Abstract
Obeticholic acid (OCA), the first FXR-targeting drug, has been claimed effective in the therapy of liver fibrosis. However, recent clinical trials indicated that OCA might not be effective against liver fibrosis, possibly due to the lower dosage to reduce the incidence of the side-effect of pruritus. Here we propose a combinatory therapeutic strategy of OCA and apoptosis inhibitor for combating against liver fibrosis. CCl4-injured mice, d-galactosamine/LPS (GalN/LPS)-treated mice and cycloheximide/TNFα (CHX/TNFα)-treated HepG2 cells were employed to assess the effects of OCA, or together with IDN-6556, an apoptosis inhibitor. OCA treatment significantly inhibited hepatic stellate cell (HSC) activation/proliferation and prevented fibrosis. Elevated bile acid (BA) levels and hepatocyte apoptosis triggered the activation and proliferation of HSCs. OCA treatment reduced BA levels but could not inhibit hepatocellular apoptosis. An enhanced anti-fibrotic effect was observed when OCA was co-administrated with IDN-6556. Our study demonstrated that OCA inhibits HSCs activation/proliferation partially by regulating BA homeostasis and thereby inhibiting activation of HSCs. The findings in this study suggest that combined use of apoptosis inhibitor and OCA at lower dosage represents a novel therapeutic strategy for liver fibrosis.
Abbreviations: ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; BA, bile acid; BrdU, bromodeoxyuridine; BSEP, bile salt export pump; CA, cholic acid; CCl4, carbon tetrachloride; CDCA, chenodeoxycholic acid; CHX, cycloheximide; Col, collagen; CYP7A1, cholesterol 7α-hydroxylase; FXR, farnesoid X receptor; GalN, d-galactosamine; H&E, hematoxylin and eosin; HPLC, high performance liquid chromatography; HSCs, hepatic stellate cells; KCs, Kupffer cells; LPS, lipopolysaccharide; OCA, obeticholic acid; PBC, primary biliary cholangitis; RT-PCR, reverse transcription polymerase chain reaction; SHP, small heterodimer partner; α-SMA, α-smooth muscle action; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinase; TNFα, tumor necrosis factor α; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
KEY WORDS: Obeticholic acid, Liver fibrosis, Bile acid, Hepatocellular apoptosis, IDN-6556, Farnesoid X receptor, Hepatic stellate cell
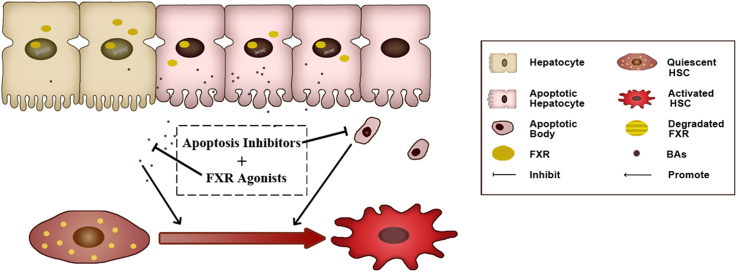
Graphical abstract
Elevated bile acids (BAs) and hepatocyte apoptosis trigger activation/proliferation of hepatic stellate cells, and ultimately promoted the development fibrosis. Treatment of obeticholic acid, a FXR agonist, reduces BA levels but could not inhibit hepatocellular apoptosis. Combined use of apoptosis inhibitor and FXR agonist represents a novel strategy for liver fibrosis.
1. Introduction
Farnesoid X receptor (FXR, NR1H4), a bile acid (BA)-responsive transcription factor1., 2., has been demonstrated to be beneficial for various liver diseases3., 4.. Thus FXR is widely accepted as a promising therapeutic target5 and various FXR modulators have been being developed6., 7., 8.. Obeticholic acid (OCA) is the first FXR modulator that has been licensed by the FDA and EMEA for primary biliary cholangitis (PBC) patients. Besides, trials of OCA in several other liver diseases, including fibrosis, are under way8., 9.. However, the high incidence side-effect of pruritus seen in clinical trials especially at 25 or 50 mg10 limited its wide use in patients. Although this side effect appears to be addressed by the use of lower dose regimes (5–10 mg), its clinical efficiency against fibrosis almost disappeared at this dosage11. Thus, it is urgent to propose a new strategy for the clinical use of OCA with effective anti-fibrotic action and minimal side effects. Previous studies have shown that OCA could prevent the development of fibrosis and cirrhosis by inhibiting hepatic stellate cells (HSCs) activation12., 13.. However, subsequent studies indicated that compared to hepatocytes, HSCs expressed very low levels of FXR14, suggesting that OCA exert anti-fibrotic effect directly through acting on HSC or indirectly through other cells in the liver.
Since HSCs are physiologically juxtaposed to hepatocytes extracellular signals from hepatocytes may also trigger the activation of HSCs. To this end, we hypothesized that OCA may regulate the factors related to hepatocytes and thereby controlling the activation of HSCs. BAs are synthesized in hepatocytes4., 15. and are known to be involved in various diseases16., 17.. More importantly, serum BAs are increased during liver fibrosis progression and may represent as biomarkers for staging the severity of liver fibrosis and cirrhosis18., 19.. However, whether and how BAs participate in the pathogenesis of liver fibrosis remains obscure. Moreover, enhanced hepatocyte apoptosis is the common feature of liver fibrosis20. Hepatocyte apoptotic body may serve as a danger signal for the activation of HSCs and thereby contributing to the ultimate development of liver fibrosis20. Therefore, anti-apoptosis has been supposed to be a strategy for the therapy of liver fibrosis.
Given this background, we hypothesized that OCA, which may exert its hepatoprotective effects by regulation of metabolic homeostasis via targeting FXR in hepatocytes, combines with apoptotic inhibitors may represent a promising strategy for the therapy of liver fibrosis. We demonstrated that OCA might inhibit HSC activation/proliferation by reducing hepatic levels of BAs. OCA is not effective against apoptotic cell death of hepatocytes. OCA combined with IDN-6556, a caspase inhibitor, additively ameliorates liver fibrosis.
2. Material and methods
2.1. Chemicals and reagents
OCA, GW4064, cycloheximide (CHX) and IDN-6556 were from MedChem Express (NJ, USA). Cholic acid (CA), chenodeoxycholic acid (CDCA), dehydrocholic acid (dhCA), lipopolysaccharide (LPS, from Escherichis coli 0111:B4), d-galactosamine (GalN), carbon tetrachloride (CCl4) and mineral oil were from Sigma–Aldrich (St. Louis, MO, USA). Recombinant human tumor necrosis factor α (TNFα) was obtained from Peprotech (Rocky Hill, USA). α-Smooth muscle action (α-SMA) antibody was purchased from Abcam (Cambridge, UK).
2.2. Animals and treatments
Specific pathogen free male C57BL/6 mice (8-week old, 20 g) were obtained from Comparative Medicine Centre of Yangzhou University, China. The animal studies were approved by the Animal Ethics Committee of China Pharmaceutical University. All animals were kept in an air-conditioned animal quarter at a temperature of 25 ± 2 °C and a relative humidity of 50 ± 10% with 12-h light/dark cycles for 1 week before experiments and allowed water and standard chow ad libitum.
To determine the effect of OCA on BA catabolism, mice were randomly divided into 3 groups and treated with vehicle or OCA at 10 mg/kg/day or 30 mg/kg/day, respectively, for 5 days.
To determine the effect of OCA on CCl4-induce liver fibrosis, mice were randomly divided into 4 groups including normal control, model, and OCA (10 mg/kg/day or 30 mg/kg/day). CCl4 (20% CCl4/mineral oil; 5 mL/kg) or mineral oil was intraperitoneally (i.p.) injected into mice twice per week for 6 weeks as previously described20. OCA was suspended in 0.5% sodium carboxymethyl cellulose and orally given once daily from the 3rd week.
To determine the effect of OCA on acute liver injury characterized with hepatocyte apoptosis, mice were randomly divided into 3 groups. Acute liver injury was developed by GalN (800 μg/g, i.p.) followed by an injection of LPS (100 ng/g, i.p.) for 6 h as previously described21 with minor modification. OCA was pre-treated for consecutive 5 days.
To determine the effect of co-administration of OCA and IDN-6556 on liver fibrosis, mice were randomly divided into 5 groups. The first group served as the normal control, receiving vehicle only. The other groups was injected with CCl4 biweekly for 6 weeks as above described. Besides, the third group was treated with OCA (10 mg/kg/day, intragastric, i.g.) and the forth group with IDN-6556 (10 mg/kg/day, i.g.), respectively, from the 3rd week. The fifth group was treated with both OCA and IDN-6556.
2.3. Cell culture and treatment
HepG2 (from Stem Cell Bank of the Chinese Academy of Sciences, Shanghai, China) and HSC-T6 (Central South University, Changsha, China) were cultured in Dulbecco׳s modified Eagle׳s medium containing 10% fetal calf serum.
A modified co-culture model20 was enrolled to investigate the effect of hepatocellular apoptosis on HSC activation. Briefly, HSC-T6 cells were plated in 12-well plates, while HepG2 cells were plated on permeable polycarbonate inserts (Transwell cell culture inserts, Millipore, USA) in another 12-well plate. HepG2 cells were incubated with CHX (40 μmol/L) for 30 min, followed by the addition of TNFα (20 ng/mL) for 12 h to induce apoptosis as previously described22. Then the cell-culture inserts containing apoptotic HepG2 cells were transferred onto the HSC-T6 cells after washed and new medium was added.
2.4. Analysis of BAs
Serum BAs were analyzed as previously described23. Briefly, 100 μL of serum samples was diluted with 500 μL of 0.01% formic acid-spiked with dhCA (internal standard), vortexed, and loaded onto Oasis-HLB cartridges, which were then washed with 1 mL H2O and eluted with 1.5 mL methanol. The elute was evaporated, and the remaining residue was reconstituted in 100 μL methanol, 5 μL of which was analyzed by a high performance liquid chromatography (HPLC) system (Shimadzu, Kyoto, Japan) and an AB-Sciex Triple TOF 5600 mass spectrometer (AB Sciex, Foster City, CA, USA). Separation was performed on a ZOEBAX Eclipse Plus C18 column. The chromatographic and mass spectrometric parameters for the quantification of BAs were same as before.
2.5. Biochemical analysis
Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by an automatic blood biochemical analyzer (Beckman Counter LX20, USA).
2.6. Histological analysis
Slices of mice livers were cut off and fixed in formalin, dehydrated in graded alcohol series, and embedded in paraffin. Liver sections were stained with hematoxylin and eosin (H&E) for histopathological evaluation. Apoptotic cells were stained by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL). Assessment of liver fibrosis was performed on Sirius red staining and Masson׳s trichrome staining. HSCs activation was detected by immunohistochemistry staining of α-SMA (Abcam, ab32575, 1:500). Kupffer cells were detected by immunohistochemistry staining of CD68 (Servicebio, GB11076, 1:500).
2.7. Reverse transcription-polymerase chain reaction (RT-PCR) analysis
RT-PCR analysis was performed following the standard protocol as previously described24. Primer sequences are listed in Supporting Information Tables S1 and S2.
2.8. Determination of HSC proliferation
Cell proliferation was measured by bromodeoxyuridine (BrdU) incorporation assay according to the manufacturer׳s protocol as previously described2.
2.9. Caspase activity assay
Activities of caspases were measured by caspase activity assay kits (Beyotime, China).
2.10. Human specimens
A total of 50 patients diagnosed with liver fibrosis and cirrhosis were recruited at The First Affiliated Hospital of Anhui Medical University (Hefei, China). Diagnoses were confirmed with liver biopsy directed by ultrasonography within 1 week after inclusion. Exclusion criteria included age younger than 15 or older than 75 years; pregnancy or breast-feeding; diagnoses of diabetes; other liver diseases; liver transplant; gastrointestinal disorders; pancreatitis; alcohol consumption within 6 months. Blood samples were obtained from patients at the time of liver biopsy, processed to plasma and stored at –80 °C until use. Demographic, clinical, and laboratory data were collected (Supporting Information Table S3). Additional, 20 healthy age-matched controls from blood bank donors without clinical signs or symptoms of liver disease, and no history of chronic illnesses, were analyzed.
The study was approved by the ethics committee of First Affiliated Hospital of Anhui Medical University (PJ2016-10-11) and all patients gave written informed consent prior to participation.
2.11. Serological detection of apoptosis
The plasma from donors was used for quantitative measurement of apoptosis-associated neo-epitope in the C-terminal domain of CK-18 with a M30-Apoptosense ELISA (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China).
2.12. Statistical analysis
Data were expressed as mean ± standard deviation (SD) and subjected to parametrical statistics. Statistical significances were evaluated using one-way analysis of variance (ANOVA), followed by the Tukey׳s multiple comparison test. Differences with P < 0.05 were considered to be statistically significant. All analyses were performed using GraphPad Prism software 6.
3. Results
3.1. OCA restores hepatic FXR signaling and BA homeostasis
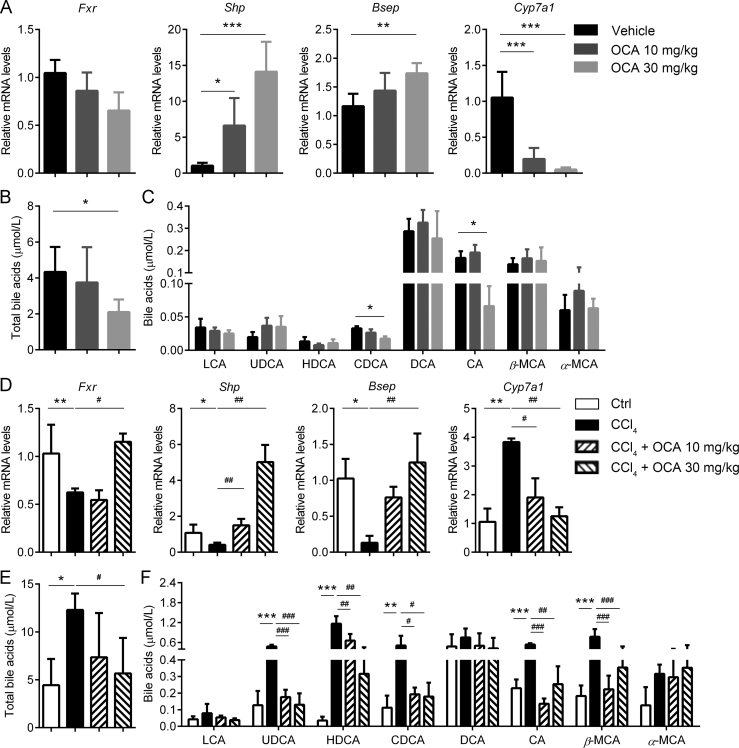
Firstly we confirmed the effect of OCA on FXR signal and the metabolic homeostasis of BAs. As expected, the mRNA levels of hepatic small heterodimer partner (Shp) and bile salt export pump (Bsep) were upregulated by OCA treatment. By contrast, hepatic Cyp7a1 (encoding cholesterol 7α-hydroxylase), a suppressive target gene of Fxr, was downregulated (Fig. 1A). Besides, serum total levels of BAs were significantly reduced upon OCA treatment (Fig. 1B). In particular, CDCA and CA were significantly decreased upon OCA treatment (Fig. 1C).
Figure 1.
OCA treatment decreases BAs levels via activating FXR signaling. (A)–(C) OCA treatment in healthy mice could successfully activate FXR signal and thereby inhibiting BA synthesis (n = 5). (A) Expression of FXR and its target genes in the liver. Serum total BA levels (B) and profiles (C). (D)–(F) OCA treatment reversed the dysregulation of FXR signal and dyshomeostasis of BAs in CCl4-treated mice (n = 5). (D) Expression of FXR and its target genes in the livers. Serum total BAs levels (E) and profiles (F). Results are mean ± SD (n = 5), *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, ###P < 0.001.
A typical liver fibrosis model induced by CCl4 injection. Upon CCl4 intoxication, hepatic FXR signal was significantly suppressed as supported by decreased expression of Fxr, Shp, Bsep and elevated expression of Cyp7a1. While treatment with OCA significantly up-regulated the expression of Fxr, Shp, Bsep, and down-regulated the expression of Cyp7a1 (Fig. 1D). Dysregulation of Fxr-Cyp7a1 signaling in fibrotic livers may result in BA accumulation. As expected, serum total levels of BAs were significantly elevated in CCl4-treated mice, which were significantly reduced by OCA treatment (Fig. 1E). Detailed BA composition analysis revealed that multiple BAs, including lithocholic acid, ursodeoxycholic acid, hyodeoxycholic acid, CDCA, deoxycholic acid, CA, β-muricholic acid, and α-muricholic acid, increased in CCl4-treated mice, and almost all these BAs levels were reduced by OCA treatment (Fig. 1F).
3.2. OCA alleviates liver fibrosis in association with attenuated HSCs activation
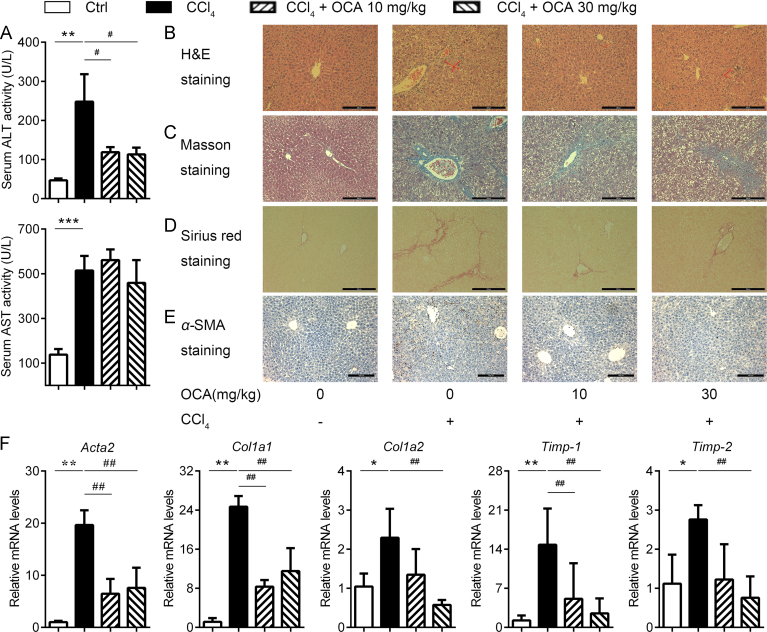
We investigate the effect of OCA on CCl4-induced fibrosis by the analysis of ALT and AST levels in serum and H&E staining of liver sections. OCA treatment significant reduced ALT levels but had little influence on AST, indicating that OCA might have limited effects on restoring liver function (Fig. 2A). Masson and Sirius Red staining of liver sections (Fig. 2C and D) revealed a significant increase in the fibrotic surface compared with control ones. The area of liver parenchyma occupied with fibrotic tissues was reduced by OCA treatment. Because HSCs activation is an important player in the process of liver fibrosis, we asked whether OCA could inhibit HSCs activation. As shown in Fig. 2E, OCA treatment significantly reduced the number of α-SMA positive cells, suggesting that OCA could attenuate activation of HSCs. Consistently, quantitative RT-PCT analysis of Acta2 (encoding α-SMA), collagen1a1 (Col1a1), Col1a2, tissue inhibitor of metalloprotease-1 (Timp-1) and Timp-2 mRNAs showed a 5- to 20-fold increase in CCl4-treated mice, while OCA administration significantly reduced the mRNA levels of all these fibrosis markers (Fig. 2F). These results indicated that OCA treatment might alleviate liver fibrosis partially via inhibiting HSCs activation.
Figure 2.
OCA treatment alleviates CCl4-induced HSCs activation and liver fibrosis. (A) Serum ALT and AST levels. (B)–(E) Histological analysis of liver sections. H&E staining (B), Masson staining (C), Sirius Red staining (D) and immunohistochemistry of α-SMA (E). (F) Expression profiling of fibrosis-related genes. Results are mean ± SD (n = 5), *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01.
3.3. OCA is not effective against hepatocellular apoptosis
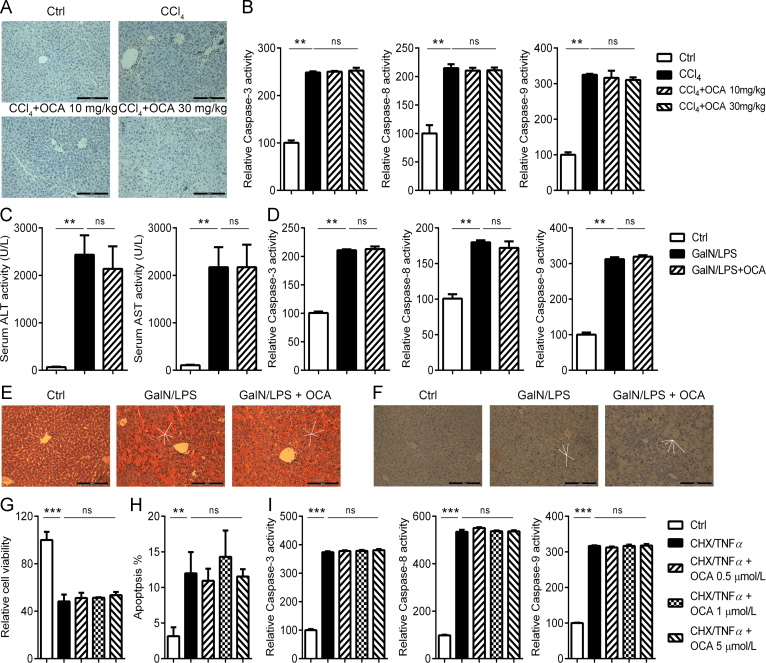
OCA has been supposed to be hepatoprotective via targeting FXR. We asked whether OCA could directly protect against apoptotic cell death of hepatocytes, which is a key event the pathological development of liver fibrosis20. In the CCl4-induced model of liver fibrosis, TUNEL-positive apoptotic hepatocytes were witnessed, and surprisingly OCA treatment had little effect in hepatocytes apoptosis (Fig. 3A). As a support, no difference was observed in the activities of caspase-3, -8, and -9, in mice treated with CCl4 alone or in combination with OCA (Fig. 3B).
Figure 3.
OCA is not effective against hepatocyte apoptosis. (A)–(B) OCA failed to prevent hepatocyte apoptosis induced by CCl4 (n = 5). (A) TUNEL staining of liver sections. (B) Caspase activities of liver homogenate. (C)–(F) OCA failed to prevent GalN/LPS-induced apoptosis (n = 5). (C) Serum ALT and AST levels. (D) Caspase activities of liver homogenate. H&E (E) and TUNEL (F) staining of liver sections. (G)–(I) OCA failed to prevent CHX/TNFα-induced HepG2 apoptosis in vitro as indicated by MTT assay (G), annexin V–PI staining (H) and caspase activities (I). Results are mean ± SD, **P < 0.01, ***P < 0.001.
To further ascertain whether OCA is effective against apoptosis, mice were treated with GalN/LPS to induce an acute fulminant liver injury which is characterized by massive apoptotic changes in the liver21. Pretreatment with OCA did not significantly impact serum transferases levels (Fig. 3C) and apoptotic/necrotic cell death as indicated by caspase activities (Fig. 3D) and histological analysis (Fig. 3E and F). In accordance with the results in vivo, OCA treatment fail to protect cultured cells from apoptosis induced by CHX/TNFα (Fig. 3G—I). Collectively, these results indicate that OCA treatment has little influence on hepatocyte apoptosis, which may explain its limited effects in liver fibrosis at lower dosage11.
3.4. BAs and apoptotic hepatocytes trigger HSC activation and proliferation
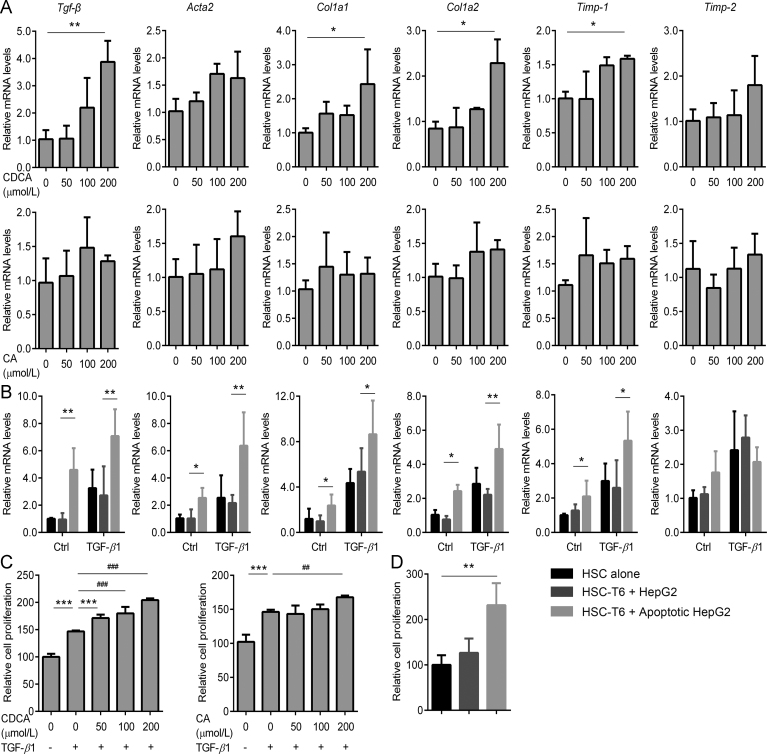
HSCs activation is a pivotal factor in triggering liver fibrosis. To provide a causal link, we asked whether accumulated BAs and apoptotic bodies, both of which are typical events witnessed in the process of liver fibrosis21, may represent the key triggers in activating HSCs. To this end, HSC-T6 cells were incubated with CDCA in the absence or presence of transforming growth factor (TGF)-β1. As shown in Fig. 4A, CDCA significantly up-regulated the mRNA levels of Tgf-β, Col1a1, Col1a2, and Timp-1, indicating that BAs indeed could stimulate HSC activation.
Figure 4.
BAs and apoptotic hepatocytes trigger HSC activation and proliferation. Effect of BAs (A) and apoptotic hepatocyte co-culture (B) on HSC activation was measured by the expression profiling of pro-fibrotic genes (n = 3). Effect of BAs (C) and apoptotic hepatocyte co-culture (D) on the proliferation of HSCs as assessed by BrdU assay (n = 3). Results are mean ± SD, *P < 0.05, **P < 0.01, ##P < 0.01, ###P < 0.001.
The effect of hepatocellular apoptosis on HSC activation was next investigated in a co-culture model. HepG2 cells, plated in cell culture inserts, were treated with CHX/TNFα to induce apoptosis. Apoptotic HepG2 cells were then transferred onto HSC-T6 cells after washed with cell culture medium. As compared with the HSC-T6 cells co-cultured with healthy HepG2 cells, HSC-T6 cells co-cultured with apoptotic HepG2 cells were characterized with elevated mRNA levels of Acta2, Col1a1, Col1a2, Timp-1 and Timp-2, indicating that apoptotic hepatocytes may trigger HSC activation (Fig. 4B).
Furthermore, BrdU incorporation analysis showed that HSC proliferation was significantly increased upon BA treatment (Fig. 4C) or apoptotic HepG2 cells co-culture (Fig. 4D). Taken together, these results indicated that the accumulated BAs and apoptotic hepatocytes may serve as potential profibrogenic factors that stimulate activation and proliferation of HSCs and ultimately promote the development of liver fibrosis.
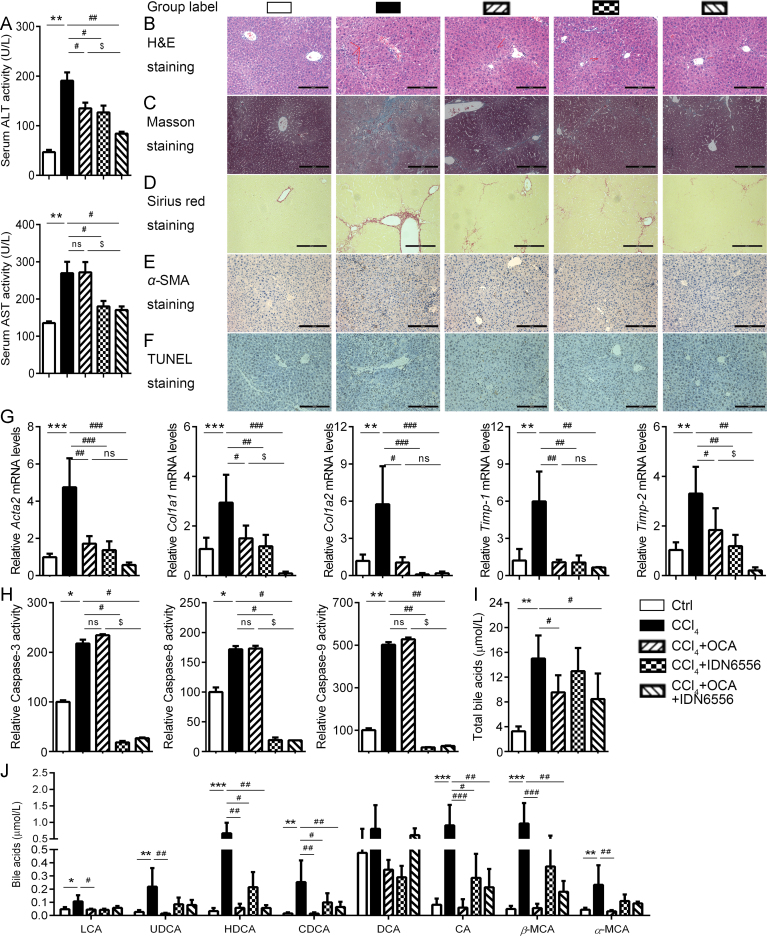
3.5. Enhanced anti-fibrotic effect of OCA when combined with IDN-6556
Given that apoptosis is a key event in the pathological development of liver fibrosis21 and OCA is not effective against apoptosis of hepatocytes, we proposed that combination of OCA and apoptosis inhibitor may exhibit enhanced benefits on liver fibrosis. Here, we investigated the anti-fibrotic effect of co-administration of OCA and IDN-655625. OCA or IDN-6556 alone reduced serum ALT levels, and the combination exhibited an enhanced reduction. Besides, IDN-6556, alone or in combination with OCA, reduced serum AST levels (Fig. 5A). These changes in serum transferase levels paralleled the histologic analysis of liver sections. OCA or IDN-6556 alone protected against liver fibrosis development as shown by histological analysis (Fig. 5B–D), while co-administration of these two agents produced an enhanced protective effect. Consistently, the combination exhibited better effect in inhibiting HSCs activation, as indicated by α-SMA staining (Fig. 5E), and much stronger effect in reducing liver expression of a number of genes involved in liver fibrosis development, including Acta2, Col1a1, Col1a2, Timp-1 and Timp-2 (Fig. 5G). We next explored hepatocellular apoptosis by TUNEL staining and caspase activities assay. As expected, IDN-6556, but not OCA alone, attenuated the percentage of TUNEL-positive staining (Fig. 5F) and reduced the activities of caspases (Fig. 5H). In contrast, OCA significant rescued the dyshomeostasis of BA caused by CCl4 (Fig. 5I and J). Taken together, combination of OCA and IDN-6556 exerted an improved protective effect against CCl4-induced fibrosis. OCA via targeting FXR may restore the homeostasis of BAs and IDN-6556 functions via inhibiting apoptotic cell death of hepatocytes and thereby additively alleviates liver fibrosis.
Figure 5.
Combined OCA and IDN-6556 treatment combats liver fibrosis. CCl4-injured mice were enrolled to test the anti-fibrotic effect of co-administration of OCA and IDN-6556. (A) Serum ALT and AST levels. (B)–(F) Histological analysis of liver sections. H&E staining (B), Masson staining (C), Sirius Red staining (D), immunohistochemistry of α-SMA (E) and TUNEL staining (F). (G) Expression profiling of fibrosis-related genes. (H) Caspase activities of liver homogenate. Serum total BAs level (I) and profiles (J). Results are mean ± SD (n = 5), *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, ###P < 0.001, $P < 0.05.
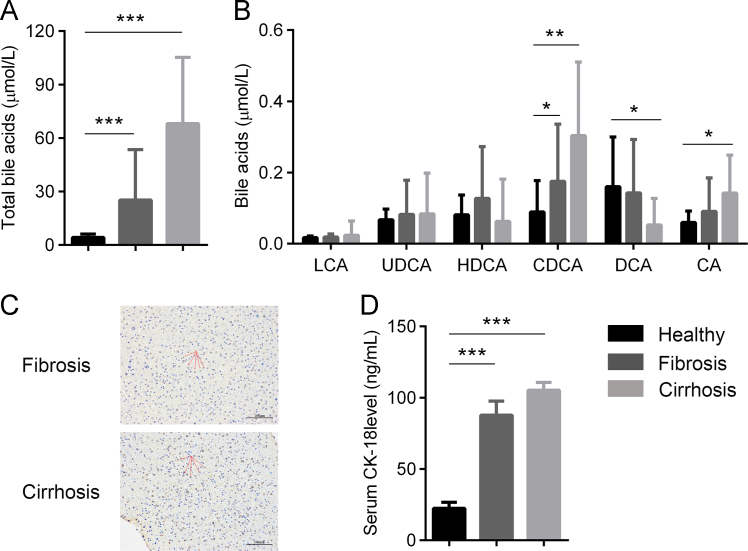
3.6. Increased serum BAs and apoptosis in patients with liver fibrosis
To validate our findings are clinically relevant, we performed a retrospective analysis of serum and liver biopsies from patients with liver fibrosis/cirrhosis. We collected a total of 20 liver biopsies from patients with liver fibrosis and 30 from liver cirrhosis. A univariate analysis revealed that the two groups did not differ significantly in gender nor body mass index (Table S3). Besides, serum samples from 20 healthy age-matched controls from blood bank donors without clinical signs or symptoms of liver disease, and no history of chronic illnesses, were collected. Notably, serum BA levels were significantly higher in fibrotic and cirrhotic patients than that in healthy controls (Fig. 6A). In particular, CDCA were significantly increased in fibrotic and cirrhotic patients compared with healthy controls (Fig. 6B). TUNEL staining of liver biopsy indicated apparent apoptotic cell death in liver sections from both fibrosis and cirrhosis patients (Fig. 6C). Additionally, serum CK-18, an apoptotic marker, was significantly higher in fibrosis and cirrhosis patients (Fig. 6D). Together, these results support that BA accumulation and apoptotic cell death are key events in the pathological development of liver fibrosis/cirrhosis.
Figure 6.
Liver fibrosis and cirrhosis are characterized with elevated BAs and hepatocyte apoptosis. Serum from liver fibrotic patients (n = 20), cirrhotic patients (n = 30) as well as healthy donors (n = 20) were collected. Serum total BA levels (A) and BA profiles as analyzed (B). Hepatocyte apoptosis was assessed by TUNEL staining of liver biopsy (C) and serum CK-18 (M30) levels (D). Results are mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
4. Discussion
HSCs activation and proliferation are key events in the development of liver fibrosis. Since liver fibrosis and cirrhosis are generally regarded to be irreversible, one of the most effective strategies for fibrosis prevention or treatment is to inhibit the activation and proliferation of HSCs. In the current study, we have identified that elevated BAs and hepatocellular apoptotic bodies are danger signals for HSC activation and proliferation. As a FXR agonist, OCA may inhibit HSCs activation via regulating BAs homeostasis; however, OCA is not effective against hepatocellular apoptosis and thus may limiting its clinical efficacy against liver fibrosis. We thereby propose and validate that the combination of OCA and an apoptosis inhibitor might be a promising strategy for combating liver fibrosis.
Liver fibrosis or cirrhosis, the pathological feature of most forms of chronic hepatic damage, is responsible for much morbidity and mortality worldwide26., 27.. The principal cell type responsible for liver fibrosis is HSCs26., 28., which express FXR at a relative low levels compared to hepatocytes (Supporting Information Fig. S1A). These cells are juxtaposed to hepatocytes and are uniquely poised to possible toxic secretion from hepatocytes or damaged hepatocytes. In accordance with previous studies18., 19., we have uncovered that liver fibrosis is characterized with accumulation of BAs. BAs are biosynthesized from cholesterol in hepatocytes, which is precisely regulated by FXR-CYP7A1 signal. We demonstrated that CDCA but not CA could induce the expression of Tgf-β and promote the phosphorylation of Smad2/3 (Fig. S1B), then triggering the transcription of several pro-fibrotic genes. Besides, both CDCA and CA can promote proliferation of HSC. These evidence indicated that BAs accumulated in the liver may serve as potential profibrogenic factor for fibrosis development. Another hallmark of liver fibrosis is increased apoptotic cell death of hepatocytes20. Furthermore, inhibition of apoptosis by pro-apoptotic gene knock-out29., 30. or caspase inhibitors31., 32. alleviate liver diseases. In the present study, we demonstrated that apoptotic hepatocytes trigger the activation and proliferation of HSCs, and proposed that apoptotic hepatocytes could be potential profibrogenic factor for fibrosis development. Liver fibrosis and cirrhosis are traditionally regarded to be irreversible and lack effective therapeutics. One possible reason is that single agents targeting one factor might not be sufficiently effective against such a complex disease. For this reason, we supposed the combined therapy might be necessary for the prevention and treatment of liver fibrosis/cirrhosis.
FXR is a member of nuclear receptor superfamily and has crucial role in controlling BA homeostasis1., 4.. Recently, this receptor has been reported be correlated with the development of fibrosis33. FXR knock-out mice are prone to probably inflammation-driven liver fibrosis with increasing age. Besides, CCl4-intoxication for up to 12 weeks resulted in more severe liver injury in FXR knock-out mice compared with wild-type controls14. Due to the potential role of FXR on the development of hepatic fibrosis, various FXR agonists were tested for the treatment of fibrosis12., 34.. However, the results and mechanisms from both the preclinical animal models and human clinical trials are somewhat controversial. Early studies indicated that FXR activation led to up-regulation of microRNA-29a in HSCs, which plays an inhibitory role in ECM production35. Besides, FXR activation in HSCs was also demonstrated to cause a SHP-dependent inhibition on pro-fibrotic gene expression13. These studies provided evidence for the direct inhibitory role of FXR agonism on HSC activation and proliferation. Since HSCs are subsequently demonstrated to express relative low level of FXR14, other cells in the liver may also contribute to the anti-fibrotic effect of FXR activation. HSC could be activated by stimulated Kupffer cells (KC) via paracrine production of pro-inflammatory cytokines36. Previously, FXR activation by OCA was demonstrated to decelerate thioacetamide-induced fibrogenesis indirectly by targeting hepatic inflammation in both KC and liver sinusoidal endothelial cells37. In line with these findings, we further confirmed that OCA treatment could inhibit KC activation and cytokines transcription caused by CCl4 injection (Supporting Information Fig. S2). More importantly, hepatocytes are highly expressed with abundant FXR compared to HSCs. Since hepatocytes may regulate the activation of HSCs via secretion of BAs and apoptotic bodies, we supposed that FXR ligands may also impede HSCs activation indirectly via targeting FXR in hepatocytes. As expected, OCA treatment significantly inhibited the synthesis of BAs, reducing the exposure of HSCs to toxic BAs. Hepatocyte apoptosis also play a key role in HSC activation and proliferation. FXR activation was previously reported to protect liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo38. However, we have not observed a significant effect of OCA against death receptors engaged apoptosis of hepatocytes, which is a dominant mechanism in promoting liver fibrosis. It seems that FXR activation by agonists might be effective against intrinsic apoptosis, as that induced by fast, but not death-receptors engaged extrinsic apoptosis. Inhibition of apoptosis is generally accepted as a promising therapeutic strategy to prevent liver fibrosis. The proof of concept of this strategy is illustrated by IDN-6556, a pan-caspase inhibitor currently undergoing phase II clinical studies39. Here in this study we revealed that treatment with IDN-6556, prevented hepatocyte apoptosis and reduced fibrosis induced by CCl4.
Although various preclinical studies suggested OCA might be effective against liver fibrosis, its clinical application might be limited by the observed serious adverse effects such as pruritus10. The rate of this adverse side-effect was found positively correlated with the dosage of OCA (15% in 10 mg vs. 38% in 50 mg)40. To avoid adverse effects and improve long term tolerance, the dosage of OCA should be reduced. However, a more recent clinical trial indicated that low dose (5–10 mg) of OCA exert little beneficial effect on liver fibrosis11. Thus, how to improve the clinical efficiency of OCA in a relative safe and low dosage is an urgent question to be solved8. In this study, we demonstrated that in combination with IDN-6556, another drug candidate for liver fibrosis, OCA could exert enhanced beneficial effect against fibrosis even at low dosage, providing a promising strategy for the therapy of liver fibrosis, and in the combination, OCA can be effective at low dosage with minimized side-effect. To provide a translational link to the clinic, we found that both BA accumulation and apoptotic death of hepatocytes were witnessed from human patients with liver fibrosis. Therefore, it is reasonable to expect that OCA in combination with apoptosis inhibitors would be a translatable strategy for the therapy of liver fibrosis.
5. Conclusions
In this study, we demonstrated that OCA inhibits HSCs activation/proliferation partially by regulating BA homeostasis and thereby inhibiting activation of HSCs. The findings in this study suggest that combined use of apoptosis inhibitor and OCA at lower dosage represents a novel therapeutic strategy for liver fibrosis.
Acknowledgment
This research was supported by National Natural Science Foundation of China (grants 81430091, 81720108032, 81421005, 91429308 and 81603194); the Project for Major New Drug Innovation and Development (grant 2015ZX09501010 and 2017ZX09101003-002-003, China); and Overseas Expertise Introduction Project for Discipline Innovation (G20582017001, China); "Double First Class" Initiative Project (CPU2018GF01 and CPU2018GF09, China); State Key Laboratory of Natural Medicines at China Pharmaceutical University (SKLNMZZCX201610 and SKLNMZZCX201801, China) and China Postdoctoral Science Foundation (grants 2016M600455 and 2017T100423).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2018.11.004.
Contributor Information
Guangji Wang, Email: gjwang@cpu.edu.cn.
Hong Wang, Email: wanghong@cpu.edu.cn.
Haiping Hao, Email: haipinghao@cpu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Peng L., Piekos S., Guo G.L., Zhong X.B. Role of farnesoid X receptor in establishment of ontogeny of phase-I drug metabolizing enzyme genes in mouse liver. Acta Pharm Sin B. 2016;6:453–459. doi: 10.1016/j.apsb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y., Wang H., Cheng X., Wu Y., Cao L., Wu M. Farnesoid X receptor activation promotes cell proliferation via PDK4-controlled metabolic reprogramming. Sci Rep. 2016;6:18751. doi: 10.1038/srep18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao H., Cao L., Jiang C., Che Y., Zhang S., Takahashi S. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–867. doi: 10.1016/j.cmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y., Liu H., Zhang M., Guo G.L. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B. 2016;6:409–412. doi: 10.1016/j.apsb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding L., Yang L., Wang Z., Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135–144. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H., Zhao Z., Zhou J., Guo Y., Wang G., Hao H. A novel intestinal-restricted FXR agonist. Bioorg Med Chem Lett. 2017;27:3386–3390. doi: 10.1016/j.bmcl.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Sepe V., Distrutti E., Fiorucci S., Zampella A. Farnesoid X receptor modulators 2014–present: a patent review. Expert Opin Ther Pat. 2018;28:351–364. doi: 10.1080/13543776.2018.1459569. [DOI] [PubMed] [Google Scholar]
- 8.Wang H., He Q., Wang G., Xu X., Hao H. FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat. 2018;28:765–782. doi: 10.1080/13543776.2018.1527906. [DOI] [PubMed] [Google Scholar]
- 9.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschfield G.M., Mason A., Luketic V., Lindor K., Gordon S.C., Mayo M. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 12.Fiorucci S., Antonelli E., Rizzo G., Renga B., Mencarelli A., Riccardi L. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Fiorucci S., Rizzo G., Antonelli E., Renga B., Mencarelli A., Riccardi L. A farnesoid X receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584–595. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- 14.Fickert P., Fuchsbichler A., Moustafa T., Wagner M., Zollner G., Halilbasic E. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol. 2009;175:2392–2405. doi: 10.2353/ajpath.2009.090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Gupte J., Gong Y., Weiszmann J., Zhang Y., Lee K.J. Chronic over-expression of fibroblast growth factor 21 increases bile acid biosynthesis by opposing FGF15/19 action. EBioMedicine. 2017;15:173–183. doi: 10.1016/j.ebiom.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Y., Yan W., Lu Y., Zhou K., Cai W. Neurotensin contributes to pediatric intestinal failure-associated liver disease via regulating intestinal bile acids uptake. EBioMedicine. 2018;35:133–141. doi: 10.1016/j.ebiom.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Golubeva A.V., Joyce S.A., Moloney G., Burokas A., Sherwin E., Arboleya S. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine. 2017;24:166–178. doi: 10.1016/j.ebiom.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Xie G., Zhao A., Zheng X., Huang F., Wang Y. Serum bile acids are associated with pathological progression of hepatitis B-induced cirrhosis. J Proteome Res. 2016;15:1126–1134. doi: 10.1021/acs.jproteome.5b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlomai A., Halfon P., Goldiner I., Zelber-Sagi S., Halpern Z., Oren R. Serum bile acid levels as a predictor for the severity of liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2013;20:95–102. doi: 10.1111/j.1365-2893.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Ge C., Zhou J., Guo Y., Cui S., Huang N. Noncanonical farnesoid X receptor signaling inhibits apoptosis and impedes liver fibrosis. EBioMedicine. 2018 doi: 10.1016/j.ebiom.2018.10.028. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F., Miao L., Sun H., Zhang Y., Bao X., Zhang D. Establishment of a new acute-on-chronic liver failure model. Acta Pharm Sin B. 2017;7:326–333. doi: 10.1016/j.apsb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill D.B., Schmidt J., Shedlofsky S.I., Cohen D.A., McClain C.J. In vitro tumor necrosis factor cytotoxicity in Hep G2 liver cells. Hepatology. 1995;21:1114–1119. [PubMed] [Google Scholar]
- 23.Zhou X., Cao L., Jiang C., Xie Y., Cheng X., Krausz K.W. PPARα-UGT axis activation represses intestinal FXR-FGF15 feedback signalling and exacerbates experimental colitis. Nat Commun. 2014;5:4573. doi: 10.1038/ncomms5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H., Yan T., Xie Y., Zhao M., Che Y., Zhang J. Mechanism-based inhibitory and peroxisome proliferator-activated receptor alpha-dependent modulating effects of silybin on principal hepatic drug-metabolizing enzymes. Drug Metab Dispos. 2015;43:444–454. doi: 10.1124/dmd.114.061622. [DOI] [PubMed] [Google Scholar]
- 25.Pockros P.J., Schiff E.R., Shiffman M.L., McHutchison J.G., Gish R.G., Afdhal N.H. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Wu X., Zhang A., Wang S., Hu C., Chen W. Targeting the PDGF-B/PDGFR-β interface with destruxin A5 to selectively block PDGF-BB/PDGFR-ββ signaling and attenuate liver fibrosis. EBioMedicine. 2016;7:146–156. doi: 10.1016/j.ebiom.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng S.Y., Wang X.Y., Vijayan S., Tang Y.L., Kim Y.O., Padberg K. IL-4 receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. Ebiomedicine. 2018;29:92–103. doi: 10.1016/j.ebiom.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Zhang H., Bai R. Advances in ultrasound-targeted microbubble-mediated gene therapy for liver fibrosis. Acta Pharm Sin B. 2017;7:447–452. doi: 10.1016/j.apsb.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatting M., Zhao G., Schumacher F., Sellge G., Al Masaoudi M., Gassler N. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189–2201. doi: 10.1002/hep.26271. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi A., De Mollerat Du Jeu X., Johnson C.D., Nektaria A., Feldstein A.E. Liver Bid suppression for treatment of fibrosis associated with non-alcoholic steatohepatitis. J Hepatol. 2016;64:699–707. doi: 10.1016/j.jhep.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratziu V., Sheikh M.Y., Sanyal A.J., Lim J.K., Conjeevaram H., Chalasani N. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anstee Q.M., Concas D., Kudo H., Levene A., Pollard J., Charlton P. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542–550. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y.X., Xu N.Y., Xu J., Kong B., Copple B., Guo G.L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology. 2014;60:919–930. doi: 10.1002/hep.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbeke L., Farre R., Trebicka J., Komuta M., Roskams T., Klein S. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Zhang Y., Kuruba R., Gao X., Gandhi C.R., Xie W. Roles of microRNA-29a in the antifibrotic effect of farnesoid X receptor in hepatic stellate cells. Mol Pharmacol. 2011;80:191–200. doi: 10.1124/mol.110.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra A., Upadhyay P.K., Nagarajan P. Immunotherapy in liver diseases: a balance between immunity and tolerance. Curr Drug Metab. 2016;17:997–1005. [PubMed] [Google Scholar]
- 37.Verbeke L., Mannaerts I., Schierwagen R., Govaere O., Klein S., Vander Elst I. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453. doi: 10.1038/srep33453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y.D., Yang F., Chen W.D., Huang X., Lai L., Forman B.M. Farnesoid X receptor protects liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo. Mol Endocrinol. 2008;22:1622–1632. doi: 10.1210/me.2007-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baskin-Bey E.S., Washburn K., Feng S., Oltersdorf T., Shapiro D., Huyghe M. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 40.Thomas H. Therapy: obeticholic acid for PBC. Nat Rev Gastroenterol Hepatol. 2016;13:558–559. doi: 10.1038/nrgastro.2016.143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material