Abstract
Growing evidence indicates that hippocampal lactate, released from astrocytes, is an important regulator of learning and memory processing. This study evaluated the selective involvement of hippocampal and striatal lactate in two object recognition tasks. The tasks tested recognition memory after a change in location of two target objects (double object location; dOL) or after replacement of familiar targets with two new objects set in the original locations (double object replacement; dOR). Rats received three study sessions across which exploration times decreased. The recognition index was the change in exploration time of both objects on a test trial from the exploration times on the final study trial. We first verified a double dissociation between hippocampus and striatum across these tasks. The sodium channel blocker, lidocaine, was infused into one of the two brain regions after the study sessions and before the test trial. To test the role of neuronal lactate in recognition memory, an inhibitor of the neuronal lactate transporter, α-cyano-4-hydroxycinnamate (4-CIN), was similarly infused. For both drugs, infusions into the hippocampus but not the striatum impaired recognition in the dOL, whereas infusions into the striatum but not hippocampus impaired recognition in the dOR. The findings obtained with 4-CIN demonstrate for the first time the importance of neuronal lactate uptake in the hippocampus and the striatum for object recognition memory processing.
Keywords: object recognition, lactate, astrocytes, 4-CIN, multiple memory systems
Epinephrine, released into blood from the adrenal medulla, enhances learning and memory across a wide range of conditions (Gold, 1995; Gold & Korol, 2012). Epinephrine does not itself readily cross from blood to brain (Weil-Malherbe, Axelrod, & Tomchick, 1959), but appears to exert its actions on cognitive functions by increasing blood glucose levels (Gold, 2014; Gold & Korol, 2014) via activation of hepatic adrenergic receptors (Sutherland & Rall, 1960). Conditions that increase circulating glucose also enhance learning and memory across a range of tasks, species, and ages (Gold, 2001; Gold & Korol, 2012; Korol, 2002; Korol & Gold, 2007; Messier, 2004; Messier, Desrochers, & Gagnon, 1999; Morris & Gold, 2013; Smith, Riby, Eekelen, & Foster, 2011; van der Zwaluw, van de Rest, Kessels, & de Groot, 2015). Direct infusions of glucose into certain brain regions improve learning and memory (McNay & Gold, 1998; Morris & Gold, 2013; Ragozzino, Pal, Unick, Stefani, & Gold, 1998; Schr-oeder & Packard, 2003; Stefani & Gold, 2001; Stefani, Nicholson, & Gold, 1999) on tasks that rely on intact functioning of those neural systems. Morever, extracellular levels of glucose in brain are not uniformly saturated but instead respond dynamically to training and memory testing with extracellular depletion seen during early phases of testing followed thereafter by return to and elevations above baseline (McNay, Fries, & Gold, 2000; McNay & Gold, 2001; McNay, McCarty, & Gold, 2001; Newman, Korol, & Gold, 2011).
In the brain, glucose might act on learning and memory as a substrate for energy metabolism by uptake into neurons (Lund-gaard et al., 2015). However, glucose may also act through astrocytic uptake and conversion to lactate, which is subsequently used by neurons under conditions of high metabolic demand such as during cognitive processing (Alberini, Cruz, Descalzi, Bessieres, & Gao, 2018; Newman et al., 2011; Steinman, Gao, & Alberini, 2016; Suzuki et al., 2011). Astrocytic production of lactate as a downstream mediator of glucose actions to enhance learning and memory is supported by findings that, like glucose, direct infusions of lactate into the hippocampus enhance memory in spatial working memory tasks (Newman et al., 2011) and for inhibitory avoidance training (Suzuki et al., 2011). Blockade of lactate transport into neurons by pharmacological administration of α-cyano-4-hydroxycinnamate (4-CIN; Newman et al., 2011), a drug that blocks the monocarboxylate 2 (MCT2) transporters on neurons (e.g., Bergersen, 2007; Brooks, 2009), or manipulations of gene expression of the MCT2 transporter (Suzuki et al., 2011) impairs memory. Interference with the MCT2 transporter attenuates the ability not only of lactate but also of glucose to enhance memory. Taken together, the results suggest that lactate uptake into neurons through MCT2 mechanisms is a necessary step in the enhancement of cognition by glucose.
Like other forms of cognition, systemic administration of glucose enhances object recognition (Messier, 1997), implicating brain lactate as a potential modulator of recognition memory. The present experiments were designed to test the importance of lactate in regulating learning and memory for object recognition tasks across multiple memory systems. Distinct brain systems are important for processing information involved in learning tasks that have different attributes, such as egocentric (response) or allocen-tric (spatial or place) properties that rely on dorsal striatum and hippocampus functions, respectively (cf. Gold, Newman, Scav- uzzo, & Korol, 2013; Kesner, Bolland, & Dakis, 1993; Korol, Gold, & Scavuzzo, 2013; Packard & Goodman, 2012, 2013; White & McDonald, 2002). Supporting this view, hippocampal lesions impair stimulus-stimulus associations and place-based learning while striatal lesions impair cued- and stimulus-response-based learning (Compton, 2004; McDonald & White, 1993). In addition, direct injections of drugs that impair the functions of these brain areas, for example, lidocaine or muscimol, also impair learning of the respective cognitive attributes (Chang & Gold, 2003a, 2004; McElroy & Korol, 2005; cf. Packard, 2009; Packard & McGaugh, 1996), whereas injections of drugs that augment function of these brain areas, for example, glutamate or glucose, enhance the classes of learning associated with each brain area (Canal, Stutz, & Gold, 2005; Packard, 1999). Furthermore, several biological measures in the hippocampus and striatum show differential responses to training, in a task by brain area manner (e.g., Colombo, 2004; Gardner et al., 2016; Gold, 2016; Gold et al., 2013; Newman, Scavuzzo, Gold, & Korol, 2017; Teather, Packard, Smith, Ellis-Behnke, & Bazan, 2005; Yagi, Chow, Lieblich, & Galea, 2016). These and other findings point to the hippocampus and striatum, among other brain areas, as parallel memory systems that process specific forms of learning and memory (Gold & Korol, 2017; White, Packard, & McDonald, 2013; Zurkovsky, Brown, Boyd, Fell, & Korol, 2007).
To examine the role of brain metabolism in different attributes of learning and memory using a multiple memory system approach, it would be useful to identify complementary hippocampus- and striatum-sensitive tasks that rely on endogenous motivators without confounds of aversive and appetitive rewards. Object recognition tasks may be particularly beneficial in this context because they circumvent the need for experimentally derived motivators; rodents explore objects in arenas spontaneously. Many neurobiological studies of object recognition tasks focus on the hippocampus as the site of action (e.g., Barker & Warburton, 2011; Cohen et al., 2013; Cohen & Stackman, 2015; Mumby, Wood, & Pinel, 1992; Steckler, Drinkenburg, Sahgal, & Aggleton, 1998a), with findings corroborating the hormonal, molecular, and pharmacological interactions with memory found using other hippocampus-dependent learning and memory tasks (e.g., Kim & Frick, 2017; Korol, 2004, 2018; Korol & Kolo, 2002; Korol & Pisani, 2015; Korol & Wang, 2018; Luine, Jacome, & Maclusky, 2003; Pisani, Neese, Katzenellenbogen, Schantz, & Korol, 2016; Sheppard, Koss, Frick, & Choleris, 2017; Walf, Rhodes, & Frye, 2006; Xu, Chen, Zhu, Shen, & Luo, 2005). However, evidence for striatum-sensitive involvement in object recognition tasks or clear dissociations of task by these two brain areas is lacking.
Here we describe double dissociations of the hippocampus and striatum in two object recognition tasks, basing differences in object recognition training procedures on past reports that showed hippocampal and nonhippocampal involvement. We first established task procedures that revealed a double dissociation of hippocampus and striatum using intrahippocampal or intrastriatal infusions of lidocaine. Lidocaine, a local anesthetic that blocks sodium channels, is often used to inactivate brain areas in many contexts, including assessments of the contributions of brain systems to learning and memory (Chang & Gold, 2003a, 2004; Gold, 2016). Subsequently, we tested the efficacy of MCT-2 blockade by 4-CIN to impair recognition memory in these hippocampus- and striatum-sensitive tasks according to the memory system involved. Thus, the second experiment tested the necessity of lactate delivery to neurons for memory enhancement across tasks and brain regions.
Method
Subjects
Three-month-old male Long-Evans rats were obtained from Harlan Laboratories (Oregon, WI). Throughout the experiment, the rats had free access to food and water and were maintained on a 12:12 hr light:dark cycle. Each rat was handled for several minutes each day for at least 4 days prior to behavioral testing. All procedures were approved by the Syracuse University Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cannula Implantation
For cannula implantations, rats were anesthetized with 2% to 4% isoflurane and placed in a stereotaxic apparatus that contained a nosepiece adapted to provide continuous isoflurane delivery throughout surgery (SomnoSuite, Kent Scientific, Torrington, CT). Rats received 100,000 U penicillin (I.M.) and 5 mg/kg Rimadyl (carprofen) preoperatively. Using standard procedures and coordinates for cranial surgery (based on Paxinos & Watson, 2005; for details see Chang & Gold, 2003a, 2004; Zurkovsky et al., 2007), guide cannulae (6 mm long, 22 gauge; Plastics One, Roanoke, VA) were positioned bilaterally into the dorsal hippocampus (coordinates: –3.6 mm from bregma; ±2.2 mm lateral; 4.0 mm ventral from skull) or dorsal striatum (coordinates: +0.3 mm from bregma; ±3.7 mm lateral; 4.0 mm ventral from skull). After surgery, rats received injections of sterile saline (10 ml, s.c.) for hydration and ibuprofen (Children’s Motrin) in drinking water (47 mg in 500 mL water bottles) for analgesic support after surgery. Rat health was monitored daily for the week after surgery.
Double Object Location and Object Replacement Recognition Tasks
At least 1 week after surgery, rats underwent object recognition training and testing within a single day. For study Sessions 1, 2, and 3 (S1, S2, and S3), rats were placed in a square, black Plexiglas arena (70 cm × 70 cm × 50 cm) with two objects, each approximately 7 cm tall, and were allowed to explore the arena. The objects were placed 40 cm apart and centered in the vertical orientation. Each study session was 5 min in duration with 3 min between sessions during which rats were returned to their holding cage. A test session was administered 30 min after S3. For the double object location task (dOL), the distance between the objects was decreased to 10 cm during the test session, as shown in Figure 1. On the test session for the double object replacement test (dOR), both objects were replaced with novel objects but kept in the same spatial locations and configuration (see Figure 1).
Figure 1.
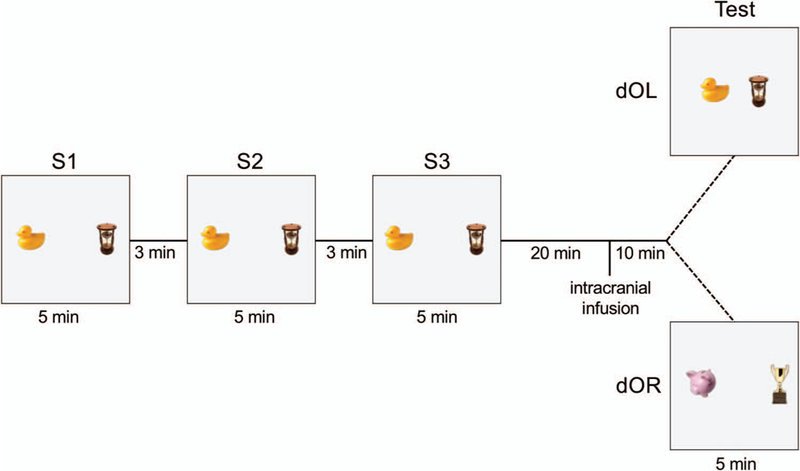
Graphic representation of double object location (dOL) and double object replacement (dOR) recognition tasks. Two 3-dimensional objects are positioned 40 cm apart in a square Plexiglas arena. During each 5-min study session (S1, S2, S3), rats are placed into the arena and allowed to explore freely the field and the two objects anchored to the floor of the arena. Between each study session, rats are removed from the arena and placed in their holding cage for a 3-min interval. Twenty minutes after S3, rats received an infusion of lidocaine or 4-CIN into either the hippocampus or striatum. Ten minutes later, a 5-min test session for dOL or dOR recognition was conducted. For dOL, the two objects were repositioned horizontally to 10 cm apart. For dOR the old objects were replaced in the original locations by two new objects that were similar in size, but different in form, color, and material. See the online article for the color version of this figure.
For each trial, the time spent exploring both objects and the total arena were recorded using videocameras and scored off-line by hand using ClickCounter (compliments of G. Dohanich). Object exploration was defined as any explicit interaction with the objects, such as whisking and sniffing. Sitting on, climbing on, or simply looking toward an object was not considered exploration unless the rat was actively engaged with the object. To reduce odor cues, objects were wiped clean with EtOH between all sessions. The configuration and testing procedures during study sessions were the same across the two tasks. Note that this recognition testing procedure involved changes in both objects and thus total time spent exploring both objects was used as the dependent measure for study and test trials. General exploratory activity was reflected in arena exploration times independent of time spent with the objects.
Drug Infusions
Drugs, purchased from Millipore Sigma (then SigmaAldrich, St. Louis, MO), were administered to the hippocampus or striatum between the last study session and the recognition test on either the object relocation or object replacement task, that is, 20 min after S3 and 10 min prior to T. Rats were assigned to one of two treatments: lidocaine hydrochloride (1%) or its vehicle (artificial cerebrospinal fluid [aCSF]) for Experiment 1, and 4-CIN (30 pmol) or its vehicle (0.9% saline [sal]) for Experiment 2. Drug or vehicle was infused bilaterally into either the hippocampus or striatum at a rate of 0.5 μl/min for 2 min. The lidocaine concentration is one used before to produce functional inactivation of the hippocampus and striatum (e.g., Chang & Gold, 2003a, 2004; Packard & McGaugh, 1996). The injection needle was left in place for 1 min after infusion to allow diffusion away from the injection needle anticipated to be ~1 mm based on previous reports (Edeline, Hars, Hennevin, & Cotillon, 2002; Myers, 1966; Walker & Gold, 1992). Except for removal during injections, each rat remained in its holding cage for the duration of the S3-test interval.
Rats were randomly assigned to one of 16 experimental groups. For each drug condition there were eight experimental groups representing two tasks (dOL, dOR), two brain sites (hippocampus, striatum), and two treatments (vehicle, drug; N = 91). Sample sizes for each group are noted in Figures 2 and 3.
Figure 2.
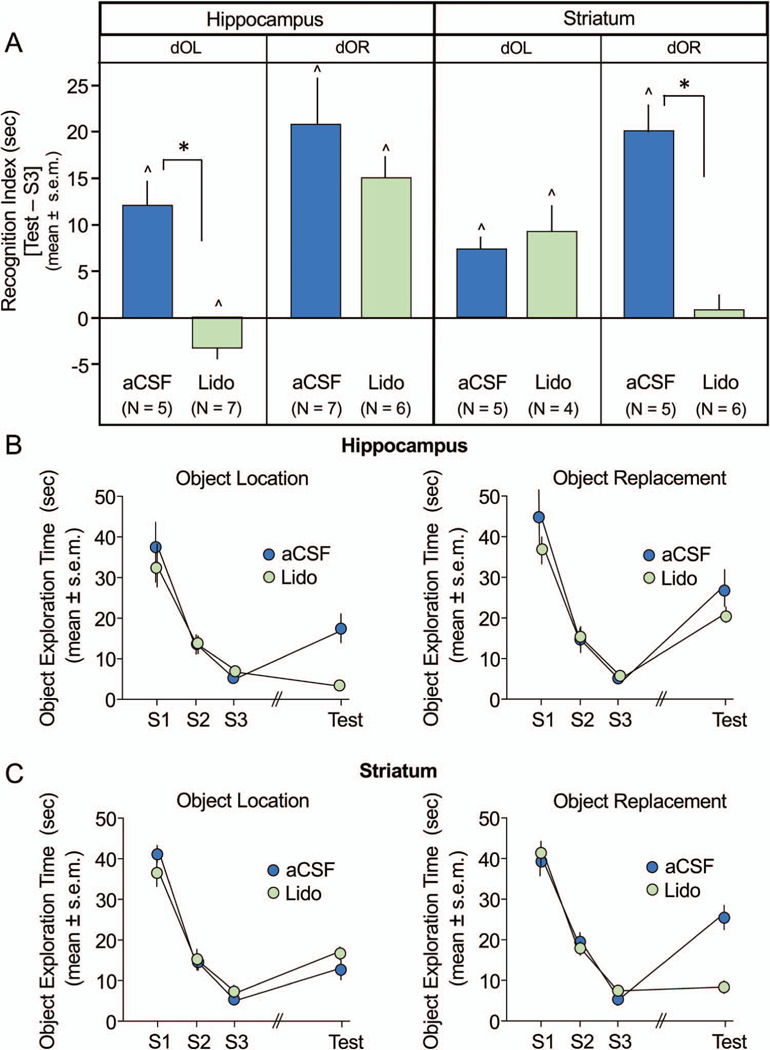
Effects of lidocaine (Lido) infusions into the hippocampus and striatum on double object location (dOL) and double object replacement (dOR) tasks. A: Recognition index scores reflecting difference in time exploring objects during test (T) and study session (S)3 (T-S3) show that compared to artificial cerebral spinal fluid (aCSF), Lido infusions into hippocampus impaired recognition for dOL but not dOR recognition (left panel), while infusions into the striatum impaired dOR but not dOL recognition (right panel). All rats treated with aCSF demonstrated significant recognition from S3. B: Time exploring both objects during S1, S2, S3, and test sessions following hippocampal infusions or striatal infusions (C). Object exploration curves reveal a decline in exploration across S1-S3. Rats that recognize change during test show increase in exploration compared to S3. * p < .0125 aCSF versus Lido; ^ p < .05 versus 0, within subjects. See the online article for the color version of this figure.
Figure 3.
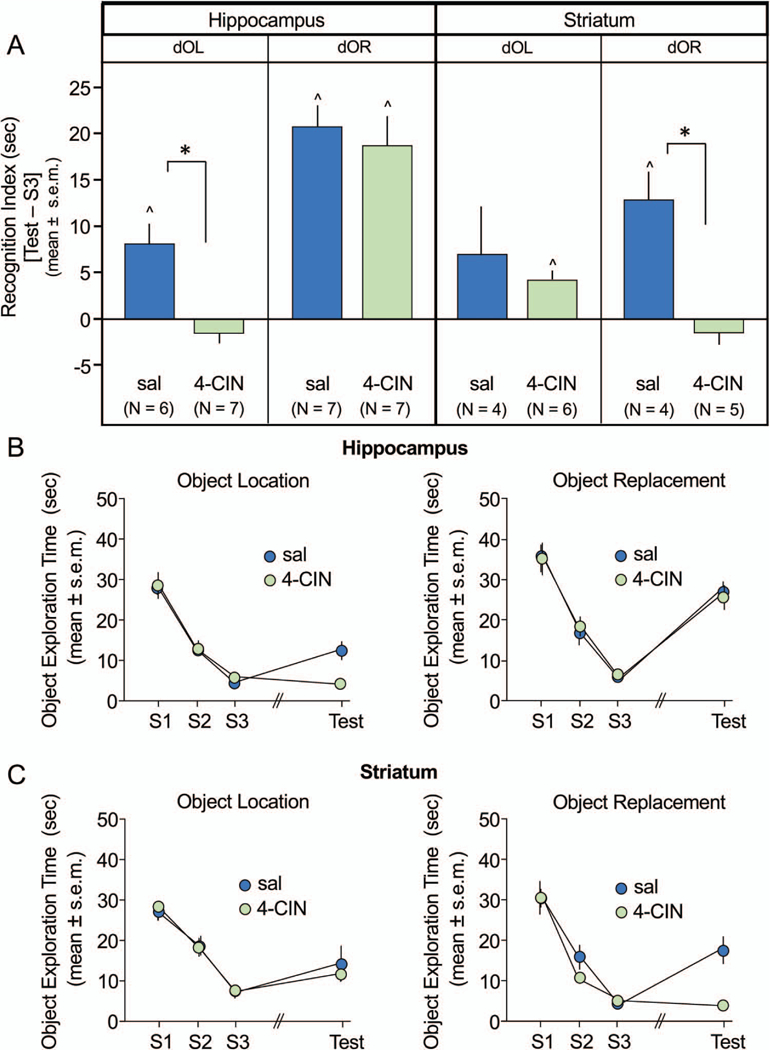
Effects of 4-CIN infusions into the hippocampus and striatum on double object location (dOL) and double object replacement (dOR). A: Recognition index scores show that 4-CIN infusions into hippocampus impaired recognition for dOL but not dOR recognition (left panel). Conversely, infusions into the striatum impaired dOR but not dOL recognition (right panel). All saline (sal)-treated rats, except those with striatal infusions tested on dOL, showed recognition of changes in objects from study session (S)3. B: Time exploring both objects during S1, S2, S3, and test sessions following hippocampal infusions or striatal infusions (C). Object exploration curves reveal a decline in exploration across S1 through S3. Rats that recognize a change during test show an increase in exploration compared to S3. * p < .0125 sal versus 4-CIN; ^ p < .05 versus 0, within subjects. See the online article for the color version of this figure.
Histology
Shortly after training and testing were complete, some rats received a pentobarbital overdose (50 mg/rat Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI) and were perfused transcardially with saline followed by paraformaldehyde. Other rats were decapitated, their brains rapidly extracted and frozen to be used for biochemical analysis in a subsequent experiment. Unfortunately, the biochemical assay failed and the data for these measures were not available. Perfused brains were removed, sectioned (40 μm) through the cannula placement site and stained with cresyl violet. Sections were examined with light microscopy to confirm accurate cannula placements and tissue integrity. During dissection and collection of flash frozen samples, brains were visually inspected to determine health of tissue and to verify cannula tracks in target structures, both of which were recorded.
Data Analysis and Statistics
Habituation to the objects across study sessions was defined as a decrease in total object exploration from S1 to S3. Four rats did not explore the arena during the study sessions, one rat failed to explore the objects, and another rat failed to show a decline in object exploration during the study phase; these rats were excluded from data analyses. The difference in object exploration times between the final study session and the test session (T-S3) was used as the index of recognition. For both tasks, this difference reflects a change in exploratory activity from the familiar condition of S3 to the novel condition of the test session. Similar discrimination indices between familiar and novel objects are often used in other object tasks to operationalize recognition memory (Cohen & Stackman, 2015).
The statistical analyses were constructed to support three main aims of this study to show (1) a double dissociation of treatment effects across task and structure (three-way analysis of variance [ANOVA]), (2) treatment effects within structure and task (pairwise planned contrasts), and (3) recognition memory within each group (paired t tests).
To identify significant interactions of Task (dOL vs. dOR) × Brain Area (hippocampus vs. striatum) × Treatment (drug or vehicle), a three-way ANOVA was performed on the recognition index values (T-S3) within each experiment, that is, lidocaine and 4-CIN. The ANOVAs were followed by nonoverlapping, planned pairwise contrasts (Field, 2009) of treatment effects within task and brain region; the Bonferroni method was used to correct for multiple comparisons (α = .0125).
The strength of recognition memory within groups was also examined. In this context, recognition memory was evaluated with paired t tests using within-subject comparisons of exploration times during S3 and test. A significant decrease in exploration from S3 to test was interpreted as continued habituation without recognition, whereas a significant increase in exploration was taken as evidence of detection of the change in object locations or new objects, that is, recognition memory as suggested by others (Goodrich-Hunsaker, Hunsaker, & Kesner, 2008).
Results
Experiment 1: Lidocaine
The results obtained using the recognition index, T-S3, are shown in Figure 2A. A double dissociation using lidocaine was confirmed with a 2 × 2 × 2 ANOVA showing a significant three-way interaction of drug treatment across brain area and task, F(1,45) = 20.9, p < .0001. Infusions of lidocaine into the hippocampus, but not striatum, impaired dOL recognition (hippocampal lidocaine vs. aCSF, t[10] = 6.09, p < .0005; striatal lidocaine vs. aCSF, t[7] = 0.67, p > .5). Conversely, infusions of lidocaine into the striatum, but not hippocampus, impaired dOR recognition (striatal lidocaine vs. aCSF, t[9] = 6.05, p < .0002; hippocampal lidocaine vs. aCSF, t[11] = 0.43, p > .6).
As shown in Figure 2B and 2C, there was a steady decrease in object exploration values during the study trials, S1 through S3, administered prior to drug treatment. Groups exhibited mean object exploration times of 35 to 45 s on S1 and ended with times of 5 to 8 s on S3. General exploratory activity was consistent across sessions and treatment groups. There were no significant effects of treatment in either task or brain region on total (Arena + Objects) exploration times (data not shown). All groups exhibited mean total exploration of the arena and objects ranging from 194 to 231 s of the 300-s test session (all ps > .25). Thus, group differences shown in Figure 2A reflect shifts in how the rats allocated their exploration time between objects and the arena on the test trial and not changes in general exploratory activity.
Strong recognition memory was demonstrated by all control, aCSF-treated rats in both dOL and dOR tasks regardless of site of infusion, as indicated by the significantly greater time spent exploring the objects during the test session than during S3 (Figure 2A; all ps < .05). Moreover, significant recognition memory was also observed in rats receiving lidocaine into the hippocampus for the dOR task and in those treated with striatal lidocaine for dOL (all ps < .05). However, lidocaine into the hippocampus for dOL and striatum for dOR prevented significant recognition, and in fact produced continued habituation in the dOL task for rats with hippocampal inactivation (see Figure 2A).
Experiment 2: 4-CIN
As shown in Figure 3A, the results seen in the recognition indices obtained with 4-CIN infusions were remarkably similar to those seen with lidocaine. The three-way ANOVA in the 4-CIN experiment revealed a significant interaction of Drug × Task × Brain Area, F(1, 44) = 4.6, p < .05. Infusions of 4-CIN into the hippocampus, but not striatum, impaired dOL recognition (hippocampal 4-CIN vs. sal, t[11] = 4.26, p < .002; striatal 4-CIN vs. sal, t[8] = 0.62, p > .5). The opposite pattern of results was seen on the dOR task. Infusions of 4-CIN into the striatum, but not hippocampus, significantly impaired dOR recognition (striatal 4-CIN vs. sal, t[7] = 3.97, p < .01; hippocampal 4-CIN vs. sal, t[12] = 0.51, p > .5).
For rats that received 4-CIN or saline infusions, the object exploration times decreased from ~25 to 40 s during S1 to 5 to 10 s during S3, that is, prior to treatments (see Figures 3A and 3B). As with lidocaine described in the preceding text, there were no significant effects of 4-CIN on total (Arena + Objects) exploration times for either the hippocampus (p > .8) or striatum (p > .1). The range of total exploration times across groups was 166 to 273 s during the 300-s test trial.
In this experiment, rats receiving saline infusions showed significant recognition memory (all ps < .05) except for rats with striatal infusions tested on dOL (p = .28; see Figure 3A). However, as described above, dOL recognition measures in these rats with striatal saline did not differ from those with striatal infusions of 4-CIN (see Figure 3A through 3C). As with lidocaine, rats with infusions of 4-CIN into hippocampus and striatum showed strong recognition memory on dOR and dOL, respectively (ps < .05), whereas those with 4-CIN infusions into the canonical structures, that is, hippocampus for dOL (p = .16) and striatum for dOR (p = .25) did not (see Figure 3A).
Histology
Complete histological assessment of cannula location was conducted in approximately one half of the rats from Experiment 1 (seven of 25 brains for hippocampal implants and 15 of 20 brains for striatal cannulae). We found 100% of the cannula placements were accurately positioned in the target structure based on light microscopic evaluation (see Figure 4). Brain samples from all rats in Experiment 2 were flash-frozen for biochemical assessment (not included). Thus, we lacked fine histological verification of cannulae for rats treated with 4-CIN. However, visual inspections of cannula tracks were recorded during brain dissections and revealed a 100% hit rate for gross placement in the target structure. Therefore, given the confirmed 100% placement rate in our samples with full histology as well as the samples using visual observations at the time of dissection, the behavioral data for all rats, even those without microscopic evaluation, were included in the final analyses. Historically, our hit rates using similar coordinates for cannula placement in hippocampus and striatum have been 95% accurate (408 hits from 428 rats total; compiled from Chang & Gold, 2004; Chang, Savage, & Gold, 2006; McNay, Canal, Sherwin, & Gold, 2006; Morris et al., 2013; Newman & Gold, 2016; Newman et al., 2011, 2017; Pych, Chang, Colon-Rivera, & Gold, 2005a; Pych, Chang, Colon-Rivera, Haag, & Gold, 2005b; Pych, Kim, & Gold, 2006; Zurkovsky, Serio, & Korol, 2011), and thus we feel confident that our findings reflect processes specific to the structures of interest.
Figure 4.
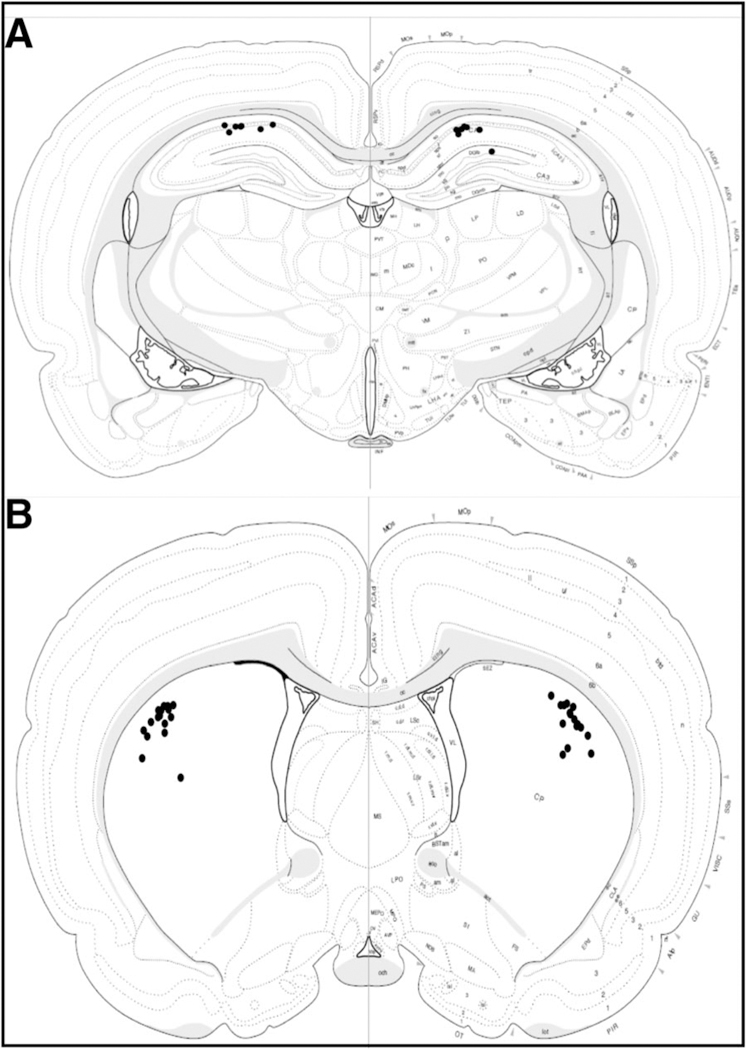
Distribution of bilateral lidocaine infusion sites targeting (A) hippocampus and (B) striatum for rats in Experiment 1. Full histological assessment was completed for hippocampal placements in 7/25 rats and for striatal placements in 15/25 rats. Note that 100% of the placements were accurate.
Discussion
We demonstrated that lidocaine and 4-CIN significantly impaired recognition memory for objects or their relative location but only when administered to the brain region believed to be necessary for that specific type of recognition. Thus, using two treatments with very different modes of action, that is, one that blocks neural activity and one that prevents lactate entry into neurons, we found a task by brain region double dissociation of recognition tasks that differed only in the type of change enacted during the recognition test, that is, change in positions versus new objects. Interference with hippocampal function disrupted dOL recognition, whereas interference with striatal function prevented dOR recognition regardless of treatment type, with the two experiments serving as an internal replication for these selective effects. It is important to note that both lidocaine and 4-CIN treatment effects were statistically significant despite the relatively small numbers of rats in each group (ns = 4–7/group). Even with the smallest sample sizes (see Figure 2A and Figure 3A), significant differences were detected for treatments into the canonical structure but not for the noncanonical structures for which the results did not approach significance. Thus, low statistical power did not likely contribute to the double dissociation.
Processing of metric relationships, consisting of quantitative measures of distances and angles, is sensitive to lesions of the hippocampus (Gallistel, 1990; Goodrich-Hunsaker, Hunsaker, & Kesner, 2005, 2008; Kuipers & Levitt, 1988; Poucet, 1993; Poucet & Herrmann, 2001). Models of hippocampus function and disruption of function following lesions suggest that the dentate gyrus of the hippocampus is engaged particularly on tasks that require distinctions between very similar spatial stimuli (Gilbert, Kesner, & Lee, 2001; Kesner, 2013; Leutgeb, Leutgeb, Moser, & Moser, 2007; Morris, Churchwell, Kesner, & Gilbert, 2012; Nakashiba et al., 2012; Rolls & Kesner, 2006). Thus, it is likely that both lidocaine and 4-CIN in the hippocampus interfered with mechanisms involved in detecting metric changes in object location. Very little, is known about the role of striatum in pattern separation and object recognition tasks, however the site selectivity seen in our results suggest that striatum engagement is not necessary for detection of change in object configurations.
The results obtained with 4-CIN that blockade of lactate transport into neurons impaired dOL and dOR are consistent with the view that lactate is a potent modulator of learning and memory processing (Alberini et al., 2018; Gao et al., 2016; Gold, 2014; Newman et al., 2011, 2017; Steinman et al., 2016; Suzuki et al., 2011; Tadi, Allaman, Lengacher, Grenningloh, & Magistretti, 2015). The impairments in learning and memory by interference with lactate transport out of astrocytes and into neurons have been found for hippocampus-sensitive abilities such as spatial working memory in spontaneous alternation tasks and memory retention in one-trial inhibitory avoidance tasks (Newman et al., 2011; Suzuki et al., 2011). However, our findings with 4-CIN in the striatum are new and suggest that provision of astrocytic lactate for neuronal use is important across multiple brain systems and different types of memory processing including recognition memory. Recently, we found that the magnitude of training-induced increases in extracellular lactate levels in the hippocampus and striatum was dissociated during place and response learning in a manner that also interacted with the nature of reward, food versus water (Newman et al., 2017). Given the striatum’s known role in reward processing (Burton, Nakamura, & Roesch, 2015), it was not surprising that the lactate response in striatum was unique to water versus food and was different from the response in the hippocampus. However, the findings do highlight the importance of controlling for reward-related effects on outcome measures that may interact with and confound experimental interventions, especially when examining differences across memory systems. One advantage of the object recognition tasks as described here is that learning is assessed under conditions of low stress and arousal in the absence of extrinsic rewards and punishments and may thus be useful in tests of neural mechanisms of memory.
Lidocaine and 4-CIN blocked detection of change in objects or object locations when injected into the striatum or hippocampus, respectively; the drugs did not significantly affect recognition on the other task or on overall locomotor activity during the test trial. These findings are therefore consistent with an extensive literature showing that the hippocampus and striatum are important components of information processing for different attributes of learning and memory. Of note, lesions and drug manipulations that disrupt function of the hippocampus and striatum impair learning and memory based on allocentric (spatial or place) or egocentric (response, habit) task features (as reviewed by Gold et al., 2013; Korol et al., 2013; Packard & Goodman, 2012, 2013; White & McDonald, 2002). These dissociations of hippocampal and striatal functions in learning are also evident when monitoring functional correlates of activity during training on similar tasks (reviewed in Colombo, 2004; Gold, 2004, 2016; Gold et al., 2013). For example, differences between hippocampus and striatum in training- related release of lactate (Newman et al., 2017) and acetylcholine (Chang & Gold, 2003b; McIntyre, Marriott, & Gold, 2003; Pych, Chang, Colon-Rivera, & Gold, 2005a; Pych, Chang, Colon-Rivera, Haag, & Gold, 2005b), and in training-related changes in levels of choline acetyltransferase (Hawley, Witty, Daniel, & Dohanich, 2015), activation of CREB, and expression of c-Fos (Colombo, 2004; Colombo, Brightwell, & Countryman, 2003), c-Jun (Teather et al., 2005), and Arc (Gardner et al., 2016) depend on whether animals used place or response strategies to solve the task. The findings of the present experiment therefore position these object recognition tasks into the broader framework of multiple memory systems, in particular compared to place versus response maze learning (Gold et al., 2013; Korol, 2018; Korol et al., 2013), win-stay versus win-shift learning (White et al., 2013), and cognitive versus habit learning (Packard & Goodman, 2013).
Although the distinction of the classes of tasks promoted by the hippocampus and striatum is clear, the application of the distinction to object recognition tasks has not always been evident. The effects of hippocampal damage on spatial and nonspatial recognition tasks are mixed (Cohen & Stackman, 2015; Steckler, Drinken- burg, Sahgal, & Aggleton, 1998b). Caudate lesions also impair spatial object recognition learning without affecting nonspatial recognition, though tests of striatal contributions to nonspatial recognition are relatively lacking (Steckler et al., 1998b). It is perhaps the mix of specific methods used, for example, delays between training and testing, time between lesions and training, mazes versus open arenas, stimulus cues, and so forth, that contribute to the considerable overlap in the neural systems involved in recognition learning (Cohen & Stackman, 2015). The present experiments attempted to minimize these differences by using the same objects and test conditions and by including habituation trials to the objects on three study sessions thereby establishing baselines from which to evaluate recognition during the test session. On the test trial for dOL task, the position of both objects and the distance between them was changed, creating a new spatial configuration. On the test for the dOR task, both objects were replaced with novel objects that were similar in many respects, that is, general size, composition, location. In this manner, the tasks differ from more common paradigms that use a single study session (Ennaceur, 2010) and that focus on exploration of the new object or position, or comparison across old and new stimuli, as the main measure of recognition.
The magnitude of change in total object exploration from study to test phases was used as the operational measure of recognition. Because the 30-min delay between S3 and test was longer than the 3-min delays used between study sessions, the increase in object exploration during test may also reflect forgetting of the previous objects or configurations. However, we found that rats continue to show habituated responses to a fourth study session where no changes are made to the objects even when given 30 min after S3 (Tunur & Korol, 2015). Thus, increases in object exploration during the test most likely reflects recognition memory that allows pattern separation and not loss of the memory for the familiar conditions.
The present design involved administration of drugs given 20 min after the last study trial and 10 min before the test trials. Therefore, the results do not distinguish between retrograde effects on memory, that is, modulation of the prior experience versus anterograde effects on memory, including actions on retrieval or other performance variables during recognition testing (Steckler et al., 1998a). Of note, however, potential anterograde effects were not evident on total arena exploration times on the test trial but were restricted to object exploration times, suggesting that the inactivated structures were independently involved in novelty detection of each attribute, object location configuration versus object replacement. Additional experiments are needed to identify more selectively the phases of recognition memory underlying the drug impairments noted here.
Our findings suggest that lactate uptake into neurons via the MCT2 transporter is a key process modulating memory in two object recognition tasks that independently engage two different neural systems, the hippocampus and striatum, involved in different types of memory processing (Bohbot, Lerch, Thorndycraft, Iaria, & Zijdenbos, 2007; Etchamendy & Bohbot, 2007; Gold & Korol, 2017; Iaria, Petrides, Dagher, Pike, & Bohbot, 2003; McDonald & White, 1993; Packard, 2009; Packard & Goodman, 2012, 2013). These tasks can be administered in a single session (as short as 25 to 30 min), and avoid experimentally derived motivation, and thus are useful for assessing underlying molecular and cellular mechanisms of learning and memory without confounding effects of food restriction or aversive stimuli. As such, they are particularly useful for identifying the role of regulators of cellular metabolism, such as lactate as seen here. The tasks diverge from more commonly used recognition tasks in that both acquisition and recognition measured during the habituation phase can be evaluated, making them useful for assessments of both learning and memory.
These two objectrecognition tasks allow formechanistic tests of cognitive dysfunction and function in many human health contexts. From a multiple memory systems perspective, a shift from one style of problem solving to another can produce both deficiencies and sparing or enhancements in function (Korol, 2018). For example, aged rats exhibit impairments on a range of hippocampus-sensitive tasks, including the dOL task, but maintained or enhanced learning of striatum-sensitive tasks, including the dOR task (Gardner et al., 2019). These findings are remarkably similar to results seen in humans, who exhibit a shift across the life span from spatial to response strategies to solve virtual mazes (Bohbot et al., 2012). The results also fit well with growing evidence revealing that losses of functions on some cognitive attributes are often accompanied by shifts to maintained brain area functions, with preserved cognitive attributes accompanying aging, menopause, neurodegenerative states including Alzheimer’s and Parkinson’s disease, and amnesia syndromes (e.g., Bohbot et al., 2012; Foerde & Shohamy, 2011; Korol & Wang, 2018; Myers et al., 2003; Poldrack et al., 2001).
Acknowledgments
This work was supported by grants from the National Science Foundation (IOS 13–18490), National Institutes of Health (NIH; AG057947), NIH AG07648, NIH P50 AT006268, National Institute on Drug Abuse (DA038798), and the Syracuse University Center for Aging and Policy Studies (NIA P30 AG034464). We thank Stephen Ajayi, Frances Batarse, Luis Castelan, Wayne Hawley, John Cote, and Emily Green for technical assistance and Lawrence Hubert for statistical advice.
Contributor Information
Donna L. Korol, Department of Biology, Syracuse University.
Robert S. Gardner, Department of Biology, Syracuse University.
Tumay Tunur, Department of Biology, Syracuse University.; Department of Kinesiology, California State University San Marcos.
Paul E. Gold, Department of Biology, Syracuse University.
References
- Alberini CM, Cruz E, Descalzi G, Bessières B, & Gao V (2018). Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia, 66, 1244–1262. 10.1002/glia.23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, & Warburton EC (2011). When is the hippocampus involved in recognition memory? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31, 10721–10731. 10.1523/JNEUR0SCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH (2007). Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience, 145, 11–19. 10.1016/j.neuroscience.2006.11.062 [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, & Zijdenbos AP (2007). Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 10078–10083. 10.1523/JNEUROSCI.1763-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, McKenzie S, Konishi K, Fouquet C, Kurdi V, Schachar R, . . . Robaey P (2012). Virtual navigation strategies from childhood to senescence: Evidence for changes across the life span. Frontiers in Aging Neuroscience, 4, 28 10.3389/fnagi.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA (2009). Cell-cell and intracellular lactate shuttles. The Journal of Physiology, 587, 5591–5600. 10.1113/jphysiol.2009.178350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, & Roesch MR (2015). From ventralmedial to dorsal-lateral striatum: Neural correlates of reward-guided decision-making. Neurobiology of Learning and Memory, 117, 51–59. 10.1016/j.nlm.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Stutz SJ, & Gold PE (2005). Glucose injections into the dorsal hippocampus or dorsolateral striatum of rats prior to T-maze training: Modulation of learning rates and strategy selection. Learning & Memory, 12, 367–374. 10.1101/lm.88205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, & Gold PE (2003a). Intra-hippocampal lidocaine injections impair acquisition of a place task and facilitate acquisition of a response task in rats. Behavioural Brain Research, 144, 19–24. 10.1016/S0166-4328(03)00063-9 [DOI] [PubMed] [Google Scholar]
- Chang Q, & Gold PE (2003b). Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 3001–3005. 10.1523/JNEUROSCI.23-07-03001.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, & Gold PE (2004). Inactivation of dorsolateral striatum impairs acquisition of response learning in cue-deficient, but not cue- available, conditions. Behavioral Neuroscience, 118, 383–388. 10.1037/0735-7044.118.2.383 [DOI] [PubMed] [Google Scholar]
- Chang Q, Savage LM, & Gold PE (2006). Microdialysis measures of functional increases in ACh release in the hippocampus with and without inclusion of acetylcholinesterase inhibitors in the perfusate. Journal of Neurochemistry, 97, 697–706. http://dx.doi.org/10.1111Zj.1471-4159.2006.03765.x [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, & Stackman RW Jr. (2013). The rodent hippocampus is essential for nonspatial object memory. Current Biology, 23, 1685–1690. 10.1016/j.cub.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, & Stackman RW Jr. (2015). Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behavioural Brain Research, 285, 105–117. 10.1016/j.bbr.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PJ (2004). Learning-induced activation of transcription factors among multiple memory systems. Neurobiology of Learning and Memory, 82, 268–277. 10.1016/j.nlm.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Brightwell JJ, & Countryman RA (2003). Cognitive strategy-specific increases in phosphorylated cAMP response element- binding protein and c-Fos in the hippocampus and dorsal striatum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 3547–3554. 10.1523/JNEUROSCI.23-08-03547.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DM (2004). Behavior strategy learning in rat: Effects of lesions of the dorsal striatum or dorsal hippocampus. Behavioural Processes, 67, 335–342. 10.1016/S0376-6357(04)00139-1 [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Hennevin E, & Cotillon N (2002). Muscimol diffusion after intracerebral microinjections: A reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiology of Learning and Memory, 78, 100–124. 10.1006/nlme.2001.4035 [DOI] [PubMed] [Google Scholar]
- Ennaceur A (2010). One-trial object recognition in rats and mice: Methodological and theoretical issues. Behavioural Brain Research, 215, 244–254. 10.1016/j.bbr.2009.12.036 [DOI] [PubMed] [Google Scholar]
- Etchamendy N, & Bohbot VD (2007). Spontaneous navigational strategies and performance in the virtual town. Hippocampus, 17, 595–599. 10.1002/hipo.20303 [DOI] [PubMed] [Google Scholar]
- Field A (2009). Discovering statistics using SPSS (3rd ed.). London, UK: SAGE. [Google Scholar]
- Foerde K, & Shohamy D (2011). The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiology of Learning and Memory, 96, 624–636. 10.1016/j.nlm.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR (1990). The organization of learning (Vol. 36). Cambridge, MA: MIT Press. [Google Scholar]
- Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, & Alberini CM (2016). Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proceedings of the National Academy of Sciences of the United States of America, 113, 8526–8531. 10.1073/pnas.1605063113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RS, Newman LA, Mohler EG, Tunur T, Gold PE, & Korol DL (2019). Not all aging is equal from a multiple memory systems viewpoint. Manuscript in preparation. [Google Scholar]
- Gardner RS, Suarez DF, Robinson-Burton NK, Rudnicky CJ, Gulati A, Ascoli GA, & Dumas TC (2016). Differential Arc expression in the hippocampus and striatum during the transition from attentive to automatic navigation on a plus maze. Neurobiology of Learning and Memory, 131, 36–45. 10.1016/j.nlm.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, & Lee I (2001). Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus, 11, 626–636. 10.1002/hipo.1077 [DOI] [PubMed] [Google Scholar]
- Gold PE (1995). Modulation of emotional and non-emotional memories: Same pharmacological systems, different neuroanatomical systems In McGaugh JL, Weinberger NM, & Lynch GS (Eds.), Brain and memory: Modulation and mediation of neural plasticity (pp. 41–74). New York, NY: Oxford University Press; 10.1093/acprof:oso/9780195082944.003.0002 [DOI] [Google Scholar]
- Gold PE (2001). Drug enhancement of memory in aged rodents and humans In Carroll ME & Overmier JB (Eds.), Animal research and human health: Advancing human welfare through behavioral science (pp. 293–304). Washington, DC: American Psychological Association; 10.1037/10441-019 [DOI] [Google Scholar]
- Gold PE (2004). Coordination of multiple memory systems. Neurobiology of Learning and Memory, 82, 230–242. 10.1016/j.nlm.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Gold PE (2014). Regulation of memory from the adrenal medulla to liver to astrocytes to neurons. Brain Research Bulletin, 105, 25–35. 10.1016/j.brainresbull.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE (2016). Balancing the contributions of multiple neural systems during learning and memory In Ragozzino ME, Jackson P, Chiba A, & Berman R (Eds.), The neurobiological basis of memory: A system, attribute, and process analysis—A Festschrift in honor of Raymond P. Kesner (pp. 261–280). New York, NY: Springer; 10.1007/978-3-319-15759-7_12 [DOI] [Google Scholar]
- Gold PE, & Korol DL (2012). Making memories matter. Frontiers in Integrative Neuroscience, 6, 116 10.3389/fnint.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, & Korol DL (2014). Forgetfulness during aging: An integrated biology. Neurobiology of Learning and Memory, 112, 130–138. 10.1016/j.nlm.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, & Korol DL (2017). Hormones and memory. Reference Module in Neuroscience and Biobehavioral Psychology, 2017, 1–8. 10.1016/B978-0-12-809324-5.00336-9 [DOI] [Google Scholar]
- Gold PE, Newman LA, Scavuzzo CJ, & Korol DL (2013). Modulation of multiple memory systems: From neurotransmitters to metabolic substrates. Hippocampus, 23, 1053–1065. 10.1002/hipo.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, & Kesner RP (2005). Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behavioral Neuroscience, 119, 1307–1315. 10.1037/0735-7044.119.5.1307 [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, & Kesner RP (2008). The interactions and dissociations of the dorsal hippocampus subregions: How the dentate gyrus, CA3, and CA1 process spatial information. Behavioral Neuroscience, 122, 16–26. 10.1037/0735-7044.122.1.16 [DOI] [PubMed] [Google Scholar]
- Hawley WR, Witty CF, Daniel JM, & Dohanich GP (2015). Choline acetyltransferase in the hippocampus is associated with learning strategy preference in adult male rats. Behavioural Brain Research, 289, 118–124. 10.1016/j.bbr.2015.04.034 [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, & Bohbot VD (2003). Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23, 5945–5952. 10.1523/JNEUROSCI.23-13-05945.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP (2013). Role of the hippocampus in mediating interference as measured by pattern separation processes. Behavioural Processes, 93, 148–154. http://dx.doi.org/10.1016Zj.beproc.2012.09.018 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Bolland BL, & Dakis M (1993). Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Experimental Brain Research, 93, 462–470. 10.1007/BF00229361 [DOI] [PubMed] [Google Scholar]
- Kim J, & Frick KM (2017). Distinct effects of estrogen receptor antagonism on object recognition and spatial memory consolidation in ovariectomized mice. Psychoneuroendocrinology, 85, 110–114. 10.1016/j.psyneuen.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Korol DL (2002). Enhancing cognitive function across the life span. Annals of the New York Academy of Sciences, 959, 167–179. 10.1111/j.1749-6632.2002.tb02091.x [DOI] [PubMed] [Google Scholar]
- Korol DL (2004). Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of Learning and Memory, 82, 309–323. 10.1016/j.nlm.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Korol DL (2018). Estrogens have their ups and downs: A multiple memory systems approach to the bidirectional effects of estrogens on learning strategy In Ball G, Balthazart J, & Nelson R (Eds.), Estrogen and memory: Basic research and clinical implications. New York, NY: Oxford University Press. [Google Scholar]
- Korol DL, & Gold PE (2007). Modulation of learning and memory by adrenal and ovarian hormones In Kesner RP & Martinez JL (Eds.), Neurobiology of learning and memory (pp. 243–268). New York, NY: Elsevier Science; 10.1016/B978-012372540-0/50008-X [DOI] [Google Scholar]
- Korol DL, Gold PE, & Scavuzzo CJ (2013). Use it and boost it with physical and mental activity. Hippocampus, 23, 1125–1135. 10.1002/hipo.22197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, & Kolo LL (2002). Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience, 116, 411–420. 10.1037/0735-7044.116.3.411 [DOI] [PubMed] [Google Scholar]
- Korol DL, & Pisani SL (2015). Estrogens and cognition: Friends or foes? Hormones and Behavior, 74, 105–115. 10.1016/j.yhbeh.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, & Wang W (2018). Viewing the effects of estrogens on cognition through a multiple memory systems lens: Implications for human health. Physiology & Behavior, 187, 67–78. 10.1016/j.physbeh.2017.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers BJ, & Levitt TS (1988). Navigation and mapping in large scale space. AI Magazine, 9, 25. [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, & Moser EI (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315, 961–966. 10.1126/science.1135801 [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, & Maclusky NJ (2003). Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology, 144, 2836–2844. 10.1210/en.2003-0004 [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, . . . Nedergaard M (2015). Direct neuronal glucose uptake heralds activity- dependent increases in cerebral metabolism. Nature Communications, 6, 6807 10.1038/ncomms7807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, & White NM (1993). A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behavioral Neuroscience, 107, 3–22. 10.1037/0735-7044.107.1.3 [DOI] [PubMed] [Google Scholar]
- McElroy MW, & Korol DL (2005). Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learning & Memory, 12, 150–158. 10.1101/lm.86205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, & Gold PE (2003). Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiology of Learning and Memory, 79, 177–183. 10.1016/S1074-7427(02)00014-X [DOI] [PubMed] [Google Scholar]
- McNay EC, Canal CE, Sherwin RS, & Gold PE (2006). Modulation of memory with septal injections of morphine and glucose: Effects on extracellular glucose levels in the hippocampus. Physiology & Behavior, 87, 298–303. 10.1016/j.physbeh.2005.10.016 [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, & Gold PE (2000). Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences of the United States of America, 97, 2881–2885. 10.1073/pnas.050583697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, & Gold PE (1998). Memory modulation across neural systems: Intra-amygdala glucose reverses deficits caused by intraseptal morphine on a spatial task but not on an aversive task. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 18, 3853–3858. 10.1523/JNEUR0SCI.18-10-03853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, & Gold PE (2001). Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 56, B66–B71. 10.1093/gerona/56.2.B66 [DOI] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, & Gold PE (2001). Fluctuations in brain glucose concentration during behavioral testing: Dissociations between brain areas and between brain and blood. Neurobiology of Learning and Memory, 75, 325–337. 10.1006/nlme.2000.3976 [DOI] [PubMed] [Google Scholar]
- Messier C (1997). Object recognition in mice: Improvement of memory by glucose. Neurobiology of Learning and Memory, 67, 172–175. 10.1006/nlme.1996.3755 [DOI] [PubMed] [Google Scholar]
- Messier C (2004). Glucose improvement of memory: A review. European Journal ofPharmacology, 490, 33–57. http://dx.doi.org/10.1016Zj.ejphar.2004.02.043 [DOI] [PubMed] [Google Scholar]
- Messier C, Desrochers A, & Gagnon M (1999). Effect of glucose, glucose regulation, and word imagery value on human memory. Behavioral Neuroscience, 113, 431–438. 10.1037/0735-7044.113.3.431 [DOI] [PubMed] [Google Scholar]
- Morris AM, Churchwell JC, Kesner RP, & Gilbert PE (2012). Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations. Neurobiology of Learning and Memory, 97, 326–331. 10.1016/j.nlm.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KA, & Gold PE (2013). Epinephrine and glucose modulate training-related CREB phosphorylation in old rats: Relationships to age-related memory impairments. Experimental Gerontology, 48, 115–127. 10.1016/j.exger.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Wood ER, & Pinel JP (1992). Object-recognition memory is only mildly impaired in rats with lesions of the hippocampus and amygdala. Psychobiology, 20, 18–27. [Google Scholar]
- Myers CE, Shohamy D, Gluck MA, Grossman S, Kluger A, Ferris S, . . . Schwartz R (2003). Dissociating hippocampal versus basal ganglia contributions to learning and transfer. Journal of Cognitive Neuroscience, 15, 185–193. 10.1162/089892903321208123 [DOI] [PubMed] [Google Scholar]
- Myers RD (1966). Injection of solutions into cerebral tissue: Relation between volume and diffusion. Physiology & Behavior, 1, 171–174. 10.1016/0031-9384(66)90064-3 [DOI] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, . . . Tonegawa S (2012). Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell, 149, 188–201. 10.1016/jxell.2012.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, & Gold PE (2016). Attenuation in rats of impairments of memory by scopolamine, a muscarinic receptor antagonist, by mecamylamine, a nicotinic receptor antagonist. Psychopharmacology, 233, 925–932. 10.1007/s00213-015-4174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Korol DL, & Gold PE (2011). Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE, 6, e28427. 10.1371/journal.pone.0028427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Scavuzzo CJ, Gold PE, & Korol DL (2017). Training-induced elevations in extracellular lactate in hippocampus and striatum: Dissociations by cognitive strategy and type of reward. Neurobiology of Learning and Memory, 137, 142–153. 10.1016/j.nlm.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG (1999). Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proceedings of the National Academy of Sciences of the United States of America, 96, 12881–12886. 10.1073/pnas.96.22.12881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG (2009). Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Research, 1293, 121–128. 10.1016/j.brainres.2009.03.029 [DOI] [PubMed] [Google Scholar]
- Packard MG, & Goodman J (2012). Emotional arousal and multiple memory systems in the mammalian brain. Frontiers in Behavioral Neuroscience, 6, 14 10.3389/fnbeh.2012.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, & Goodman J (2013). Factors that influence the relative use of multiple memory systems. Hippocampus, 23, 1044–1052. 10.1002/hipo.22178 [DOI] [PubMed] [Google Scholar]
- Packard MG, & McGaugh JL (1996). Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory, 65, 65–72. 10.1006/nlme.1996.0007 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2005). The ratbrain in stereotaxic coordinates.San Diego, CA: Elsevier. [Google Scholar]
- Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, & Korol DL (2016). Estrogen receptor selective agonists modulate learning in female rats in a dose- and task-specific manner. Endocrinology, 157, 292–303. 10.1210/en.2015-1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Paré-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, & Gluck MA (2001). Interactive memory systems in the human brain. Nature, 414, 546–550. 10.1038/35107080 [DOI] [PubMed] [Google Scholar]
- Poucet B (1993). Spatial cognitive maps in animals: New hypotheses on their structure and neural mechanisms. Psychological Review, 100, 163–182. 10.1037/0033-295X.100.Z163 [DOI] [PubMed] [Google Scholar]
- Poucet B, & Herrmann T (2001). Exploratory patterns of rats on a complex maze provide evidence for topological coding. Behavioural Processes, 53, 155–162. 10.1016/S0376-6357(00)00151-0 [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, & Gold PE (2005a). Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiology of Learning and Memory, 8–4, 93–101. http://dx.doi.org/10.1016Zj.nlm.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Haag R, & Gold PE (2005b). Acetylcholine release in the hippocampus and striatum during place and response training. Learning & Memory, 12, 564–572. 10.1101/lm.33105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pych JC, Kim M, & Gold PE (2006). Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behavioural Brain Research, 167, 373–378. 10.1016/j.bbr.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, & Gold PE (1998). Modulation of hippocampal acetylcholine release and of memory by intrahippocampal glucose injections. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 18, 1595–1601. 10.1523/JNEUR0SCI.18-04-01595.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, & Kesner RP (2006). A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology, 79, 1–48. 10.1016/j.pneurobio.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Schroeder JP, & Packard MG (2003). Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. The European Journal of Neuroscience, 17, 1482–1488. 10.1046/j.1460-9568.2003.02578.x [DOI] [PubMed] [Google Scholar]
- Sheppard PA, Koss WA, Frick KM, & Choleris E (2017). Rapid actions of oestrogens and their receptors on memory acquisition and consolidation in females. Journal of Neuroendocrinology, 30, e12485. 10.1111/jne.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Riby LM, Eekelen JA, & Foster JK (2011). Glucose enhancement of human memory: A comprehensive research review of the glucose memory facilitation effect. Neuroscience and Biobehavioral Reviews, 35, 770–783. http://dx.doi.org/10.1016Zj.neubiorev.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WH, Sahgal A, & Aggleton JP (1998a). Recognition memory in rats—I. Concepts and classification. Progress in Neurobiology, 54, 289–311. 10.1016/S0301-0082(97)00060-9 [DOI] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WH, Sahgal A, & Aggleton JP (1998b). Recognition memory in rats—II. Neuroanatomical substrates. Progress in Neurobiology, 54, 313–332. 10.1016/S0301-0082(97)00061-0 [DOI] [PubMed] [Google Scholar]
- Stefani MR, & Gold PE (2001). Intrahippocampal infusions of k-atp channel modulators influence spontaneous alternation performance: Relationships to acetylcholine release in the hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21, 609–614. 10.1523/JNEUROSCI.21-02-00609.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Nicholson GM, & Gold PE (1999). ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: A potential mechanism for glucose-mediated memory enhancement. Neuroscience, 93, 557–563. 10.1016/S0306-4522(99)00128-1 [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Gao V, & Alberini CM (2016). The role of lactate- mediated metabolic coupling between astrocytes and neurons in longterm memory formation. Frontiers in Integrative Neuroscience, 10, 10 10.3389/fnint.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland EW, & Rall TW (1960). The relation of adenosine-3’, 5’-phosphate and phosphorylase to the actions of catecholamines and other hormones. Pharmacological Reviews, 12, 265–299. [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, & Alberini CM (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell, 144, 810–823. 10.1016/j.cell.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadi M, Allaman I, Lengacher S, Grenningloh G, & Magistretti PJ (2015). Learning-induced gene expression in the hippocampus reveals a role of neuron-astrocyte metabolic coupling in long term memory. PLoS ONE, 10, e0141568. 10.1371/journal.pone.0141568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Smith DE, Ellis-Behnke RG, & Bazan NG (2005). Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiology of Learning and Memory, 84, 75–84. 10.1016/j.nlm.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Tunur T, & Korol DL (2015, June). Opposing effects of estrogens on two different pattern separation tasks. Paper presented at the 19th Annual Meeting of the Society for Behavioral Neuroendocrinology, Pacific Grove, CA. [Google Scholar]
- van der Zwaluw NL, van de Rest O, Kessels RP, & de Groot LC (2015). Effects of glucose load on cognitive functions in elderly people. Nutrition Reviews, 73, 92–105. 10.1093/nutrit/nuu002 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, & Frye CA (2006). Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory, 86, 35–46. http://dx.doi.org/10.1016Zj.nlm.2006.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, & Gold PE (1992). Impairment of spontaneous alternation performance by an NMDA antagonist: Attenuation with non- NMDA treatments. Behavioral and Neural Biology, 58, 69–71. 10.1016/0163-1047(92)90952-Z [DOI] [PubMed] [Google Scholar]
- Weil-Malherbe H, Axelrod J, & Tomchick R (1959). Blood-brain barrier for adrenaline. Science, 129, 1226–1227. 10.1126/science.129.3357.1226 [DOI] [PubMed] [Google Scholar]
- White NM, & McDonald RJ (2002). Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory, 77, 125–184. 10.1006/nlme.2001.4008 [DOI] [PubMed] [Google Scholar]
- White NM, Packard MG, & McDonald RJ (2013). Dissociation of memory systems: The story unfolds. Behavioral Neuroscience, 127, 813–834. 10.1037/a0034859 [DOI] [PubMed] [Google Scholar]
- Xu SJ, Chen Z, Zhu LJ, Shen HQ, & Luo JH (2005). Visual recognition memory is related to basic expression level of NMDA receptor NR1/NR2B subtype in hippocampus and striatum of rats. Acta Pharmacologica Sinica, 26, 177–180. 10.1111/j.1745-7254.2005.00532.x [DOI] [PubMed] [Google Scholar]
- Yagi S, Chow C, Lieblich SE, & Galea LA (2016). Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus, 26, 87–101. 10.1002/hipo.22493 [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, & Korol DL (2007). Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience, 144, 26–37. 10.1016/j.neuroscience.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Serio SJ, & Korol DL (2011). Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Hormones and Behavior, 60, 470–477. 10.1016/j.yhbeh.2011.07.014 [DOI] [PubMed] [Google Scholar]


