Abstract
Background: The objective of this study was to evaluate the clinical efficacy of stereotactic body radiotherapy (SBRT) and surgical treatment for stage I–II non-small cell lung cancer (NSCLC). Methods: This retrospective analysis included 879 patients with primary NSCLC who underwent SBRT or surgical treatment in Zhejiang Cancer Hospital, Hangzhou, China from January 2012 to December 2017. Results: Propensity score matching (PSM) analysis was performed between the two groups. Each group included 66 patients who met the inclusion criteria. The median follow-up in the SBRT and surgery groups was 30.8 and 48.4 months, respectively. In the SBRT group, the 1- and 3-year overall survival rates were 98.5 and 83.9%, respectively. In the surgery group, these rates were 98.5 and 89.4%, respectively (P = .248). The 3-year cancer-specific survival rates in the SBRT and surgery groups were 89.1 and 95.2%, respectively (P = .056). Conclusions: In these propensity score matched early-stage NSCLC patients, the 1- and 3-year overall survival rates associated with SBRT were similar to those observed with surgery. In addition, there was no significant difference in cancer-specific survival between the two groups.
Introduction
Surgical anatomic resection with lymph node evaluation remains the standard of care for operable patients with early-stage non-small cell lung cancer [1], [2]. However, 20% to 30% of patients are inoperable due to advanced age or the presence of comorbidities [3], [4]. Stereotactic body radiotherapy delivers high radiation doses to restricted volumes through multiple precisely aimed radiotherapy beams [5], [6]. This approach is currently considered as the preferred treatment option in patients unfit for surgery or at high risk for the occurrence of postoperative complications [7]. One of the potential indications of SBRT is operable patients. Several retrospective studies and prospective trials have shown that overall survival (OS) after SBRT was comparable with that reported after surgical resection [8], [9], [10], [11], [12]. However, a retrospective series showed that there are important differences between patients treated with surgery and those who received SBRT in terms of age, performance status, comorbid medical conditions, etc. These differences render direct comparisons between these two approaches problematic. Moreover, thus far, there are no phase III prospective randomized trials comparing the two treatment modalities have completed.
Propensity score matching analysis allows for matching across a broad range of baseline factors, producing two similar groups for comparison [13], [14]. In this study, we performed a comprehensive PSM analysis designed to compare the outcomes of two potentially curative approaches for stage I–II NSCLC using uniform definitions of recurrence and survival from recently completed and ongoing clinical trials. We hypothesized that locoregional control (LRC) and cancer-specific survival (CSS) in early-stage NSCLC patients undergoing surgery or SBRT may be comparable.
Materials and Methods
Study Population
Patients with cytologically/histologically or clinically confirmed NSCLC from January 2012 to December 2017 were eligible for inclusion in the study. They were staged according to the findings of chest computed tomography (CT). Patients with radiologically suspicious lymph nodes underwent endobronchial ultrasonography or mediastinoscopy. In addition, patients underwent bone imaging and brain magnetic resonance imaging or fludeoxyglucose-positron-emission tomography (18FDG-PET) to identify the presence of T1–2 N0 M0. Disease staging was performed using the Unite and Support the Cancer Community (UICC) TNM-7th edition. The indications were fully examined and discussed among patients, surgeons, radiation oncologists, and diagnostic radiologists. All multidisciplinary consultations were recorded in detail. Patients with adequate pulmonary function to tolerate at least a lobectomy and absence of other contraindicating medical comorbidity—according to the thoracic surgeon—were selected for lobar resection. Radical lymph node dissection was performed in accordance with the current guidelines [15]. Inoperable patients—according to the thoracic surgeon—and those who refused sublobar resection were selected for SBRT. Target coverage, conformality, and normal tissue constraints were investigated according to the protocol for the clinical trial Radiotherapy Oncology Group (RTOG) 0236 [16]. Treatment plans were optimized to limit the administration of high doses to regions of organs at risk. This was achieved using more fractions and a lower dose per fraction for larger tumors and those adjacent to critical organs [17]. Biological effective dose (BED) was calculated using BEDα/β = nd(1+ d/α/β), where n = number of fractions, d = dose per fraction, and α/β = 10 for the tumor in line with prior reports [18]. Patients received a median BED10 of 100 Gy (range: 71–120 Gy). Exclusion criteria included patients with Karnofsky Performance Status ≤60; other antitumor therapy within 3 months prior to surgery or SBRT; local recurrence and distant metastasis within 3 months after surgery or SBRT; and lost to follow-up.
Data Collection
Clinical information was obtained from the electronic file database of Zhejiang Cancer Hospital, Hangzhou, China. Comorbidity scores were recorded using the Charlson Comorbidity Index (CCI) [19]. Toxicity in the SBRT group and complications in the surgery group were scored according to the Common-Terminology-Criteria-for-Adverse-Events version 4.0 to eliminate historic discrepancies in definitions of failure between surgery and SBRT. LRC was defined as the absence of any recurrence in the ipsilateral lung, the bronchial stump/suture line, and N1–N3 nodal areas. Locoregional failure was defined as disease recurrence in or adjacent to the planning target volume (for SBRT patients), resection margins (for surgery patients), and ipsilateral regional lymph nodes. Distant failure indicated recurrence other than locoregional failure. The rates of LRC were determined as the date of treatment to the date of first locoregional failure. The overall survival time was defined as the period from the date of treatment initiation to the date of death or last follow-up. CSS was defined as death due to lung cancer or treatment-related mortality.
Post-treatment follow-up generally consisted of a contrast-enhanced CT scan of the thorax and abdomen performed every 1–3 months for the first 2 years and once every 6 to 10 months thereafter. A PET/CT scan was performed in patients with suspicion of disease recurrence; censored at any other recurrence or at last follow up. Biopsies were performed following CT and PET evidence of progression or recurrence.
PSM
The propensity score was calculated using multivariable logistic regression to model a dichotomous outcome of surgery or SABR for the cohort of 879 patients. The details of patients were accessed through a database. An initial PSM analysis was performed to compare patients in the SBRT and surgery groups based on age, gender, tumor diameter, pathology, Karnofsky Performance Status score, forced expiratory volume in 1 second (FEV1), FEV1 and forced vital capacity ratio (FEV1/FVC%), and CCI. A propensity score difference of 0.10 was used as a maximum caliper width for matching the two treatment groups. All matching was performed in a 1:1 ratio.
Study Outcome
The main purpose of the study was to determine the OS, CSS, LRC, and distant control after treatment with SBRT or surgery in patients with early-stage NSCLC. Every recurrence was confirmed through CT that showed an increase in its longest axis and persisted for ≥6 months or confirmed by biopsy or 18FDG-PET imaging and discussed in a multidisciplinary team.
Statistical Analysis
The Kaplan–Meier method was used to calculate the OS and LRC rates. The Wilcoxon rank-sum test was used for medians. The two-tailed t test was used for continuous variables unless the data were non-normally distributed. For such cases, we used the Mann–Whitney U test for comparison. The χ2 test was used for categorical variables. All statistical tests were two-sided using an α = 0.05 level of significance. PSM was performed using the SPSS for Windows version-24.0 (SPSS Inc., Chicago, IL, USA). The GraphPad Prism-7.0 for Windows (GraphPad Software, San Diego, CA, USA) was used to construct the Kaplan–Meier survival curves.
Results
Patient Characteristics
A total of 879 patients were selected for matching. The baseline characteristics of the patients prior to PSM are listed in Table 1. There were no significant differences between the SBRT and surgery groups in terms of age, gender, tumor size, and pathology. However, patients who received SBRT exhibited significantly poorer FEV1.0 and CCI than those who underwent surgery (P < .01). PSM was performed to reduce these selection biases and identified 66 patients from each treatment group with similar characteristics for further analysis (Table 2). The absolute standardized differences for all measured covariates were< 10%. The eligible patients were similar in terms of age (median: 68 years), gender, tumor size (2.0 vs. 2.2 cm, respectively), and FEV1.0 (1.60 vs. 1.67 L, respectively). The median follow-up time was 30.5 and 48.3 months, respectively. The clinical stage of T and N was determined using 18FDG-PET and CT in 40 and 11 patients who underwent SBRT or surgery, respectively (P < .01). Both groups of patients successfully completed treatment.
Table 1.
Characteristics of the Entire Patient Cohort
| Factor | SBRT | Surgery | P-value |
|---|---|---|---|
| Number | 107 | 772 | |
| Age (years) | .00 | ||
| Median (range) | 72 (49–88) | 60 (33–83) | |
| Gender | .00 | ||
| Male | 79 | 396 | |
| Female | 28 | 376 | |
| Tumor size (cm) | .03 | ||
| Median (range) | 2.1 (0.7–5.3) | 2.0 (0.4–5.5) | |
| Pathology | .00 | ||
| Ade | 50 (47) | 626 (81) | |
| SCC | 38 (35) | 125 (16) | |
| Others | 19 (18) | 21 (3) | |
| FEV1 | .00 | ||
| Median (range) | 1.47 (0.44–2.73) | 2.15 (0.77–3.93) | |
| FEV1/FVC (%) | .00 | ||
| Median (range) | 94 (31–129) | 107 (54–190) | |
| CCI (%) | .00 | ||
| 0 | 42 (39) | 561 (73) | |
| 1 | 32 (30) | 107 (14) | |
| 2 | 23 (21) | 76 (10) | |
| 3 | 4 (3) | 17 (2) | |
| 4 | 6 (7) | 10 (1) | |
| ≥5 | 0 (0) | 1 (0) | |
| KPS | .22 | ||
| Median (range) | 90 (70–100) | 90 (70–100) |
Table 2.
Characteristics of the Propensity Score-Matched Patients
| Factor | SBRT | Surgery | P-value |
|---|---|---|---|
| Number | 66 | 66 | |
| Age (years) | .83 | ||
| Median (range) | 68 (49–85) | 68 (40–83) | |
| Gender | .86 | ||
| Male | 43 | 42 | |
| Female | 23 | 24 | |
| Tumor size (cm) | .47 | ||
| Median (range) | 2.0 (0.7–5.3) | 2.2 (0.8–4.0) | |
| Pathology | .96 | ||
| Ade | 38 (57) | 38 (57) | |
| SCC | 21 (32) | 20 (31) | |
| Others | 7 (11) | 8 (12) | |
| FEV1 | .15 | ||
| Median (range) | 1.60 (0.44–2.73) | 1.67 (0.77–3.25) | |
| FEV1/FVC (%) | .81 | ||
| Median (range) | 102 (55–129) | 101 (54–123) | |
| CCI (%) | .64 | ||
| 0 | 37 (56) | 38 (58) | |
| 1 | 12 (18) | 12 (18) | |
| 2 | 13 (20) | 8 (12) | |
| 3 | 2 (3) | 4 (6) | |
| 4 | 2 (3) | 4 (6) | |
| ≥5 | 0 (0) | 0 (0) | |
| KPS | .27 | ||
| Median (range) | 90 (70–100) | 90 (80–100) |
SBRT: stereotactic body radiotherapy, Ade: adenocarcinoma, SCC: squamous cell carcinoma, FEV1: forced expiratory volume in 1 second, FEV1/FVC%: FEV1 and forced vital capacity ratio, CCI: Charlson comorbidity index, KPS: Karnofsky performance status.
Survival
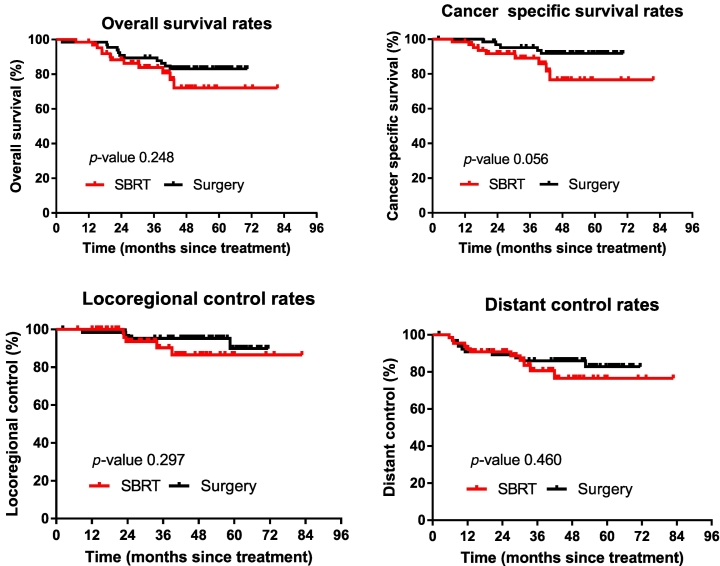
The 1- and 3-year OS rates in patients who received SBRT were 98.5 and 83.9%, respectively. In the surgery group, these rates were 98.5 and 89.4%, respectively. The survival was not significantly different between the two groups (P = .25). Kaplan–Meier plots comparing patterns of survival for the entire cohort of patients are presented in Figure 1.
Figure 1.
Comparison of overall survival, cancer-specific survival, locoregional control, and distant control rates of patients after surgery or SBRT following propensity score matching.
The 90-day mortality rate after surgery was 1.5%. There were no deaths attributable to SBRT (P = .079). Nine patients (i.e., three patients in the SBRT group and six patients in the surgery group) expired due to non-tumor factors (e.g., cerebral infarction, heart disease) during the follow-up period. The CSS was similar between the two treatment groups (P = .056). The corresponding 3-year CSS in the SBRT and surgery group was 89.1 and 95.2%, respectively.
Locoregional recurrence occurred in nine patients, (i.e., five in the SBRT group and four in the surgery group). The LRC rates did not differ significantly between the groups (P = .297). Among patients who received SBRT, the LRC rates at 1 and 3 years were 100.0 and 90.3%, respectively. In the surgery group, these rates were 98.5 and 95.2%, respectively.
Distant metastasis was reported in 21 patients (i.e., 11 in the SBRT group and 10 in the surgery group). Differences in the rates of distant control were not statistically significant (P = .460). In the SBRT group, the rates at 1 and 3 years were 92.4 and 80.6%, respectively. In the surgery group, these rates were 90.8 and 86.0%, respectively.
Treatment Toxicity
In the SBRT group, none of the patients experienced grade 4–5 toxic events. Systemic reactions were mainly fatigue, anorexia, and dyspnea during treatment. Most of these reactions resolved after symptomatic treatment. Side effects within 6 weeks after SBRT were observed in 20 (30%) patients. Grade 1–3 radioactive pneumonitis—according to the RTOG—was observed in 14 (70%), five (25%), and one (5%) patients, respectively. These adverse events improved after symptomatic treatment or administration of a glucocorticoid. Late grade 1–2 pulmonary fibrosis was observed in 11 (17%) patients. There were no deaths attributable to SBRT.
In the surgery group, grade 2–3 complications were observed in 14 (21%) and six (9%) patients respectively. Of note, four (6%) patients experienced grade 4 toxic events. One (2%) patient expired due to pulmonary infection within 90 days after treatment.
Discussion
In recent years, the use of SBRT has achieved great progress in the treatment of early-stage NSCLC [20], [21]. Currently, it is the standard treatment option for inoperable patients with early-stage NSCLC or those who reject surgery. Several clinical trials have shown encouraging efficacy of SBRT with an acceptable toxicity profile [16], [22], [23], [24]. Although the current consensus guidelines established by the American Society of Radiation Oncology do not recommend the use of SBRT in patients who are suitable for lobectomy [20]. The investigation of the feasibility of SBRT in operable patients was based on the hypothesis that the outcomes associated with SBRT may be equivalent to those observed after surgery. However, unlike those who underwent surgery, most patients who received SBRT were inoperable. There are important differences in terms of age, cardiopulmonary function, performance status, etc. between these two groups. Unfortunately, retrospective studies cannot overcome all uncertainties associated with the absence of potential variables, though the findings are encouraging [11], [12], [25], [26]. This underscores the need for randomized trials that are not subject to the biases that underlie decisions to treat patients with surgery or SBRT in this setting. It is regrettable that thus far, there are no phase III prospective trials comparing these two treatment modalities.
Retrospective reviews, such as the present study, may provide clues regarding the appropriate management of patients with early-stage lung cancer. Our intention was to compare the efficacy of surgery and SBRT using propensity scores to control for selection bias and a coherent definition of failure in both groups. To the best of our knowledge, this is the first report using PSM to compare the efficacy of surgery and SBRT in patients with early-stage NSCLC in China. The results were noted in light of a good balance between demographic and tumor factors in the examined groups. The results of this study reveal that the 3-year OS, LRC, and distant control associated with SBRT in patients with early-stage NSCLC is comparable with those reported after surgery. The OS and LRC in these two groups were consistent with those previously reported [27], [28], [29]. After 3 years, there seems to be a trend toward improved CSS for patients who undergo surgery.
A possible explanation for this trend may be the variation in practice for the removal of suspicious lymph node zones during surgery. A key advantage of surgery in stage I–II NSCLC is the ability to invasively stage lymph nodes. For patients with recurrent or metastatic disease, the pathology obtained through surgery renders the planning of treatment more accurate. Hence, the development of targeted therapies and chemotherapy regimens should be based on pathology and, in particular, genotyping. Another explanation is that the majority of patients in the SBRT group were inoperable, despite the accurate matching of the cohorts. Considering that this was a retrospective study, other factors not included in the matching process may be responsible for the observed differences in outcome.
One matched comparison of patients with T1-2 N0 M0 NSCLC who underwent SBRT or sublobar resection reported improved LRC in the former group [30]. In 2015, Chang et al. conducted a matched analysis of cases included in the STARS (NCT00840749) and ROSEL (NCT00687986) projects. The estimated 3-year OS was 95% in the SBRT (31 cases) group versus 79% in the surgery group (27 cases) (P = .037), potentially owing to surgery-associated morbidity/mortality [8]. However, other recent studies [31], [32], [33] using PSM reported the superiority of surgery in operable stage I NSCLC patients. Further robust studies are warranted to demonstrate that SBRT may achieve comparable results with those reported after surgery. Unfortunately, the randomized trials investigating surgery and SBRT (i.e., RTOG1021, STARS and ROSEL) have been beset by poor enrollment. RTOG 0618 was a single-arm phase II trial that included 23 patients with T1 tumors and three patients with T2 tumors (<5 cm). The tumors had to be located ≥2 cm away (in all directions) from the proximal bronchial tree. Patients were deemed operable by meeting all of the following baseline criteria: FEV1 and predicted diffusing capacity of the lung for carbon monoxide >35%, PaO2 >60 mmHg, PaCO2 <50 mmHg, and absence of severe medical problems. They were administered a dose of 54 Gy in three fractions. During the long-term follow-up, only one patient exhibited primary tumor recurrence. The estimated 4-year rate of both primary tumor and local control was 96%. In addition, the estimated 4-year rates of disease-free and OS were 57 and 56%, respectively. Toxicity remained acceptable, with two patients experiencing grade 3 toxicity, and absence of grade 4–5 toxicity. These results illustrate that SBRT may be a viable alternative to surgical resection for the treatment of operable patients [10].
Systemic reactions during SBRT were mainly anorexia and dyspnea, with the majority resolving after treatment. Only one patient developed a grade 3 complication (radioactive pneumonitis) and there were no deaths attributable to SBRT. In the surgery group, one patient expired due to pulmonary infection and grade 3 complications were observed in six patients. The difference of both treatment complications is one of the most important reference indicators for discussing treatment options with patients.
A strength of the present study is that the demographic and tumor matching factors were comprehensive, with limited variability at baseline. All patients were treated in a single hospital institution with minimal variability in radiotherapy techniques and surgical conditions. Another advantage is the strict unified definition of recurrence and survival, rendering the comparison between the two groups more valuable. In addition, longer follow-up is one of our highlights.
The limitations of this study must be acknowledged. Although the cohorts were accurately matched, this study was a retrospective study; thus, factors not included in the matching process may be responsible for the observed differences in outcome. Therefore, randomized comparisons between surgery and SBRT are warranted to avoid imbalances and selection biases. In addition, the relatively small sample size of the patient cohort is another limitation.
Conclusion
Lobectomy remains the standard of care for early-stage NSCLC. However, there is no direct evidence of its superiority compared with SBRT. Further studies are warranted to confirm that SBRT is associated with similar or even higher OS rates compared with surgery. The recommendation for the treatment plan of an individual patient should be comprehensive, taking into consideration the size and location of the primary tumor, age, complications, and clinical evidence that can be referenced. For this reason, randomized controlled trials are warranted to investigate the role of SBRT and surgery as treatment options for operable patients with stage I–II NSCLC. The high local control rates combined with a low-toxicity profile observed in inoperable stage I NSCLC patients after SBRT indicate that this treatment modality is worthy of further investigation.
Acknowledgement
This study was supported by grant 2017C33092 from public welfare funds of Zhejiang province.
Contributor Information
Ming Chen, Email: chenming@zjcc.org.cn.
Jianping Cao, Email: jpcao@suda.edu.cn.
References
- 1.Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ, Putnam JB., Jr. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest. 2011;139(5):1124–1129. doi: 10.1378/chest.10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–622. doi: 10.1016/0003-4975(95)00537-u. (discussion 622-3) [DOI] [PubMed] [Google Scholar]
- 3.Iyengar P, Timmerman RD. Stereotactic ablative radiotherapy for non-small cell lung cancer: rationale and outcomes. J Natl Compr Cancer Netw. 2012;10(12):1514–1520. doi: 10.6004/jnccn.2012.0157. [DOI] [PubMed] [Google Scholar]
- 4.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 5.Guckenberger M, Klement RJ, Allgauer M, Appold S, Dieckmann K, Ernst I, Ganswindt U, Holy R, Nestle U, Nevinny-Stickel M. Applicability of the linear-quadratic formalism for modeling local tumor control probability in high dose per fraction stereotactic body radiotherapy for early stage non-small cell lung cancer. Radiother Oncol. 2013;109(1):13–20. doi: 10.1016/j.radonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozzi A, Iftode C, Reggiori G, Tomatis S, Infante M. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC) Radiother Oncol. 2013;107(3):414–418. doi: 10.1016/j.radonc.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palma DA, Senan S. Improving outcomes for high-risk patients with early-stage non-small-cell lung cancer: insights from population-based data and the role of stereotactic ablative radiotherapy. Clin Lung Cancer. 2013;14(1):1–5. doi: 10.1016/j.cllc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, Straube WL, Nedzi LA, McGarry RC, Robinson CG. Stereotactic body radiation therapy for operable early- stage lung cancer: findings from the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018;4(9):1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Karasawa K, Hayakawa K, Niibe Y, Takai Y. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011;81(5):1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 12.Louie AV, Rodrigues G, Hannouf M, Zaric GS, Palma DA, Cao JQ, Yaremko BP, Malthaner R, Mocanu JD. Stereotactic body radiotherapy versus surgery for medically operable Stage I non-small-cell lung cancer: a Markov model-based decision analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):964–973. doi: 10.1016/j.ijrobp.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123(1):8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 14.Mokhles MM, Kortke H, Stierle U, Wagner O, Charitos EI, Bogers AJ, Gummert J, Sievers HH, Takkenberg JJ. Survival comparison of the Ross procedure and mechanical valve replacement with optimal self-management anticoagulation therapy: propensity-matched cohort study. Circulation. 2011;123(1):31–38. doi: 10.1161/CIRCULATIONAHA.110.947341. [DOI] [PubMed] [Google Scholar]
- 15.De Leyn P, Lardinois D, Van Schil PE, Rami-Porta R, Passlick B, Zielinski M, Waller DA, Lerut T, Weder W. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32(1):1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 16.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurkmans CW, Cuijpers JP, Lagerwaard FJ, Widder J, van der Heide UA, Schuring D, Senan S. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol. 2009;4(1) doi: 10.1186/1748-717X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen JR, Robinson CG, El Naqa I, Creach KM, Drzymala RE, Bloch C, Parikh PJ, Bradley JD. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e299–e303. doi: 10.1016/j.ijrobp.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, Lally BE, Latzka K, Lo SS, Moghanaki D. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(5):295–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Verma V, Shostrom VK, Zhen W, Zhang M, Braunstein SE, Holland J, Hallemeier CL, Harkenrider MM, Iskhanian A, Jabbour SK. Influence of fractionation scheme and tumor location on toxicities after stereotactic body radiation therapy for large (>/=5 cm) non-small cell lung cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;97(4):778–785. doi: 10.1016/j.ijrobp.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timmerman RD, Hu C, Michalski JM, Bradley JC, Galvin J, Johnstone DW, Choy H. Long-term results of stereotactic body radiation therapy in medically inoperable Stage I non-small cell lung cancer. JAMA Oncol. 2018;4(9):1287–1288. doi: 10.1001/jamaoncol.2018.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.B Sun ED, Brooks RU, Komaki Z, Liao MD, Jeter MF, McAleer PK, Allen PA, Balter JD. Welsh, MS O'Reilly, et al., 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 2017;123(16):3031–3039. doi: 10.1002/cncr.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GM Videtic C Hu, Singh AK, Chang JY, Parker W, Olivier KR, Schild SE, Komaki R, Urbanic JJ, Timmerman RD. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG Oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015;93(4):757–764. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94(1):1–11. doi: 10.1016/j.radonc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, Slotman BJ, Paul MA, Smit EF, Senan S. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 27.Verstegen NE, Oosterhuis JW, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, Mollema R, van Tets WF, Warner A, Joosten JJ. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013;24(6):1543–1548. doi: 10.1093/annonc/mdt026. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Zhu F, Ma X, Tian Y, Cao D, Luo S, Xuan Y, Liu L, Wei Y. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;112(2):250–255. doi: 10.1016/j.radonc.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo Y, Chen F, Hamaji M, Kawaguchi A, Ueki N, Nagata Y, Sonobe M, Morita S, Date H, Hiraoka M. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer. 2014;50(17):2932–2938. doi: 10.1016/j.ejca.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Grills IS, Mangona VS, Welsh R, Chmielewski G, McInerney E, Martin S, Wloch J, Ye H, Kestin LL. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 31.Yerokun BA, Yang CJ, Gulack BC, Li X, Mulvihill MS, Gu L, Wang X, Harpole DH, D'Amico TA, Berry MF. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. 2017;154(2):675–686e4. doi: 10.1016/j.jtcvs.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 32.Hamaji M, Chen F, Matsuo Y, Kawaguchi A, Morita S, Ueki N, Sonobe M, Nagata Y, Hiraoka M, Date H. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg. 2015;99(4):1122–1129. doi: 10.1016/j.athoracsur.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Rosen JE, Salazar MC, Wang Z, Yu JB, Decker RH, Kim AW, Detterbeck FC, Boffa DJ. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2016;152(1):44–54e9. doi: 10.1016/j.jtcvs.2016.03.060. [DOI] [PubMed] [Google Scholar]