. [Google Scholar]
- [10].Palmerini T, Biondi-Zoccai G, DellaRiva D, Mariani A, Sabaté M, Smits PC, Kaiser C, D’Ascenzo F, Frati G, Mancone M, Genereux P, et al. Clinical Outcomes With Bioabsorbable Polymer–Versus Durable Polymer-Based Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2014;63(4):299–307. doi: 10.1016/j.jacc.2013.09.061. [DOI] [PubMed] [Google Scholar]
- [11].Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- [12].Camenzindm E, Steg PG, Wijns W. Stent Thrombosis Late After Implantation of First-Generation Drug-Eluting Stents: A Cause for Concern. Circulation. 2007;115(11):1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- [13].Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological Mechanisms of Fatal Late Coronary Stent Thrombosis in Humans. Circulation. 2003;108(14):1701–1706. doi: 10.1161/01.CIR.0000091115.05480.B0. [DOI] [PubMed] [Google Scholar]
- [14].Popma JJ, Weiner B, Cowley MJ, Simonton C, McCormick D, Feldman T. FDA Advisory Panel on the Safety and Efficacy of Drug-Eluting Stents: Summary of Findings and Recommendations. J Intervention Cardiol. 2007;20(6):425–446. doi: 10.1111/j.1540-8183.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- [15].National Library of Medicine. ClinicalTrials.gov. U.S. National Institutes of Health; Bethesda, MD: 2015. http://www.clinicaltrials.gov/ [Google Scholar]
- [16].ACC. Definition of Stent Thrombosis: Proposed by Academic Research Consortium, American College of Cardiology Foundation. American College of Cardiology; Washington, DC: 2007. http://www.cardiosource.com/pops/imagepop.asp?imgid1⁄417564. [Google Scholar]
- [17].Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Genereux P, Branzi A, Stone GW. Stent Thrombosis With Drug-Eluting Stents: Is the Paradigm Shifting? J Am Coll Cardiol. 2013;62(21):1915–1921. doi: 10.1016/j.jacc.2013.08.725. [DOI] [PubMed] [Google Scholar]
- [18].Rodriguez AE, Mieres J, Fernandez-Pereira C, Vigo CF, Rodriguez-Alemparte M, Berrocal D, Grinfeld L, Palacios I. Coronary Stent Thrombosis in the Current Drug-Eluting Stent Era: Insights From the ERACI III Trial. J Am Coll Cardiol. 2006;47(1):36–38. doi: 10.1016/j.jacc.2005.10.016. [DOI] [PubMed] [Google Scholar]
- [19].Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G, Hellige G, van Domburg RT, et al. Early and Late Coronary Stent Thrombosis of Sirolimus-Eluting and Paclitaxel-Eluting Stents in Routine Clinical Practice: Data From a Large Two-Institutional Cohort Study. Lancet. 2007;369(9562):667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- [20].Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, et al. Incidence, Predictors, and Outcome of Thrombosis After Successful Implantation of Drug-Eluting Stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- [21].Ong ATL, Hoye A, Aoki J, van Mieghem CAG, Rodriguez Granillo GA, Sonnenschein K, Regar E, McFadden EP, Sianos G, van der Giessen WJ, de Jaegere PPT, et al. Thirty-Day Incidence and Six-Month Clinical Outcome of Thrombotic Stent Occlusion After Bare-Metal, Sirolimus, or Paclitaxel Stent Implantation. J Am Coll Cardiol. 2005;45(6):947–953. doi: 10.1016/j.jacc.2004.09.079. [DOI] [PubMed] [Google Scholar]
- [22].Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich K-L, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent Thrombogenicity Early in High-Risk Interventional Settings is Driven by Stent Design and Deployment and Protected by Polymer-Drug Coatings. Circulation. 2011;123(13):1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alfonso F, Suárez A, Angiolillo DJ, Sabaté M, Escaned J, Moreno R, Hernández R, Bañnuelos C, Macaya C. Findings of Intravascular Ultrasound During Acute Stent Thrombosis. Heart. 2004;90(12):1455–1459. doi: 10.1136/hrt.2003.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B, Windecker S. Incomplete Stent Apposition and Very Late Stent Thrombosis After Drug-Eluting Stent Implantation. Circulation. 2007;115(18):2426–2434. doi: 10.1161/CIRCULATIONAHA.106.658237. [DOI] [PubMed] [Google Scholar]
- [25].Acharya G, Park K. Mechanisms of Controlled Drug Release From Drug-Eluting Stents. Adv Drug Delivery Rev. 2006;58(3):387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [26].Tanner FC, Yang Z-Y, Duckers E, Gordon D, Nabel GJ, Nabel EG. Expression of Cyclin-Dependent Kinase Inhibitors in Vascular Disease. Circ Res. 1998;82(3):396–403. doi: 10.1161/01.res.82.3.396. [DOI] [PubMed] [Google Scholar]
- [27].Babapulle MN. Coated Stents for the Prevention of Restenosis: Part II. Circulation. 2002;106(22):2859–2866. doi: 10.1161/01.cir.0000038984.30279.89. [DOI] [PubMed] [Google Scholar]
- [28].Topol EJ, Serruys PW. Frontiers in Interventional Cardiology. Circulation. 1998;98(17):1802–1820. doi: 10.1161/01.cir.98.17.1802. [DOI] [PubMed] [Google Scholar]
- [29].McGinty S, McKee S, Wadsworth RM, McCormick C. Modelling Drug-Eluting Stents. Math Med Biol. 2011;28(1):1–29. doi: 10.1093/imammb/dqq003. [DOI] [PubMed] [Google Scholar]
- [30].Khan W, Farah S, Domb AJ. Drug Eluting Stents: Develop-ments and Current Status. J Controlled Release. 2012;161(2):703–712. doi: 10.1016/j.jconrel.2012.02.010. [DOI] [PubMed] [Google Scholar]
- [31].Bittl JA. Deconstructing Stent Polymers. J Am Coll Cardiol. 2014;63(4):308–309. doi: 10.1016/j.jacc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- [32].van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmesm DR, Ellis SG, Topol EJ. Marked Inflammatory Sequelae to Implantation of Biodegradable and Nonbiodegradable Polymers in Porcine Coronary Arteries. Circulation. 1996;94(7):1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- [33].Boden M, Richard R, Schwarz MC, Kangas S, Huibregtse B, Barry JJ. In Vitro and In Vivo Evaluation of the Safety and Stability of the TAXUS Paclitaxel-Eluting Coronary Stent. J Mater Sci Mater Med. 2009;20(7):1553–1562. doi: 10.1007/s10856-009-3705-5. [DOI] [PubMed] [Google Scholar]
- [34].Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug Eluting Stents: An Updated Meta-Analysis of Randomised Controlled Trials. Heart. 2006;92(5):641–649. doi: 10.1136/hrt.2005.061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ako J, Bonneau HN, Honda Y, Fitzgerald PJ. Design Criteria for the Ideal Drug-Eluting Stent. Am J Cardiol. 2007;100(8B):3M–9M. doi: 10.1016/j.amjcard.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [36].Grube E, Buellesfeld L. Rapamycin Analogs for Stent-Based Local Drug Delivery. Everolimus- and Tacrolimus-Eluting Stents. Herz. 2004;29(2):162–166. doi: 10.1007/s00059-004-2556-6. [DOI] [PubMed] [Google Scholar]
- [37].Schmidt H, Menning M. Wet Coating Technologies for Glass. 2000 SolGel.com. http://www.solgel.com/articles/nov00/mennig.htm.
- [38].Jang H-S, Nam HY, Kim J-M, Hahm D-H, Nam SH, Kim KL, Joo J-R, Suh W, Park J-S, Kim DK, Gwon H-C. Effects of Curcumin for Preventing Restenosis in a Hypercholesterolemic Rabbit Iliac Artery Stent Model. Catheter Cardiovasc Intervention. 2009;74(6):881–888. doi: 10.1002/ccd.22047. [DOI] [PubMed] [Google Scholar]
- [39].Acharya G, Lee CH, Lee Y. Optimization of Cardiovascular Stent Against Restenosis: Factorial Design-Based Statistical Analysis of Polymer Coating Conditions. PLoS One. 2012;7(8):e43100. doi: 10.1371/journal.pone.0043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Song S-J, Park YJ, Park J, Cho MD, Kim J-H, Jeong MH, Kim YS, Cho DL. Preparation of a Drug-Eluting Stent Using a TiO2 Film Deposited by Plasma Enhanced Chemical Vapour Deposition as a Drug-Combining Matrix. J Mater Chem. 2010;20(23):4792–4801. [Google Scholar]
- [41].Park S, Bhang SH, La W-G, Seo J, Kim B-S, Char K. Dual Roles of Hyaluronic Acids in Multilayer Films Capturing Nanocarriers for Drug-Eluting Coatings. Biomaterials. 2012;33(21):5468–5477. doi: 10.1016/j.biomaterials.2012.04.005. [DOI] [PubMed] [Google Scholar]
- [42].Bakhshi R, Edirisinghe MJ, Darbyshire A, Ahmad Z, Seifalian AM. Electrohydrodynamic Jetting Behaviour of Polyhedral Oligomeric Silsesquioxane Nanocomposite. J Biomater Appl. 2009;23(4):293–309. doi: 10.1177/0885328208089125. [DOI] [PubMed] [Google Scholar]
- [43].Levy Y, Mandler D, Weinberger J, Domb AJ. Evaluation of Drug-Eluting Stents’ Coating Durability–Clinical and Regulatory Implications. J Biomed Mater Res B: Appl Biomater. 2009;91(1):441–451. doi: 10.1002/jbm.b.31420. [DOI] [PubMed] [Google Scholar]
- [44].Okner R, Shaulov Y, Tal N, Favaro G, Domb AJ, Mandler D. Electropolymerized Tricopolymer Based on N-Pyrrole Derivatives as a Primer Coating for Improving the Performance of a Drug-Eluting Stent. ACS Appl Mater Interfaces. 2009;1(4):758–767. doi: 10.1021/am800139s. [DOI] [PubMed] [Google Scholar]
- [45].Shaulov Y, Okner R, Levi Y, Tal N, Gutkin V, Mandler D, Domb AJ. Poly(Methyl Methacrylate) Grafting Onto Stainless Steel Surfaces: Application to Drug-Eluting Stents. ACS Appl Mater Interfaces. 2009;1(11):2519–2528. doi: 10.1021/am900465t. [DOI] [PubMed] [Google Scholar]
- [46].Ammam M. Electrophoretic Deposition Under Modulated Electric Fields: A Review. RSC Adv. 2012;2(20):7633–7646. [Google Scholar]
- [47].Wang Y, Zhang W, Zhang J, Sun W, Zhang R, Gu H. Fabrication of a Novel Polymer-Free Nanostructured Drug-Eluting Coating for Cardiovascular Stents. ACS Appl Mater Interfaces. 2013;5(20):10337–10345. doi: 10.1021/am403365j. [DOI] [PubMed] [Google Scholar]
- [48].Nukala RK, Boyapally H, Slipper IJ, Mendham AP, Douroumis D. The Application of Electrostatic Dry Powder Deposition Technology to Coat Drug-Eluting Stents. Pharm Res. 2010;27(1):72–81. doi: 10.1007/s11095-009-0008-y. [DOI] [PubMed] [Google Scholar]
- [49].Liu Y, Wang W, Acharya G, Shim Y-B, Choe ES, Lee CH. Advanced Stent Coating for Drug Delivery and In Vivo Biocompatibility. J Nanopar Res. 2013;15(10):1962–1978. [Google Scholar]
- [50].Hagiwara K, Hasebe T, Hotta A. Effects of Plasma Treatments on the Controlled Drug Release From Poly(Ethylene-co-Vinyl Acetate) Surf Coat Technol. 2013;216:318–323. [Google Scholar]
- [51].Gallo A, Mani G. A Stent for Co-Delivering Paclitaxel and Nitric Oxide From Abluminal and Luminal Surfaces: Preparation, Surface Characterization, and In Vitro Drug Release Studies. Appl Surf Sci. 2013;279:216–232. [Google Scholar]
- [52].Su L-C, Chen Y-H, Chen M-C. Dual Drug-Eluting Stents Coated With Multilayers of Hydrophobic Heparin and Sirolimus. ACS Appl Mater Interfaces. 2013;5(24):12944–12953. doi: 10.1021/am403615q. [DOI] [PubMed] [Google Scholar]
- [53].Huang Y, Venkatraman SS, Boey FYC, Lahti EM, Umashankar PR, Mohanty M, Arumugam S, Khanolkar L, Vaishnav S. In Vitro and In Vivo Performance of a Dual Drug-Eluting Stent (DDES) Biomaterials. 2010;31(15):4382–4391. doi: 10.1016/j.biomaterials.2010.01.147. [DOI] [PubMed] [Google Scholar]
- [54].Thakkar A, Raval A, Mandal R, Parmar S, Jariwala A, Tailor J, Mehta A. Development and Evaluation of Drug Eluting Stent Having Biphasic Release From a Single Layer of Biodegradable Polymer. ASME J Med Devices. 2013;7(1) 011005. [Google Scholar]
- [55].Petersen S, Hussner J, Reske T, Grabow N, Senz V, Begunk R, Arbeiter D, Kroemer HK, Schmitz K-P, Meyer zu Schwabedissen HE, Sternberg K. In Vitro Study of Dual Drug-Eluting Stents With Locally Focused Sirolimus and Atorvastatin Release. J Mater Sci -Mater Med. 2013;24(11):2589–2600. doi: 10.1007/s10856-013-5001-7. [DOI] [PubMed] [Google Scholar]
- [56].Raval A, Parikh J, Engineer C. Dexamethasone Eluting Biodegradable Polymeric Matrix Coated Stent for Intravascular Drug Delivery. Chem Eng Res Des. 2010;88(11):1479–1484. [Google Scholar]
- [57].Raval A, Parikh J, Engineer C. Mechanism and In Vitro Release Kinetic Study of Sirolimus From a Biodegradable Polymeric Matrix Coated Cardiovascular Stent. Ind Eng Chem Res. 2011;50(16):9539–9549. [Google Scholar]
- [58].Engineer C, Parikh J, Raval A. Effect of Copolymer Ratio on Hydrolytic Degradation of Poly(Lactide-co-Glycolide) From Drug Eluting Coronary Stents. Chem Eng Res Des. 2011;89(3):328–334. [Google Scholar]
- [59].Liu S-J, Hsiao C-Y, Chen J-K, Liu K-S, Lee C-H. In-Vitro Release of Anti-Proliferative Paclitaxel From Novel Balloon-Expandable Polycaprolactone Stents. Mater Sci Eng C. 2011;31(5):1129–1135. [Google Scholar]
- [60].Shanshan C, Lili T, Yingxue T, Bingchun Z, Ke Y. Study of Drug-Eluting Coating on Metal Coronary Stent. Mater Sci Eng, C. 2013;33(3):1476–1480. doi: 10.1016/j.msec.2012.12.049. [DOI] [PubMed] [Google Scholar]
- [61].Kim DM, Lee BS, Kang JH, Choi J, Park K, Son T-I, Jeong MH, Han DK. Fabrication and Controlled Release of Electrosprayed ReoPro-Loaded Metal Surface for Vascular Stent. Macromol Res. 2011;19(5):501–506. [Google Scholar]
- [62].Krishna OD, Jeon OC, Kim K, Byun Y, Moon HT. Drug Release From a Chemically-Anchored PEG/Phospholipid Monolayer Onto Polymer-Coated Metallic Stents. J Biomater Sci Polym Ed. 2010;21(6):789–802. doi: 10.1163/156856209X445294. [DOI] [PubMed] [Google Scholar]
- [63].Bian H, Zhou S, Liang X, Li Q, Han W. In Vitro Study of Poly(Ethylene Carbonate) as a Drug-Eluting Stent Coating. Prog Nat Sci Mater Int. 2012;22(4):295–302. [Google Scholar]
- [64].Yuk SH, Oh KS, Park J, Kim S-J, Kim JH, Kwon IK. Paclitaxel-Loaded Poly(Lactide-co-Glycolide)/Poly(Ethylene Vinyl Acetate) Composite for Stent Coating by Ultrasonic Atomizing Spray. Sci Technol Adv Mater. 2012;13(2) doi: 10.1088/1468-6996/13/2/025005. 025005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Choi J, Cho SB, Lee BS, Joung YK, Park K, Han DK. Improvement of Interfacial Adhesion of Biodegradable Polymers Coated on Metal Surface by Nanocoupling. Langmuir. 2011;27(23):14232–14239. doi: 10.1021/la2030318. [DOI] [PubMed] [Google Scholar]
- [66].Wang GX, Luo LL, Yin TY, Li Y, Jiang T, Ruan CG, Guidoin R, Chen YP, Guzman R. Ultrasonic Atomization and Subsequent Desolvation for Monoclonal Antibody (mAb) to the Glycoprotein (GP) IIIa Receptor Into Drug Eluting Stent. J Microencapsul. 2010;27(2):105–114. doi: 10.1080/02652040903046798. [DOI] [PubMed] [Google Scholar]
- [67].Onuma Y, Serruys PW. Bioresorbable Scaffold: The Advent of a New Era in Percutaneous Coronary and Peripheral Revascularization? Circulation. 2011;123(7):779–797. doi: 10.1161/CIRCULATIONAHA.110.971606. [DOI] [PubMed] [Google Scholar]
- [68].Serruys PW, Onuma Y, Dudek D, Smits PC, Koolen J, Chevalier B, de Bruyne B, Thuesen L, McClean D, van Geuns R-J, Windecker S, et al. Evaluation of the Second Generation of a Bioresorbable Everolimus-Eluting Vascular Scaffold for the Treatment of de Novo Coronary Artery Stenosis: 12-Month Clinical and Imaging Outcomes. J Am Coll Cardiol. 2011;58(15):1578–1588. doi: 10.1016/j.jacc.2011.05.050. [DOI] [PubMed] [Google Scholar]
- [69].Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The Roles of Tissue Engineering and Vascularisation in the Development of Micro-Vascular Networks: A Review. Biomaterials. 2005;26(14):1857–1875. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- [70].Kannan RY, Salacinski HJ, Ghanavi J, Narula A, Odlyha M, Peirovi H, Butler PE, Seifalian AM. Silsesquioxane Nanocomposites as Tissue Implants. Plast Reconstr Surg. 2007;119(6):1653–1662. doi: 10.1097/01.prs.0000246404.53831.4c. [DOI] [PubMed] [Google Scholar]
- [71].Ahmed M, Hamilton G, Seifalian AM. Viscoelastic Behaviour of a Small Calibre Vascular Graft Made From a POSS-Nanocomposite. 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Buenos Aires; Aug. 31–Sept. 4; 2010. pp. 251–254. [DOI] [PubMed] [Google Scholar]
- [72].Cottone RJ, Thatcher GL, Parker SP, Hanks L, Kujawa DA, Rowland SM, Costa M, Schwartz RS, Onuma Y. OrbusNeich Fully Absorbable Coronary Stent Platform Incorporating Dual Partitioned Coatings. EuroIntervention. 2009;5(Suppl. 2009):F65–F71. doi: 10.4244/EIJV5IFA11. [DOI] [PubMed] [Google Scholar]
- [73].Curcio A, Torella D, Indolfi C. Mechanisms of Smooth Muscle Cell Proliferation and Endothelial Regeneration After Vascular Injury and Stenting. Circ J. 2011;75(6):1287–1296. doi: 10.1253/circj.cj-11-0366. [DOI] [PubMed] [Google Scholar]