Abstract
Purpose of review:
Numerous B-cell abnormalities in HIV-1 infection have been described over the past three decades yet have remained poorly defined mechanistically. We review recent studies that describe mechanisms of B-cell dysregulation in chronic HIV-1 infection associated with IgG3 and T-bet.
Recent findings:
HIV-1 infection causes hypergammaglobulinemia and dysregulation of B-cell populations, including the expansion during chronic viremia of functionally impaired tissue-like memory (TLM) B cells. TLM B cells and B cells in other conditions of chronic activation and inflammation with similar phenotypes are characterized by increased expression of the transcription factor T-bet and preferential immunoglobulin class-switching to IgG3. However, defects in B-cell function during chronic HIV-1 viremia are also associated with the binding of soluble IgG3 to IgM-expressing B cells, with highest intensities observed on TLM B cells. The consequence of IgG3 binding to TLM B cells is increased clustering of the IgM B-cell receptor and decreased response to stimulation.
Summary:
The identification of T-bet and IgG3 as the regulators of B-cell function in chronic HIV-1 viremia could provide new targets for therapeutic intervention aimed at reversing the damaging effects of HIV-1-associated chronic immune activation.
Keywords: B cells, chronic immune activation, IgG3, T-bet
INTRODUCTION
Over the past few years, HIV-1 research regarding B cells and antibody responses has seen considerable progress, as evidenced by the topics covered in this edition. Our longstanding interest is in delineating pathogenic mechanisms associated with B cells in HIV-1 infection and how such mechanisms relate to other chronic infectious and non-infectious diseases where B cells are dysregulated. We focus this review on two related areas of recent advancement in these disease settings: the role of IgG3 in regulating B-cell function and the role of T-bet in regulating B-cell differentiation.
ROLE OF IGG3 IN HIV-1 INFECTION
Abnormal activation of B cells in HIV-1-infected individuals results in increased concentration of circulating IgG, a phenomenon known as hypergammaglobulinemia (1, 2). HIV-1-induced hypergammaglobulinemia is dominated by IgG1, distantly followed by IgG3 (3, 4). Among the four human IgG subclasses, IgG3 is the most polymorphic (5), and has the longest, most flexible hinge region (6). It also has higher affinities for Fc receptors and C1q, the first component of the classical complement pathway, than other IgG subclasses (7, 8). As a result, IgG3 possesses strong effector functions, including Fc-mediated antibody-dependent cellular cytotoxicity and capacity to fix complement (7). Furthermore, the hinge and Fc regions of IgG3 have been associated respectively with increased HIV-neutralizing potency (9) and superior internalization of infectious virions (10), when compared to that of IgG1. The greater flexibility in the hinge region of IgG3 may increase access to HIV-1 epitopes and as a result of enhanced virion and C1q binding, promote formation of immune complexes that then enhance effector function. These properties may also help explain why the vast majority of broadly HIV-1- neutralizing antibodies (bNAbs) directed against membrane proximal external region (MPER) of HIV-1 gp41, a region difficult to access, are IgG3 while very few of the other bNAbs isolated thus far are IgG3 (11).
Despite its many advantages, IgG3 has until recently rarely been considered for immunotherapy, due at least in part to its relatively short half-live (12). In this regard, HIV-1-specific IgG3 responses have been shown to arise early, consistent with the effect of proximity to IgM during class-switch recombination (13), but wane rapidly over the course of HIV-1 infection or following immunization (14), likely as a result of the relatively short half-life. In the RV144 vaccine trial, where a significant yet transient reduction in HIV-1 acquisition was observed, non-neutralizing V1V2-specific IgG3 antibodies with polyfunctionalities involving Fc-mediated effector responses were one of few correlates of protection identified (15). However, more recent systems serology studies have suggested that the IgG3 response in the RV144 trial may have been a surrogate rather than a driver of protection (16).
T-BET IN RELATION TO IGG3 AND DYSREGULATED B-CELL POPULATIONS
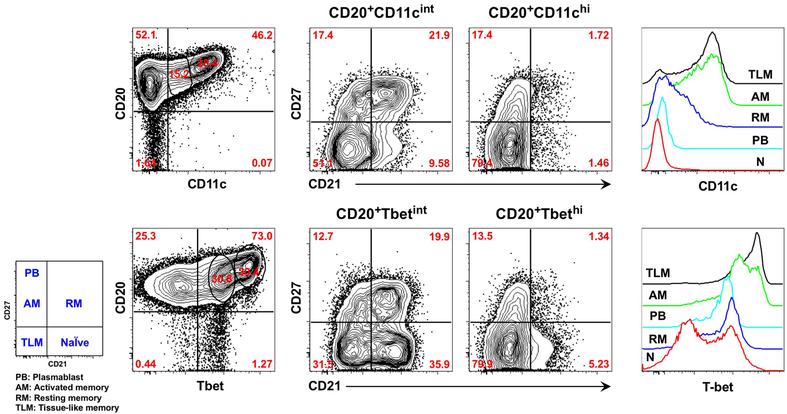
Chronic HIV-1 viremia is associated with the expansion of several aberrant B-cell subpopulations, including functionally exhausted tissue-like memory (TLM) B cells, that are normally present at low frequencies in the peripheral blood of uninfected individuals (17). These B cells exhibit increased expression of multiple inhibitory receptors, as well as the inflammatory chemokine receptor CXCR3 and integrin receptor CD11c. Functionally, TLM B cells respond poorly to stimulation through the B cell receptor (BCR) and display a reduced ability to proliferate, undergo affinity maturation and secrete cytokines or antibodies (17, 18). These defects can be reversed, as demonstrated ex vivo by targeting inhibitory receptors using an siRNA-based approach, thus confirming a role for these receptors in B-cell exhaustion (19). Similar defects have been recently reported in other disease conditions that cause chronic immune activation and inflammation, although the precise inhibitory receptors and affected responses may vary (20, 21). However, two elements appear to be consistent across disease conditions: cell-surface expression of CD11c and increased expression of the transcription factor T-bet. As depicted in Figure 1, these features are mutually enriched among TLM B cells in chronic HIV-1 infection (22) and among similar B cells in other disease settings (20, 21, 23). T-bet, master regulator of T helper 1 (Th1) lineage commitment, also regulates immunoglobulin class-switching, preferentially IgG2a/c in mice and IgG1 and IgG3 in humans, under IFN-γ-dependent conditions (24). In HIV-1 infection, T-bet expression has been associated with preferential switching to IgG1 and IgG3 (22), whereas in malaria, IFN-γ-mediated T-bet expression has predominantly been associated with switching to IgG3 (21). The link between IgG3 and Th1 cytokines and T-bet is illustrated in complement C3-deficient patients (25), and age-related effects of streptococcal infection (26).
Figure 1.
The integrin receptor CD11c and the transcription factor T-bet show similar patterns of expression among major B-cell populations that circulate in the blood of HIV-1-infected individuals with chronic viremia. Increasing intensities of CD11c and T-bet are associated with enrichment of TLM B cells, as defined by the reduced expression of complement receptor CD21 and classic marker of memory CD27. Int, intermediate; N, naïve.
Under various conditions of lymphadenopathy, including in HIV-1 disease, the expression of T-bet has been associated with a unique lymphoid tissue population, referred to as monocytoid B cells, that reside primarily outside germinal centers (27). Monocytoid B cells can be distinguished from similarly distributed marginal zone B cells by the low expression of CD21 and high expression of T-bet and the immunoregulatory receptor FCRL4 (28), previously known as IRTA1. In 2001, IRTA1 was identified in the breakpoint region of chromosomal rearrangement in a myeloma cell line and thus named immune receptor translocation associated protein 1 (29). In 2005, FcRH4, another name for FCRL4, was the defining marker of a distinct population of tonsil-derived memory B cells, with features that included low expression of CD21 and the classic marker of human B-cell memory, CD27, as well as functional properties that suggested FCRL4 played an immunoregulatory role by restricting BCR responses to various stimuli (30). We later identified similar B cells in the peripheral blood of HIV-1-infected individuals, hence the term tissue-like memory B cells (17). FCRL4 is over-expressed on TLM B cells of HIV-1-infected individuals, along with several other inhibitory receptors, many of which have been associated with similar B cells in other diseases (31–37), and shown to participate in the restricting B-cell responses (19).
BINDING OF IGG3 TO B CELLS OF HIV-1-INFECTED INDIVIDUALS
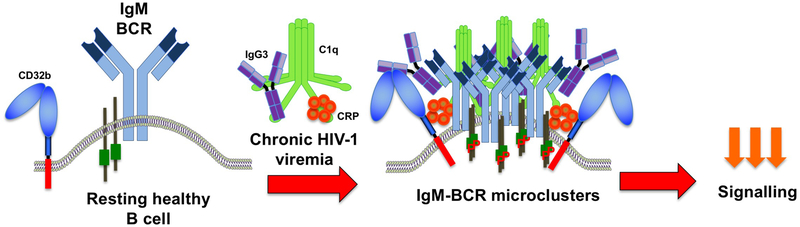
Given the effects of T-bet on immunoglobulin class-switching and our observations of a strong association between T-bet expression and TLM B cells in HIV-1-infected individuals (Figure 1), we began to survey our large and diverse cohort of HIV-1-infected individuals to delineate how IgG subclass expression was associated with HIV-1 disease status. Unexpectedly, we discovered a novel and unique mechanism of B-cell regulation in HIV-1 infection mediated by the binding of soluble IgG3 to IgM-BCR expressing B cells (38). This does not negate an IgG3-skewing effect of T-bet expression, to the contrary, the individuals identified with IgG3-bound B cells (referred to as affected individuals or B cells) also had higher serum concentrations of IgG3 and greater ratios of IgG3 to IgG2 compared to those who did not have affected B cells, indicating that IgG3-expressing and -secreting B cells were being selectively induced. While the source and nature of the soluble IgG3 is currently unknown, binding to B cells of affected HIV-1-infected individuals is mediated by direct glycan-dependent interactions with the IgM-BCR and is dependent on the presence of other factors (Figure 2). The binding of IgG3 to B cells is almost exclusively observed during chronic HIV-1 viremia, absent in HIV-1-uninfected healthy individuals, and is more prevalent in individuals who are African-American or of black African descent, suggesting that genetics may influence the ability of IgG3 to bind B cells. Given the high degree of polymorphism in IgG3, a genetic influence may not be unexpected, although variations in the other factors involved in the binding of IgG3 to IgM-BCR may also explain the wide spectrum of IgG3 intensities observed on B cells of HIV-1-infected individuals (38). It is notable that in acute HIV-1 viremia, IgG3-bound B cells are rarely observed, despite high viral burdens, and conversely, suppression of chronic HIV-1 viremia with ART reverses the binding of IgG3 to B cells, suggesting that factors associated with persistent HIV-1 viremia are needed for binding to occur. While the specificity or specificities of the IgG3 bound to B cells remain unknown, there is no evidence that these are HIV-1-specific. As far as we can determine using conventional methods of detection, there is no increase in IgG3-associated HIV-1 specificity, either when compared to other IgG isotypes in individuals with high intensity IgG3-bound B cells or compared to IgG3- associated HIV-1 specificity of infected individuals with low or no IgG3 on their B cells. In a majority of affected individuals, the B-cell bound IgG3 is polyclonal, as evidenced by the pattern of Ig light chain double-positivity on IgG3-bound B cells.
Figure 2.
Depiction of a resting healthy IgM-expressing B cell in an uninfected individual and changes that occur during chronic HIV-1 infection in affected individuals. During chronic HIV-1 viremia, inhibitory Fc receptor CD32b expression increases and becomes associated with IgG3-IgM-BCR microclusters, along with C1q and CRP. The clustering occurs predominantly in TLM B cells and involves direct interactions between IgG3 and the IgM-BCR. P, phosphorylated.
In addition to differences in IgG3 B-cell binding profiles across groups of infected individuals, we also observe differences across B-cell populations within an affected individual. The majority of affected cells are naïve and TLM B cells, with the former typically displaying higher frequencies of IgG3-bound B cells, not unexpected given that the binding partner, IgM-BCR, is expressed on all naïve but only a fraction of TLM and even less of activated (AM) and resting (RM) memory B cells. However, TLM B cells bind higher intensities of IgG3 than naïve B cells and manifest stronger clustering of IgG3 with IgM-BCR. As a result, TLM B cells are more adversely affected functionally than naïve B cells. Consistent with the highly clustered IgM-BCR observed by high-resolution microscopy in our studies and reported in other conditions of chronic signaling (39), basal levels of phosphorylated signaling intermediates are increased on IgG3-bound TLM B cells (38). However, these affected B cells also respond poorly to stimulation through the IgM-BCR, as measured by induced phosphorylation of downstream intermediates and intracellular calcium mobilization, and this unresponsiveness is most pronounced on IgG3-bound TLM B cells whereas the binding of IgG3 to naïve B cells does not alter their capacity to signal.
The affinity of IgG for CD32b, the primary Fc receptor expressed on B cells, is typically below the threshold for binding, unless the IgG is multimerized in an immune complex or aggregate (8). A multimeric form of IgG3 interacting with B cells of affected individuals was initially considered given that the bound IgG3 could be displaced with antagonistic anti-CD32b antibodies (38). However, further analyses conducted on IgG3 isolated from the serum of affected individuals were inconsistent with an aggregated form of binding. After determining that IgG3 isolated from the serum of affected but not unaffected individuals could be transferred to unaffected B cells, thus providing us with material that could easily be fractionated, we used size exclusion chromatography to demonstrate that the main binding entity was monomeric rather than multimeric (38). Given the improbability of that single ligand-receptor binding dynamics could explain the high prevalence and high frequencies of IgG3 B-cell binding observed, we considered additional binding partners for IgG3. We considered potential factors that were present in both IgG3 isolated from serum of affected individuals and on their B cells, particularly on TLM B cells where IgG3 binding was most intense. This approach revealed two additional binding partners, the first component of the complement cascade, C1q, and the second, the inflammatory biomarker c-reactive protein (CRP). These proteins were present in fractions of IgG3 isolated from sera of affected individuals that were able to bind B cells of unaffected individuals and the binding could be blocked with C1q or CRP or CD32b-specific agonistic antibodies. CRP, which has been shown to interact with and activate Fc receptors, including CD32b (40), can also associate with C1q, which in turn is known to interact strongly with Fc regions of both IgG3 and IgM (41). Both C1q and CRP were detected on B cells of HIV-1-infected chronically-viremic individuals, enriched on TLM B cells and together with bound IgG3, were associated with the most pronounced reduction in response to IgM-BCR-triggering. It is notable that this reduced responsiveness to stimulation was observed both among TLM B cells as well as the small fraction of naïve B cells that bound all three soluble proteins (38), suggesting that their regulatory effects are not restricted by the population to which they are bound.
With strong evidence that the IgG3 bound to B cells of affected individuals was not mediated by immune complexes, we considered other binding mechanisms that would also involve CD32b. The first clue came from total internal reflection fluorescence (TIRF) microscopy where co-localization between IgG3 and the IgM-BCR of affected B cells was found to be consistently stronger than between IgG3 and CD32b. This led us to consider a direct interaction between IgG3 and IgM-BCR, which was strongly suggested when the addition of exogenous IgG3 diminished the colocalization between IgG3 and IgM-BCR but not CD32b. The confirmation for a direct interaction between IgG3 and the IgM-BCR was made by immunoprecipitating IgG3 in affected B-cell lysates and revealing the presence of IgM in the precipitate (38). An important lesson was also learned while performing these pull-down assays: while we initially assumed that the presence of IgM would most likely to be revealed in cells with the highest intensities of bound IgG3, the cells that ultimately revealed IgG3-IgM interactions had intermediate intensities of IgG3. The strong clustering of IgM-BCR on high IgG3 intensity B cells likely increased IgM insolubility in cell lysates and decreased the yield of detectable IgG3-IgM conjugates. While not helpful for immunoprecipitation, the presence of IgG3-IgM-BCR microclusters helped explain how CD32b may both mediate binding of IgG3 and contribute to the reduction in BCR signaling. Studies have shown that the clustering of the BCR following antigen-induced signaling involves changes in the local microenvironment of the plasma membrane to enable other proteins, including inhibitory receptors such as CD32b, to co-aggregate and be in sufficiently close proximity to reduce BCR responses (42). Thus, as illustrated in Figure 2, the IgM-BCR of TLM B cells of affected individuals is likely to be regulated by IgG3 through direct interactions in highly clustered formations that also include C1q, CRP and CD32b.
There are very likely other modes of soluble factor-receptor interactions that regulate B cells in chronic infectious diseases, including HIV-1 infection. The C1q that is present on B cells, especially TLM B cells is not always associated with IgG3 and may be binding to other receptors that are known to be expressed on B cells (43), including LAIR-1 which we found to be over-expressed on TLM B cells (17). Other mechanisms of soluble immunoglobulin binding and regulation are likely to manifest under different conditions and in different environments and compartments. In this regard, we have observed increased binding of soluble IgM on TLM B cells, in conjunction with increased expression of the IgM Fc receptor TOSO (unpublished observations) that recently has been shown to be a regulator of BCR signaling (44).
CONCLUSION
Further investigations are needed to identify the source and BCR specificity of B cells that secrete the IgG3 that in turn binds IgM-expressing B cells of affected individuals and to further delineate the nature of interaction between soluble IgG3 and the IgM-BCR. It is also unclear whether IgG3 binding to B cells drives TLM B-cell differentiation and whether T-bet regulates either the B cells secreting the IgG3 or those binding IgG3. While frequencies of TLM correlate with those of IgG3-bound B cells (38), this could simply reflect that both arise from the underlying chronic activation associated with persistent HIV-1 viremia. Further delineation of the nature and role of IgG3 and other similar mechanisms of B-cell regulation may also provide opportunities for therapeutic intervention to reverse the damaging effects of excessive immune activation that occurs in HIV-1 and other chronic infectious diseases.
KEY POINTS.
In early HIV-1 infection, IgG3 is involved in rapid yet transient antibody responses against the virus.
In chronic HIV-1 infection, IgG3 regulates B-cell function in viremic individuals.
In HIV-1 infection and other chronic diseases, T-bet is associated with B-cell dysregulation.
Acknowledgements:
We thank Dr. Clarisa Buckner for critical reading of the manuscript.
Funding: Intramural Research Program of the National Institutes of Health
Financial support and sponsorship: This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Conflicts of interest: None
REFERENCES AND RECOMMENDED READING
- 1.Lane HC, Masur H, Edgar LC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309(8):453–8. [DOI] [PubMed] [Google Scholar]
- 2.De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103(6):2180–6. [DOI] [PubMed] [Google Scholar]
- 3.Kekow J, Hobusch G, Gross WL. Predominance of the IgG1 subclass in the hypergammaglobulinemia observed in pre-AIDS and AIDS. Cancer Detect Prev. 1988;12(1–6):211–6. [PubMed] [Google Scholar]
- 4.Buckner CM, Moir S, Ho J, et al. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol. 2013;87(10):5800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefranc MP, Lefranc G. Human Gm, Km, and Am allotypes and their molecular characterization: a remarkable demonstration of polymorphism. Methods Mol Biol. 2012;882:635–80. [DOI] [PubMed] [Google Scholar]
- 6.Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159(7):3372–82. [PubMed] [Google Scholar]
- 7.Michaelsen TE, Sandlie I, Bratlie DB, et al. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol. 2009;70(6):553–64. [DOI] [PubMed] [Google Scholar]
- 8.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–25. [DOI] [PubMed] [Google Scholar]
- 9.Scharf O, Golding H, King LR, et al. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J Virol. 2001;75(14):6558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay MZ, Liu P, Williams LD, et al. Antibody-Mediated Internalization of Infectious HIV-1 Virions Differs among Antibody Isotypes and Subclasses. PLoS Pathog. 2016;12(8):e1005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molinos-Albert LM, Clotet B, Blanco J, Carrillo J. Immunologic Insights on the Membrane Proximal External Region: A Major Human Immunodeficiency Virus Type-1 Vaccine Target. Front Immunol. 2017;8:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena A, Wu D. Advances in Therapeutic Fc Engineering - Modulation of IgG-Associated Effector Functions and Serum Half-life. Front Immunol. 2016;7:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitaura K, Yamashita H, Ayabe H, et al. Different Somatic Hypermutation Levels among Antibody Subclasses Disclosed by a New Next-Generation Sequencing-Based Antibody Repertoire Analysis. Front Immunol. 2017;8:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates NL, Liao HX, Fong Y, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung AW, Ghebremichael M, Robinson H, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra38. [DOI] [PubMed] [Google Scholar]
- 16.Chung AW, Kumar MP, Arnold KB, et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell. 2015;163(4):988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meffre E, Louie A, Bannock J, et al. Maturational characteristics of HIV-specific antibodies in viremic individuals. JCI Insight. 2016;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kardava L, Moir S, Wang W, et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121(7):2614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton AR, Pallett LJ, McCoy LE, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest. 2018;128(10):4588–603.** This study demonstrates impaired B-cell immunity in blood and liver of individuals with chronic hepatitis B virus disease associated with expansion of atypical CD11c/T-bet-expressing B cells.
- 21.Obeng-Adjei N, Portugal S, Holla P, et al. Malaria-induced interferon-gamma drives the expansion of Tbethi atypical memory B cells. PLoS Pathog. 2017;13(9):e1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knox JJ, Buggert M, Kardava L, et al. T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight. 2017;2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenks SA, Cashman KS, Zumaquero E, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49(4):725–39.** This study identifies dysregulated pathway of B-cell response in systemic lupus erythematous associated with expansion of autoreactive CD11c/T-bet-expressing B cells.
- 24.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13(11):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekkarinen PT, Heikkila N, Kisand K, et al. Dysregulation of adaptive immune responses in complement C3-deficient patients. Eur J Immunol. 2015;45(3):915–21. [DOI] [PubMed] [Google Scholar]
- 26.Mortensen R, Nissen TN, Blauenfeldt T, et al. Adaptive Immunity against Streptococcus pyogenes in Adults Involves Increased IFN-gamma and IgG3 Responses Compared with Children. J Immunol. 2015;195(4):1657–64. [DOI] [PubMed] [Google Scholar]
- 27.Johrens K, Anagnostopoulos I, Durkop H, Stein H. Different T-bet expression patterns characterize particular reactive lymphoid tissue lesions. Histopathology. 2006;48(4):343–52. [DOI] [PubMed] [Google Scholar]
- 28.Johrens K, Shimizu Y, Anagnostopoulos I, et al. T-bet-positive and IRTA1-positive monocytoid B cells differ from marginal zone B cells and epithelial-associated B cells in their antigen profile and topographical distribution. Haematologica. 2005;90(8):1070–7. [PubMed] [Google Scholar]
- 29.Hatzivassiliou G, Miller I, Takizawa J, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14(3):277–89. [DOI] [PubMed] [Google Scholar]
- 30.Ehrhardt GR, Hsu JT, Gartland L, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202(6):783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knox JJ, Kaplan DE, Betts MR. T-bet-expressing B cells during HIV and HCV infections. Cell Immunol. 2017;321:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portugal S, Obeng-Adjei N, Moir S, et al. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol. 2017;321:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karnell JL, Kumar V, Wang J, et al. Role of CD11c+ T-bet+ B cells in human health and disease. Cell Immunol. 2017;321:40–5. [DOI] [PubMed] [Google Scholar]
- 34.Pupovac A, Good-Jacobson KL. An antigen to remember: regulation of B cell memory in health and disease. Curr Opin Immunol. 2017;45:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winslow GM, Papillion AM, Kenderes KJ, Levack RC. CD11c+ T-bet+ memory B cells: Immune maintenance during chronic infection and inflammation? Cell Immunol. 2017;321:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frasca D, Diaz A, Romero M, et al. Aging effects on T-bet expression in human B cell subsets. Cell Immunol. 2017;321:68–73. [DOI] [PubMed] [Google Scholar]
- 37.Myles A, Gearhart PJ, Cancro MP. Signals that drive T-bet expression in B cells. Cell Immunol. 2017;321:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kardava L, Sohn H, Youn C, et al. IgG3 regulates tissue-like memory B cells in HIV-infected individuals. Nat Immunol. 2018;19(9):1001–12.** This study identifies IgG3 as a regulator of B-cell function in HIV-1 infection by demonstrating that soluble IgG3 binds to IgM-expressing B cells, along with C1q and CRP and inhibits further activation.
- 39.Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13(8):578–91. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Marnell LL, Marjon KD, et al. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456(7224):989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorman A, Zhang L, Ding Z, Heyman B. How antibodies use complement to regulate antibody responses. Mol Immunol. 2014;61(2):79–88. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Won Sohn H, Tolar P, et al. Antigen-induced oligomerization of the B cell receptor is an early target of Fc gamma RIIB inhibition. J Immunol. 2010;184(4):1977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Son M, Diamond B, Santiago-Schwarz F. Fundamental role of C1q in autoimmunity and inflammation. Immunol Res. 2015;63(1–3):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen TT, Klasener K, Zurn C, et al. The IgM receptor FcmuR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol. 2017;18(3):321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]