Abstract
Many signaling pathways, including the JAK/STAT3 pathway, are aberrantly activated and associated with ovarian cancer growth and progression. However, inhibition of STAT3 pathway alone was not sufficient to effectively block human ovarian cancer cell survival in vitro, which could be due to the activation and compensation of multiple survival pathways. In this study, we investigated a strategy that can enhance antitumor activity of JAK/STAT3 inhibitor by combining with inhibitors targeting other growth and survival pathways. We found that the in vitro activity of JAKi was remarkably increased when additional survival pathway was blocked. Blocking SRC pathway with SRC inhibitor (SRCi) increased the efficacy of JAKi more effectively than blocking AKT or MAPK pathway. The increased activity of JAKi in combination with SRCi is synergistic and associated with attenuation of p-STAT3, p-SRC, p-AKT and p-MAPK and increased inhibition of p-AKT. Simultaneous blockade of multiple survival pathways by combining JAKi with both AKT inhibitor (AKTi) and MEK inhibitor (MEKi) also resulted in a synergistic inhibition of cell survival. Furthermore, the combined treatment of JAKi and SRCi led to an increased apoptosis and greater inhibition of tumor growth and ascites formation. Taken together, our results demonstrate that the antitumor efficacy of JAKi is improved most effectively when combined with SRCi, providing a potential combination strategy for the treatment of advanced ovarian cancer.
Introduction
The JAK/STAT3 pathway has emerged as an attractive target for cancer treatment. STAT3 is a member of the STAT family of transcription factors that mediate cellular responses to cytokines and growth factors. In healthy tissue, STAT3 is predominantly located in the cytoplasm in an inactive form. However, in response to cytokine stimulation, STAT3 is phosphorylated at Tyr705 by Janus family kinases (JAK) [1], [2] and translocated into the nucleus where it binds DNA and activates the transcription of various genes that regulate vital cellular functions, including cell survival, proliferation, angiogenesis, and tumor evasion [3]. In contrast to normal cells, where activation of STAT3 is tightly regulated and transient, STAT3 is frequently constitutively activated in cancer cells [3], [4], [5], [6], [7]. Persistent activation of STAT3 is associated with a poor prognosis in cancer patients, including ovarian cancer patients [8], [9].
Several recent studies have demonstrated a critical role of STAT3 in ovarian cancer growth and progression. For example, elevated levels of IL-6 in serum, ascites, and malignant cancer cells correlate with poor overall patient survival [8], [10], [11], [12], [13]. Additionally, inhibition of STAT3 activation leads to reduced tumor growth, decreased peritoneal dissemination, and diminished ascites production in a peritoneal ovarian tumor model [14], [15], [16]. Although JAK/STAT3 pathway can be effectively suppressed with JAK inhibitor (JAKi) at doses lower than100 nM, the effect of JAKi on cell survival was relatively weaker [15]. One possible explanation for this is that multiple survival pathways are activated in ovarian cancer cells, and therefore, suppressing a single pathway may not be sufficient to suppress cell growth in vitro due to compensation by other survival pathways.
In this study, we studied the contribution of multiple survival pathways to ovarian cancer cell viability in response to STAT3 inhibition. Our results demonstrate that the limited in vitro activity of JAK inhibitor (JAKi) in ovarian cancer cells can be enhanced through combination with inhibitors of other survival pathways. We find that blocking the SRC pathway increased antitumor activity of JAKi more effectively than blocking AKT or MAPK pathway.
Materials and Methods
Reagents
JAKi, AZD1480, was kindly provided by AstraZeneca. SRCi, saracatinib, and dasatinib were purchased from LC lab. MK2206 and AZD6244 were purchased from Selleck Chemicals. Antibodies against p-STAT3 (Y705), STAT3, p-MAPK (T202/Y204), MAPK, p-AKT (S473), p-SRC (Y416), SRC, p-JAK2 (Y1007/1008), JAK2, PARP, Caspase 3, and GAPDH were obtained from Cell Signaling Technology (Danvers, MA). The antibody against AKT was purchased from Santa Cruz Biotechnology (Dallas, TX). The antibody against actin was purchased from Sigma (St. Louis, MO).
Cell Culture
SKOV3, MDAH2774, CaOV3, and OVCAR 3 cells were obtained from ATCC. OVCAR-8 cells were obtained from the National Cancer Institute. SKOV3 and MDAH2774 cells were cultured in DMEM. OVCAR3, CaOV3, and OVCAR-8 cells were cultured in RPMI1640 medium. Culture media were supplemented with 10% FBS and 1% penicillin/streptomycin (P/S). All cells were grown in 5% (v/v) CO2 at 37 °C.
Cell Viability Assays
Cells (4000 per well for all cells except 7000 per well for MDAH2774) were plated in 96-well plate format in 100 μl growth medium. Cells were treated with DMSO or drugs the next day at the indicated concentrations and incubated for an additional 2-3 days. Viable cells were determined either by the MTS assay (Promega, Madison, WI) or the acid phosphatase assay as described previously [17]. For the MTS assay, 25 μl MTS solution was added directly into each well according to the manufacturer's instructions. For the acid phosphatase assay, all media were removed; p-nitrophenyl phosphate substrate (10 mM 100 μl) was added into each well and incubated at 37 °C for 45 minutes. NaOH was added to stop the reaction, and the absorbance was read at 415 nM. The IC50 was determined using Calcusyn software (Biosoft, Ferguson, MO).
Determination of Combination Index (CI)
Statistical analysis of synergy was used to evaluate the effect of combined treatment. A CI for synergy was determined by comparing the antiproliferative effect of JAKi or SRCi alone with that of the combination of both drugs. CI was determined using the Chou-Talalay method [18] using the Calcusyn software (Biosoft). CI values were calculated for the effective doses ED50, ED75, and ED90. A CI <1 indicates synergy (CI = 0.3-0.7, synergy; CI = 0.1-0.3, strong synergy; CI<0.1, very strong synergy), CI >1 indicates antagonistic interactions, and a CI value = 1 indicates additive effect.
Western Blot Analysis
Western blots were performed as described previously [15], [19]. Cells were grown in complete medium overnight and treated with DMSO or drugs at various concentrations for 24 hours. Cells were washed in ice-cold PBS and lysed in RIPA lysis buffer (Thermo Scientific) containing Halt protease and phosphatase inhibitors (Thermo Scientific). Proteins were quantified using BCA protein assay reagent (Thermo Scientific). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and incubated with total and phosphorylated protein-specific antibodies. Binding of primary antibody was detected using a horseradish peroxidase–conjugated secondary antibody and chemiluminescent substrates (Thermo Scientific).
Annexin V Staining
Apoptosis was measured using the annexin V apoptosis detection kit (BD Bioscience). Briefly, ovarian cancer cells were treated with JAKi, SRCi, or both compounds. After 48 hours, both floating and attached cells were collected and stained with FITC-annexin V and PI (propidium iodide). The staining intensity was then quantified using fluorescence-activated cell sorting.
Transfection with Small Interfering RNA (siRNA)
RNAiMAX (Invitrogen) was used to transfect siRNA according to the manufacturer's instructions. After 24 hours of incubation with RNAi (Santa Cruz Biotechnology), cells were treated with drugs as described in Results.
Animal Models
All animal studies were carried out under protocols approved by the Institutional Animal Care and Use Committee at City of Hope in accordance with all applicable federal, state, and local requirements and institutional guidelines. MDAH2774 cells (5 × 106 in 100 μl) were inoculated into peritoneal cavity of 6- to 8-week-old female athymic nude mice (National Cancer Institute). Animals were randomized into groups with 10 mice for each group starting 1 week after inoculation. Mice were then treated by oral gavage with vehicle, AZD1480 (20 mg/kg daily), dasatinib (15 mg/kg daily), or a combination of both agents. The doses for these two drugs were chosen based on previously published studies [15], [20]. The mice were monitored for ascites production and any adverse effect, then euthanized 3-5 weeks after cell inoculation. Visible tumor nodules were excised and weighed, and the ascites fluid was collected and measured for volume.
Statistical Analysis
Data are presented as mean ± S.D. Student's t test was used to compare the means of two groups. All experiments were carried out at least in triplicate. P < .05 was considered statistically significant.
Results
Effects of JAKi on Signaling Pathways in Human Ovarian Cancer Cells
When JAK inhibitor (JAKi) AZD1480 was tested for its effect on cell viability in various cancer cells, including human ovarian cancer cell lines MDAH2774, OVCAR 8, and SKOV3, the IC50s were found to range from 1.57 μM to 14.96 μM for inhibition of cell viability, much higher than the IC50 for inhibition of STAT3 phosphorylation [15].
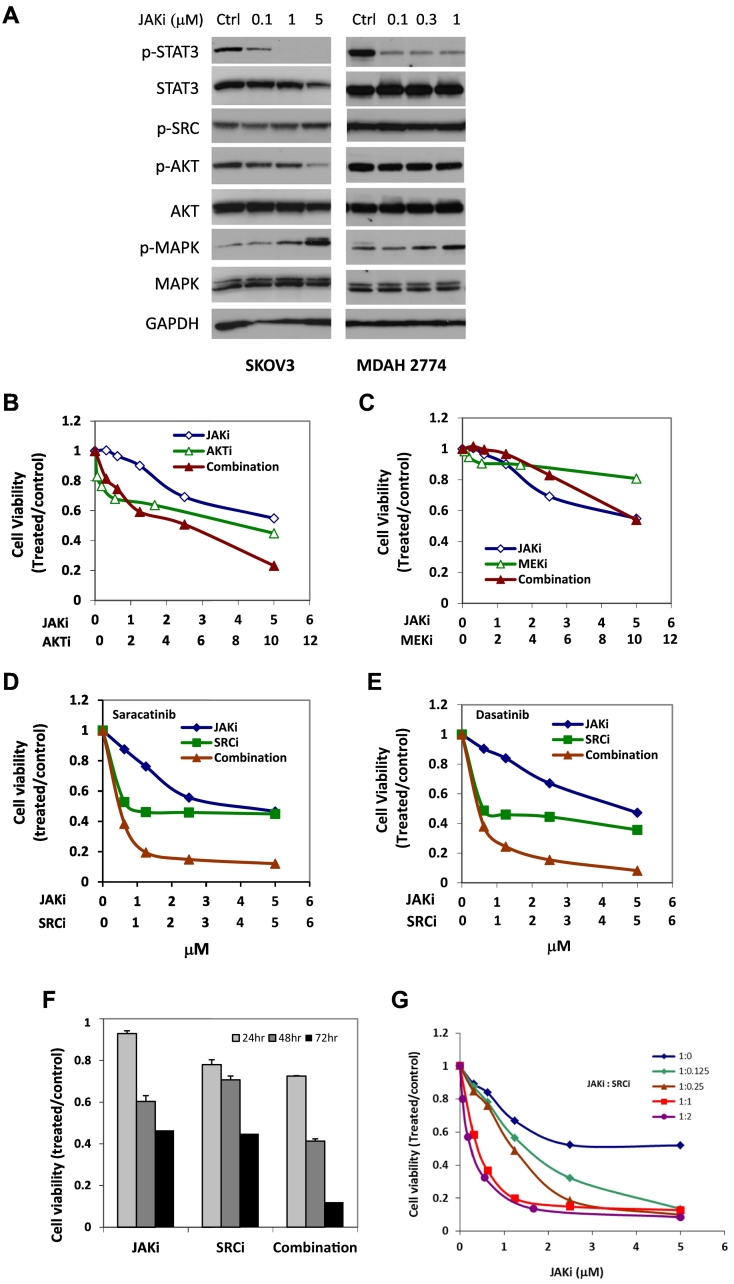
The limited inhibitory effect of JAKi on cell survival could be due to the activation and compensation of multiple survival pathways in vitro. A number of survival pathways are persistently activated in ovarian cancer cells, including AKT, MAPK, SRC, and STAT3 signaling [21]. To understand the effect of inhibiting STAT3 pathway on these signaling pathways in ovarian cancer cells, we incubated SKOV3 and MDAH2774 cells with increasing concentrations of JAKi (0.1-5 μM) followed by Western blot analysis. Our results demonstrated that JAKi significantly decreased phosphorylation of STAT3 in a dose-dependent manner, but we also observed increased phosphorylation of MAPK in both cell lines. Phosphorylation of AKT was decreased in SKOV3 cells but not in MDAH2774 cells. Phosphorylation of SRC remained unchanged in both cell lines (Figure 1A). These results suggest that the efficacy of JAKi in human ovarian cancer cells could be attenuated by other survival pathways.
Figure 1.
Suppressing additional survival pathway enhances the antitumor activity of JAKi in human ovarian cancer cells. (A) Effect of JAKi on downstream signaling pathways in ovarian cancer cells. Human ovarian cancer cells, SKOV3 and MDAH2774, were treated with the indicated concentrations of JAKi for 24 hours and tested for the expression of p-STAT3, p-AKT, p-SRC, and p-MAPK by Western blot. GAPDH and actin were used as a loading control. (B-E) Combination of JAKi and a small molecule inhibitor of other survival pathways in SKOV3 cells. SKOV3 cells were treated with JAK inhibitor (AZD1480) or AKT inhibitor (MK2206), MEK inhibitor (AZD6244), and SRC inhibitor (dasatinib and saracatinib), either alone or in combination, at various concentrations. Cell viability was determined 72 hours later. (F) SKOV3 cells were treated with JAKi (5 μM) or SRCi (5 μM) either as single agent or in combination. Cell viability was determined 24 hours, 48 hours, and 72 hours later. (G) SKOV3 cells were treated with JAKi or SRCi, alone or in combination, at various molar ratios. Cell viability was determined 72 hours later. Results are representative of three or more preparations.
Combination Treatment by Targeting JAK/STAT3 Pathway with Other Survival Pathways
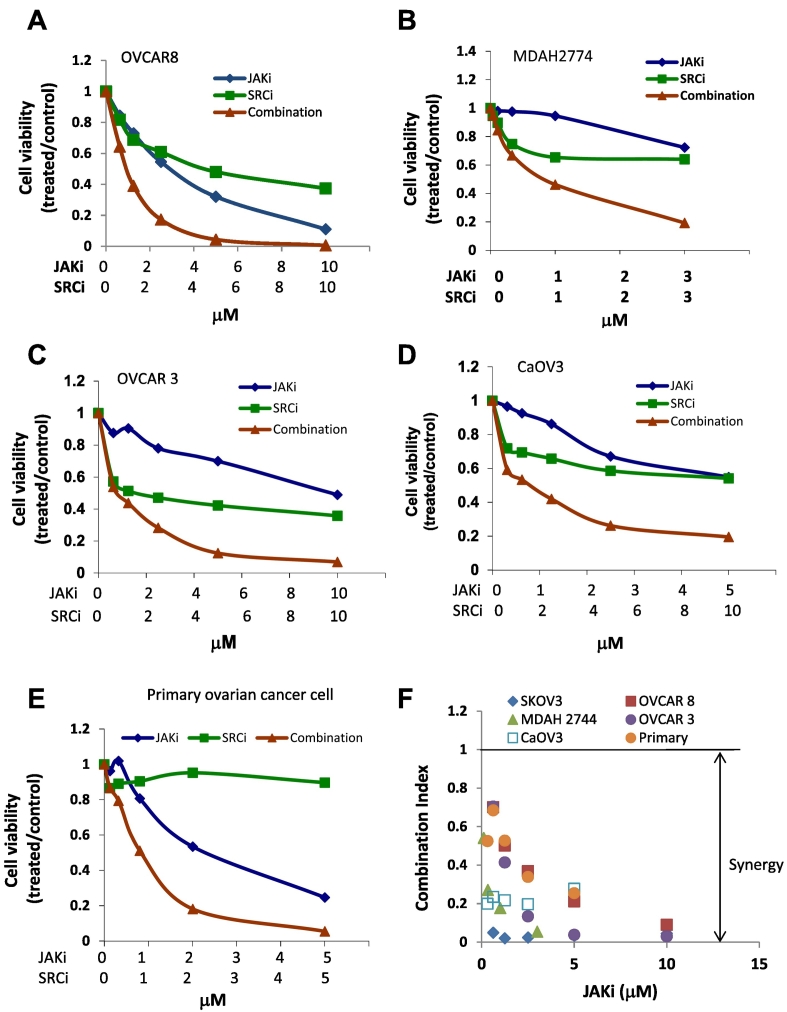
To understand whether inhibiting other survival pathways could increase the sensitivity of human ovarian cancer cells to JAKi, we tested the effect of JAKi on cell viability in SKOV3 ovarian cancer cells, either alone or in combination with AKT inhibitor (MK2206), MEK inhibitor (AZD6244), or SRC inhibitor (dasatinib and saracatinib), at various concentrations in a fixed molar ratio. The CI was determined using the Chou-Talalay method (CI = 1, additive effect, CI < 1, synergism; CI > 1, antagonism). The combination treatment of JAKi and SRCi decreased cell viability much more robustly than either agent alone with synergy to very strong synergy (CI < 0.1), but inhibition of MEK with AZD2206 did not significantly reduce the IC50 of JAKi in SKOV3 cells. Although MK2206, an AKT inhibitor, also synergistically increased JAKi sensitivity in SKOV3 cells, this inhibitor was less effective than SRCi (Figure 1, B-E, Table 1). The concentration of JAKi that gave 50% inhibition in SKOV3 cells decreased by about 25-fold and 43-fold, respectively, by including dasatinib or saracatinib in the treatment (Figure 1 and Table 1). These results suggest that inhibiting the SRC pathway was more effective than inhibiting AKT and MEK signaling pathways in sensitizing these human ovarian cancer cells to JAKi. The enhanced antitumor activity by combining JAKi and SRCi was time-dependent, with the strongest inhibition seen at 72 hours (Figure 1F), and ratio dependent, with strongest inhibition and synergy seen at 1:1 or 1:2 JAKi:SRCi molar ratio (Figure 1G, Table 2) in SKOV3 cells. The combined treatment of JAKi and SRCi was also investigated in other human ovarian cancer cells, including OVCAR 8, MDAH2774, OVCAR 3, CaOV3, as well as primary ovarian cancer cells isolated from ascites of ovarian cancer patients [15]; synergy to very strong synergy (CI < 0.1) was observed in these cell lines (Figure 2, Table 1).
Table 1.
Effects of Combining JAK Inhibitor (AZD1480) with SRC Inhibitor (Dasatinib and Saracatinib), AKT Inhibitor (MK2204), and MEK Inhibitor (AZD6244) on the Viability of Ovarian Cancer Cells
| Inhibitors Combined with JAKi | Fold Reduction |
CI |
||||
|---|---|---|---|---|---|---|
| IC50 | IC90 | ED50 | ED75 | ED90 | ||
| SKOV3 | SRCi (Dasatinib) | 25.32 | 31.81 | 0.53 | 0.08 | 0.03 |
| SRCi (Saracatinib) | 43.17 | 29.62 | 0.27 | 0.03 | 0.03 | |
| AKTi (MK2206) | 4.24 | 1.31 | 0.48 | 0.72 | 1.67 | |
| mTORi (RAD001) | 6.29 | 1.86 | 0.19 | 0.29 | 0.54 | |
| MEKi (AZD6244) | 0.50 | 0.85 | 2.21 | 2.33 | 2.54 | |
| OVCAR8 | SRCi (Dasatinib) | 2.26 | 2.53 | 0.62 | 0.59 | 0.56 |
| SRCi (Saracatinib) | 2.54 | 4.52 | 0.59 | 0.37 | 0.25 | |
| AKTi (MK2206) | 2.07 | 1.24 | 0.72 | 0.90 | 1.13 | |
| MEKi (AZD6244) | 1.77 | 1.08 | 0.57 | 0.72 | 0.92 | |
| MDAH2774 | SRCi (Dasatinib) | 8.41 | 1.24 | 0.27 | 0.47 | 0.80 |
| SRCi (Saracatinib) | 4.09 | 5.55 | 0.29 | 0.34 | 0.39 | |
| AKTi (MK2206) | 2.18 | 2.39 | 0.63 | 0.47 | 0.42 | |
| MEKi (AZD6244) | 9.33 | 3.31 | 0.21 | 0.18 | 0.30 | |
Table 2.
Synergistic Interaction Between JAKi and SRCi in Variety of Molar Ratios on the Viability of SKOV3 Cells
| Ratio (JAKi:SRCi) | CI |
IC50 (μM) |
IC90(μM) |
||||
|---|---|---|---|---|---|---|---|
| ED50 | ED75 | ED90 | JAKi | SRCi | JAKi | SRCi | |
| 1:2 | 0.63 | 0.061 | 0.055 | 0.26 | 0.52 | 3.19 | 6.38 |
| 1:1 | 0.46 | 0.087 | 0.086 | 0.35 | 0.35 | 4.96 | 4.96 |
| 4:1 | 0.58 | 0.153 | 0.082 | 1.11 | 0.28 | 4.74 | 1.19 |
| 8:1 | 0.55 | 0.208 | 0.119 | 1.42 | 0.18 | 6.89 | 0.86 |
Figure 2.
Suppressing the SRC pathway enhances the antitumor activity of JAKi in several human ovarian cancer cell lines. OVCAR8 (A), MDAH2774 (B), OVCAR 3 cells (C), CaOV3 cells (D), and primary ovarian cancer cells (E) were treated with JAKi or SRCi (saracatinib) either alone or in combination. Cell viability was determined 72 hours later. (F) Synergistic interaction between JAKi and SRCi on the viability of various ovarian cancer cells.
Effects of Combination Treatment with JAKi and SRCi on Apoptosis
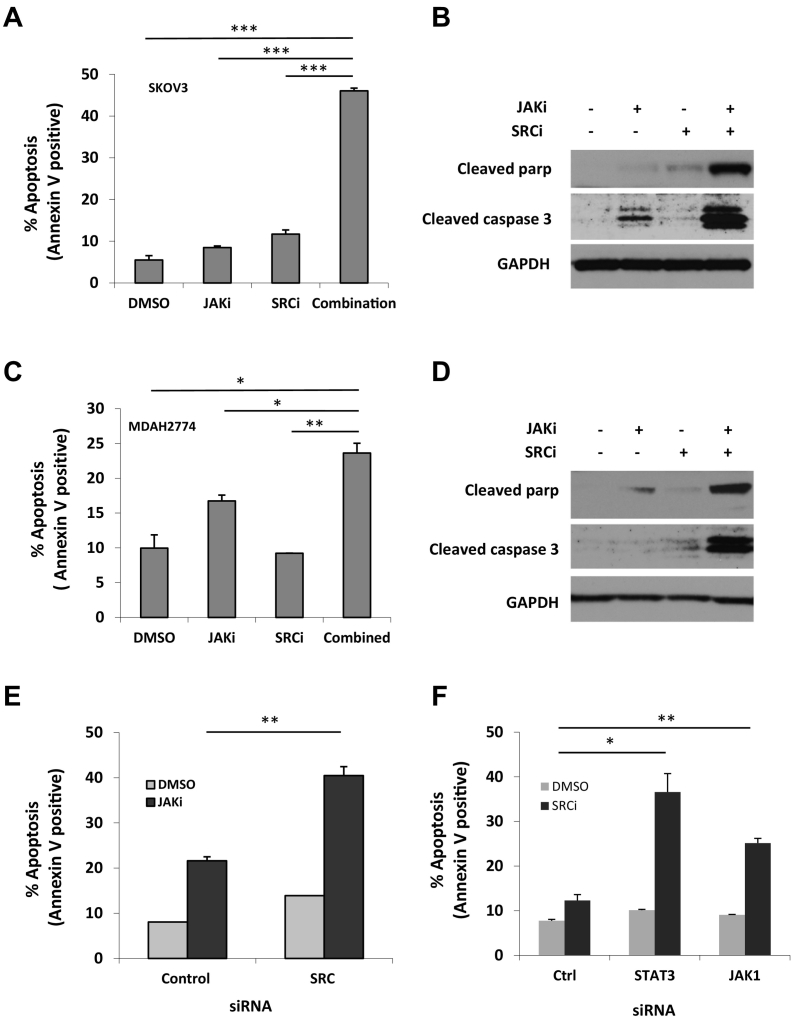
Next, we determined whether the synergistic effects of JAKi and SRCi were due to the induction of apoptosis. We treated cells with JAKi and SRCi either alone or in combination for 48 hours and determined the number of apoptotic cells by annexin V staining. As shown in Figure 3, JAKi-induced apoptosis increased from 8.5% to 46.1% in SKOV3 cells and from 16.1% to 24.6% in MDAH2774 cells when combined with SRCi (Figure 3, A and C). Consistent with the annexin V staining results, more cleaved caspase 3 and cleaved poly-ADP ribose polymerase (PARP), two molecular markers for apoptosis, were generated in cells that were treated with both JAKi and SRCi (Figure 3, B and D). These results indicate that inhibition of the SRC pathway could effectively enhance the sensitivity of these human ovarian cancer cells to JAKi by promoting apoptosis.
Figure 3.
Suppressing SRC signaling enhances JAKi-induced apoptosis in human ovarian cancer cells. SKOV3 (A & B) and MDAH2774 (C & D) cells were treated with JAKi and SRCi, either alone or in combination, for 48 hours. Apoptosis was determined by flow cytometry using Annexin V and PI staining (A & C) or by Western blot for cleaved poly-ADP ribose polymerase (PARP) and cleaved caspase-3 by (B & D). *, P < .05, **, P < .005; ***, P < .0005, combination vs. JAKi alone or SRCi alone. (E) SKOV3 cells were transfected with siRNA against SRC or control siRNA and treated with JAKi (5 μM) or (F) transfected with siRNA against JAK1 or STAT3 and treated with SRCi (saracatinib,10 μM). After 48 hours, cells were harvested and evaluated for apoptosis using annexin V staining. *, P < .05; **, P < .005, vs. control siRNA.
Effect of JAKi on the Viability of Cells with SRC Knockdown
To further understand whether inhibition of the SRC pathway could increase the sensitivity of human ovarian cancer cells to JAKi, we tested the effect of JAKi on apoptosis in SKOV3 cells in which SRC was knocked down by siRNA. As shown in Figure 3E, JAKi-induced apoptosis increased from 21.6% to 40.5% when cells were transfected with SRC siRNA. This result further demonstrates that inhibition of SRC pathway is an effective way to enhance JAKi activity in ovarian cancer.
Similarly, we also investigated whether knockdown of JAK/STAT3 expression could increase SRCi-induced apoptosis. Our previous studies showed that depletion of JAK1, but not JAK2, abolished phosphorylation of STAT3 in SKOV3 and MDAH2774 cells, suggesting that JAK1 is a major kinase responsible for STAT3 phosphorylation in these two cell lines [15]. Here, we examined the sensitivity of ovarian cancer cells to SRCi when the JAK/STAT3 pathway was depleted either with JAK1 siRNA or STAT3 siRNA. In response to SRCi treatment, the number of apoptotic cells significantly increased from 12.3% in cells transfected with a control siRNA to 22.9% or 30.1% in cells transfected with siRNA against JAK1 or STAT3, respectively (Figure 3F), suggesting that inhibition of JAK1 could potentiate SRCi-induced apoptosis in ovarian cancer.
Effects of Combined JAKi and SRCi Treatment on Downstream Signaling Pathways
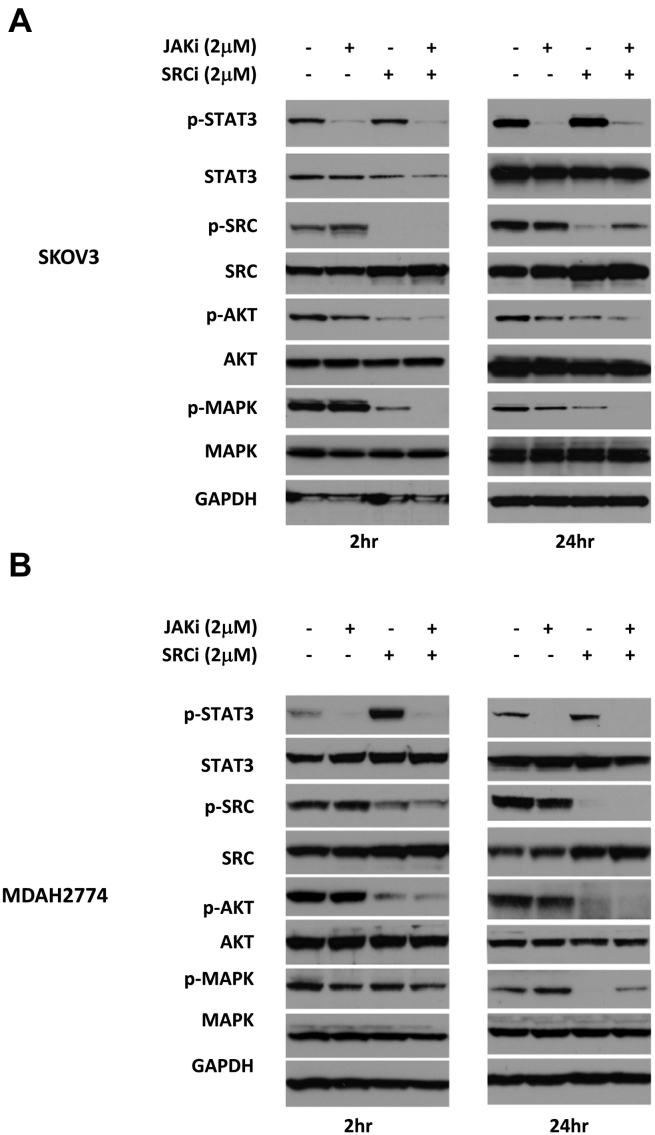
To understand the molecular mechanism underlying this synergistic effect, we evaluated the molecular changes in ovarian cancer cells in response to treatment with JAKi and SRCi either alone or in combination. A number of signaling pathways, including MEK/MAPK, SRC, PI3K/AKT, and JAK/STAT3 pathways, are constitutively activated and play important roles in the growth and progression of ovarian cancer. To study the effect of JAKi and SRCi on these signaling pathways, we treated SKOV3 cells and MDAH2774 cells with JAKi and SRCi either alone or in combination for 2 hours or 24 hours, and tested for the expression of p-STAT3, p-SRC, p-AKT, and p-MAPK by Western blot. As shown in Figure 4, treatment with JAKi alone inhibited p-STAT3 as expected in both cell lines at 2 hours and 24 hours but had little or no inhibitory effect on p-SRC, p-AKT, or p-MAPK. Treatment with SRCi alone resulted in a decreased level of p-SRC, p-AKT, and p-MAPK in both cell lines at 2 hours and 24 hours. The combined treatment with both JAKi and SRCi led to inhibition of p-STAT3, p-SRC, p-AKT, and p-MAPK (Figure 4). The inhibition of p-AKT caused by combined treatment was considerably greater compared to any single treatment in both cell lines. Taken together, these results demonstrate that combined targeting of both JAK/STAT3 and SRC pathways inhibit multiple survival pathways and result in a greater inhibition of p-AKT.
Figure 4.
Combination treatment with JAKi and SRCi attenuates of multiple signaling pathways. SKOV3 (A) and MDAH2774 (B) cells were treated with JAKi, SRCi, or the combination for 2 hours and 24 hours and analyzed by Western blot. GAPDH was used as a loading control.
Effects of Combined JAKi, AKTi, and MEKi Treatment on Cell Viability
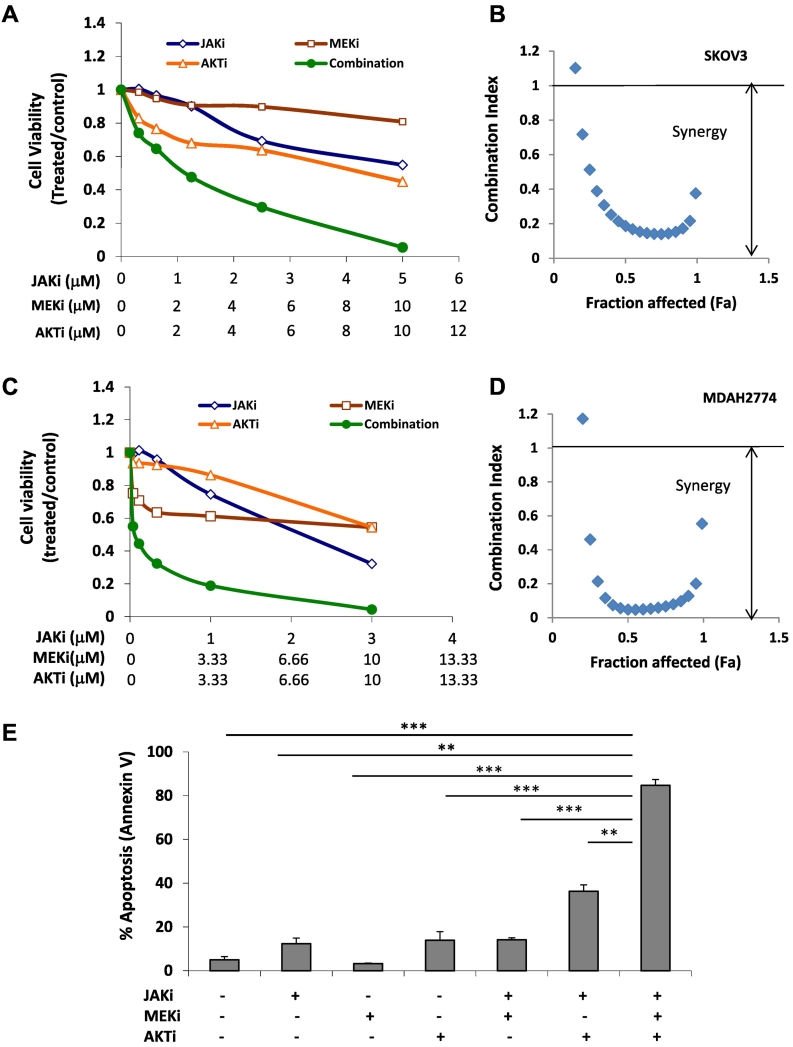
Because the combination of JAKi and SRCi led to simultaneous inhibition of AKT and MEK pathways, we next investigated whether the combined targeting of these three pathways, JAK/STAT3, AKT, and MEK/MAPK, could achieve a similar result. We treated SKOV3 and MDAH2774 cells with JAKi either alone or in combination with both AKT inhibitor (MK2206) and MEK inhibitor (AZD6244) at various concentrations. As shown in Figure 5, combined treatment of all three inhibitors led to a stronger inhibition of cell viability in both cells line with very strong synergy (CI < 0.1). To further study the effect of triple treatment on cell viability, we treated SKOV3 cells with JAKi along with both AKTi and MEKi. The number of apoptotic cells was determined by annexin V staining 48 hours later. JAKi-induced apoptosis increased from 12.34% to 36.32% when combined with AKTi, from 12.34% to 14.16% when combined to MEKi, and from 12.34% and to 84.73% when combined with both AKTi and MEKi. These results further support that inhibition of multiple survival pathways, such as JAK/STAT3, AKT and MEK/MAPK, in ovarian cancer cells is more potent than inhibition of any single pathway.
Figure 5.
The antitumor activity of JAKi in combination with both AKT and MEK inhibitor is enhanced human ovarian cancer cells. SKOV3 (A and B) and MDAH2774 (C and D) cells were treated with JAKi (AZD1480), AKTi (MK2206), or MEKi (AZD6244), either alone or in combination, at various concentrations. Cell viability was determined 72 hours later. (B and D) Synergistic interaction between JAKi and AKTi /MEKi on the viability of ovarian cancer cells. (E) SKOV3 cells were treated with JAKi (AZD1480, 2 μM), AKTi (MK2206, 10 μM), or MEKi (AZD6244, 10 μM), either alone or together, for 48 hours. Apoptosis was determined by flow cytometry using annexin V and PI staining. **, P < .005; ***, P < .0005, combination vs. control, alone or combination of two.
Effect of Combination Treatment on Ovarian Cancer Growth in Mice
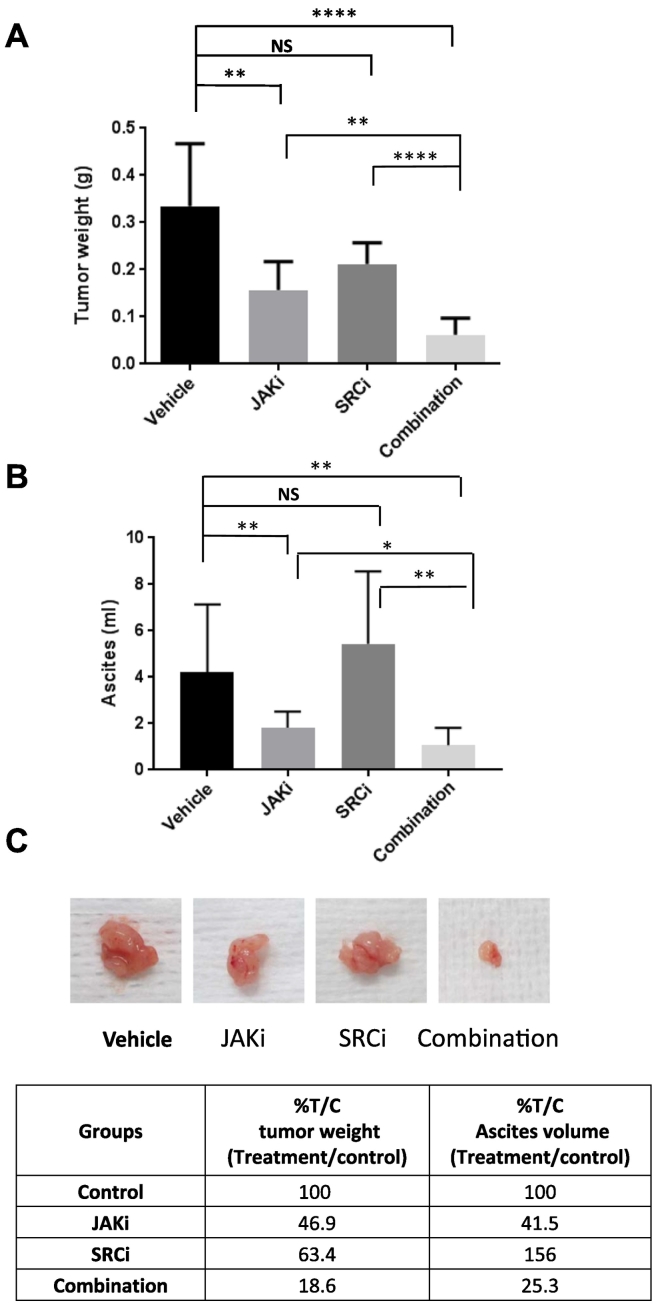
Next, we investigated whether the combination treatment could suppress tumor growth in vivo more effectively than either treatment alone. NSG mice were inoculated intraperitoneally with MDAH274 ovarian cancer cells. One week after inoculation, we randomized mice into four groups and treated with vehicle control, JAKi, SRCi (dasatinib), or JAKi plus SRCi through oral gavage. No toxicity was observed in mice with any of the treatments, whether the drugs were used alone or in combination, as indicated by absence of significant (>5%) change in body weight (not shown). Treatment with SRCi alone was not very effective; the tumor weight was reduced from 0.33 g to 0.21 g, but not significantly. In fact, the ascites volume increased from 4.21 ml to 6.58 ml (Figure 6). Treatment with JAKi alone at a daily dose of 20 mg/kg decreased the tumor burden from 0.33 g to 0.16 g and ascites volume from 4.21 ml to 1.75 ml. However, the combination treatment further decreased the tumor weight to 0.06 g and ascites volume to 1.07 ml (Figure 6A), suggesting that the combination treatment was more effective than any single treatment.
Figure 6.
SRCi increases the antitumor activity of JAKi in mice. (A-C) MDAH2774 cells were implanted into the peritoneal cavity of female athymic nude mice. Tumors were treated daily with vehicle, JAKi (20 mg/kg), SRCi (15 mg/kg), or their combination by oral gavage. Mice were euthanized 4 weeks later. The tumor nodules throughout the peritoneal cavity were excised and weighed (A). Ascites were collected, and the volumes were measured (B). Representative tumors were photographed (C). The percentage of treated compared to vehicle controlled were calculated (C). Data represents means ± SD (n = 4-10).*, P < .05, **, P < .005, ****, P < .0001, NS, not significant.
Discussion
Inhibition of STAT3 pathway alone does not appear to be sufficient to effectively suppress human ovarian cancer cell viability in vitro [15]. This could be due to the compensation by alternative growth/survival pathways that are often activated in ovarian cancer cells. In this study, we investigated a strategy that can enhance antitumor activity of JAK/STAT3 inhibitor by combining with inhibitors targeting other growth and survival pathways, such as SRC, AKT, and MAPK. Our results demonstrated that blocking the SRC pathway with SRC inhibitors, dasatinib or saracatinib, more effectively increased the antitumor activity of JAK/STAT3 inhibitor than blocking the AKT or MAPK pathways. Combination treatment of SRCi and JAKi resulted in greater inhibition of AKT, indicating the critical role of these interconnected signaling proteins.
SRC family kinases (SFK) are a family of nonreceptor tyrosine kinases that includes nine members: C-SRC, FYN, YES, LYN, LCK, HCK, BLK, EGR, and YRK [22]. In healthy tissue, SRC is predominantly inactive. SRC becomes active in response to cytokine stimulation, and its activation is tightly regulated and transient. However, in cancer cells, SRC can become constitutively active [22], [23], [24]. Increased SRC activity has been found in ovarian cancer cell lines and late-stage, poor-prognosis ovarian tumors [25], [26]. The aberrant activated SRC is important in tumor growth and metastasis by promoting cell proliferation, survival, migration, invasion, and angiogenesis via multiple signaling pathways, including PI3K/AKT, MEK/MAPK, and STAT3 [23], [27]. Preclinical studies have demonstrated antitumor activity of SRC inhibitors in a number of tumors, including prostate, colon, breast, and ovarian [20], [28], [29]. Several pharmacologic SRC inhibitors, dasatinib (BMS-354825; Bristol-Myers Squibb), saracatinib (AZD0530; AstraZeneca), and bosutinib (SKI-606; Wyeth/Pfizer), have been evaluated in clinical trials for the treatment of various solid tumors including ovarian cancer, either as a single agent or in combination with carboplatin and paclitaxel [26], [30], [31]. However, these inhibitors have shown limited activity [32]. Dasatinib has shown little activity as a single agent for recurrent high-grade serous ovarian cancer, with only 21% of patients progression-free after 6 months [33], [34]. Saracatinib has shown no additive benefit when combined chemotherapy in recurrent platinum-resistant ovarian cancer. The limited antitumor activity of SRC inhibitor could be due to the compensation of other survival pathways [24]. It has been shown that the sustained inhibition of SRC results in reactivation of STAT3 after initial inhibition in multiple cancer cell types, including advanced head and neck squamous cell carcinoma, mesothelioma, squamous cell skin carcinoma cell lines, and non–small cell lung cancer. Suppression of STAT3 reactivation increases cell death induced by SRC inhibition in these cells [35], [36], [37], [38]. It has also been shown that inhibition of SRC leads to the activation of MEK/MAPK pathway, and dual inhibition of both SRC and MEK has a more effective antitumor activity in ovarian cancer [39]. Here, we demonstrated that inhibition of JAK/STAT3 pathway increases sensitivity of ovarian cancer cells to SRC inhibition. In fact, blocking JAK/STAT3 pathway increases antitumor activity of SRCi more effectively than blocking AKT or MAPK pathway in human ovarian cancer cells.
In human ovarian cancer cells, the phosphorylation of STAT3 can be inhibited by JAK inhibitor, but not by SRC inhibitor (dasatinib), or other kinase inhibitors, such as EGFR inhibitor (gefitinib), multiple kinase inhibitor (sunitinib) and mTOR inhibitor (RAD001) [15]. Our previous results demonstrated that JAK1 is a critical kinase responsible for the persistent activation of STAT3 in human ovarian cancer cells. Consistent with previous results, the present study also demonstrated that inhibition of SRC pathway had negligible effect on STAT3 phosphorylation, and inhibition of STAT3 has negligible effect on SRC pathway either. Combined treatment with both JAKi and SRCi leads to the inhibition of both pathways and much stronger antitumor activity in human ovarian cancer cells.
Mutations in ovarian cancer are rare [40], [41], [42]. Instead, the concurrent activation of multiple signaling pathways, including PI3/AKT, SRC, MEK/MAPK and JAK/STAT3, appears to be more common in ovarian cancer and may play an important role in the ovarian tumor growth. Inhibition of each single pathway was not sufficient to effectively block ovarian cancer growth and survival. Although JAKi blocks activation of STAT3, other survival pathways remain active, which could compromise the antitumor activity of JAKi in vitro. Combination treatment targeting JAK/STAT3 pathway with one of other survival pathways, including AKT/mTOR, MEK/MAPK, or SRC, results in an increased antitumor activity compared to targeting JAK/STAT3 alone. The combined targeting of both JAK/STAT3 and SRC pathways results in blockade of multiple survival pathways, including p-STAT3, p-SRC, p-AKT, and p-MAPK, and dramatically increased antitumor activity both in vitro and in vivo. Consistent with this result, combined targeting of three pathways, JAK/STAT3, AKT, and MEK/MAPK, also causes a greater antitumor activity with a very strong synergy (CI < 0.1). Therefore, concurrent activation of multiple signaling pathways might be the critical force that drives ovarian cancer cells to proliferate and survive. If this is the case, simultaneous blockade of multiple survival pathways may be required to achieve maximum antitumor activity.
Although our present study suggests a possible mechanism for limited activity of inhibiting the STAT3 pathway in vitro, suppression of the JAK/STAT3 pathway could inhibit tumor progression through regulating the tumor microenvironment in vivo. Activation of STAT3 has been shown to play a critical role in promoting immunosuppressive tumor microenvironment during tumor progression [3], [43]. Myeloid-derived suppressor cells and M2 tumor-associated macrophages are often linked to STAT3 mediated immunosuppressive tumor microenvironment [3]. Endothelial cells are another important cell type in the microenvironment [44]. It has been reported that activated STAT3 upregulates VEGF levels in human cancer cells and induce angiogenesis, the formation of new blood vessel, which is critical for tumors to grow and progress [4].
Conclusions
Taken together, our results demonstrate that the antitumor activity of JAK/STAT3 inhibitor can be improved with blockade of additional survival pathways. Blocking SRC pathway with SRCi increased the efficacy of JAKi more effectively than blocking AKT or MAPK pathway. The increased activity of JAKi in combination with SRCi is synergistic, leading to simultaneous attenuation of multiple survival pathways, increased inhibition of p-AKT and a greater inhibition of tumor growth and ascites formation. This study may provide a potential combination therapeutic strategy for the treatment of advanced ovarian cancer.
Acknowledgments
Acknowledgements
We thank AstraZeneca for providing AZD1480; Dr. Chris Gandhi for critical reading of this manuscript; and Lucy Brown of the Analytical Cytometry Core, the Animal Tumor Models Program, for their technical assistance.
Funding
This work was supported by National Institutes of Health grant R01 CA11567405 to R. J. Research reported in this publication included work performed in the Analytical Cytometry, Animal Tumor Models Program, and Small Animal Image Cores supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interest
None.
Contributor Information
Wei Wen, Email: jyim@coh.org.
John H. Yim, Email: jyim@coh.org.
References
- 1.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 2.Darnell, Jr JE. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 6.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 7.Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 8.Rosen DG, Mercado-Uribe I, Yang G, Bast RC, Jr., Amin HM, Lai R, Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–2740. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 9.Anglesio MS, George J, Kulbe H, Friedlander M, Rischin D, Lemech C, Power J, Coward J, Cowin PA, House CM. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res. 2011;17:2538–2548. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 10.Scambia G, Testa U, Benedetti Panici P, Foti E, Martucci R, Gadducci A, Perillo A, Facchini V, Peschle C, Mancuso S. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer. 1995;71:354–356. doi: 10.1038/bjc.1995.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol. 1997;66:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 12.Lane D, Matte I, Rancourt C, Piche A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yigit R, Figdor CG, Zusterzeel PL, Pots JM, Torensma R, Massuger LF. Cytokine analysis as a tool to understand tumour-host interaction in ovarian cancer. Eur J Cancer. 2011;47:1883–1889. doi: 10.1016/j.ejca.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Zhang X, Xu X, Shen L, Yao Y, Yang Z, Liu P. STAT3 decoy oligodeoxynucleotides-loaded solid lipid nanoparticles induce cell death and inhibit invasion in ovarian cancer cells. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0124924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen W, Liang W, Wu J, Kowolik CM, Buettner R, Scuto A, Hsieh MY, Hong H, Brown CE, Forman SJ. Targeting JAK1/STAT3 signaling suppresses tumor progression and metastasis in a peritoneal model of human ovarian cancer. Mol Cancer Ther. 2014;13:3037–3048. doi: 10.1158/1535-7163.MCT-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gritsina G, Xiao F, O'Brien SW, Gabbasov R, Maglaty MA, Xu R-H, Thapa RJ, Zhou Y, Nicolas E, Litwin S. Targeted blockade of JAK/STAT3 signaling inhibits ovarian carcinoma growth. Mol Cancer Ther. 2015;14:1035–1047. doi: 10.1158/1535-7163.MCT-14-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang TT, Sinai P, Kain SR. An acid phosphatase assay for quantifying the growth of adherent and nonadherent cells. Anal Biochem. 1996;241:103–108. doi: 10.1006/abio.1996.0383. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Zhang K, Nam S, Anderson RA, Jove R, Wen W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis. 2010;31:481–488. doi: 10.1093/carcin/bgp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S, Yen Y, Lee F, Yu H, Wen W. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–935. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan S, Coward JI, Bast RC, Jr., Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 23.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33:122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, Bast RC, Mills GB, Gallick GE. Activated Src protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88:73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 26.Konecny GE, Glas R, Dering J, Manivong K, Qi J, Finn RS, Yang GR, Hong KL, Ginther C, Winterhoff B. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer. 2009;101:1699–1708. doi: 10.1038/sj.bjc.6605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C, Meyer D, Campbell G, Larner A, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 28.Wiener JR, Nakano K, Kruzelock RP, Bucana CD, Bast RC, Gallick GE. Decreased Src tyrosine kinase activity inhibits malignant human ovarian cancer tumor growth in a nude mouse model. Clin Cancer Res. 1999;5:2164–2170. [PubMed] [Google Scholar]
- 29.Han LY, Landen CN, Trevino JG, Halder J, Lin YG, Kamat AA, Kim T-J, Merritt WM, Coleman RL, Gershenson DM. Antiangiogenic and antitumor effects of src inhibition in ovarian carcinoma. Cancer Res. 2006;66:8633–8639. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teoh D, Ayeni TA, Rubatt JM, Adams DJ, Grace L, Starr MD, Barry WT, Berchuck A, Murphy SK, Secord AA. Dasatinib (BMS-35482) has synergistic activity with paclitaxel and carboplatin in ovarian cancer cells. Gynecol Oncol. 2011;121:187–192. doi: 10.1016/j.ygyno.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpkins F, Hevia-Paez P, Sun J, Ullmer W, Gilbert CA, da Silva T, Pedram A, Levin ER, Reis IM, Rabinovich B. Src Inhibition with saracatinib reverses fulvestrant resistance in ER-positive ovarian cancer models in vitro and in vivo. Clin Cancer Res. 2012;18:5911–5923. doi: 10.1158/1078-0432.CCR-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campone M, Bondarenko I, Brincat S, Hotko Y, Munster PN, Chmielowska E, Fumoleau P, Ward R, Bardy-Bouxin N, Leip E. Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann Oncol. 2012;23:610–617. doi: 10.1093/annonc/mdr261. [DOI] [PubMed] [Google Scholar]
- 33.Schilder RJ, Brady WE, Lankes HA, Fiorica JV, Shahin MS, Zhou XC, Mannel RS, Pathak HB, Hu W, Alpaugh RK. Phase II evaluation of dasatinib in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127:70–74. doi: 10.1016/j.ygyno.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Secord AA, Teoh DK, Barry WT, Yu M, Broadwater G, Havrilesky LJ, Lee PS, Berchuck A, Lancaster J, Wenham RM. A phase I trial of dasatinib, an Src-family kinase inhibitor, in combination with paclitaxel and carboplatin in patients with advanced or recurrent ovarian cancer. Clin Cancer Res. 2012;18:5489–5498. doi: 10.1158/1078-0432.CCR-12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson FM, Saigal B, Tran H, Donato NJ. Abrogation of signal transducer and activator of transcription 3 reactivation after Src kinase inhibition results in synergistic antitumor effects. Clin Cancer Res. 2007;13:4233–4244. doi: 10.1158/1078-0432.CCR-06-2981. [DOI] [PubMed] [Google Scholar]
- 36.Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, Cascone T, Mills GB, Heymach JV, Johnson FM. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15:6852–6861. doi: 10.1158/1078-0432.CCR-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69:1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lue H-W, Cole B, Rao SAM, Podalak J, Gaest A, King C, Eide CA, Wilmot B, Xue C, Spellman P. 2015. Src and STAT3 inhibitors synergize to promote tumor inhibition in renal cell carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahani VM, Yue P, Haftchenary S, Zhao W, Lukkarila JL, Zhang X, Ball D, Nona C, Gunning PT, Turkson J. Identification of purine-scaffold small-molecule inhibitors of Stat3 activation by QSAR studies. ACS Med Chem Lett. 2011;2:79–84. doi: 10.1021/ml100224d. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Hart S, Goh KC, Novotny-Diermayr V, Hu CY, Hentze H, Tan YC, Madan B, Amalini C, Loh YK, Ong LC. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25:1751–1759. doi: 10.1038/leu.2011.148. [DOI] [PubMed] [Google Scholar]
- 41.Jung J-G, Shih I-M, Park JT, Gerry E, Kim TH, Ayhan A, Handschuh K, Davidson B, Fader AN, Selleri L. Ovarian cancer chemoresistance relies on the stem cell reprogramming factor PBX1. Cancer Res. 2016;76:6351–6361. doi: 10.1158/0008-5472.CAN-16-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brasseur K, Gevry N, Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. 2017;8:4008–4042. doi: 10.18632/oncotarget.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. Jak-Stat. 2013;2 doi: 10.4161/jkst.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]