Abstract
Limited knowledge is currently available about alterations of retinal blood flow (F), oxygen delivery (DO2), oxygen metabolism (MO2), oxygen extraction fraction (OEF), or thickness after the ophthalmic blood vessels have been closed for a substantial interval and then reopened. We ligated the ophthalmic vessels for 120 minutes in one eye of 17 rats, and measured these variables within 20 minutes after release of the ligature in the 10 rats which had immediate reflow. F, DO2 and MO2 were 5.2 ± 3.1 μL/min, 428 ± 271 nL O2/min, and 234 ± 133 nL O2/min, respectively, that is, to 58%, 46% and 60% of values obtained from normal fellow eyes (P < 0.004). OEF was 0.65 ± 0.23, 148% of normal (P = 0.03). Inner and total retinal thicknesses were 195 ± 24 and 293 ± 20 μm, respectively, 117% and 114% of normal, and inversely related to MO2 (P ≤ 0.02). These results reflect how much energy is available to the retina immediately after an interval of nonperfusion for 120 minutes. Thus, they elucidate aspects of the pathophysiology of nonperfusion retinal injury and may improve therapy in patients with retinal artery or ophthalmic artery obstructions.
Subject terms: Retinal diseases, Blood flow
Introduction
Inner retinal ischemia is a major pathophysiologic factor in retinal vascular occlusions, diabetic retinopathy1–4, sickle cell retinopathy5, and has been implicated in glaucoma6–10. Much important information about ischemic retinal injury, and pharmacological modification of it, has been obtained from animal models of retinal ischemia. In most of these models, retinal blood flow is interrupted, and, since the baseline oxygen metabolic rate normally is high11,12 and oxygen stores are minimal13, oxygen metabolism rapidly ceases. In many of the models, obstruction of the retinal blood supply can be reversed after some time period, for example, by release of a ligature or by normalizing the intraocular pressure14–23. In that case, improvement of retinal blood flow and inner retinal oxygen metabolism can occur. However, upon reflow, reperfusion injury is known to develop in neural tissue24,25, and it may be difficult to separate the effects of hypoperfusion and reperfusion on the ultimate tissue outcome. In fact, reports on retinal ischemia often equivocate on this point and describe their observations as being on ischemia-reperfusion injury.
In these reperfusion models, knowledge is limited about the improvement of blood flow and oxygen metabolism after reversible retinal ischemia because these outcomes usually have not been measured. However, post-ischemic hyperemia has been observed in the retina of cats and rats after 60 minutes or less of ischemia26–29. On the other hand, incomplete normalization of retinal blood flow by obstruction of the microvasculature with leukocytes after ischemia has been reported30,31, consistent with the “no reflow phenomenon” observed in the brain24,32–35. Since the eventual outcome following severe ischemia is known to be highly dependent on the duration of ischemia between about 60 and 120 minutes (i.e., improvement is nearly complete with durations less than 60 minutes, and improvement is minimal at durations beyond 120 minutes)16,18,20,22,23,36, it is difficult to extrapolate from the above results how inner retinal blood flow would improve immediately after severe retinal ischemia at durations toward the end of this interval. Furthermore, essentially no information is available about the improvement of oxygen delivery and metabolism shortly after retinal ischemia. It is known, however, that ischemia can result in anatomic and physiologic injury to mitochondria that limits resumption of normal oxygen metabolism37–39.
Since retinal function requires energy, it is dependent on a continuous supply of oxygen and nutrients. Thus, the ideal therapy for severely impaired blood flow beyond the 60 minute duration would appear to be to 1) re-establish perfusion and oxygenation, and 2) suppress reperfusion injury. Consequently, it is important to characterize how blood flow and oxygen metabolism improve as part of the overall enterprise to maximize retinal survival after an ischemic insult. Since there is evidence for post-ischemic hyperemia, we hypothesized that immediately after an interval in which retinal and choroidal blood flow had been eliminated by ophthalmic vessel occlusion (OVO)20 the improvement of blood flow, and, consequently, oxygen delivery, would be greater than that of oxygen metabolism. We tested this hypothesis using methods we have developed previously to measure inner retinal blood flow (F), oxygen delivery (DO2), oxygen metabolism (MO2), and the oxygen extraction fraction (OEF) in rats21,40. In addition, since the retina thickens after ischemia41–43 and there is a lack of information about any role of MO2 in this, we tested the hypothesis that there is a relationship between retinal thickening and the reduction in MO2 immediately following an interval of nonperfusion.
Results
Retinal nonperfusion with prompt reperfusion
The retina reperfused immediately in 10 rats after 120 minutes of nonperfusion. Table 1 shows retinal arterial and venous PO2 (PO2A and PO2V, respectively), arterial and venous diameter (DA and DV, respectively), venous velocity (V), and F measurements in these rats. PO2A and PO2V were lower in eyes with OVO compared to those in fellow eyes (P ≤ 0.01). DA was lower in eyes with OVO relative to that in their fellow eyes (P < 0.001), while the difference in DV between eyes with OVO and their fellow eyes was not statistically significant (P = 0.09). Measurements of V and F were reduced in eyes with OVO compared to those in fellow eyes (P ≤ 0.002). F in eyes with OVO improved to 58% of that in their fellow eyes.
Table 1.
Retinal Arterial and Venous Oxygen Tension (PO2A and PO2V, respectively), Arterial and Venous Diameter (DA and DV, respectively), Venous Blood Velocity (V) and Venous Blood Flow (F) in Rat Eyes with Ophthalmic Vessel Occlusion (OVO) and Fellow Eyes after 2 Hours of Nonperfusion (N = 10). Statistically significant P values in bold.
| Eyes with OVO (Mean ± SD) | Fellow Eyes (Mean ± SD) | P value | |
|---|---|---|---|
| PO2A (mm Hg) | 37 ± 6 | 45 ± 7 | 0.004 |
| PO2V (mm Hg) | 20 ± 9 | 31 ± 5 | 0.01 |
| DA (μm) | 40 ± 6 | 47 ± 5 | <0.001 |
| DV (μm) | 60 ± 10 | 54 ± 4 | 0.09 |
| V (mm/s) | 4.0 ± 2.0 | 9.7 ± 2.0 | <0.001 |
| F (μL/min) | 5.2 ± 3.1 | 9.0 ± 2.1 | 0.002 |
Table 2 displays mean inner retinal DO2, MO2, and OEF measured after 120 minutes of nonperfusion and prompt reperfusion. DO2 was lower in eyes with OVO (428 ± 271 nL O2/min) compared to that in their fellow eyes (924 ± 284 nL O2/min) (P = 0.001), an improvement to only 46% of that in the fellow eye. Similarly, MO2 was lower in eyes with OVO (234 ± 133 nL O2/min) relative to that in their fellow eyes (389 ± 87 nL O2/min) (P = 0.004), improving to 60% of what was present in the fellow eye. OEF was higher in eyes with OVO (0.65 ± 0.23) compared to that in their fellow eyes (0.44 ± 0.08) (P = 0.03), 48% greater than in fellow eyes.
Table 2.
Retinal Oxygen Metabolism (MO2), Oxygen Delivery (DO2) and Oxygen Extraction Fraction (OEF) in Rat Eyes with Ophthalmic Vessel Occlusion (OVO) and Fellow Eyes after 2 Hours of Nonperfusion (N = 10). Statistically significant P values in bold.
| Eyes with OVO (Mean ± SD) | Fellow Eyes (Mean ± SD) | P value | |
|---|---|---|---|
| DO2 (nL O2/min) | 428 ± 271 | 924 ± 284 | 0.001 |
| MO2 (nL O2/min) | 234 ± 133 | 389 ± 87 | 0.004 |
| OEF | 0.65 ± 0.23 | 0.44 ± 0.08 | 0.03 |
Table 3 shows inner retinal thickness (IRT), outer retinal thickness (ORT) and total retinal thickness (TRT) measured after 120 minutes of nonperfusion with prompt reperfusion (thickness data were not available from one rat). IRT was higher in eyes with OVO (195 ± 24 μm) compared to that in the fellow eyes (166 ± 12 μm)(P = 0.009), an increase of 17% compared to that of the fellow eyes. ORT was not significantly different in eyes with OVO (98 ± 11 μm) compared to that in their fellow eyes (90 ± 7 μm) (P = 0.07). TRT was higher in eyes with OVO (293 ± 20 μM) compared to that in their fellow eyes (256 ± 13 μm) (P < 0.001), increasing by 14% compared to their fellow eyes.
Table 3.
Inner Retinal Thickness (IRT), Outer Retinal Thickness (ORT), and Total Retinal Thickness (TRT) in Rat Eyes with Ophthalmic Vessel Occlusion (OVO) and Fellow Eyes after 2 Hours of Nonperfusion (N = 9). Statistically significant P values in bold.
| Eyes with OVO Mean ± SD) | Fellow Eyes (Mean ± SD) | P value | |
|---|---|---|---|
| IRT (μm) | 195 ± 24 | 166 ± 12 | 0.009 |
| ORT (μm) | 98 ± 11 | 90 ± 7 | 0.07 |
| TRT (μm) | 293 ± 20 | 256 ± 13 | <0.001 |
The mixed linear model revealed that IRT was linearly related to MO2 (β = −0.09 µm * nL O2−1 * min; P = 0.02, Fig. 1). In addition, a linear relationship between TRT and MO2 (β = −0.12 µm * nL O2−1 * min, P = 0.006, Fig. 2) was found. No significant relationship between ORT and MO2 was observed (P = 0.3).
Figure 1.
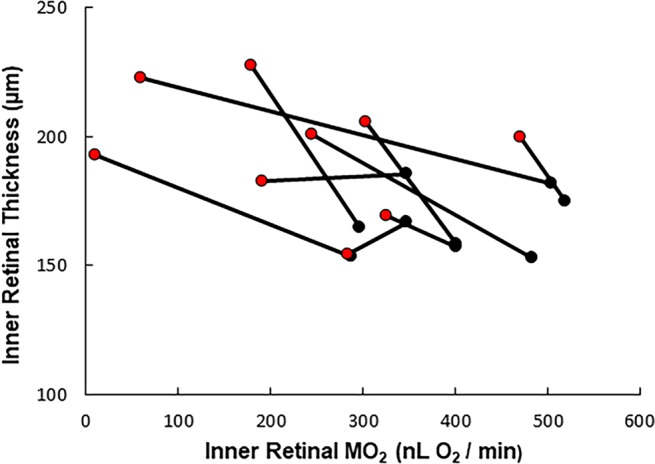
Relationship between inner retinal thickness and inner retinal oxygen metabolism (MO2) in rats with normal fellow and nonperfusion eyes. Data from the normal fellow eyes (black) are connected to the corresponding data obtained from the eyes immediately after 120 minutes of total ocular nonperfusion (red). An inverse linear relationship was found between increases in inner retinal thickness values and nonperfusion-induced reductions in MO2 values (β = −0.093 µm * min * nL O2−1, P = 0.019, N = 9).
Figure 2.
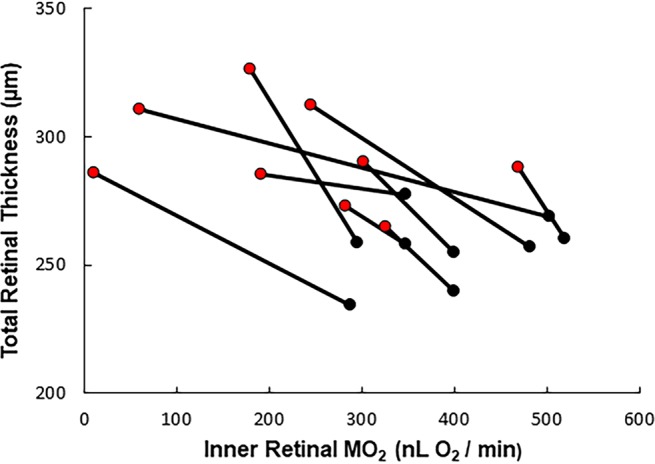
Relationship between total retinal thickness and inner retinal oxygen metabolism (MO2) in rats with normal fellow and nonperfusion eyes. Data from the normal fellow eyes (black) are connected to the corresponding data obtained from the eyes immediately after 120 minutes of total ocular nonperfusion (red). An inverse linear relationship was found between increases in total retinal thickness values and nonperfusion-induced reductions in MO2 values (β = −0.12 µm * nL O2−1 * min, P = 0.006, N = 9).
Retinal nonperfusion without prompt reperfusion
In seven of the 17 eyes undergoing OVO, reflow did not occur immediately. In five of these, reflow had not occurred by one hour after release of the ligature, which precluded blood flow measurements, and the experiments were concluded. We could not interpret the results in the other two eyes with confidence.
Discussion
The present study had several major findings. First, we rejected the hypothesis that with prompt reperfusion after total ocular nonperfusion for a substantial period of time (at least 120 minutes) the improvement of F and, consequently, DO2 would be robust, exceeding that of MO2. Instead, with respect to the normal fellow eyes, the percentage improvement in F, and especially DO2, did not exceed that of MO2, the values being 58%, 46% and 60%, respectively, after release of the ligature. Of note, F, DO2 and MO2 values in fellow eyes were similar to those previously published in healthy control eyes44.
While post-ischemic hyperemia has been well documented after shorter durations of retinal ischemia26–29, we did not observe this phenomenon with 120 minutes of nonperfusion. Not only did F fail to completely improve immediately, but in seven of the 17 eyes there was no improvement immediately, and in five eyes no reflow had occurred by one hour after release of the ligature. This “no reflow phenomenon”24,30,32–35,45 occurs particularly after substantial periods in which F is completely interrupted. It appears to result from small vessel obstruction by leukocytes30,31,35, erythrocytes34, microthrombi33, pericyte constriction45, as well as other possible contributing factors34. These factors will variably affect the level of improvement of F, and, therefore, quantifying a nonperfusion insult merely by the duration that the investigator imposes the occlusive intervention may often underestimate persisting, potentially injurious F reduction. Monitoring F after reversing the occlusive intervention is likely to better quantify the nonperfusion insult and reduce the variability of its effect on the outcome under study.
The second major finding was that DO2 did not improve as much as F within 20 minutes of reperfusion. In many situations DO2 is dominated by F because oxygen in the retinal arteries near the optic nerve has not been exposed significantly to oxygen extraction by the retinal tissue. In the current experiments, while F improved roughly to the same extent as MO2, this did not enable comparable improvement in oxygen availability because PO2A, and consequently the oxygen concentrations in the arteries (O2A) were reduced. We tentatively interpreted this to be the result of reduced arterial velocity, so that there was more time for oxygen to be extracted from the blood across the arterial walls prior to arriving at the measurement sites near the optic nerve head. A reduction in arterial blood velocity can be deduced from our data on V, DA and DV. That DO2 did not improve as much as F indicates that there are limits to inferring DO2 from measurements of F alone.
The third major finding was that OEF was increased in OVO eyes after reperfusion as compared to fellow eyes. OEF is the ratio of MO2 to DO2 and, accordingly, quantifies the adequacy of oxygen supply relative to the tissue’s metabolic demand. Thus, the increase in OEF was a consequence of the fact that changes in MO2 exceeded those of DO2 after release of the ligature. OEF has been shown in the brain to be of prognostic value for predicting ischemic stroke46–48. We have previously reported increased retinal OEF in rats under systemic hypoxia and ischemia, and in mice with diabetes21,49,50. OEF may prove to have prognostic value in retinal ischemic conditions, as well.
The fourth major finding was that MO2 did not completely improve in the first 20 minutes after release of the ligature as compared to the level found in the normal fellow eye. We believe this to be the first report of MO2 after a substantial interval of retinal nonperfusion. If there had been complete recovery in the eyes with OVO, the value of MO2 would have been essentially the same as that in the normal fellow eyes (389 nL O2/min). However, this value is less than DO2 in the eyes with OVO (428 nL O2/min). This indicates that the incomplete improvement in MO2 only to 234 nL O2/min was not caused by the overall unavailability of oxygen to the tissue from the vasculature. There are at least two explanations for this. First, nonperfusion may have induced maldistribution of microvascular blood flow. Some inner retinal areas may have received low enough levels of flow that oxygen metabolism was limited to less than normal values for the corresponding tissue, whereas other areas may have received more flow than was needed to deliver the normal amount of oxygen to the corresponding tissue (that is, shunting). Second, nonperfusion may have injured the capacity of the tissue to utilize oxygen. We previously found that with short periods of graded reductions of retinal blood flow, the starving retina can draw out all of the delivered oxygen and raise OEF to its theoretical maximum value of one, that is, where MO2 equals DO221. If the capacity to metabolize oxygen (and blood flow distribution) were normal, the reperfused retina would have been able to reach the normal MO2 value despite the reduced DO2 and produce an OEF of about 0.91. Thus, the MO2 of 234 nL O2/min and OEF of 0.65 after reperfusion are consistent with nonperfusion-induced injury to the metabolic machinery underlying MO2. Indeed, in the brain, and presumably in the comparable neural retina, ischemia has significant injurious effects on mitochondria. These include excitotoxic calcium entry, stimulation of degradative enzymes, production of reactive oxygen species, alterations in membrane permeability, increases in volume, loss of cytochrome c, impairment of energy generation and, eventually, cell death37–39. Further studies are needed to determine the relative contributions to our results of maldistribution of blood flow and impaired capacity to utilize oxygen.
While much remains to be discovered about MO2 in retinal ischemia, considerable information has been obtained about the rate of oxygen metabolism in the ischemic brain using positron emission tomography and MRI methods (these rates are referred to as rCMRO2 or OMI, which are similar to MO2). Threshold values for these variables have been found that correlate well with tissue that will not or has not survived, and they are superior to OEF and other parameters for this purpose51–54. It is reasonable to suppose that MO2 may also prove to have similar utility with further studies of its role in retinal ischemia. However, whether MO2 or OEF will prove to be the more valuable factor in retinal ischemia remains to be determined, and important roles for both MO2 (similar to oxygen extraction55 and uptake56) and OEF may be identified now that they can be evaluated in human disease55–58.
The fifth major finding was that we accepted the hypothesis that there is a relationship between retinal thickening and the reduction in MO2 immediately following an interval of nonperfusion. The significant relationships found were linear and inverse between both IRT and TRT and MO2, that is, the more severe the impairment of MO2, the thicker the retina. Retinal thickening has been observed previously both in patients with retinal arterial occlusions41 and in experimental retinal ischemia42,43. It is thought predominately to be the result of cytotoxic edema related to cell volume increases rather than to vasogenic edema related to blood-retinal barrier breakdown59–61. The regulation of cell volume is related to sodium transport, and this has been shown to account for about half of the energy used by the retina62. Since the cellular functions that are most energy-dependent would tend to be impaired preferentially in ischemia, it makes sense that cytotoxic cellular edema and ensuing increased IRT and TRT would be associated with retinal ischemia-induced reduction in MO2. In fact, in the brain, which is similar to the retina, it is known that shortly after ischemia there is marked Na+ influx, K+ efflux and excessive water entry into the intracellular space associated with failure of the Na+/K+ -ATPase pump resulting in cytotoxic edema63,64. Nonetheless, there is a paucity of information about the relationship between retinal thickness and MO2 in retinal ischemia. Accordingly, our findings are of interest because they provide empirical support for the role of nonperfusion-induced reduction in MO2 in the development of retinal thickening in the context of acute nonperfusion. Furthermore, once there is retinal thickening, MO2 can be further impaired (and retinal thickening exacerbated) by increases in the distance for oxygen to diffuse from the vasculature to the cells. The lack of a significant relationship between ORT and MO2 presumably is explained by the minimal contribution of the retinal vasculature to the oxygen supply of the outer retina.
Our results of retinal thickening and reduced MO2 are in agreement with other retinal assessments that were obtained under similar conditions. Histopathology in rats 30 minutes after 90 minutes of ischemia revealed marked edema43, and observations obtained 240 minutes after 60 minutes of ischemia showed edema, cytoplasmic vacuolization and condensed nuclei42. Fluorescein angiography performed on rhesus monkeys shortly after 135 minutes of ischemia showed only mild leakage65. These results are consistent with the predominance of cytotoxic edema with disordered cellular ionic transport secondary to the decreased MO262. Electroretinography performed within several minutes after 105–120 minutes of ischemia in rhesus and squirrel monkeys showed a small amount of improvement shortly after reflow, and improvement still was not complete after more than two additional hours66,67. Tissue obtained after the effects of an interval of ischemia of about two hours duration had had time to develop revealed substantial damage on histopathology16,20,23,67.
This study had limitations. First, as with all animal experiments, the results in rats may not be generalizable to other species. Second, the OVO method induced nonperfusion in both the retinal and choroidal circulations, whereas in clinical retinal vascular occlusions the choroid is not involved. However, OVO does mimic carotid occlusive disease and ophthalmic artery occlusion, and it likely resembles retinal vascular occlusion in some respects. Third, the calculation of PO2 from phosphorescence lifetime used oxyphor constants that were derived from ex vivo experiments, which may be different from those under in vivo conditions. This may affect absolute PO2 values, but not the results of comparing measurements between fellow eyes. Fourth, we did not measure the pH of the blood, which can be reduced in ischemia. Lowering the pH shifts the oxygen hemoglobin dissociation curve to the right and causes SO2 to become lower for a given PO2 value. While our SO2 and O2 concentration values may be somewhat high, it is unlikely that this would have a major impact on the overall relationships among the oxygen-related variables. Fifth, our measurements of MO2 and OEF assumed that the retinal circulation supplied the same tissue with and without nonperfusion. Future studies are needed to measure retinal tissue PO2 in order to document accurately the retinal and choroidal vascular sources of oxygen before, during and after OVO.
In conclusion, immediately after a substantial interval of retinal nonperfusion, the improvements of F and MO2 were partial, but greater than that of DO2, which led to an elevation of OEF. Furthermore, reduction in MO2 was related to thickening of the retina. Since these variables reflect how much energy is available to be utilized by the retina for cellular maintenance and visual processing immediately after an ischemic interval, they may prove to be of great value for clarifying the pathophysiology of nonperfusion retinal injury, predicting tissue outcome and identifying a window of opportunity for clinical intervention to limit retinal damage.
Methods
Animals
The study was performed in 17 Long Evans pigmented rats (314–590 g). All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the study was approved by the Animal Care Committee at the University of Illinois at Chicago. The rats were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (5 mg/kg) and were given additional doses to maintain anesthesia as needed. The femoral artery was cannulated and a catheter was attached. Both pupils were dilated with 2.5% phenylephrine and 1% tropicamide. Ocular nonperfusion was induced using OVO, and the surgical procedure was performed according to Lafuente and co-workers20. Briefly, a longitudinal midline incision over the cranium was performed, and the right orbit was entered subperiosteally to expose the optic nerve. A slit was made in the meningeal sheath, and the exposed optic nerve was gently displaced through the slit. An 8–0 nylon suture was passed between the optic nerve and the meningeal sheath, which contains the ophthalmic vessels68. The suture then was passed the rest of the way around the sheath and tied tightly with a slip knot to ligate the vessels. The rats were placed on an animal holder that incorporated copper tubing perfused with heated water to maintain the body temperature. A glass cover slip with 1% hydroxypropyl-methylcellulose was applied to the corneas to minimize their refractive powers and prevent dehydration for imaging. The retina of the eye with OVO was imaged every 15 minutes to document nonperfusion as evidenced by visualization of extremely narrowed arteries and/or segmented blood columns and a pale optic nerve head. After 120 minutes, the ligature was released. Imaging was performed on both eyes of the rats within 20 minutes of release of the ligature (10 minutes of imaging required per eye). The fellow eye served as a paired control. As described elsewhere40, an oxygen-sensitive molecular probe, Pd-porphine (Frontier Scientific, Logan, UT), was administered through the femoral arterial catheter (20 mg/kg) for PO2 imaging, and 2-µm polystyrene fluorescent microspheres (Invitrogen, Grand Island, NY) were injected through the catheter for retinal blood flow imaging.
Imaging
Retinal blood flow was measured using our previously reported imaging system40. Measurements were made between one half to one optic disc diameter from the edge of the optic disc. Red-free retinal images were analyzed to determine retinal arterial (DAind) and venous (DVind) diameter for each major vessel. Image sequences that displayed motion of fluorescent microspheres were analyzed to provide measurements of blood velocity (Vind) in each major vein. F was calculated by the product of Vind, π and (DVind)2/4, summed over all major veins. For each rat, taking the mean of DAind, DVind, and Vind gave DA, DV, and V, respectively.
Retinal vascular PO2 was measured using our previously reported phosphorescence lifetime system69. Briefly, a modulated laser slit at 532 nm wavelength was projected at an oblique angle on the retina for excitation of the oxyphor. A high pass filter (>650 nm) was placed in the phosphorescence imaging path. An electron multiplied intensified charge-coupled device camera with modulated sensitivity acquired phase-delayed optical section phosphorescence images. Images were analyzed to determine phosphorescence lifetime with a frequency domain approach, and PO2 in each major artery (PO2Aind) and vein (PO2Vind) surrounding the optic nerve was calculated by the Stern-Volmer equation69. A mean PO2A and PO2V was calculated for each eye. O2A and the oxygen concentrations in the veins (O2V) were calculated from PO2A and PO2V respectively using the oxygen-hemoglobin dissociation curve in rats70 and values of hemoglobin measured in previous experiments (13.8 g/dL). DO2 was calculated by F * O2A, and MO2 was calculated by F * (O2A − O2V). OEF was calculated as MO2/DO2.
Retinal thickness was measured in a region nasal to the optic nerve using a commercially available spectral-domain optical coherence tomography instrument (Spectralis, Heidelberg Engineering). The instrument’s commercial software was used to automatically measure IRT from the inner limiting membrane to external limiting membrane, ORT from external limiting membrane to Bruch’s membrane, and TRT from internal limiting membrane to Bruch’s membrane.
Data Analysis
Data were compared between eyes with OVO and fellow eyes using paired t-tests. The relationships of IRT, ORT and TRT with MO2 were determined by mixed linear models with individual rats considered as a random variable. Statistical analyses were performed using SSPS Statistics, Version 24 (IBM Armonk, New York). Significance was accepted at P ≤ 0.05.
Acknowledgements
This study was supported by research grants from the National Eye Institute, Bethesda, MD, EY017918 and EY001792, and Research to Prevent Blindness, New York, NY, Senior Scientific Investigator award (MS) and an unrestricted departmental award. We thank Tara Nguyen for animal care and surgical assistance.
Author Contributions
M.S. and N.P.B. planned the study. N.P.B., M.R.T. and A.E.F. performed the experiments. M.S., N.P.B. and M.R.T. analyzed the data. N.P.B. wrote the main manuscript, but it was also reviewed in detail and revised by M.S. and A.E.F. M.S. and N.P.B. prepared the figures.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bresnick GH, De Venecia G, Myers FL, Harris JA, Davis MD. Retinal ischemia in diabetic retinopathy. Archives of ophthalmology. 1975;93:1300–1310. doi: 10.1001/archopht.1975.01010020934002. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–612. doi: 10.1016/S0161-6420(81)34983-5. [DOI] [PubMed] [Google Scholar]
- 3.Silva PS, et al. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology. 2015;122:2465–2472. doi: 10.1016/j.ophtha.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Stefansson E. Oxygen and diabetic eye disease. Graefe’s archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1990;228:120–123. doi: 10.1007/BF02764304. [DOI] [PubMed] [Google Scholar]
- 5.Do BK, Rodger DC. Sickle cell disease and the eye. Curr Opin Ophthalmol. 2017;28:623–628. doi: 10.1097/ICU.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 6.Marangoni D, et al. Subfoveal choroidal blood flow and central retinal function in early glaucoma. Acta ophthalmologica. 2012;90:e288–294. doi: 10.1111/j.1755-3768.2011.02340.x. [DOI] [PubMed] [Google Scholar]
- 7.Nicolela MT, Hnik P, Drance SM. Scanning laser Doppler flowmeter study of retinal and optic disk blood flow in glaucomatous patients. American journal of ophthalmology. 1996;122:775–783. doi: 10.1016/S0002-9394(14)70373-3. [DOI] [PubMed] [Google Scholar]
- 8.Hafez AS, Bizzarro RL, Lesk MR. Evaluation of optic nerve head and peripapillary retinal blood flow in glaucoma patients, ocular hypertensives, and normal subjects. American journal of ophthalmology. 2003;136:1022–1031. doi: 10.1016/S0002-9394(03)00632-9. [DOI] [PubMed] [Google Scholar]
- 9.Michelson G, Langhans MJ, Harazny J, Dichtl A. Visual field defect and perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open-angle glaucoma. Graefe’s archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1998;236:80–85. doi: 10.1007/s004170050046. [DOI] [PubMed] [Google Scholar]
- 10.Hafez AS, Bizzarro RL, Rivard M, Lesk MR. Changes in optic nerve head blood flow after therapeutic intraocular pressure reduction in glaucoma patients and ocular hypertensives. Ophthalmology. 2003;110:201–210. doi: 10.1016/S0161-6420(02)01716-5. [DOI] [PubMed] [Google Scholar]
- 11.Ames A, 3rd, Maynard KI, Kaplan S. Protection against CNS ischemia by temporary interruption of function-related processes of neurons. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1995;15:433–439. doi: 10.1038/jcbfm.1995.54. [DOI] [PubMed] [Google Scholar]
- 12.Braun RD, Linsenmeier RA, Goldstick TK. Oxygen consumption in the inner and outer retina of the cat. Investigative ophthalmology & visual science. 1995;36:542–554. [PubMed] [Google Scholar]
- 13.Ames, A., 3rd. In Biochemistry of the Retina. (ed C.N. Graymore) 22 (Academic Press, Inc 1965).
- 14.Lelong DC, et al. Novel mouse model of monocular amaurosis fugax. Stroke. 2007;38:3237–3244. doi: 10.1161/STROKEAHA.107.499319. [DOI] [PubMed] [Google Scholar]
- 15.Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M. Retinal ischemia induced by the intraluminal suture method in rats. Neurosci Lett. 1997;232:45–48. doi: 10.1016/S0304-3940(97)00575-2. [DOI] [PubMed] [Google Scholar]
- 16.Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1991;203:138–147. doi: 10.1159/000310240. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Lam TT, Edward DP, Buchi ER, Tso MO. Protective effects of flunarizine on ischemic injury in the rat retina. Archives of ophthalmology. 1992;110:862–870. doi: 10.1001/archopht.1992.01080180134041. [DOI] [PubMed] [Google Scholar]
- 18.Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87:75–78. doi: 10.1016/S0161-6420(80)35283-4. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Nun J, Alder VA, Cringle SJ, Constable IJ. A new method for oxygen supply to acute ischemic retina. Investigative ophthalmology & visual science. 1988;29:298–304. [PubMed] [Google Scholar]
- 20.Lafuente MP, et al. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience. 2002;109:157–168. doi: 10.1016/S0306-4522(01)00458-4. [DOI] [PubMed] [Google Scholar]
- 21.Blair NP, Felder AE, Tan MR, Shahidi M. A Model for Graded Retinal Ischemia in Rats. Transl Vis Sci Technol. 2018;7:10. doi: 10.1167/tvst.7.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair NP, et al. Limitation of retinal injury by vitreoperfusion initiated after onset of ischemia. Archives of ophthalmology. 1991;109:113–118. doi: 10.1001/archopht.1991.01080010115042. [DOI] [PubMed] [Google Scholar]
- 23.Hughes WF. Quantitation of ischemic damage in the rat retina. Experimental eye research. 1991;53:573–582. doi: 10.1016/0014-4835(91)90215-Z. [DOI] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nature medicine. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1:185–199. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostwald P, Goldstein IM, Pachnanda A, Roth S. Effect of nitric oxide synthase inhibition on blood flow after retinal ischemia in cats. Investigative ophthalmology & visual science. 1995;36:2396–2403. [PubMed] [Google Scholar]
- 27.Li G, Kiel JW, Cardenas DP, De La Garza BH, Duong TQ. Postocclusive reactive hyperemia occurs in the rat retinal circulation but not in the choroid. Investigative ophthalmology & visual science. 2013;54:5123–5131. doi: 10.1167/iovs.13-12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostwald P, Park SS, Toledano AY, Roth S. Adenosine receptor blockade and nitric oxide synthase inhibition in the retina: impact upon post-ischemic hyperemia and the electroretinogram. Vision Res. 1997;37:3453–3461. doi: 10.1016/S0042-6989(96)00222-2. [DOI] [PubMed] [Google Scholar]
- 29.Roth S. Post-ischemic hyperemia in the cat retina: the effects of adenosine receptor blockade. Current eye research. 1995;14:323–328. doi: 10.3109/02713689509033533. [DOI] [PubMed] [Google Scholar]
- 30.Hatchell DL, Wilson CA, Saloupis P. Neutrophils plug capillaries in acute experimental retinal ischemia. Microvascular research. 1994;47:344–354. doi: 10.1006/mvre.1994.1027. [DOI] [PubMed] [Google Scholar]
- 31.Tsujikawa A, Ogura Y. Evaluation of leukocyte-endothelial interactions in retinal diseases. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2012;227:68–79. doi: 10.1159/000332080. [DOI] [PubMed] [Google Scholar]
- 32.Ames A, 3rd, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. The American journal of pathology. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- 33.Heye N, Paetzold C, Cervos-Navarro J. The role of microthrombi and microcirculatory factors in localization and evolution of focal cerebral ischemia. Neurosurg Rev. 1991;14:7–16. doi: 10.1007/BF00338186. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Connor J, Peterson S, Shuttleworth CW, Liu KJ. Direct visualization of trapped erythrocytes in rat brain after focal ischemia and reperfusion. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:1222–1230. doi: 10.1097/01.wcb.0000037998.34930.83. [DOI] [PubMed] [Google Scholar]
- 35.Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.STR.23.5.712. [DOI] [PubMed] [Google Scholar]
- 36.Blair NP, Baker DS, Rhode JP, Solomon M. Vitreoperfusion. A new approach to ocular ischemia. Archives of ophthalmology. 1989;107:417–423. doi: 10.1001/archopht.1989.01070010427039. [DOI] [PubMed] [Google Scholar]
- 37.MacDougall G, Anderton RS, Mastaglia FL, Knuckey NW, Meloni BP. Mitochondria and neuroprotection in stroke: Cationic arginine-rich peptides (CARPs) as a novel class of mitochondria-targeted neuroprotective therapeutics. Neurobiol Dis. 2018;121:17–33. doi: 10.1016/j.nbd.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochimica et biophysica acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanek J, Teng PY, Albers J, Blair NP, Shahidi M. Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomedical optics express. 2011;2:2562–2568. doi: 10.1364/BOE.2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritter M, et al. In vivo identification of alteration of inner neurosensory layers in branch retinal artery occlusion. The British journal of ophthalmology. 2012;96:201–207. doi: 10.1136/bjo.2010.198937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam TT, Abler AS, Tso MO. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Investigative ophthalmology & visual science. 1999;40:967–975. [PubMed] [Google Scholar]
- 43.Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion-induced histologic changes in the rat retina. Demonstration of a free radical-mediated mechanism. Investigative ophthalmology & visual science. 1991;32:1471–1478. [PubMed] [Google Scholar]
- 44.Blair NP, Wanek J, Teng PY, Shahidi M. The effect of intravitreal vascular endothelial growth factor on inner retinal oxygen delivery and metabolism in rats. Experimental eye research. 2016;143:141–147. doi: 10.1016/j.exer.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yemisci M, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nature medicine. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 46.Grubb RL, Jr., et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi H, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996;61:18–25. doi: 10.1136/jnnp.61.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamauchi H, et al. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive diseases. J Nucl Med. 1999;40:1992–1998. [PubMed] [Google Scholar]
- 49.Teng PY, Wanek J, Blair NP, Shahidi M. Inner retinal oxygen extraction fraction in rat. Investigative ophthalmology & visual science. 2013;54:647–651. doi: 10.1167/iovs.12-11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair NP, et al. Inner Retinal Oxygen Delivery, Metabolism, and Extraction Fraction in Ins2Akita Diabetic Mice. Investigative ophthalmology & visual science. 2016;57:5903–5909. doi: 10.1167/iovs.16-20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An H, et al. Defining the ischemic penumbra using magnetic resonance oxygen metabolic index. Stroke. 2015;46:982–988. doi: 10.1161/STROKEAHA.114.008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin W, Powers WJ. Oxygen metabolism in acute ischemic stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2018;38:1481–1499. doi: 10.1177/0271678X17722095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powers WJ, Grubb RL, Jr., Darriet D, Raichle ME. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1985;5:600–608. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- 54.Touzani O, Young AR, Derlon JM, Baron JC, MacKenzie ET. Progressive impairment of brain oxidative metabolism reversed by reperfusion following middle cerebral artery occlusion in anaesthetized baboons. Brain Res. 1997;767:17–25. doi: 10.1016/S0006-8993(97)00515-5. [DOI] [PubMed] [Google Scholar]
- 55.Palkovits S, et al. Regulation of retinal oxygen metabolism in humans during graded hypoxia. American journal of physiology. Heart and circulatory physiology. 2014;307:H1412–1418. doi: 10.1152/ajpheart.00479.2014. [DOI] [PubMed] [Google Scholar]
- 56.Tayyari F, et al. Retinal Blood Flow and Retinal Blood Oxygen Saturation in Mild to Moderate Diabetic Retinopathy. Investigative ophthalmology & visual science. 2015;56:6796–6800. doi: 10.1167/iovs.15-17481. [DOI] [PubMed] [Google Scholar]
- 57.Shahidi M, Felder AE, Tan O, Blair NP, Huang D. Retinal Oxygen Delivery and Metabolism in Healthy and Sickle Cell Retinopathy Subjects. Investigative ophthalmology & visual science. 2018;59:1905–1909. doi: 10.1167/iovs.17-23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felder AE, Wanek J, Blair NP, Shahidi M. Inner Retinal Oxygen Extraction Fraction in Response to Light Flicker Stimulation in Humans. Investigative ophthalmology & visual science. 2015;56:6633–6637. doi: 10.1167/iovs.15-17321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroll AJ. Experimental central retinal artery occlusion. Archives of ophthalmology. 1968;79:453–469. doi: 10.1001/archopht.1968.03850040455014. [DOI] [PubMed] [Google Scholar]
- 60.Bringmann A, et al. Neuronal versus glial cell swelling in the ischaemic retina. Acta ophthalmologica Scandinavica. 2005;83:528–538. doi: 10.1111/j.1600-0420.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 61.Stefansson E, et al. Quantitative measurements of retinal edema by specific gravity determinations. Investigative ophthalmology & visual science. 1987;28:1281–1289. [PubMed] [Google Scholar]
- 62.Ames A, 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na + transport. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu HJ, Song M. Disrupted Ionic Homeostasis in Ischemic Stroke and New Therapeutic Targets. J Stroke Cerebrovasc Dis. 2017;26:2706–2719. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Song M, Yu SP. Ionic regulation of cell volume changes and cell death after ischemic stroke. Transl Stroke Res. 2014;5:17–27. doi: 10.1007/s12975-013-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. I. Ophthalmoscopic and fluorescein fundus angiographic studies. The British journal of ophthalmology. 1980;64:896–912. doi: 10.1136/bjo.64.12.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamasaki DI, Kroll AJ. Experimental central retinal artery occlusion. An electrophysiological study. Archives of ophthalmology. 1968;80:243–248. doi: 10.1001/archopht.1968.00980050245019. [DOI] [PubMed] [Google Scholar]
- 67.Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Experimental eye research. 2004;78:723–736. doi: 10.1016/S0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 68.Sugiyama K, Gu ZB, Kawase C, Yamamoto T, Kitazawa Y. Optic nerve and peripapillary choroidal microvasculature of the rat eye. Investigative ophthalmology & visual science. 1999;40:3084–3090. [PubMed] [Google Scholar]
- 69.Shahidi M, Wanek J, Blair NP, Mori M. Three-dimensional mapping of chorioretinal vascular oxygen tension in the rat. Investigative ophthalmology & visual science. 2009;50:820–825. doi: 10.1167/iovs.08-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cartheuser CF. Standard and pH-affected hemoglobin-O2 binding curves of Sprague-Dawley rats under normal and shifted P50 conditions. Comparative biochemistry and physiology. Comparative physiology. 1993;106:775–782. doi: 10.1016/0300-9629(93)90396-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


