Abstract
Background
The antiretroviral drug efavirenz is widely used during breastfeeding. Evaluating its safety requires an understanding of its breast milk pharmacokinetics, level of breastfed infants’ exposure and potential influence of polymorphisms in drug disposition genes.
Methods
For this observational study, we investigated plasma and breast milk pharmacokinetics of efavirenz and breastfed infants’ exposure in HIV positive nursing mothers and their breastfed infants. We also evaluated potential variability due to genetic polymorphisms in CYP2B6, NR1I3, CYP2A6, ABCB1, ABCB5 and ABCG2.
Results
CYP2B6 516G>T was independently associated with efavirenz concentrations in maternal plasma, breast milk and infant plasma (n = 134). When stratified based on CYP2B6 516G>T (n = 29 ; 11 GG, 10 GT and 8 TT), EFV pharmacokinetic parameters in plasma and breast milk differed significantly between patient groups. The median time averaged milk-to-plasma concentration ratio was 1.10 (range: 0.57-1.71). The estimated maximum infant efavirenz dose from breast milk was 809 µg/kg/day (215-2760) and paediatric dose weight-adjusted exposure index was 4.05% (1.08-13.8). Infant plasma concentrations did not change significantly during the dosing interval, 157 ng/mL (28.6-1360) in pooled analysis and 315 ng/mL (108-1360) in CYP2B6 516TT group. Infant plasma concentrations were highest up to 8 days of age at 1590 ng/mL (190-4631) and decreased by about 90% in the age stratum day 9 to 3 months. No efavirenz related toxicity was reported.
Conclusions
Most breastfed infants are exposed to less than 10% of the weight-adjusted therapeutic paediatric dose, the safety threshold for exposure to maternal drugs from breast milk.
Keywords: efavirenz, breast milk, pharmacokinetics, infant exposure, CYP2B6 516G>T
Exclusive breastfeeding for the first six months of life, continued for up to two years with gradual introduction of safe and nutritionally adequate replacement feeding, is the recommended feeding option in the context of HIV/AIDS in resource-limited settings [1–3]. The potential benefits of avoiding or early cessation of breastfeeding in this population are largely offset by increased infant morbidity/mortality from causes other than HIV/AIDS, including malnutrition, diarrhoea and pneumonia [4–7]. Mother-to-child transmission (MTCT) during this period is prevented by maternal antiretroviral drugs (ARVs) started during pregnancy and continued until breastfeeding ends (Option B) or for life (Option B+) [8, 9]. The infant is given daily nevirapine post-exposure prophylaxis (PEP) from birth until 4-6 weeks old [8], which reduces MTCT to less than 5% in these settings from the baseline 20-45% without intervention [10].
The current WHO guidelines recommend efavirenz (EFV) as the preferred non-nucleoside reverse transcriptase inhibitor component of first-line antiretroviral therapy (ART) for adults across different patient populations, including nursing mothers [11]. However, EFV is not licensed for use in children < 3 months old or < 3.5 kg because optimal dosing and safety have not been evaluated [12]. However, it is increasingly used by nursing mothers and its presence in breast milk and breastfed infants’ plasma have been reported [13, 14], but in limited numbers of mother-infant pairs and at single time points after maternal dose. Milk production, composition, and infant feeding patterns change during the dosing interval and may cause variations in the milk-to-plasma (M/P) concentration ratio, making single point estimates unreliable [15, 16]. In addition, the only study that reported EFV concentration in breast milk used skimmed milk [13], which often yields lower drug concentrations than whole milk. A recently developed method for the quantification of EFV in dried breast milk spots [17] has now extended our ability to study the pharmacokinetics of EFV in whole milk during an entire dosing interval.
The influence of single nucleotide polymorphisms (SNPs) in drug metabolizing enzymes, transporter and nuclear receptor genes on plasma EFV concentration is well established [18]. The interindividual variability observed in EFV plasma pharmacokinetics in different populations has been associated with such SNPs [19]. We previously hypothesised that SNPs may affect EFV excretion into breast milk and transfer to breastfed infants [20]. Understanding the pharmacokinetics of EFV in human breast milk during an entire dosing interval and potential differences introduced by genetic polymorphisms are crucial for an accurate estimation of infant exposure.
In the present study, associations between EFV concentrations in plasma and breast milk of nursing mother-infant pairs and SNPs in CYP2B6, NR1I3, CYP2A6, ABCB1, ABCB5 and ABCG2 genes were explored. We then investigated EFV plasma and breast milk pharmacokinetics and breastfed infants’ exposure in genetically-defined subgroups, stratified by the SNP with the highest predictive power.
Methods
Patients
HIV positive nursing mothers and their breastfed infants were recruited from three hospitals in Benue State, Nigeria: Bishop Murray Medical Centre, Makurdi; St Mary’s Hospital, Okpoga; and St Monica’s Hospital, Adikpo. Potentially eligible subjects were identified using the current PMTCT delivery register and invited to participate after an information session conducted in English and the local Tiv language. All participants gave prior written informed consent. Once subjects had signed a consent form, we ascertained eligibility by examining case notes and conducting a brief interview. The inclusion criteria included HIV positive and breastfeeding, enrolled in the PMTCT programme and started EFV-containing regimen during pregnancy. Exclusion criteria (assessed at enrolment) included exclusive formula feeding, mixed feeding in infants less than 6 months old, opportunistic infections (e.g. tuberculosis, pneumonia), severe maternal or infant illness, and maternal or infant treatment with drugs or herbal medication with known or uncertain interaction with EFV. The protocol and the materials transfer agreement were approved by the National Health Research Ethics Committee (NHREC), Abuja, Nigeria and Ethics and Research Committee, Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria. Clinicaltrials.gov ID: NCT02269462.
Study Design
This was an observational study conducted in two phases. In the preliminary phase, we explored associations between 12 SNPs in drug disposition genes and mid-dose plasma and breast milk EFV concentrations in an unselected cohort of HIV positive nursing mothers and their breastfed infants. In the intensive pharmacokinetic phase, the SNP independently associated with the highest predictive power was used to stratify mother-infant pairs into three groups: non-carriers, heterozygotes, and homozygotes. Randomly selected mother-infant pairs from each group were re-recruited and invited for the intensive pharmacokinetic phase.
Samples Collection
In the preliminary phase, paired dried blood spots (DBS) and dried breast milk spots samples were collected from mothers and infants at a single, recorded time point post-dose. In the intensive pharmacokinetic phase, maternal DBS and dried breast milk spots were collected at 0.5, 1, 2, 4, 8, 12 and 24 hours after an observed evening dose of 600 mg EFV and stored as previously described [17]. DBS samples were collected from infants at 2 h and 8 h after maternal EFV dose in the intensive pharmacokinetic phase. To reflect real-life situations, infant feeding times were not controlled; all infants were breastfed on demand. In addition, mothers took standard local meals about 30 min before drug administration. Samples were shipped at ambient temperature to the University of Liverpool, UK for analysis.
DNA Extraction and SNP Genotyping
Genomic DNA was extracted using E.Z.N.A.® Blood DNA Mini Kit (Omega Bio-Tek, Inc., Norcross, GA, USA) in accordance with the manufacturer’s protocol. DNA was quantified spectrophotometrically using NanoDrop® (Thermo Fisher Scientific Inc., Wilmington, DE, USA) before storage at -20°C. Genotyping was performed by real-time PCR on a DNA Engine Chromo4 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The Supplementary Material contains further details about TaqMan® SNP Genotyping assays.
EFV Quantification and Pharmacokinetic Analysis
EFV in DBS and dried breast milk spots was quantified using previously described LC-MS/MS methods [17, 21]. Plasma concentrations were determined using [DBS[EFV]/(1-HCT)]*0.995, where DBS[EFV] is EFV concentration in DBS, HCT is the patient-specific haematocrit and 0.995 is the fraction of EFV bound to plasma protein [22]. Minimum (Cmin) and maximum (Cmax) plasma concentrations were determined by direct inspection. The area under the concentration-time curve during the dosing interval (AUC0-24) was calculated using the trapezoidal rule and the apparent clearance (Cl/F) was calculated by dividing dose by AUC0-24. Maximum (InfantDosemax) and average (InfantDoseavg) infant EFV doses from breast milk were calculated using equations (1) and (2), respectively. Paediatric dose (for children aged ≥ 3 months and weighing ≥ 3.5 kg [12]) weight-adjusted exposure index (EIpaediatric) and maternal dose weight-adjusted exposure index (EImaternal) were calculated using equations (3) and (4), respectively.
| Equation (1) |
| Equation (2) |
| Equation (3) |
| Equation (4) |
where 150 is the average volume of infant daily milk intake in mL/kg/day ; MilkCmax is the maximum EFV concentration in breast milk (µg/mL); MilkAUC0-24 is EFV breast milk AUC0-24 (µg.h/mL); 24 is the dosing interval in h; Dosepaediatric is the weight-adjusted licensed paediatric dose of EFV (µg/kg/day); Dosematernal is the weight-adjusted licensed adult dose of EFV (µg/kg/day).
Statistical Analysis
Compliance with Hardy Weinberg Equilibrium was tested as previously described [23]. Data were subjected to Kolmogorov-Smirnov normality test prior to statistical analysis. Relationships between continuous variables were tested by Pearson or Spearman correlation. Univariate linear regression analysis was conducted to identify variables associated with EFV concentrations in maternal plasma, infant plasma and breast milk. Bonferroni correction was used to adjust for multiple testing. Independent variables with Bonferroni P value ≤ 0.1 in the univariate analysis were included in a multivariate stepwise linear regression analysis. Differences in EFV concentrations and pharmacokinetic parameters between patient groups were investigated using one-way analysis of variance (ANOVA) and Kruskal-Wallis or Mann Whitney U test. Trend across groups was investigated using Cuzick's test on StatDirect (StatsDirect Ltd, Altrincham, Cheshire, UK). Post hoc analysis of statistical power achieved was conducted using G*Power version 3.9.1.2 (Heinrich-Heine-University, Düsseldorf, Germany). All other analyses were conducted using IBM ®SPSS® Statistics version 20.0 (IBM, Armonk, NY, USA) and GraphPad Prism® (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patients’ Characteristics
Between December 2012 and October 2013 134 eligible HIV positive nursing mothers and their breastfed infants were recruited. Maternal and infant characteristics are summarised in Table 1. The mean (SD) duration on current ARV regimen was 17.6 (14.1) months, starting with baseline CD4 count of 380 (217) c/mm3. Most patients were taking EFV, emtricitabine and tenofovir (65%; 87/134) or EFV, lamivudine and zidovudine (32%; 43/134). Infants less than 6 weeks of age (22%; 29/134) were taking nevirapine PEP. Genotype frequencies are summarised in Table 1. All twelve SNPs were in Hardy Weinberg equilibrium.
Table 1. Characteristics of nursing mother-infant pairs.
| Mothers (n = 134)a | |
| Age (y) | 29 (18-44) |
| Weight (kg) | 57 (39-80) |
| Time since diagnosis (months) | 21.1 (1.3-68) |
| Infants (n = 134)a | |
| Age (weeks) | 20.0 (0.29-75) |
| Weight (kg) | 5.8 (2.2-10) |
| Gender (Female) | 52% (70/134) |
| Maternal drug regimen and CD4 counta | |
| TDF/FTC/EFV | 65% (87/134) |
| 3TC/AZT/EFV | 32% (43/134) |
| 3TC/TDF/EFV | 2% (3/134) |
| 3TC/ABC/EFV | 1% (1/134) |
| Time post dose (h)b | 11.8 (5.5) |
| Duration on regimen (months)b | 17.6 (14.1) |
| Baseline CD4 count (c/mm3)b | 380 (217) |
| CD4 change (c/mm3)b | 177 (217) |
| Maternal genotype frequency: | |
| CYP2B6 516G>T (rs3745274) | GG, 0.36; GT, 0.45; TT, 0.19 |
| CYP2B6 983T>C (rs28399499) | TT, 0.79; CT, 0.21; CC, 0.00 |
| CYP2B6 c.485-18C>T (rs4803419) | CC, 0.79; CT, 0.21; TT, 0.00 |
| NR1I3 c.540C>T (rs2307424) | CC, 0.77; CT, 0.23; TT, 0.00 |
| NR1I3 c.152-1089T>C (rs3003596) | TT, 0.19; CT, 0.54; CC, 0.27 |
| ABCB1 4046A>G (rs1045642) | AA, 0.75; AG, 0.25; GG, 0.00 |
| ABCB1 4036A>G (rs3842) | AA, 0.65; AG, 0.35; GG, 0.01 |
| ABCB1 1236C>T (rs1128503) | CC, 0.76; CT, 0.23; TT, 0.02 |
| CYP2A6 48T>G (rs28399433) | TT, 0.93; GT, 0.07; GG, 0.01 |
| ABCB5 c.2908G>A (rs6461515) | GG, 0.45; GA, 0.45; AA, 0.10 |
| ABCG2 c.1728-46G>A (rs2231164) | GG, 0.68; GA, 0.30; AA, 0.02 |
| ABCG2 78551A>G (rs2622604) | AA, 0.84; AG, 15; GG, 0.01 |
| Infants’ genotype frequency: | |
| CYP2B6 516G>T (rs3745274) | GG, 0.45; GT, 0.67; TT, 0.18 |
Unless otherwise indicated, values are expressed as median (range) or % (number) of subjects;
mean (standard deviation).
Factors Associated With Plasma and Breast Milk EFV Concentrations
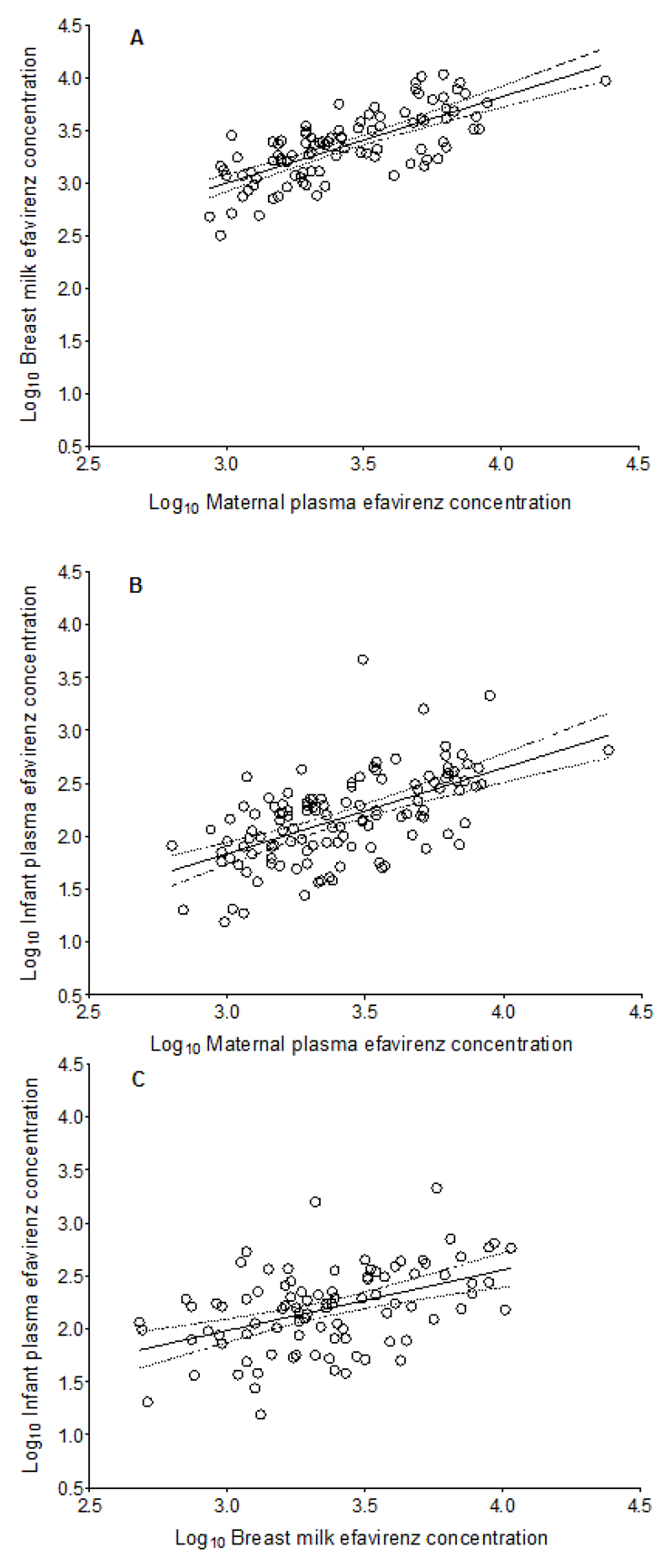
In the preliminary phase, samples were collected at 14.00 h (0.50-21.5) post maternal EFV dose and median (range) maternal plasma, breast milk and infant plasma EFV concentrations were 2310 ng/mL (632-8880), 2280 ng/mL (475-10800) and 173 ng/mL (46.0-4630), respectively (Table 3). There were significant correlations in EFV concentrations between maternal plasma and breast milk, p < 0.0001, Pearson’s r = 0.73 (0.62, 0.81); maternal plasma and infant plasma, p < 0.0001; Pearson’s r = 0.58 (0.45, 0.69); and breast milk and infant plasma, p < 0.0001; Pearson’s r = 0.46 (0.28, 0.61) (Figure 1).
Table 3. EFV pharmacokinetic parameters in plasma and breast milk and breastfed infants’ exposure.
| Pooled | CYP2B6 516GG | CYP2B6 516GT | CYP2B6 516TT | |
| Preliminary phase pharmacokinetic data | n = 117 | n = 42 | n = 52 | n = 23 |
| Maternal plasma EFV conc. (ng/mL) | 2310 (632-8880) | 1660 (632-3610) | 2390 (951-8880) | 5400 (1920-8110) |
| Breast milk EFV conc. (ng/mL) | 2280 (475-10800) | 1610 (475-5580) | 2370 (713-10300) | 4070 (995-10800) |
| Infant plasma EFV conc. (ng/mL) | 173 (46.0-4630) | 124 (46.0-4630) | 164 (48.5-2150) | 333 (75.5-1590) |
| Intensive pharmacokinetic parameters | n = 29 | n = 10 | n = 11 | n = 8 |
| Plasma | ||||
| Cl/F (L/h)a | 9.89 (3.39-22.4) | 12.2 (9.37-22.4) | 10.0 (4.71-20.7) | 4.64 (3.39-5.35) |
| AUC0-24 (ng.h/mL)a | 60700 (26800-177000) | 49400 (26800-64000) | 59700 (29000-128000) | 130000 (112000-177000) |
| Cmax (ng/mL)a | 4630 (2050-9760) | 3220 (2310-4630) | 4750 (2050-6780) | 6940 (5560-9760) |
| Cmin (ng/mL)a | 2030 (755-6740) | 1640 (861-2310) | 1580 (755-4860) | 5150 (3830-6740) |
| Breast milk | ||||
| AUC0-24 (ng.h/mL)b | 68500 (26300-257000) | 55000 (29200-105000) | 60600 (26300-206000) | 105000 (68100-257000) |
| Cmax (ng/mL)c | 5390 (1430-18400) | 4020 (2400-8450) | 4540 (1430-9220) | 8920 (5810-18400) |
| Cmin (ng/mL)d | 1680 (316-9570) | 1120 (534-2430) | 1500 (316-6070) | 2480 (1500-9570) |
| Time averaged M/P conc. ratio | 1.10 (0.57-1.71) | 1.22 (0.61-1.71) | 1.08 (0.57-1.57) | 0.98 (0.59-1.66) |
| M/P AUC0-24 ratio | 1.13 (0.50-1.93) | 1.23 (0.71-1.93) | 1.18 (0.73-1.73) | 0.95 (0.57-1.66) |
| Infant exposure | ||||
| Average infant EFV dose (µg/kg/day) | 428 (164-1610) | 344 (182-656) | 379 (164-1290) | 656 (426-1610) |
| Maximum infant EFV dose (µg/kg/day) | 809 (215-2760) | 603 (360-1270) | 681 (215-1380) | 1340 (872-2760) |
| Average EIpaediatrice (%) | 2.14 (0.82-8.05) | 1.72 (0.91-3.28) | 1.90 (0.82-6.45) | 3.28 (2.13-8.05) |
| Maximum EIpaediatrice (%) | 4.05 (1.08-13.8) | 3.02 (1.80-6.35) | 3.41 (1.08-6.90) | 6.70 (4.36-13.8) |
| EImaternale (%) | 7.69 (2.04-26.2) | 5.73 (3.42-12.1) | 6.47 (2.04-13.1) | 12.7 (8.28-26.2) |
| Infant plasma EFV conc. 1f(ng/mL) | 173 (27.2-1590) | 166.10 (27.3-208) | 88.53 (37.7-273) | 293 (103-1590) |
| Infant plasma EFV conc. 2f (ng/mL) | 146 (29.9-1130) | 133.54 (30.0-223) | 85.83 (45.2-313) | 342 (113-1130) |
Values are presented as median (range).
a, b and c: Significant differences between genotype groups (Kruskal-Wallis test) at 0.001, 0.05, 0.01 and levels, respectively
no significant difference between genotype groups (Kruskal-Wallis test)
EIpaediatric and EImaternal represent paediatric and maternal dose weight-adjusted exposure indices, respectively (average EIpaediatric was calculated by replacing InfantDosemax with InfantDoseavg in equation 3)
Infant plasma EFV conc. 1 and 2 represent infant plasma concentrations 2 h and 8 h after maternal dose, respectively.
Figure 1.
Correlations between EFV concentrations in maternal plasma and breast milk; p < 0.0001; Pearson’s r = 0.73 (0.62, 0.81) (A), maternal plasma and infant plasma; p < 0.0001; Pearson’s r = 0.58 (0.45, 0.69) (B), and breast milk and infant plasma; p < 0.0001; Pearson’s r = 0.46 (0.28, 0.61) (C). Solid lines represent mean values and broken lines represent 95% confidence intervals.
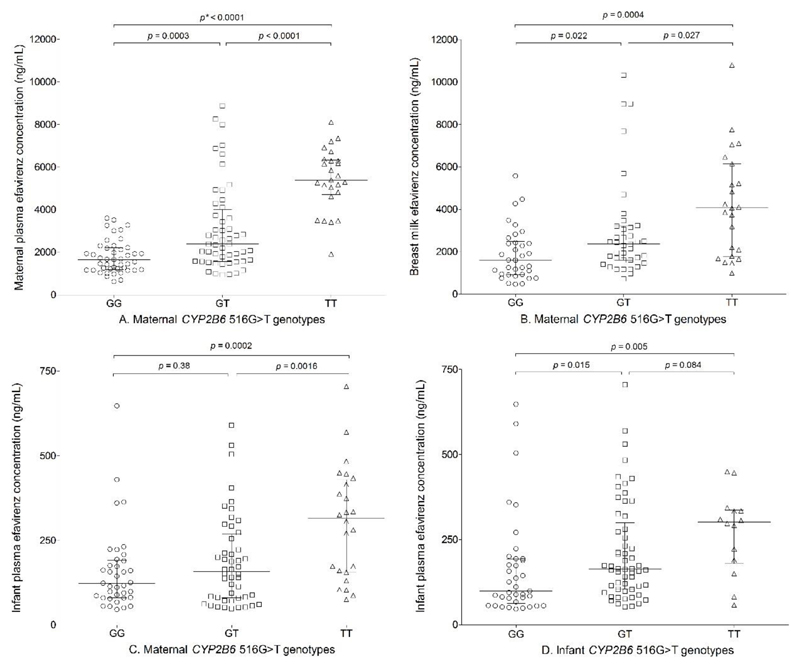
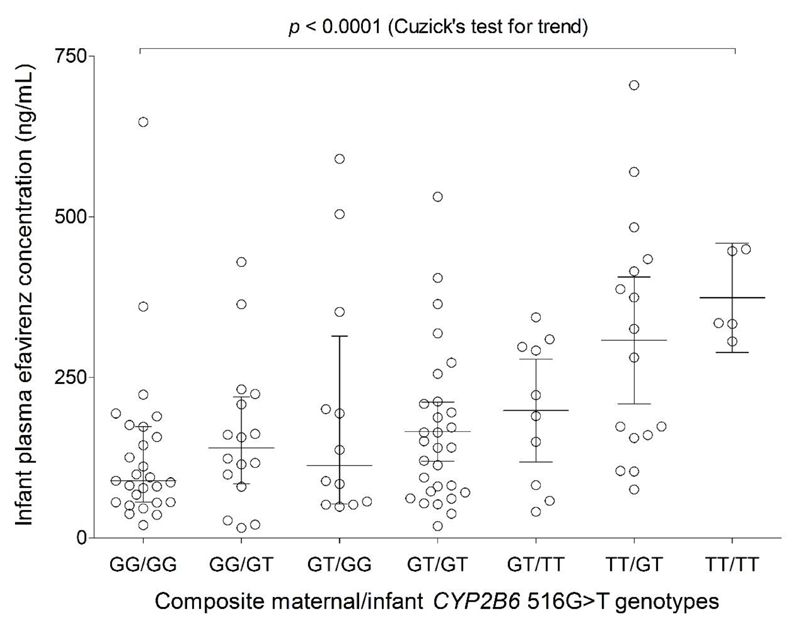
Of the 12 SNPs, only maternal CYP2B6 516G>T (rs3745274) was independently associated with EFV concentrations in maternal plasma, breast milk and infant plasma. In addition, infant age and time post maternal EFV dose were independently associated with infant plasma EFV concentration. The regression coefficients (β), which represents incremental change in log10 EFV concentration per unit change in patient characteristics, are presented in Table 2. In a separate analysis, both infant CYP2B6 516G>T (p = 0.019) and composite infant/maternal CYP2B6 516G>T genotypes (p = 0.006) were independently associated with infant log10 EFV concentration after adjusting for breast milk concentration and infant age with β values of 0.105 (0.018, 0.193) and 0.033 (0.010, 0.057), respectively. More than 99% statistical power was achieved in the multiple linear regressions for plasma (maternal and infant) and breast milk concentrations. Significant differences were observed in maternal plasma EFV concentrations based on CYP2B6 516G>T genotypes (p < 0.0001): GG (n = 42), 1660 ng/mL (632-3610); GT (n = 52), 2390 ng/mL (951-8880); and TT (n = 23), 5400 ng/mL (1920-8110). Breast milk concentrations also varied based on CYP2B6 516G>T genotypes (p = 0.0002): GG, 1610 ng/mL (475-5580); GT, 2370 ng/mL (713-10300); and TT, 4070 ng/mL (995-10800). A similar trend was observed for infant plasma concentrations based on both maternal and infant CYP2B6 516G>T genotypes (Table 3 and Figure 2). After excluding infants less than 10 days old with residual intrauterine exposure, plasma EFV concentrations in infants based on maternal CYP2B6 516G>T genotypes were: GG: 120 ng/mL (46.0-429); GT: 157 ng/mL (48.5-590); and TT: 329 ng/mL (75.5-705). There was a significant trend towards higher infant plasma EFV concentration from GG/GG to TT/TT composite maternal/infant CYP2B6 516G>T genotype, Cuzick’s test for trend p value < 0.0001 (Figure 3).
Table 2. Linear regression analysis showing associations of patient characteristics and maternal single nucleotide polymorphisms in drug disposition genes with log10 EFV plasma concentrations in maternal plasma, breast milk and infant plasma.
| Univariate linear regression | Multivariate linear regression | |||||
|---|---|---|---|---|---|---|
| Patient characteristic | βa (log10 EFV conc., 95% CI) | p value | Bonferroni p value |
Ba (log10 EFV conc., 95% CI) | p value | Bonferroni p value |
| Mother (n = 134) | ||||||
| Weight (kg) | ||||||
| Maternal plasma | -0.002 (-0.011, 0.008) | 0.73 | ||||
| Breast milk | -0.005 (-0.013, 0.004) | 0.30 | ||||
| Infant plasma | -0.004 (-0.013, 0.004) | 0.32 | ||||
| Maternal age (y) | ||||||
| Maternal plasma | -0.0003 (-0.012, 0.012) | 0.96 | ||||
| Breast milk | 0.006 (-0.007, 0.019) | 0.089 | ||||
| Infant plasma | -0.002 (-0.014, 0.01) | 0.71 | ||||
| Infant age (months) | ||||||
| Maternal plasma | 0.002 (-0.016, 0.02) | 0.80 | ||||
| Breast milk | 0.003 (-0.021, 0.027) | 0.80 | ||||
| Infant plasma | -0.026 (-0.043, -0.009) | 0.004 | 0.064 | -0.032 (-0.047, -0.016) | 9.9 x 10-5 | 0.0016 |
| Time post-dose (h) | ||||||
| Maternal plasma | 0.002 (-0.01, 0.014) | 0.78 | ||||
| Breast milk | 0.011 (-0.006, 0.027) | 0.20 | ||||
| Infant plasma | 0.016 (-0.005, 0.028) | 0.006 | 0.096 | 0.017 (0.007, 0.027) | 0.001 | 0.016 |
|
CYP2B6
516G>T (rs3745274) |
||||||
| Maternal plasma | 0.24 (0.17, 0.31) | 1.9 x 10-9 | 3.0 x 10-8 | 0.24 (0.17, 0.31) | 1.9 x 10-9 | 3.0 x 10-8 |
| Breast milk | 0.18 (0.098, 0.27) | 4.7 x 10-5 | 7.5 x 10-4 | 0.18 (0.098, 0.27) | 4.7 x 10-5 | 7.5 x 10-4 |
| Infant plasma | 0.18 (0.10, 0.26) | 2.3 x 10-5 | 3.7 x 10-4 | 0.19 (0.11, 0.27) | 6.0 x 10-6 | 3.7 x 10-4 |
|
CYP2B6
983T>C (rs28399499) |
||||||
| Maternal plasma | 0.061 (-0.098, 0.22) | 0.45 | ||||
| Breast milk | 0.043 (-0.13, 0.22) | 0.63 | ||||
| Infant plasma | -0.051 (-0.21, 0.11) | 0.53 | ||||
|
CYP2B6
c.485-18C>T (rs4803419) |
||||||
| Maternal plasma | -1.3E-5 (-0.17, 0.17) | 1.00 | ||||
| Breast milk | 0.008 (-0.18, 0.2) | 0.93 | ||||
| Infant plasma | -0.038 (-0.21, 0.14) | 0.67 | ||||
|
NR1I3
c.540C>T (rs2307424) |
||||||
| Maternal plasma | -0.091 (-0.24, -0.061) | 0.24 | ||||
| Breast milk | 0.025 (-0.14, 0.19) | 0.76 | ||||
| Infant plasma | -0.044 (-0.20, 0.11) | 0.57 | ||||
|
NR1I3
c.152-1089T>C (rs3003596) |
||||||
| Maternal plasma | -0.009 (-0.11, 0.087) | 0.85 | ||||
| Breast milk | -0.020 (-0.12, 0.08) | 0.69 | ||||
| Infant plasma | 0.042 (-0.055, 0.14) | 0.39 | ||||
|
ABCB1
4046A>G (rs1045642) |
||||||
| Maternal plasma | 0.12 (-0.026, 0.27) | 0.11 | ||||
| Breast milk | -0.011 (-0.17, 0.15) | 0.89 | ||||
| Infant plasma | -0.095 (-0.24, 0.053) | 0.21 | ||||
|
ABCB1
4036A>G (rs3842) |
||||||
| Maternal plasma | 0.037 (-0.1, 0.18) | 0.59 | ||||
| Breast milk | 0.071 (-0.075, 0.22) | 0.34 | ||||
| Infant plasma | 0.084 (-0.054, 0.22) | 0.23 | ||||
|
ABCB1
1236C>T (rs1128503) |
||||||
| Maternal plasma | 0.067 (-0.066, 0.2) | 0.32 | ||||
| Breast milk | -0.030 (-0.18, 0.12) | 0.69 | ||||
| Infant plasma | -0.052 (-0.19, 0.082) | 0.44 | ||||
|
CYP2A6
48T>G (rs28399433) |
||||||
| Maternal plasma | 0.058 (-0.22, 0.34) | 0.68 | ||||
| Breast milk | 0.11 (-0.40, 0.18) | 0.44 | ||||
| Infant plasma | 0.019 (-0.26, 0.30) | 0.89 | ||||
|
ABCB5
c.2908G>A (rs6461515) |
||||||
| Maternal plasma | 0.043 (-0.053, 0.14) | 0.38 | ||||
| Breast milk | 0.070 (-0.034, 0.18) | 0.18 | ||||
| Infant plasma | -0.039 (-0.14, 0.057) | 0.42 | ||||
|
ABCG2
c.1728-46G>A (rs2231164) |
||||||
| Maternal plasma | -0.020 (-0.15, 0.11) | 0.76 | ||||
| Breast milk | 0.11 (-0.050, 0.27) | 0.18 | ||||
| Infant plasma | 0.010 (-0.12, 0.14) | 0.88 | ||||
|
ABCG2
78551A>G (rs2622604) |
||||||
| Maternal plasma | -0.029 (-0.19, 0.13) | 0.72 | ||||
| Breast milk | -0.065 (-0.24, 0.11) | 0.47 | ||||
| Infant plasma | -0.11 (-0.27, 0.047) | 0.16 | ||||
β is regression coefficient, which represents incremental change in log10 EFV concentration per unit change in a patient characteristic.
Figure 2.
Associations between maternal CYP2B6 516G>T genotype (GG, 42; GT, 52, and TT, 23) and EFV concentrations in maternal plasma (A), breast milk (B), infant plasma (C), and infant CYP2B6 516G>T genotype (GG, 45; GT, 67; and TT, 18) and EFV concentrations in infant plasma (D). Bars represent median and interquartile range (IQR) and p values are for Mann Whitney U test.
Figure 3.
Effect of composite maternal/infant CYP2B6 516G>T genotype on infant plasma EFV concentration. A significant trend towards increasing infant plasma EFV concentration from GG/GG to TT/TT composite maternal/infant CYP2B6 516G>T genotype was observed.
Only maternal CYP2B6 516G>T (rs3745274) was used to stratify mother-infant pairs and a total of 29 (GG, 10; GT, 11; TT, 8) were invited for the intensive pharmacokinetic phase.
Full Pharmacokinetic Profiles of EFV in Plasma and Breast Milk
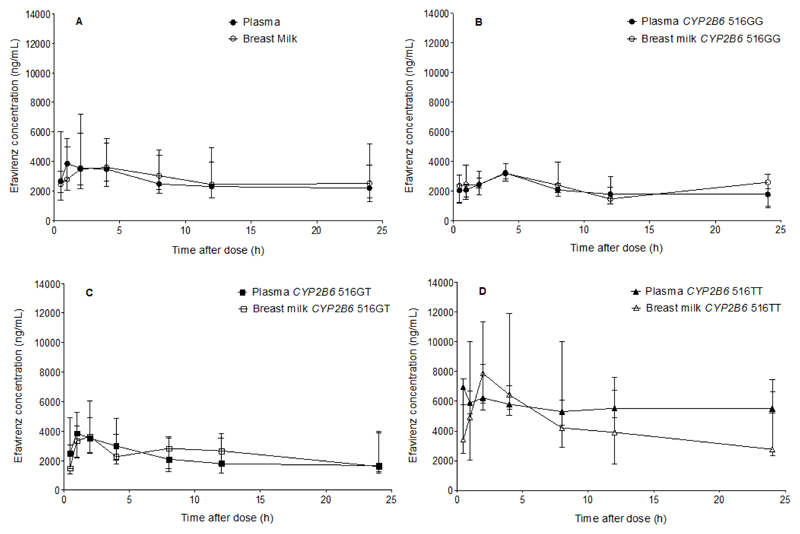
EFV concentration-time profiles in maternal plasma and breast milk in the entire population and in CYP2B6 516GG, CYP2B6 516GT, and CYP2B6 516TT groups are presented in Figure 4. The corresponding pharmacokinetic parameters are presented in Table 3. In pooled analysis, median (range) pharmacokinetic parameters in maternal plasma versus breast milk were: AUC0-24, 60700 ng.h/mL (26800-177000) versus 68500 ng.h/mL (26300-257000); Cmax, 4630 ng/mL (2050-9760) versus 5390 ng/mL (1430-18400); and Cmin, 2030 ng/mL (755-6740) versus 1680 ng/mL (316-9570). Wide intra- and inter-individual variability in the M/P ratio during the dosing interval were observed. The time averaged M/P concentration ratio was 1.10 (0.57-1.71) and M/P AUC0-24 ratio was 1.13 (0.50-1.93) (Table 3 and Figure 4). EFV pharmacokinetic parameters in plasma and breast milk differed significantly between patients stratified by CYP2B6 516G>T genotypes (p <0.05). As with plasma, EFV breast milk AUC0-24, Cmax and Cmin were significantly lower in nursing mothers with CYP2B6 516GG compared with CYP2B6 516GT or CYP2B6 516TT genotypes (Table 3 and Figure 4). The study was sufficiently powered to detect differences between GG vs TT (100% power) and GT vs TT (99.9% power), but not between GG vs GT groups (26% power).
Figure 4.
EFV concentration-time profiles in plasma and breast milk of nursing mothers: in pooled analysis (n = 29) (A); with CYP2B6 GG genotype (n = 10) (B); with CYP2B6 516GT genotype (n = 11) (C); and with CYP2B6 516TT genotype (n = 8) (D). Values are plotted as median (IQR). Details are presented in Table 3.
Breastfed Infants’ Exposure to EFV From Breast Milk
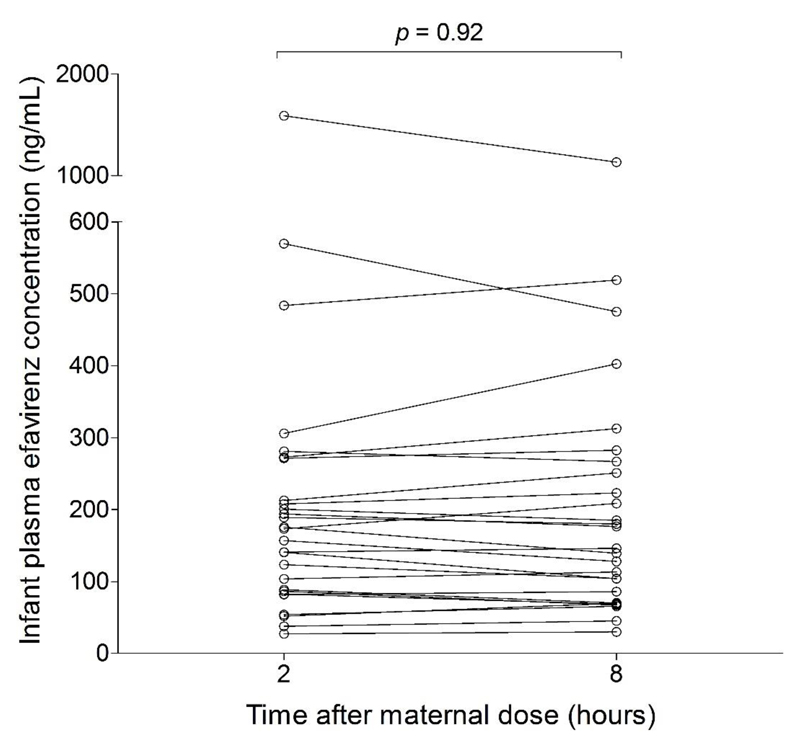
The average infant EFV dose from breast milk, calculated using AUC0-24-derived average milk concentration during the dosing interval and 150 mL/kg/day as average milk intake, was 428 µg/kg/day (164-1610). Using EFV Cmax in breast milk as the maximum breast milk concentration, the maximum EFV dose from breast milk was 809 µg/kg/day (215-2760). In pooled analysis, paediatric dose (for children aged ≥ 3 months and weighing ≥ 3.5 kg [12]) weight-adjusted exposure index was 4.05% (1.08-13.8) and maternal dose weight-adjusted exposure index was 7.69% (2.04-26.2). When stratified by maternal CYP2B6 516G>T, paediatric and maternal dose weight-adjusted exposure indices were highest in CYP2B6 516TT group with values of 6.70 (4.36-13.8) and 12.7 (8.28-26.2) respectively. Infant plasma concentrations did not change significantly during the dosing interval, with no significant differences (p >0.05) between concentrations at 2 hours and 8 hours after maternal EFV dose, with an average of 157 ng/mL (28.6-1360), highest in infants of CYP2B6 516TT mothers with a value of 315 ng/mL (108-1360) (Table 3 and Figure 5). Infant EFV concentration decreased from 1590 ng/mL (190-4631) in 2-8 days old infants, to 194 ng/mL (51.9-705) in 9 days-3 months old, 149 ng/mL (51.8-325) in > 3-6 months old, and 102 ng/mL (40.8-590) in > 6 months old. All infants in this cohort achieved EFV concentrations above the IC50 of 0.51ng/mL for wild-type HIV-1 in protein-free medium, and more than 75% achieved greater than 100 x IC50 [24]. About 96% (129/134) achieved the protein binding-corrected IC50 of 36 ng/mL reported by Acosta et al [25], but only 57% (76/134) achieved the corresponding IC95 of 126 ng/mL.
Figure 5.
Infants’ plasma EFV concentrations during dosing interval. In pooled analysis and when stratified based on maternal genotype, there were no significant differences in infant plasma EFV concentrations at 2 h and 8 h after maternal dose.
Discussion
To our knowledge, this is the first study describing EFV pharmacokinetics in human breast milk during the entire dosing interval in genetically-defined subgroups of nursing mothers. This approach highlights possible worst case scenarios of infant exposure to drugs from breast milk in terms of maternal drug metabolism capacity and infant feeding time. It is also the largest study of EFV excretion into human breast milk and transfer to breastfed infants. Findings indicated that maternal CYP2B6 516G>T was associated with EFV concentrations in maternal plasma, breast milk and infant plasma. With a paediatric dose weight-adjusted exposure index of 4.05% (1.08-13.8), the overall infant exposure to maternal EFV from breast milk was relatively low, highest in infants of mothers with the CYP2B6 516TT genotype at 6.70% (4.36-13.8). The infant plasma EFV concentrations also varied depending on maternal and infant CYP2B6 516G>T genotypes, suggesting a role for CYP2B6 SNPs in EFV disposition in neonates and supporting a recent FDA recommendation for CYP2B6 516G>T-guided dosing of EFV in children between 3 months and 3 years old [12]. CYP2B6 is known to be expressed in infants and to contribute to EFV pharmacokinetic variability in children [26, 27]. The relatively high infant plasma EFV concentrations in the early neonatal period which rapidly declined after day 8 is consistent with observations of in utero exposure to EFV and persistence in plasma up to day 7 after delivery [28, 29]. Significant increases in the expression of CYP2B6 after the neonatal period may also play a role [30].
The EFV dose below which there is no clinically significant effect in infants is unknown. An exposure index of no more than 10% weight-adjusted therapeutic paediatric dose has been proposed as a safety threshold for infant exposure to maternal drugs from breast milk [16]. There was no report of drug-related adverse events in any infants in the present study, suggesting EFV ingestion through breast milk is unlikely to result in toxicity. However, exposure to subtherapeutic EFV concentrations through breast milk raises concerns about potential development of resistance in the rare event of PMTCT failure as reported for other drugs [31, 32]. About 96% and 57% of infants achieved plasma EFV concentrations above the published protein binding-corrected IC50 and IC95, respectively, for wild-type HIV-1 [25].
Studies in chronically infected patients have demonstrated limited compartmentalization and clonal amplification of functional HIV-1 in human breast milk, suggesting ongoing blood-to-breast milk seeding of virus, followed by transient local replication in breast milk [33, 34]. Therefore, therapeutic concentrations of EFV in breast milk may play an important role in preventing ongoing replication of HIV in mammary glands and development of resistance [35], which may otherwise be passed to infants if PMTCT fails [36, 37]. These findings support recent updates in treatment guidelines recommending the use of EFV-based ART started during pregnancy and continued until breastfeeding ends (Option B) or for life (Option B+) for PMTCT [11].
These findings should be interpreted in the context of certain limitations. The study was not adequately powered to detect differences in pharmacokinetic parameters between the GG (n = 10) vs GT (n = 11) groups; only 26% statistical power was achieved. Although we observed higher EFV concentrations in 22% of the infants receiving nevirapine prophylaxis (data not shown), we are unable to make firm conclusions about the interaction since the prophylaxis is given to younger infants who also have residual EFV from intrauterine exposure. As a result of practical challenges associated with plasma collection in resource-limited settings, samples were collected as DBS. However, the negative bias associated with EFV quantification in DBS was adequately compensated using a validated method correcting for patient haematocrit and protein binding [38]. Dried breast milk spot allowed for EFV quantification in whole milk [17], a major difficulty associated with liquid milk [39].
In conclusion, most breastfed infants are exposed to less than 10% of weight-adjusted licensed paediatric dose of EFV through breast milk. Further studies to monitor the long-term safety, including possible emergence of resistance in infants who may become infected if PMTCT fails, are now warranted.
Supplementary Material
Article’s main point.
EFV permeates into human breast milk with milk-to-plasma ratio of 1.10. Most infants are exposed to less than 10% of licensed paediatric dose through breast milk. Maternal CYP2B6 516G>T was associated with EFV concentrations in plasma and breast milk.
Acknowledgments
We would like to thank the participating patients and staff at collaborating clinical sites in Benue State, Nigeria for their support.
Financial support. This work was supported by a grant from Tertiary Education Trust Fund of Nigeria for AOlagunju's PhD at the University of Liverpool, United Kingdom. CW was funded by an Academy of Medical Sciences Starter Grant for Clinical Lecturers subsequently by a Wellcome Clinical Postdoctoral Training Fellowship WT104422MA. The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Potential conflicts of interest. We declare that we have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organisation. Rapid advice: infant feeding in the context of HIV. Geneva: WorldHealth Organisation; 2009. [Accessed 30 September 2014]. Available at: http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf. [Google Scholar]
- 2.Cames C, Saher A, Ayassou KA, Cournil A, Meda N, Simondon KB. Acceptability and feasibility of infant-feeding options: experiences of HIV-infected mothers in the World Health Organization Kesho Bora mother-to-child transmission prevention (PMTCT) trial in Burkina Faso. Maternal & child nutrition. 2010;6:253–65. doi: 10.1111/j.1740-8709.2009.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Guidelines on HIV and infant feeding. Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: World Health Organisation; 2010. [Accessed 30 September 2014]. Available at: http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf. [Google Scholar]
- 4.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471–6. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 5.Ngwende S, Gombe NT, Midzi S, Tshimanga M, Shambira G, Chadambuka A. Factors associated with HIV infection among children born to mothers on the prevention of mother to child transmission programme at Chitungwiza Hospital, Zimbabwe, 2008. BMC Public Health. 2013;13:1181. doi: 10.1186/1471-2458-13-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kafulafula G, Hoover DR, Taha TE, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 7.Asbjornsdottir KH, Slyker JA, Weiss NS, et al. Breastfeeding is associated with decreased pneumonia incidence among HIV-exposed, uninfected Kenyan infants. AIDS. 2013;27:2809–15. doi: 10.1097/01.aids.0000432540.59786.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: programmatic update. [Accessed 30 September 2014]; Available at: http://whqlibdoc.who.int/hq/2012/WHO_HIV_2012.6_eng.pdf.
- 9.Centers for Disease Control and Prevention. Impact of an Innovative Approach to Prevent Mother-to-Child Transmission of HIV - Malawi, July 2011-September 2012. MMWR Morbidity and mortality weekly report. 2013;62:148–51. [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation. PMTCT strategic vision 2010-2015. Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. [Accessed 30 September 2014]. Available at: http://www.who.int/entity/hiv/pub/mtct/strategic_vision.pdf. [Google Scholar]
- 11.World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [Accessed 30 September 2014]. Available at: http://www.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [Google Scholar]
- 12.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Rockville: U.S. Department of Health and Human Services (HHS); 2014. [Accessed 30 September 2014]. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. [Google Scholar]
- 13.Schneider S, Peltier A, Gras A, et al. Efavirenz in human breast milk, mothers', and newborns' plasma. J Acquir Immune Defic Syndr. 2008;48:450–4. doi: 10.1097/QAI.0b013e31817bbc21. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi M, Mwesigwa J, Aweeka F, et al. Hair and Plasma Data Show That Lopinavir, Ritonavir, and Efavirenz All Transfer From Mother to Infant In Utero, But Only Efavirenz Transfers via Breastfeeding. J Acquir Immune Defic Syndr. 2013;63:578–84. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JT, Brown RD, Cherek DR, et al. Drug excretion in human breast milk: principles, pharmacokinetics and projected consequences. Clin Pharmacokinet. 1980;5:1–66. doi: 10.2165/00003088-198005010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118–26. doi: 10.1056/NEJM200007133430208. [DOI] [PubMed] [Google Scholar]
- 17.Olagunju A, Bolaji OO, Amara A, et al. Development, validation and clinical application of a novel method for the quantification of efavirenz in dried breast milk spots using LC-MS/MS. J Antimicrob Chemother. 2015;70(2):555–61. doi: 10.1093/jac/dku420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakhmanina NY, van den Anker JN. Efavirenz in the therapy of HIV infection. Expert Opin Drug Metab Toxicol. 2010;6:95–103. doi: 10.1517/17425250903483207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreiro P, Fernandez-Montero JV, de Mendoza C, Labarga P, Soriano V. Pharmacogenetics of antiretroviral therapy. Expert Opin Drug Metab Toxicol. 2014;10(8):1119–30. doi: 10.1517/17425255.2014.930128. [DOI] [PubMed] [Google Scholar]
- 20.Olagunju A, Owen A, Creesey TR. Potential effect of pharmacogenetics on maternal, fetal and infant antiretroviral drug exposure during pregnancy and breastfeeding. Pharmacogenomics. 2012;13:1501–22. doi: 10.2217/pgs.12.138. [DOI] [PubMed] [Google Scholar]
- 21.Amara AB, Else LJ, Tjia J, et al. A validated method for quantification of efavirenz in dried blood spots using high-performance liquid chromatography-mass spectrometry. Ther Drug Monit. 2015;37(2):220–8. doi: 10.1097/FTD.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 22.Kromdijk W, Mulder JW, Rosing H, Smit PM, Beijnen JH, Huitema AD. Use of dried blood spots for the determination of plasma concentrations of nevirapine and efavirenz. J Antimicrob Chemother. 2012;67:1211–6. doi: 10.1093/jac/dks011. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. American journal of epidemiology. 2009;169:505–14. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48:437–43. doi: 10.1128/AAC.48.2.437-443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta EP, Limoli KL, Trinh L, et al. Novel Method To Assess Antiretroviral Target Trough Concentrations Using In Vitro Susceptibility Data. Antimicrob Agents Chemother. 2012;56:5938–45. doi: 10.1128/AAC.00691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E. Human hepatic CYP2B6 developmental expression: The impact of age and genotype. Biochemical pharmacology. 2009;78(2):184–90. doi: 10.1016/j.bcp.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 27.ter Heine R, Scherpbier HJ, Crommentuyn KML, et al. A pharmacokinetic and pharmacogenetic study of efavirenz in children: dosing guidelines can result in subtherapeutic concentrations. Antiviral Therapy. 2008;13(6):779–87. [PubMed] [Google Scholar]
- 28.Cressey TR, Stek A, Capparelli E, et al. Efavirenz Pharmacokinetics During the Third Trimester of Pregnancy and Postpartum. J Acquir Immune Defic Syndr. 2012;59:245–52. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dooley KE, Denti P, Martinson N, et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis. 2015;211(2):197–205. doi: 10.1093/infdis/jiu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E. Human hepatic CYP2B6 developmental expression: The impact of age and genotype. Biochem Pharmacol. 2009;78:184–90. doi: 10.1016/j.bcp.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Palombi L, Pirillo MF, Andreotti M, et al. Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antivir Ther. 2012;17(8):1511–9. doi: 10.3851/IMP2315. [DOI] [PubMed] [Google Scholar]
- 32.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8(3):e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar-Gonzalez JF, Salazar MG, Learn GH, et al. Origin and evolution of HIV-1 in breast milk determined by single-genome amplification and sequencing. J Virol. 2011;85:2751–63. doi: 10.1128/JVI.02316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gantt S, Carlsson J, Heath L, et al. Genetic Analyses of HIV-1 env Sequences Demonstrate Limited Compartmentalization in Breast Milk and Suggest Viral Replication within the Breast That Increases with Mastitis. J Virol. 2010;84:10812–9. doi: 10.1128/JVI.00543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Perre P, Rubbo PA, Viljoen J, et al. HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Science translational medicine. 2012;4:143sr3. doi: 10.1126/scitranslmed.3003327. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn L, Hunt G, Technau KG, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014;28(11):1673–8. doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micek MA, Dross S, Blanco AJ, et al. Transmission of nevirapine-resistant HIV type 1 via breast milk to infants after single-dose nevirapine in Beira, Mozambique. J Infect Dis. 2014;210:641–5. doi: 10.1093/infdis/jiu130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kromdijk W, Mulder JW, Rosing H, Smit PM, Beijnen JH, Huitema AD. Use of dried blood spots for the determination of plasma concentrations of nevirapine and efavirenz. J Antimicrob Chemother. 2012;67(5):1211–6. doi: 10.1093/jac/dks011. [DOI] [PubMed] [Google Scholar]
- 39.Rezk NL, Abdel-Megeed MF, Kashuba AD. Development of a highly efficient extraction technique and specific multiplex assay for measuring antiretroviral drug concentrations in breast milk. Ther Drug Monit. 2007;29(4):429–36. doi: 10.1097/FTD.0b013e318074db39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.