Abstract
Long non-coding RNA (lncRNA) FEZF1 antisense RNA 1 (FEZF1-AS1) has been shown to be up-regulated in tumor tissues and cells, and exerts oncogenic effects on various types of malignancies. However, the expression and function of FEZF1-AS1 was still fully unclear in retinoblastoma. The purpose of our study was to investigate the expression and clinical value of FEZF1-AS1 in retinoblastoma patients, and explore the effect of FEZF1-AS1 on retinoblastoma cell proliferation, migration and invasion. In our results, levels of FEZF1-AS1 expression were elevated in retinoblastoma tissue specimens and cell lines compared with adjacent normal retina tissue specimens and human retinal pigment epithelial cell line, respectively. The correlation analysis indicated that high FEZF1-AS1 expression was significantly correlated with present choroidal invasion and optic nerve invasion. Survival analysis suggested that retinoblastoma patients in high FEZF1-AS1 expression group had obviously short disease-free survival (DFS) compared with retinoblastoma patients in low FEZF1-AS1 expression group, and high FEZF1-AS1 expression was an independent unfavorable prognostic factor for DFS in retinoblastoma patients. Loss-of-function study indicated silencing FEZF1-AS1 expression inhibited retinoblastoma cell proliferation, invasion and migration. In conclusion, FEZF1-AS1 functions as an oncogenic lncRNA in retinoblastoma.
Keywords: biomarkers, cancer, FEZF1-AS1, large intervening non-coding RNA, retinoblastoma
Introduction
Retinoblastoma originated from the retinal photoreceptor precursor cells, and is the most common childhood intraocular cancer accounting for 3% of all pediatric cancers [1,2]. The incidence of retinoblastoma is about 1 case in every 15000–20000 live births [3]. Although the survival rate of retinoblastoma patients is up to 95% in developed countries, the survival rate of patients with retinoblastoma is still less than 70% in developing countries and less developed countries [4,5]. The tumorigenesis of retinoblastoma is a highly complicated process, which has close relationship with gene mutation [6,7], oncogene aberrant expression [8,9], and activation of oncogenic signaling pathways [10,11]. The molecular mechanisms underlying retinoblastoma occurrence and development remain unknown. Therefore, it is very useful to investigate the molecular mechanisms of retinoblastoma initiation to develop new targeted therapy and improving clinical outcome.
Long non-coding RNAs (lncRNAs) are identified as RNA transcripts greater than 200 nts in length and limited protein-coding potential [12]. More and more reports have suggested that lncRNAs play key roles in the development and progression of retinoblastoma, such as PlncRNA-1 [13], ANRIL [14], THOR [15], AFAP1-AS1 [16], XIST [17] and so on. lncRNA FEZF1 antisense RNA 1 (FEZF1-AS1) is localized at the human chromosome 7q31.32, and is transcribed in the opposite direction of FEZF1 gene. FEZF1-AS1 has been shown to be up-regulated in tumor tissues and cells, and exert oncogenic effects on lung cancer [18–21], breast cancer [22], liver cancer [23,24], gastric cancer [25–27], colorectal cancer [28,29], pancreatic cancer [30,31], ovarian cancer [32], cervical cancer [33], osteosarcoma [34], nasopharyngeal carcinoma [35] and multiple myeloma [36]. However, the expression and function of FEZF1-AS1 was still fully unclear in retinoblastoma. The purpose of our study was to investigate the expression and clinical value of FEZF1-AS1 in retinoblastoma patients, and explore the effect of FEZF1-AS1 on retinoblastoma cell proliferation, migration and invasion.
Materials and methods
Clinical specimens
Total 60 fresh retinoblastoma tissue specimens and 30 adjacent normal retina tissue specimens were obtained from retinoblastoma patients who did not receive any preoperative anti-tumor treatment and underwent surgery at Qingyang People’s Hospital, Xi’an No.4 Hospital, Shaanxi Ophthalmic Medical Center or Affiliated Guangren Hospital School of Medicine Xi’an Jiaotong University. Adjacent normal retina tissues were at least 1 cm away from the edge of the primary tumor with no obvious tumor cells. All clinical specimens were immediately frozen in liquid nitrogen after surgery, and pathologically confirmed by at least two pathologists. This research was conducted with the approval of the Ethics Review Committee of Qingyang People’s Hospital, Xi’an No.4 Hospital, Shaanxi Ophthalmic Medical Center and Affiliated Guangren Hospital School of Medicine Xi’an Jiaotong University. The written informed consents were gained from patients or their guardians.
RNA isolation and qRT-PCR
Total RNAs were isolated from tissue specimens or cell lines by using TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.), and were used for template to reverse-transcribe into cDNAs by using PrimeScript RT reagent Kit (Takara Biomedical Technology, Beijing, China). The qRT-PCR was performed through utilizing One Step TB Green PrimeScript RT-PCR Kit II (Takara Biomedical Technology, Beijing, China) according to the manufacturer’s protocols. The specific primers were: FEZF1-AS1, forward, 5′-AGAGGCTATGACTCAGGGTT-3′ and reverse, 5′-TGTTGCTCCACAGTAAAGGT-3′; GAPDH, forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′. Relative FEZF1-AS1 expression was normalized to GAPDH.
Cell lines and cell transfection
Human retinoblastoma cell lines (Weri-RB-1, Y79 and RBL-13) were grown in Roswell Park Memorial Institute (RPMI)-1640 medium (HyClone, Logan, UT, U.S.A.) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT, U.S.A.). Human retinal pigment epithelial cell line (ARPE-19) was grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS (HyClone, Logan, UT, U.S.A.). All cells were maintained in a humidified incubator with 5% CO2 at 37°C. To silence FEZF1-AS1 expression, three individual siRNAs (FEZF1-AS1-siRNA#1, FEZF1-AS1-siRNA#2, and FEZF1-AS1-siRNA#3) and an extra negative control siRNA (NC-siRNA) were designed and optimized by GenePharma Corporation. The sequences of siRNA-FEZF1-AS1s were shown as follows: FEZF1-AS1-siRNA#1 (sense, 5′-AAACAUGGCAGCUACAAGACGGGUC-3′ and antisense, 5′-GACCCGUCUUGUAGCUGCCAUGUUU-3′); FEZF1-AS1-siRNA#2 (sense, 5′-CAGGUACCACAAAGCCACUAGUGCA-3′ and antisense, 5′-UGCACUAGUGGCUUUGUGGUACCUG-3′); FEZF1-AS1-siRNA#3 (sense, 5′-CCAGGACUGGGCAGUGCAUUCUUUA-3′ and antisense, 5′-UAAAGAAUGCACUGCCCAGUCCUGG-3′). The siRNAs were transfected into retinoblastoma cells through using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s protocols. The qRT-PCR was utilized to confirm the transfection efficiencies of FEZF1-AS1-siRNAs in retinoblastoma cells.
Cell counting kit-8 assay
Cell counting kit-8 (CCK-8) assay was used to estimate the cell proliferation ability of retinoblastoma cells. Briefly, transfected retinoblastoma cells (1 × 104 per well) were seeded into 96-well plate (Corning, New York, NY, U.S.A.) and maintained at 37°C in humidified incubator for 24, 48, 72 and 96 h. Whereafter, 10 μl CCK-8 solution was added into each well and the retinoblastoma cells were maintained at 37°C for 1 h. The absorbance at 450 nm was detected by using a Microplate Reader (Bio-Rad, Hercules, CA, U.S.A.).
Transwell migration and invasion assays
The 24-well transwell chambers (8-μm pore size; Corning, Franklin Lakes, NJ, U.S.A.) were used to conduct transwell migration and invasion assays. Briefly, 1 × 105 retinoblastoma cells at 200 μl FBS-free RPMI-1640 medium were seeded into the upper chambers. Then, 500 μl RPMI-1640 medium with 20% FBS was added into bottom chambers. After incubation at 37°C for 24 h, the retinoblastoma cells on the upper chambers were removed, and the retinoblastoma cells on the lower chambers were fixed by 4% paraformaldehyde for 1 h and stained with 1% Crystal Violet for 15 min. Finally, the number of cells was counted under an inverted microscopy in five randomly chosen fields. The transwell invasion assay was performed similarly with transwell migration assay, except that chambers were pre-coated with Matrigel Basement Membrane Matrix (BD Biosciences, San Jose, CA, U.S.A.).
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (Chicago, IL, U.S.A.). The significance of the differences between two independent groups was estimated using Student’s t test. Correlations between FEZF1-AS1 expression and each clinicopathological parameter were evaluated using chi-square test. The disease-free survival (DFS) rate was calculated from the date of surgery to the date of progression. Survival curves were drawn by Kaplan–Meier method and estimated by log-rank test. Univariate and multivariate Cox proportional-hazard models were used for analyzing variables on DFS. P<0.05 was considered to indicate a statistically significant difference.
Results
FEZF1-AS1 expression is up-regulated in retinoblastoma tissues and cells
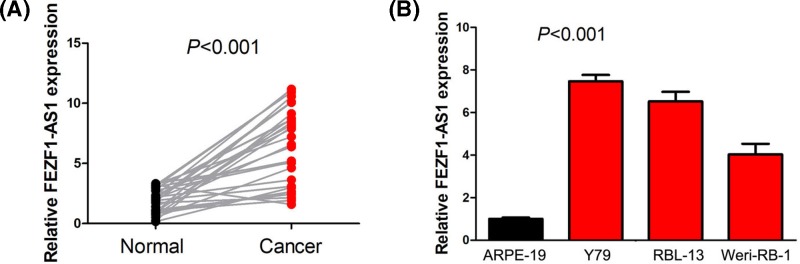
To investigate the expression pattern of FEZF1-AS1 in retinoblastoma, we first detected FEZF1-AS1 expression in retinoblastoma and adjacent normal retina tissue specimens. The results suggested that retinoblastoma tissue specimens exhibited higher FEZF1-AS1 expression than adjacent normal retina tissue specimens (Figure 1A). Furthermore, we detected levels of FEZF1-AS1 expression in human retinoblastoma cell lines (Y79, RBL-13 and Weri-RB-1) and human retinal pigment epithelial cell line (ARPE-19), and found FEZF1-AS1 expression was obviously increased in human retinoblastoma cell lines compared with human retinal pigment epithelial cell line (Figure 1B).
Figure 1. FEZF1-AS1 expression is up-regulated in retinoblastoma tissues and cells.
(A) Retinoblastoma tissue specimens exhibited higher FEZF1-AS1 expression than adjacent normal retina tissue specimens. (B) FEZF1-AS1 expression was obviously increased in human retinoblastoma cell lines (ERI-RB-1, Y79 and RBL-13) compared with human retinal pigment epithelial cell line (ARPE-19).
FEZF1-AS1 overexpression is associated with the malignant status of retinoblastoma patients
For exploring the clinical significance of FEZF1-AS1 in retinoblastoma patients, all retinoblastoma patients were divided into two groups (high FEZF1-AS1 expression group and low FEZF1-AS1 expression group) according to the median FEZF1-AS1 expression level. Then, we analyzed the relationship between FEZF1-AS1 expression and the clinicopathological features of retinoblastoma patients, and found high FEZF1-AS1 expression was significantly correlated with present choroidal invasion and optic nerve invasion (Table 1). However, FEZF1-AS1 expression had no statistical correlation with age, gender, laterality and pathologic grade in retinoblastoma patients (Table 1).
Table 1. Correalations between FEZF1-AS1 and clinicopathological features in retinoblastoma.
| Features | n | High FEZF1-AS1 expression | Low FEZF1-AS1 expression | P |
|---|---|---|---|---|
| Age (years) | ||||
| ≤2 | 31 | 15 | 16 | 0.796 |
| >2 | 29 | 15 | 14 | |
| Gender | ||||
| Male | 35 | 15 | 20 | 0.190 |
| Female | 25 | 15 | 10 | |
| Choroidal invasion | ||||
| No | 36 | 11 | 25 | <0.001 |
| Yes | 24 | 19 | 5 | |
| Optic nerve invasion | ||||
| No | 37 | 13 | 24 | 0.003 |
| Yes | 23 | 17 | 6 | |
| Laterality | ||||
| Unilateral | 43 | 19 | 24 | 0.152 |
| Bilateral | 17 | 11 | 6 | |
| Pathologic grade | ||||
| Well differentiated | 21 | 11 | 10 | 0.787 |
| Poorly differentiated | 39 | 19 | 20 |
FEZF1-AS1 overexpression is associated with unfavorable prognosis in retinoblastoma patients
In order to estimate the prognostic value of FEZF1-AS1 in retinoblastoma patients, we analyzed the relationship between FEZF1-AS1 expression and DFS. We found that retinoblastoma patients in high FEZF1-AS1 expression group had obviously shorter DFS compared with retinoblastoma patients in low FEZF1-AS1 expression group (Figure 2). Furthermore, we estimated that potential prognostic factors for DFS in retinoblastoma patients through univariate and multivariate Cox proportional-hazard models. The results of univariate Cox proportional-hazard models showed choroidal invasion, optic nerve invasion and FEZF1-AS1 expression were prognostic factors in retinoblastoma patients (Table 2). Moreover, multivariate Cox proportional-hazard models indicated high FEZF1-AS1 expression was an independent unfavorable prognostic factor for DFS in retinoblastoma patients (Table 2).
Figure 2. FEZF1-AS1 overexpression is associated with unfavorable prognosis in retinoblastoma patients.
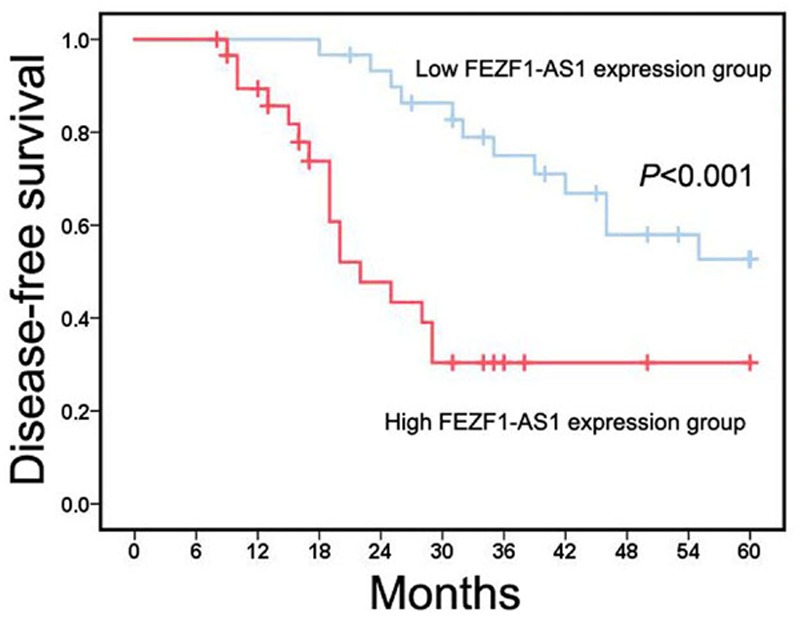
The relationship between FEZF1-AS1 expression and DFS was evaluated through Kaplan–Meier method and log-rank test.
Table 2. Univariate and multivariate Cox regression of prognostic factors for DFS in retinoblastoma patients.
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (years) | ||||||
| ≤2 vs. >2 | 1.072 | 0.517–2.224 | 0.851 | |||
| Gender | ||||||
| Male vs. Female | 1.059 | 0.506–2.218 | 0.879 | |||
| Choroidal invasion | ||||||
| No vs. Yes | 3.218 | 1.476–7.018 | 0.003 | 1.464 | 0.543–3.946 | 0.452 |
| Optic nerve invasion | ||||||
| No vs. Yes | 3.150 | 1.493–6.646 | 0.003 | 2.315 | 1.024–5.230 | 0.044 |
| Laterality | ||||||
| Unilateral vs. Bilateral | 1.972 | 0.918–4.235 | 0.082 | |||
| Pathologic grade | ||||||
| Well differentiated vs. Poorly differentiated | 0.562 | 0.269–1.176 | 0.126 | |||
| FEZF1-AS1 | ||||||
| Low vs. High | 3.703 | 1.703–8.052 | 0.001 | 2.618 | 1.008–6.802 | 0.048 |
Abbreviations: HR, hazard ratio; 95%CI, 95% confidence interval.
Silencing FEZF1-AS1 expression inhibits retinoblastoma cell proliferation, invasion and migration
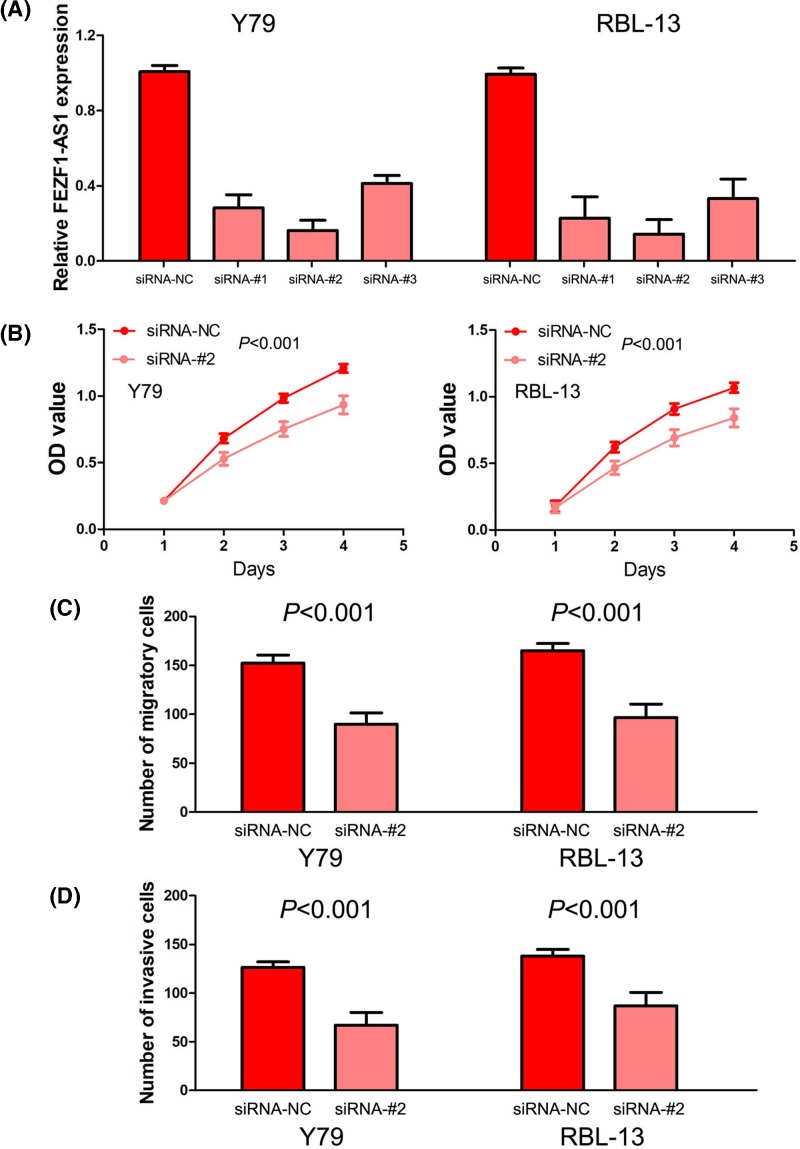
For exploring the effects of FEZF1-AS1 on cell’s biological behavior, we performed loss-of-function study in retinoblastoma cells through siRNA transfection. After transfection with FEZF1-AS1-siRNA#1, FEZF1-AS1-siRNA#2, and FEZF1-AS1-siRNA#3, we found FEZF1-AS1-siRNA#2 was the most effective siRNA and chosen for following experiments in vitro (Figure 3A). The effects of FEZF1-AS1 on retinoblastoma cell proliferation were evaluated by CCK-8 assay. The results showed silencing FEZF1-AS1 expression remarkably suppressed cell proliferation ability of retinoblastoma cells (Figure 3B). In addition, transwell migration and invasion assays were used to assess the influence of FEZF1-AS1 on retinoblastoma cell invasion and migration. We found silencing FEZF1-AS1 expression dramatically repressed retinoblastoma cell invasion and migration abilities of retinoblastoma cells (Figure 3C,D).
Figure 3. Silencing FEZF1-AS1 expression inhibits retinoblastoma cell proliferation, invasion and migration.
(A) The qRT-PCR was utilized to confirm the transfection efficiencies of FEZF1-AS1-siRNAs in retinoblastoma cells. (B) The effects of FEZF1-AS1 on retinoblastoma cell proliferation were evaluated by CCK-8 assay. (C,D) Transwell migration and invasion assays were used to assess the influences of FEZF1-AS1 on retinoblastoma cell invasion and migration.
Discussion
In recent years, increasing studies suggested that FEZF1-AS1 was significantly overexpressed in many types of human cancers, such as lung cancer [18–21], breast cancer [22], liver cancer [23,24], gastric cancer [25–27], colorectal cancer [28,29], pancreatic cancer [30,31], ovarian cancer [32], cervical cancer [33], osteosarcoma [34], nasopharyngeal carcinoma [35] and multiple myeloma [36]. However, the expression of FEZF1-AS1 was still unclear in retinoblastoma. In our study, we first detected FEZF1-AS1 expression in retinoblastoma tissue specimens and cells, and found levels of FEZF1-AS1 expression were elevated in retinoblastoma tissue specimens and cell lines compared with adjacent normal retina tissue specimens and human retinal pigment epithelial cell line, respectively. Furthermore, we analyzed the relationship between FEZF1-AS1 expression and the clinicopathological features for exploring the clinical significance of FEZF1-AS1 in retinoblastoma patients. The correlation analysis indicated that high FEZF1-AS1 expression was significantly correlated with present choroidal invasion and optic nerve invasion. In lung cancer patients, three clinical studies congruously showed that high FEZF1-AS1 expression was correlated with advanced clinical stage [18,19,21]. Besides, Zhang et al. [22] found high FEZF1-AS1 expression was related with advanced TNM stage, lymphatic metastasis, distant metastasis, positive HER2 expression and ER expression in breast cancer patients. In addition, Wang et al. [24] reported FEZF1-AS1 overexpression was associated with large tumor size, and late TNM stage and present venous invasion in patients with hepatocellular carcinoma. In gastric cancer patients, Liu et al. [25] and Wu et al. [27] both showed high FEZF1-AS1 expression was often observed in patients with advanced clinical stage, large tumor size or poor tumor grade. Additionally, the similar clinical significance of FEZF1-AS1 was also observed in colorectal cancer [28,29], pancreatic cancer [30,31], cervical cancer [33], osteosarcoma [34] and nasopharyngeal carcinoma [35].
High FEZF1-AS1 expression in tumor tissues has been suggested to be an independent unfavorable prognostic predictor in several types of tumors. In lung cancer, high FEZF1-AS1 expression was associated with poor overall survival, and acted as an independent prognostic factor for overall survival in lung adenocarcinoma patients [20,21]. Similar results in colorectal cancer patients were reported, these studies indicated that FEZF1-AS1 expression was negatively correlated with overall survival time, and FEZF1-AS1 overexpression served as an independent poor prognostic factor for overall survival [28,29]. In cervical cancer patients, Zhang and Li [33] suggested high-level expression of FEZF1-AS1 was correlated with poor prognosis, and was considered an unfavorable independent prognostic biomarker for overall survival. In addition, there was negative correlation between FEZF1-AS1 expression and clinical outcome in breast cancer [22], liver cancer [23,24], gastric cancer [25,27], pancreatic cancer [30,31], ovarian cancer [32], osteosarcoma [34] and nasopharyngeal carcinoma [35]. The prognostic value of FEZF1-AS1 expression was still unknown in retinoblastoma patients. In our research, we found retinoblastoma patients in high FEZF1-AS1 expression group had obviously shorter DFS compared with retinoblastoma patients in low FEZF1-AS1 expression group, and high FEZF1-AS1 expression was an independent unfavorable prognostic factor for DFS in retinoblastoma patients, which was similar to the prognostic value of FEZF1-AS1 expression in other types of human cancers.
Based on the above clinical study about the role of FEZF1-AS1 expression in retinoblastoma patients, we guessed that FEZF1-AS1 functions as an oncogenic lncRNA to regulate biological behavior in retinoblastoma. Thus, we performed loss-of-function study in retinoblastoma cells, and found silencing FEZF1-AS1 expression inhibited retinoblastoma cell proliferation, invasion and migration. In addition, FEZF1-AS1 was suggested to promote tumor cell proliferation, migration and invasion through activating Wnt/β-catenin signaling pathway in lung cancer [18], gastric cancer [27] and nasopharyngeal carcinoma [35]. Besides, FEZF1-AS1 acted as sponge toward miR-30a/Nanog [22], miR-107/ZNF312B [30], miR-142/HIF-1α [31], miR-133a/EGFR [31], miR-4443/NUPR1 [34] and miR-610/Akt3 [36]. Regrettably, the limit of our study lacked the molecular mechanism of FEZF1-AS1 in retinoblastoma.
In conclusion, FEZF1-AS1 was up-regulated in retinoblastoma tissues and cells, and correlated with aggressive phenotypes and poor clinical outcome in retinoblastoma patients. Silencing FEZF1-AS1 expression inhibits retinoblastoma cell proliferation, invasion and migration.
Abbreviations
- CCK-8
cell counting kit-8
- DFS
disease-free survival
- FBS
fetal bovine serum
- FEZF1-AS1
FEZF1 antisense RNA 1
- lncRNA
long non-coding RNA
- RPMI
Roswell Park Memorial Institute
- TNM
tumor node metastasis
Author Contribution
Lian-Jiao Quan and Wen-Jun Wang designed research, collected the samples and performed the experiments and prepared the manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
References
- 1.Benavente C.A. and Dyer M.A. (2015) Genetics and epigenetics of human retinoblastoma. Annu. Rev. Pathol. 10, 547–562 10.1146/annurev-pathol-012414-040259 [DOI] [PubMed] [Google Scholar]
- 2.Singh L. and Kashyap S. (2018) Update on pathology of retinoblastoma. Int. J. Ophthalmol. 11, 2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao R. and Honavar S.G. (2017) Retinoblastoma. Indian J. Pediatr. 84, 937–944 10.1007/s12098-017-2395-0 [DOI] [PubMed] [Google Scholar]
- 4.Fabian I.D., Onadim Z., Karaa E., Duncan C., Chowdhury T., Scheimberg I.. et al. (2018) The management of retinoblastoma. Oncogene 37, 1551–1560 10.1038/s41388-017-0050-x [DOI] [PubMed] [Google Scholar]
- 5.Jain M., Rojanaporn D., Chawla B., Sundar G., Gopal L. and Khetan V. (2019) Retinoblastoma in Asia. Eye 33, 87–96 10.1038/s41433-018-0244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojanaporn D., Boontawon T., Chareonsirisuthigul T., Thanapanpanich O., Attaseth T., Saengwimol D.. et al. (2018) Spectrum of germline RB1 mutations and clinical manifestations in retinoblastoma patients from Thailand. Mol. Vis. 24, 778–788 [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman S.E., Racher H., Zhang C., MacDonald H. and Gallie B.L. (2017) Genetics and molecular diagnostics in retinoblastoma-an update. Asia Pac. J. Ophthalmol. (Phila.) 6, 197–207 10.22608/APO.201711 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Xue C., Cui H. and Huang Z. (2015) High expression of TAZ indicates a poor prognosis in retinoblastoma. Diagn. Pathol. 10, 187 10.1186/s13000-015-0415-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y., Su Y., Deng L. and Wang W. (2018) Ginsenoside-Rg5 inhibits retinoblastoma proliferation and induces apoptosis through suppressing BCL2 expression. Chemotherapy 63, 293–300 10.1159/000495575 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Yuan J., Yang L., Wang P., Wang X., Wu Y.. et al. (2018) Inhibition of migration and invasion by berberine via inactivation of PI3K/Akt and p38 in human retinoblastoma cell line. Adv. Clin. Exp. Med. 27, 899–905 10.17219/acem/70418 [DOI] [PubMed] [Google Scholar]
- 11.Liao Y., Yin X., Deng Y. and Peng X. (2018) MiR-140-5p suppresses retinoblastoma cell growth via inhibiting c-Met/AKT/mTOR pathway. Biosci. Rep. 38, pii:BSR20180076 10.1042/BSR20180776 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhu C.H., Xiao D.H., Dai L.G., Xu H.G., Jiang Y.H. and Zheng Z.J. (2018) Highly expressed lncRNA FAL1 promotes the progression of gastric cancer by inhibiting PTEN. Eur. Rev. Med. Pharmacol. Sci. 22, 8257–8264 [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Liu J., Yang Y., Hao F. and Zhang L. (2018) PlncRNA-1 is overexpressed in retinoblastoma and regulates retinoblastoma cell proliferation and motility through modulating CBR3. IUBMB Life 70, 969–975 10.1002/iub.1886 [DOI] [PubMed] [Google Scholar]
- 14.Yang Y. and Peng X.W. (2018) The silencing of long non-coding RNA ANRIL suppresses invasion, and promotes apoptosis of retinoblastoma cells through the ATM-E2F1 signaling pathway. Biosci. Rep. 38, pii:BSR20180558 10.1042/BSR20180558 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Shang Y. (2018) LncRNA THOR acts as a retinoblastoma promoter through enhancing the combination of c-myc mRNA and IGF2BP1 protein. Biomed. Pharmacother. 106, 1243–1249 10.1016/j.biopha.2018.07.052 [DOI] [PubMed] [Google Scholar]
- 16.Hao F., Mou Y., Zhang L., Wang S. and Yang Y. (2018) LncRNA AFAP1-AS1 is a prognostic biomarker and serves as oncogenic role in retinoblastoma. Biosci. Rep. 38, pii:BSR20180384. 10.1042/BSR20180384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu K., Zhang N., Ma J., Huang J., Chen J., Wang L.. et al. (2018) Long noncoding RNA FAL1 promotes proliferation and inhibits apoptosis of human colon cancer cells. IUBMB Life 70, 1093–1100 10.1002/iub.1880 [DOI] [PubMed] [Google Scholar]
- 18.He R., Zhang F.H. and Shen N. (2017) LncRNA FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 95, 331–338 10.1016/j.biopha.2017.08.057 [DOI] [PubMed] [Google Scholar]
- 19.Gong W., Cao Y., Wang Y., Yang L., Su W., Qiu F.. et al. (2018) Upregulation of LncRNA FEZF-AS1 is associated with advanced clinical stages and family history of cancer in patients with NSCLC. Pathol. Res. Pract. 214, 857–861 10.1016/j.prp.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 20.Liu Z., Zhao P., Han Y. and Lu S. (2018) lncRNA FEZF1-AS1 is associated with prognosis in lung adenocarcinoma and promotes cell proliferation, migration, and invasion. Oncol. Res. 27, 39–45 10.3727/096504018X15199482824130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin S., Chen S., Ma Y., Yang B. and Liu Y. (2017) LincRNA FEZF1-AS1 contributes to the proliferation of LAD cells by silencing p57 expression. Oncotarget 8, 103004–103013 10.18632/oncotarget.21265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Sun L., Zhang Y., Lu G., Li Y. and Wei Z. (2018) Long non-coding RNA FEZF1-AS1 promotes breast cancer stemness and tumorigenesis via targeting miR-30a/Nanog axis. J. Cell. Physiol. 233, 8630–8638 10.1002/jcp.26611 [DOI] [PubMed] [Google Scholar]
- 23.Gong J., Wang J., Liu T., Hu J. and Zheng J. (2018) lncRNA FEZF1AS1 contributes to cell proliferation, migration and invasion by sponging miR4443 in hepatocellular carcinoma. Mol. Med. Rep. 18, 5614–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Zhao Z., Zhang S., Li Z., Li D., Yang S.. et al. (2018) LncRNA FAL1 is a negative prognostic biomarker and exhibits pro-oncogenic function in osteosarcoma. J. Cell. Biochem. 119, 8481–8489 10.1002/jcb.27074 [DOI] [PubMed] [Google Scholar]
- 25.Liu Y.W., Xia R., Lu K., Xie M., Yang F., Sun M.. et al. (2017) LincRNAFEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation. Mol. Cancer 16, 39 10.1186/s12943-017-0588-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu J., Li Y., Fan L., Zhao Q., Tan B., Hua K.. et al. (2017) Identification of aberrantly expressed long non-coding RNAs in stomach adenocarcinoma. Oncotarget 8, 49201–49216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X., Zhang P., Zhu H., Li S., Chen X. and Shi L. (2017) Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of gastric cancer and promotes tumorigenesis via activation of Wnt signaling pathway. Biomed. Pharmacother. 96, 1103–1108 10.1016/j.biopha.2017.11.113 [DOI] [PubMed] [Google Scholar]
- 28.Chen N., Guo D., Xu Q., Yang M., Wang D., Peng M.. et al. (2016) Long non-coding RNA FEZF1-AS1 facilitates cell proliferation and migration in colorectal carcinoma. Oncotarget 7, 11271–11283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian Z., Zhang J., Li M., Feng Y., Wang X., Zhang J.. et al. (2018) LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin. Cancer Res. 24, 4808–4819 10.1158/1078-0432.CCR-17-2967 [DOI] [PubMed] [Google Scholar]
- 30.Ye H., Zhou Q., Zheng S., Li G., Lin Q., Ye L.. et al. (2018) FEZF1-AS1/miR-107/ZNF312B axis facilitates progression and Warburg effect in pancreatic ductal adenocarcinoma. Cell Death Dis. 9, 34 10.1038/s41419-017-0052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou Z.L., Zhang M., Ji L.D., Luo Z., Han T., Lu Y.B.. et al. (2019) Long noncoding RNA FEZF1-AS1 predicts poor prognosis and modulates pancreatic cancer cell proliferation and invasion through miR-142/HIF-1alpha and miR-133a/EGFR upon hypoxia/normoxia. J. Cell. Physiol., in press 10.1002/jcp.28188 [DOI] [PubMed] [Google Scholar]
- 32.Zhao X., Cheng Z. and Wang J. (2018) Long noncoding RNA FEZF1-AS1 promotes proliferation and inhibits apoptosis in ovarian cancer by activation of JAK-STAT3 pathway. Med. Sci. Monit. 24, 8088–8095 10.12659/MSM.911194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H.H. and Li A.H. (2018) Long non-coding RNA FEZF1-AS1 is up-regulated and associated with poor prognosis in patients with cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 22, 3357–3362 [DOI] [PubMed] [Google Scholar]
- 34.Zhou C., Xu J., Lin J., Lin R., Chen K., Kong J.. et al. (2018) Long non-coding RNA FEZF1-AS1 promotes osteosarcoma progression by regulating miR-4443/NUPR1 axis. Oncol. Res. 26, 1335–1343 10.3727/096504018X15188367859402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y. (2019) FEZF1-AS1 is a key regulator of cell cycle, epithelial-mesenchymal transition and Wnt/beta-catenin signaling in nasopharyngeal carcinoma cells. Biosci. Rep. 39, pii:BSR20180906 10.1042/BSR20180906 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Li Q.Y., Chen L., Hu N. and Zhao H. (2018) Long non-coding RNA FEZF1-AS1 promotes cell growth in multiple myeloma via miR-610/Akt3 axis. Biomed. Pharmacother. 103, 1727–1732 10.1016/j.biopha.2018.04.094 [DOI] [PubMed] [Google Scholar]